Perovskite Thin Film Consisting with One-Dimensional Nanowires
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
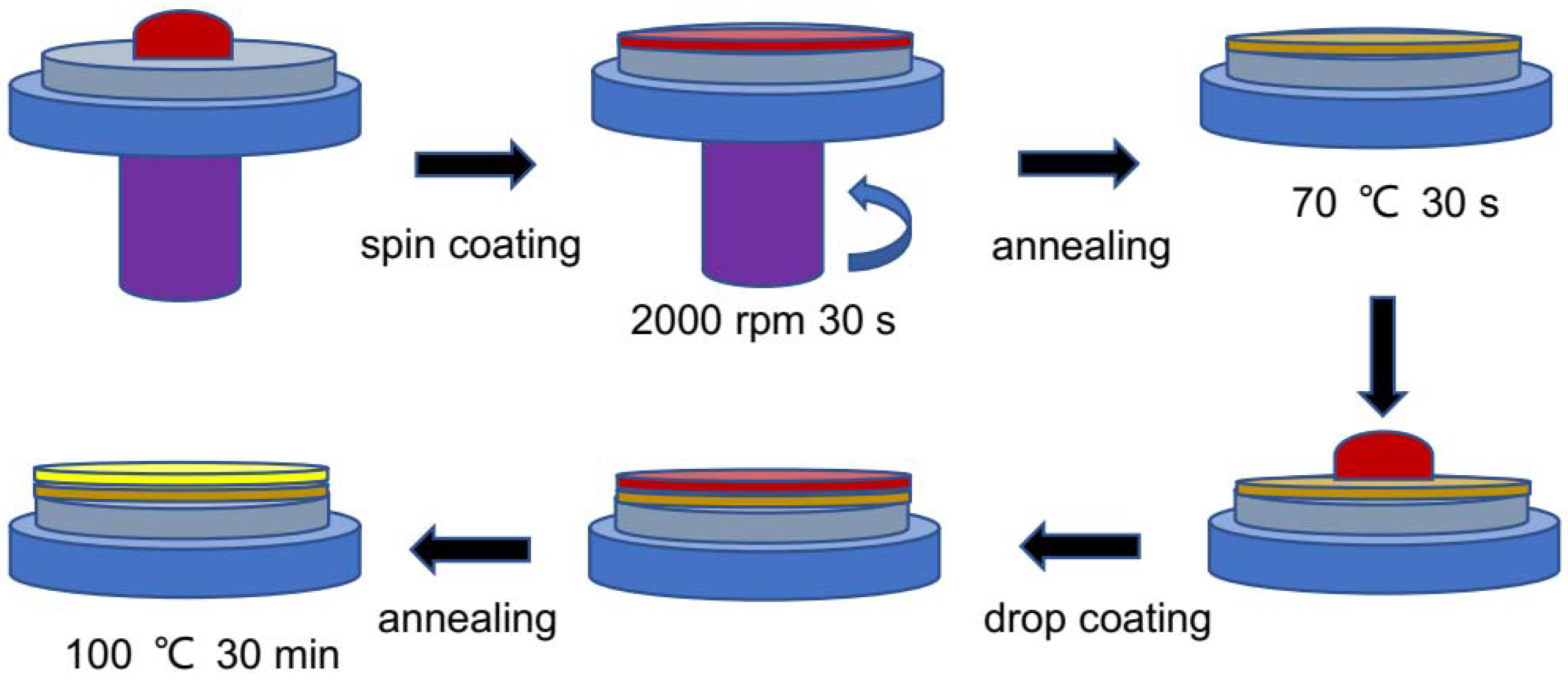
2.2. Perovskite Thin Films and Solar Cells Fabrication
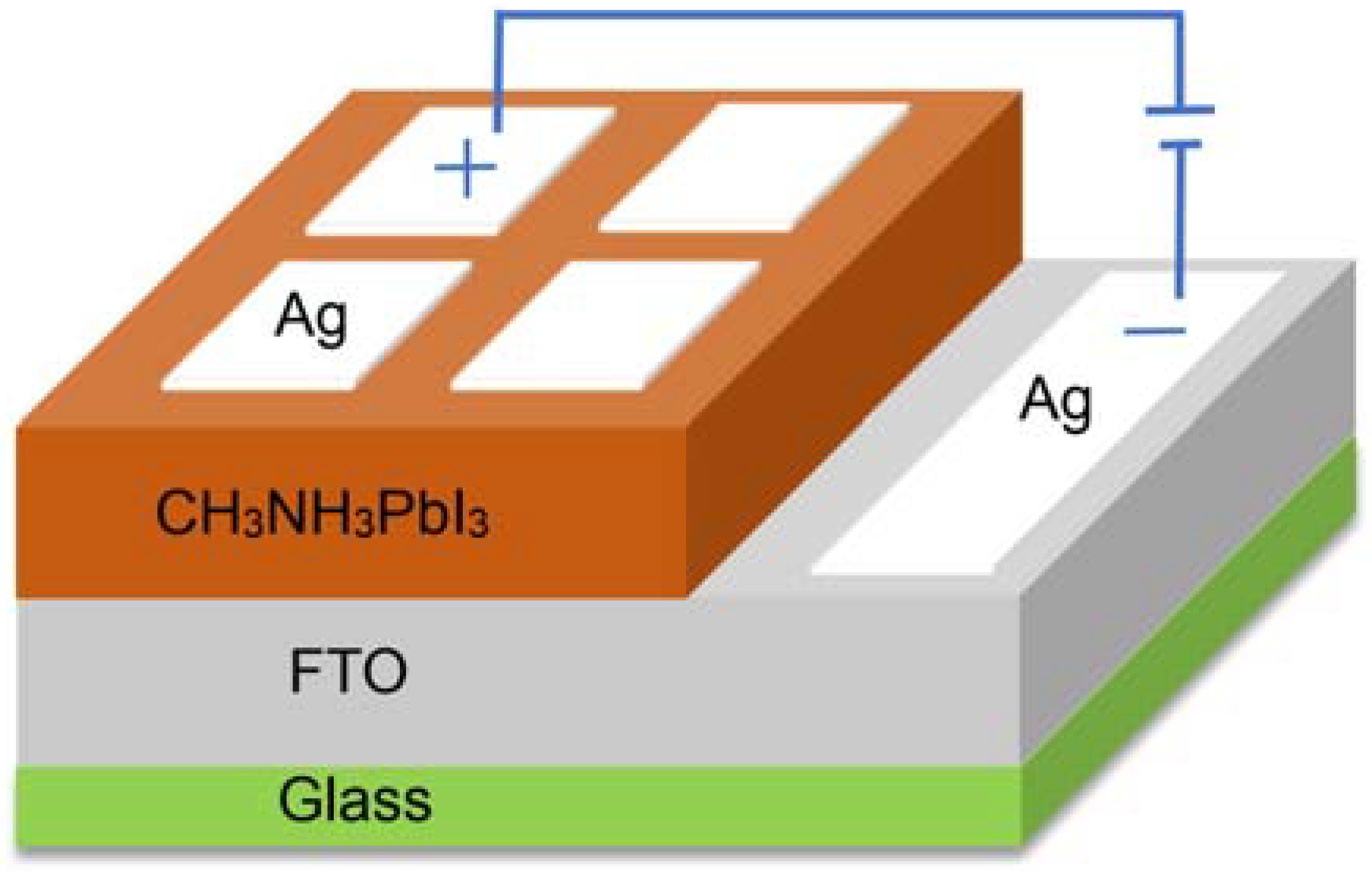
