Developing a New Generation of Therapeutic Dental Polymers to Inhibit Oral Biofilms and Protect Teeth
Abstract
1. Introduction
2. Antibacterial Polymeric Dental Composites
3. Antibacterial Dental Bonding Agents
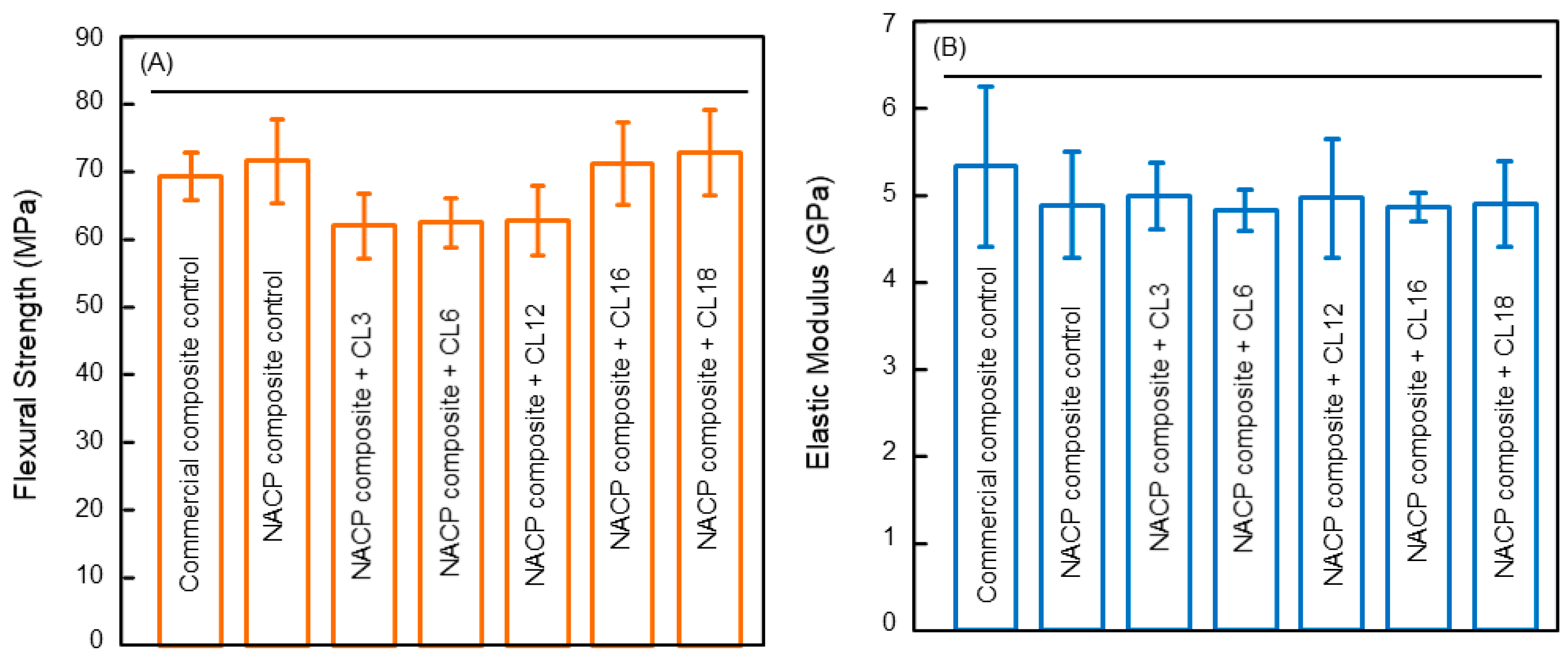