2.3. Material and Device Characterization
3. Results and Discussion
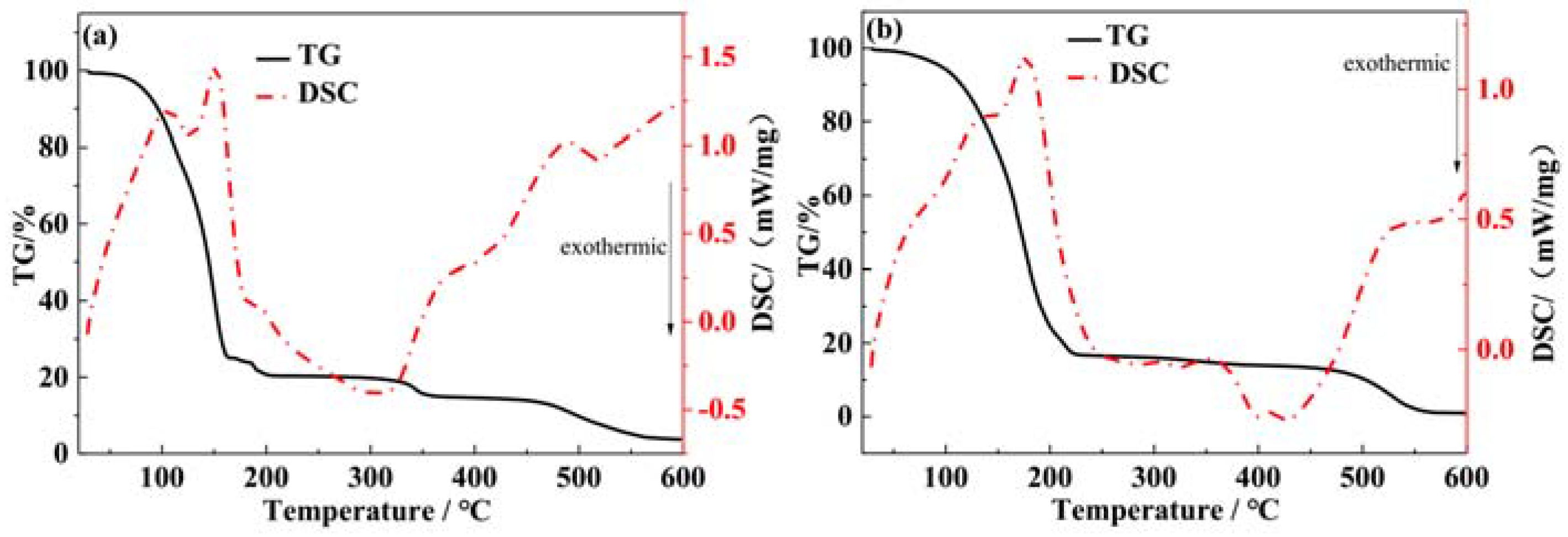
3.1. TG-DSC Analysis
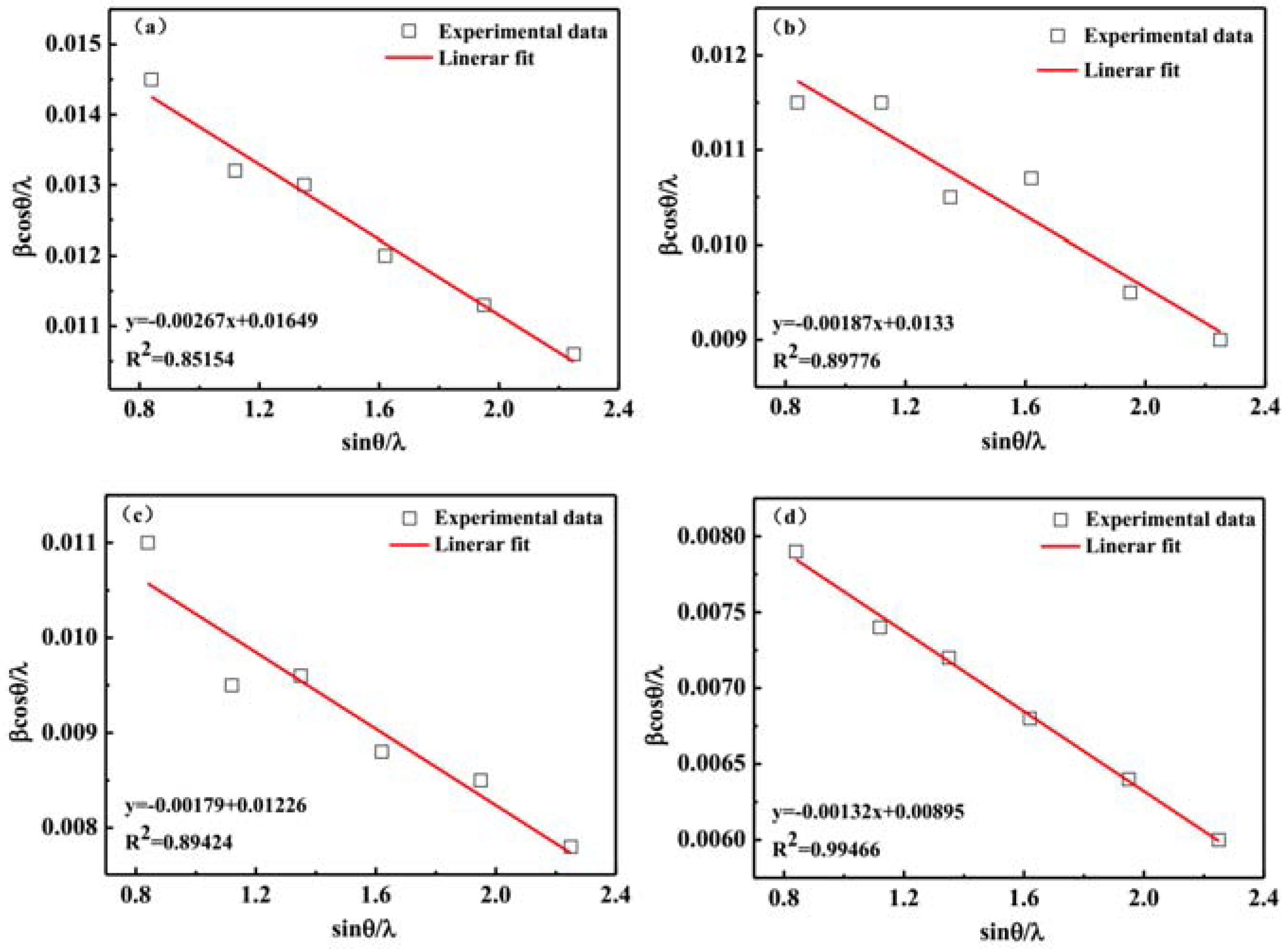
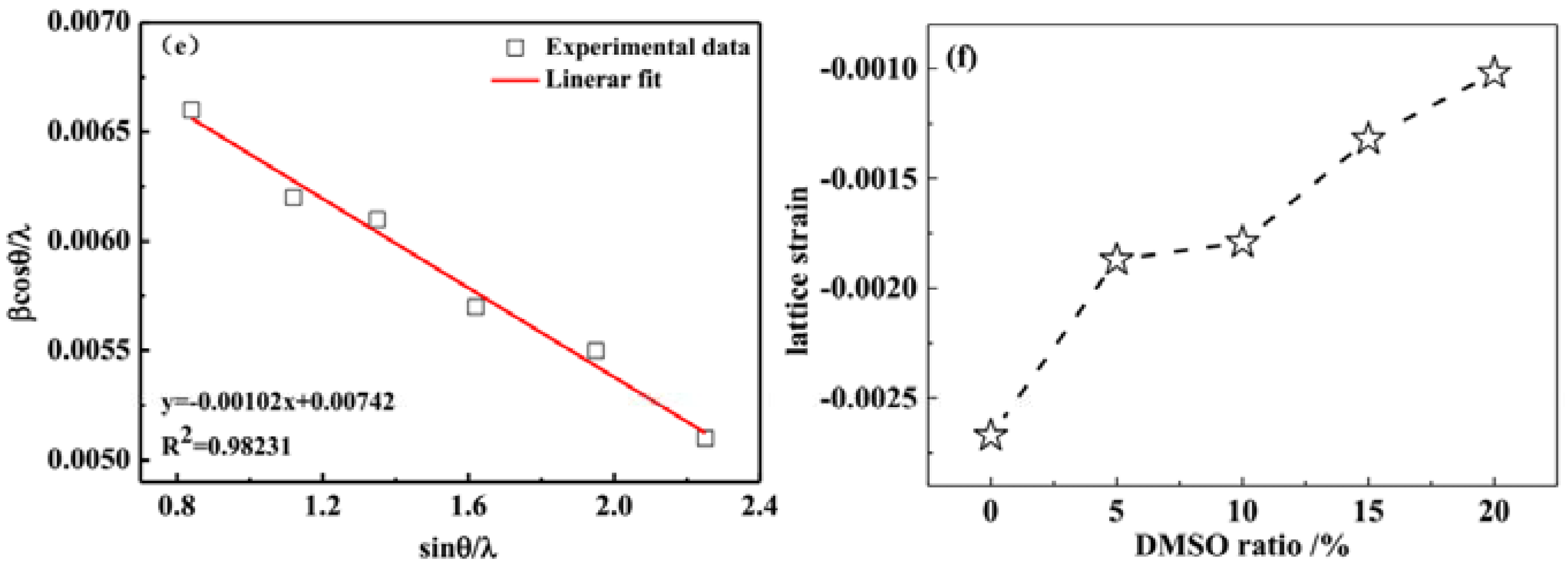
3.2. X-ray Diffraction Analysis
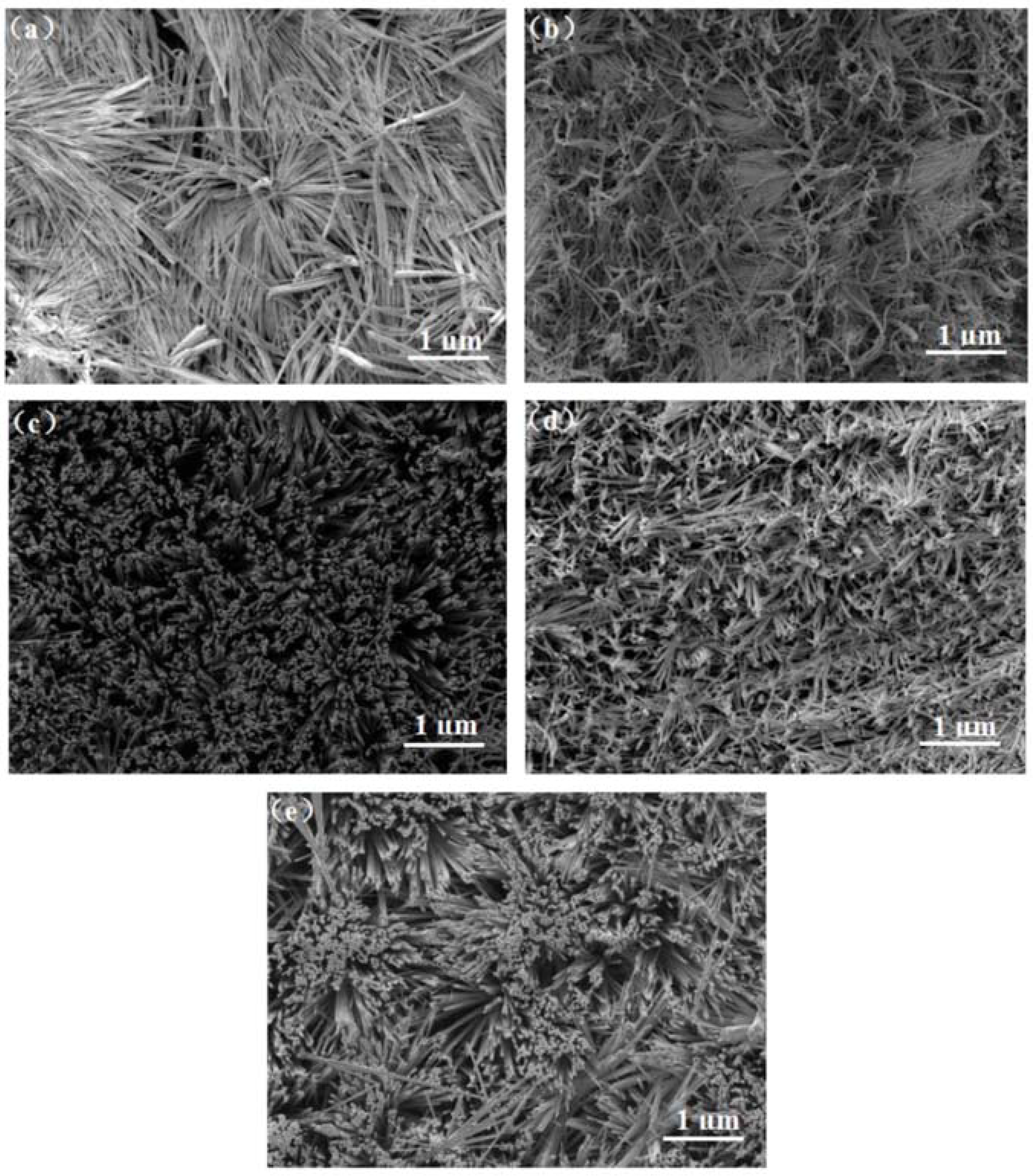
3.3. Scanning Electron Microscopy Analysis
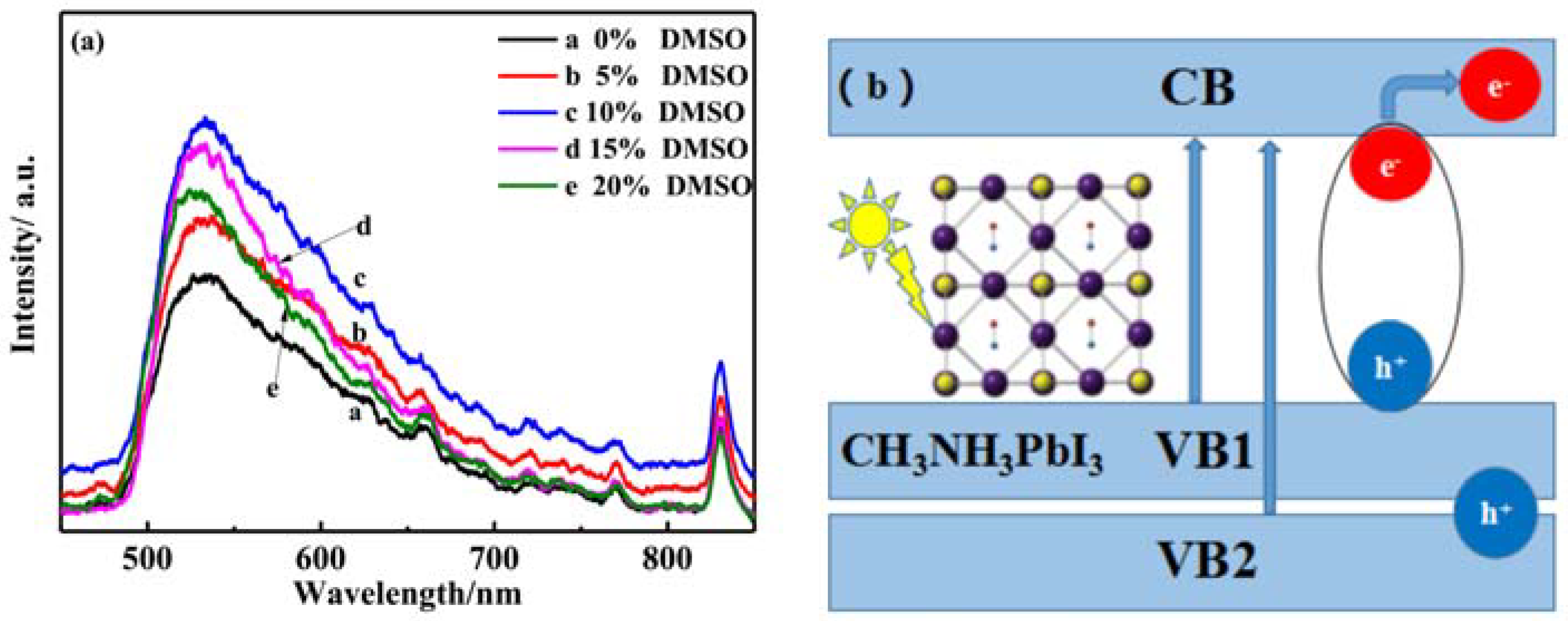
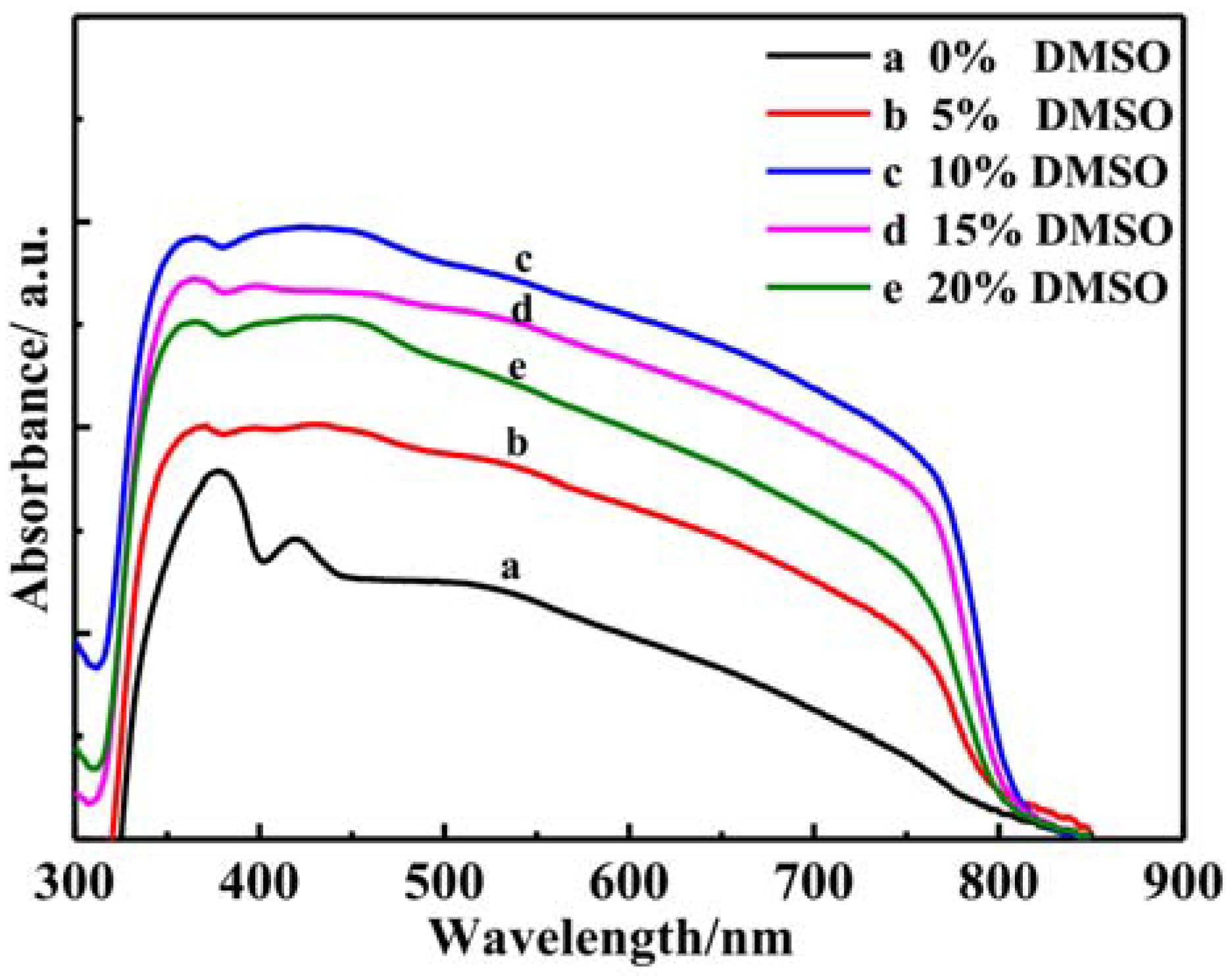
3.4. Optical Performannce Analysis
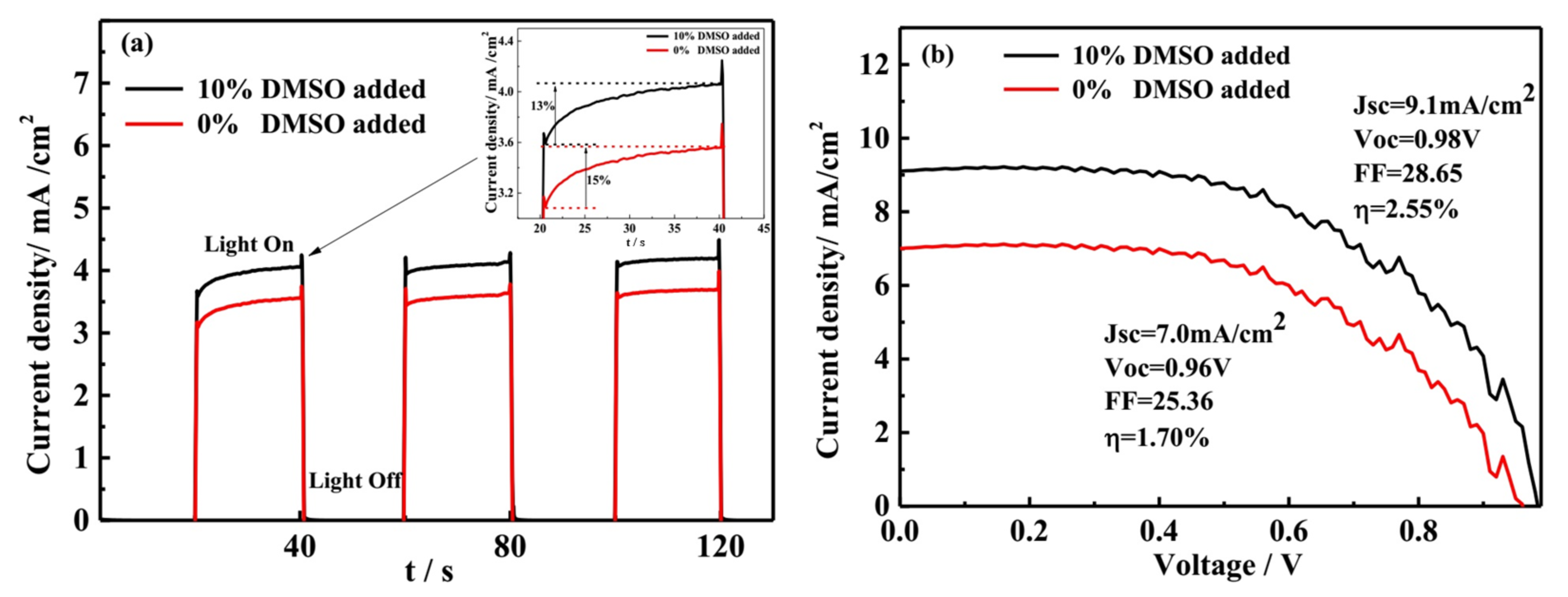
3.5. The Transient Photocurrent and J-V Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, Z.B.; Rajagopal, A.; Jen, A.K.Y. Ideal bandgap organic-inorganic hybrid perovskite solar cells. Adv. Mater. 2017, 29, 1704418. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.P.; Yu, J.G.; Fan, K.; Zou, H.Y.; He, B.W. Nanosheet-based printable perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 159, 518–525. [Google Scholar] [CrossRef]
- Park, N.G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Chart of Best Research-Cell Efficiencies Provided by NREL. Available online: https://www.nrel.gov/pv/assets/images/efficiency-chart-20180716.jpg (accessed on 17 September 2018).
- Dubey, A.; Kantack, N.; Adhikari, N.; Reza, K.M.; Venkatesan, S.; Kumar, M.; Khatiwada, D.; Darling, S.; Qiao, Q.Q. Room temperature air crystallized perovskite film for high performance solar cells. J. Mater. Chem. A 2016, 4, 10231–10240. [Google Scholar] [CrossRef]
- Wang, B.B.; Zhang, Z.G.; Ye, S.Y.; Rao, H.X.; Bian, Z.Q.; Huang, C.H.; Li, Y.F. Room-temperature water-vapor annealing for high performance planar perovskite solar cells. J. Mater. Chem. A 2016, 4, 17267–17273. [Google Scholar] [CrossRef]
- Fang, X.; Wu, Y.H.; Lu, Y.T.; Sun, Y.; Zhang, S.; Zhang, J.; Zhang, W.H.; Yuan, N.Y.; Ding, J.N. Annealing-free perovskite films with solvent engineering for efficient solar cells. J. Mater. Chem. C 2017, 5, 842–847. [Google Scholar] [CrossRef]
- Noel, N.K.; Severin, N.; Wenger, H.B.; Klug, M.T.; Horantner, M.T.; Johnston, M.B.; Nicholas, R.J.; Moore, D.T.; Snaith, H.J. A low viscosity, low boiling point, clean solvent system for the rapid crystallisation of highly specular perovskite films. Energy Environ. Sci. 2017, 10, 145–152. [Google Scholar] [CrossRef]
- Yin, M.S.; Xie, F.X.; Chen, H.; Yang, X.D.; Ye, F.; Bi, E.B.; Wu, Y.Z.; Cai, M.L.; Han, L.Y. Annealing-free perovskite films by instant crystallization for efficient solar cells. J. Mater. Chem. A 2016, 4, 8548–8553. [Google Scholar] [CrossRef]
- Zhou, Z.M.; Wang, Z.W.; Zhou, Y.Y.; Pang, S.P.; Wang, D.; Xu, H.X.; Liu, Z.H.; Padture, N.P.; Cui, G.L. Methylamine-gas induced defect-healing behavior of CH3NH3PbI3 thin films for perovskite solar cells. Angew. Chem. Int. Ed. 2015, 54, 9705–9709. [Google Scholar] [CrossRef] [PubMed]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Masuda, T.; Miyano, K. Enhancement in efficiency and optoelectronic quality of perovskite thin films annealed in MACl vapor. Sustain. Energy Fuels 2017, 1, 755–766. [Google Scholar] [CrossRef]
- Han, Q.; Bai, Y.; Liu, J.; Du, K.; Li, T.; Ji, D.; Zhou, Y.; Can, C.; Shin, D.; Ding, J.; et al. Additive engineering for high-performance room-temperature processed perovskite absorbers with micron-size grains and microsecond-range carrier lifetimes. Energy Environ. Sci. 2017, 10, 2365–2371. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, Q.; Xia, Y.; Yu, H.; Zhang, K.; Liu, X.; Guo, X.; Zhou, Y.; Zhang, M.; Song, B. Copolymers based on thiazolothiazole-dithienosilole as hole transporting materials for high efficient perovskite solar cells. Org. Electron. 2016, 33, 142–149. [Google Scholar] [CrossRef]
- Xiao, M.D.; Huang, F.Z.; Huang, W.C.; Dkhissi, Y.; Zhu, Y.; Etheridge, J.; Gray-Weale, A.; Bach, U.; Cheng, Y.; Spiccia, L. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew. Chem. Int. Ed. 2015, 53, 9898–9903. [Google Scholar] [CrossRef] [PubMed]
- Ahn, N.; Son, D.Y.; Jang, I.H.; Kang, S.M.; Choi, M.; Park, N.G. Highly reproducible perovskite solar cells with average efficiency of 18.3% and best efficiency of 19.7% fabricated via lewis base adduct of lead(II) iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Xue, Y.Z.; Wang, Z.Y.; Xu, Z.Q.; Zheng, C.X.; Weber, B.; Song, J.C.; Wang, Y.S.; Lu, Y.R.; Zhang, Y.P.; et al. Two-dimensional CH3NH3PbI3 perovskite: Synthesis and optoelectronic application. ACS Nano 2016, 10, 3536–3542. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J. Continuous-time random-walk model of electron transport in nanocrystalline TiO2 electrodes. Phys. Rev. B 1999, 59, 15374–15380. [Google Scholar] [CrossRef]
- Niu, W.; Eiden, A.; Prakash, G.V.; Baumberg, J.J. Exfoliation of self-assembled 2D organic-inorganic perovskite semiconductors. Appl. Phys. Lett. 2014, 104, 171111. [Google Scholar]
- Im, J.H.; Luo, J.; Franckevičius, M.; Pellet, N.; Gao, P.; Moehl, T.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Grätzel, M.; Park, N.G. Nanowire perovskite solar cell. Nano Lett. 2015, 15, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hao, H.; Dong, J.; Cheng, F.; Hao, J.; Xing, J.; Liu, H. Fast extraction of electron across the interface of nanowire CH3NH3PbI3/ZnO on flexible PET substrate. Mater. Lett. 2017, 197, 139–142. [Google Scholar] [CrossRef]
- Xing, J.; Liu, X.F.; Zhang, Q.; Ha, S.T.; Yuan, Y.W.; Shen, C.; Sum, T.C.; Xiong, Q. Vapor phase synthesis of organometal halide perovskite nanowires for tunable room-temperature nanolasers. Nano Lett. 2015, 15, 4571–4577. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fu, Y.; Meng, F.; Wu, X.; Gong, Z.; Ding, Q.; Gustafsson, M.V.; Tuan Trinh, M.; Zhu, X.Y. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 2015, 14, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Eaton, S.W.; Yu, Y.; Dou, L.; Yang, P. Solution-phase synthesis of cesium lead halide perovskite nanowires. J. Am. Chem. Soc. 2015, 137, 9230–9233. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Dong, D.; Qiao, K.; Bu, L.; Li, B.; Yang, D.; Wang, H.E.; Cheng, Y.; Zhao, Z.; Tang, J. Growth, patterning and alignment of organolead iodide perovskite nanosires for optoelectronic devices. Nanoscale 2015, 7, 4163–4170. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Yan, S.; Wang, M.; Chang, L.; Wang, J.L.; Wang, Z. Construction of nanowire CH3NH3PbI3-based solar cells with 17.62% efficiency by solvent etching technique. Sol. Energy Mater. Sol. Cells 2017, 167, 173–177. [Google Scholar] [CrossRef]
- Liang, P.W.; Liao, C.Y.; Chueh, C.C.; Zuo, F.; Williams, S.T.; Xin, X.K.; Lin, J.; Jen, A.K.Y. Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells. Adv. Mater. 2014, 26, 3748–3754. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Lee, S.; Kang, R.; Kim, J.E.; Yeo, J.S.; Lee, S.H.; Kim, S.S.; Yun, J.M.; Kim, D.Y. Planar heterojunction perovskite solar cells with superior reproducibility. Sci. Rep. 2014, 4, 6953. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Gu, S.; Shen, X.; Xu, N.; Tan, Y.; Zhuang, S.; Deng, Y.; Lu, Z.; Wang, Z.; Zhu, J. Direct conversion of perovskite thin films into nanowires with kinetic control for flexible optoelectronic devices. Nano Lett. 2016, 16, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Men, L.; Guo, Y.; Zhu, Q.; Bhattacharjee, U.; Goodwin, P.M.; Petrich, J.W.; Smith, E.A.; Vela, J. Shape evolution and single particle luminescence of organometal halide perovskite nanocrystals. ACS Nano 2015, 9, 2948–2959. [Google Scholar] [CrossRef] [PubMed]
- Horváth, E.; Spina, M.; Szekrényes, Z.; Kamarás, K.; Gaal, R.; Gachet, D.; Forró, L. Nanowires of methylammonium lead iodide (CH3NH3PbI3) prepared by low temperature solution-mediated crystallization. Nano Lett. 2014, 14, 6761–6766. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Li, L.H.; Ma, Z.H.; Huang, J.L.; Ren, F.Z. Low-cost synthesis, fluorescent properties, growth mechanism and structure of CH3NH3PbI3 with millimeter grains. Opt.-Int. J. Light Electron Opt. 2017, 142, 293–300. [Google Scholar] [CrossRef]
- Li, X.L.; Li, L.H.; Ma, Z.H.; Lu, J.X.; Volinsky, A.A.; Ren, F.Z. Boron doping effects on microcrystalline silicon film roughness studied by spectroscopic ellipsmetry. J. Alloys Compd. 2016, 684, 582–586. [Google Scholar] [CrossRef]
- Dai, S.M.; Tian, H.R.; Zhang, M.L.; Xing, Z.; Wang, L.Y.; Wang, X.; Wang, T.; Deng, L.L.; Xie, S.Y.; Huang, R.B.; et al. Pristine fullerenes mixed by vacuum-free solution process: Efficient electron transport layer for planar perovskite solar cells. J. Power Sources 2017, 339, 27–32. [Google Scholar] [CrossRef]
- Runa, A.; Feng, S.; Wen, G.; Feng, F.; Wang, J.; Liu, L.; Su, P.Y.; Yang, H.B.; Fu, W.Y. Highly reproducible perovskite solar cells based on solution coating from mixed solvents. J. Mater. Sci. 2018, 53, 3590–3602. [Google Scholar] [CrossRef]
- Thomas, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar]
- Yeo, J.S.; Kang, R.; Lee, S.; Jeon, Y.J.; Myoung, N.S.; Lee, C.L.; Kim, D.Y.; Yun, J.M.; Seo, Y.H.; Kim, S.K.; et al. Highly efficient and stable planar perovskite solar cells with reduced graphene oxide nanosheets as electrode interlayer. Nano Energy 2015, 12, 96–104. [Google Scholar] [CrossRef]
- Cao, J.; Jing, X.; Yan, J.; Hu, C.; Chen, R.; Yin, J.; Li, J.; Zheng, N. Identifying the molecular structures of intermediates for optimizing the fabrication of high-quality perovskite films. J. Am. Chem. Soc. 2016, 138, 9919–9926. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Xu, X.; Zhao, Y.H.; Shi, J.J.; Xu, Y.Z.; Luo, Y.H.; Li, D.M.; Wu, H.J.; Meng, Q.B. DMF as an additive in two-step spin coating method for 20% conversion efficiency perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 26937–26947. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.S.; Hao, Y.C.; Dong, Q.F. Organometal trihalide perovskite single crystals: A next wave of materials for 25% efficiency photovoltaics and applications beyond? J. Phys. Chem. Lett. 2015, 6, 3218–3277. [Google Scholar] [CrossRef]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Ryan, J.W.; Miyano, K. Exploring the effects of interfacial carrier transport layers on device performance and optoelectronic properties of planar perovskite solar cells. J. Mater. Chem. C 2017, 5, 8819–8827. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electron- and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, Y.; Li, L.; Huang, J. Perovskite Thin Film Consisting with One-Dimensional Nanowires. Materials 2018, 11, 1759. https://doi.org/10.3390/ma11091759
Li X, Chen Y, Li L, Huang J. Perovskite Thin Film Consisting with One-Dimensional Nanowires. Materials. 2018; 11(9):1759. https://doi.org/10.3390/ma11091759
Chicago/Turabian StyleLi, Xinli, Yongchao Chen, Lihua Li, and Jinliang Huang. 2018. "Perovskite Thin Film Consisting with One-Dimensional Nanowires" Materials 11, no. 9: 1759. https://doi.org/10.3390/ma11091759
APA StyleLi, X., Chen, Y., Li, L., & Huang, J. (2018). Perovskite Thin Film Consisting with One-Dimensional Nanowires. Materials, 11(9), 1759. https://doi.org/10.3390/ma11091759




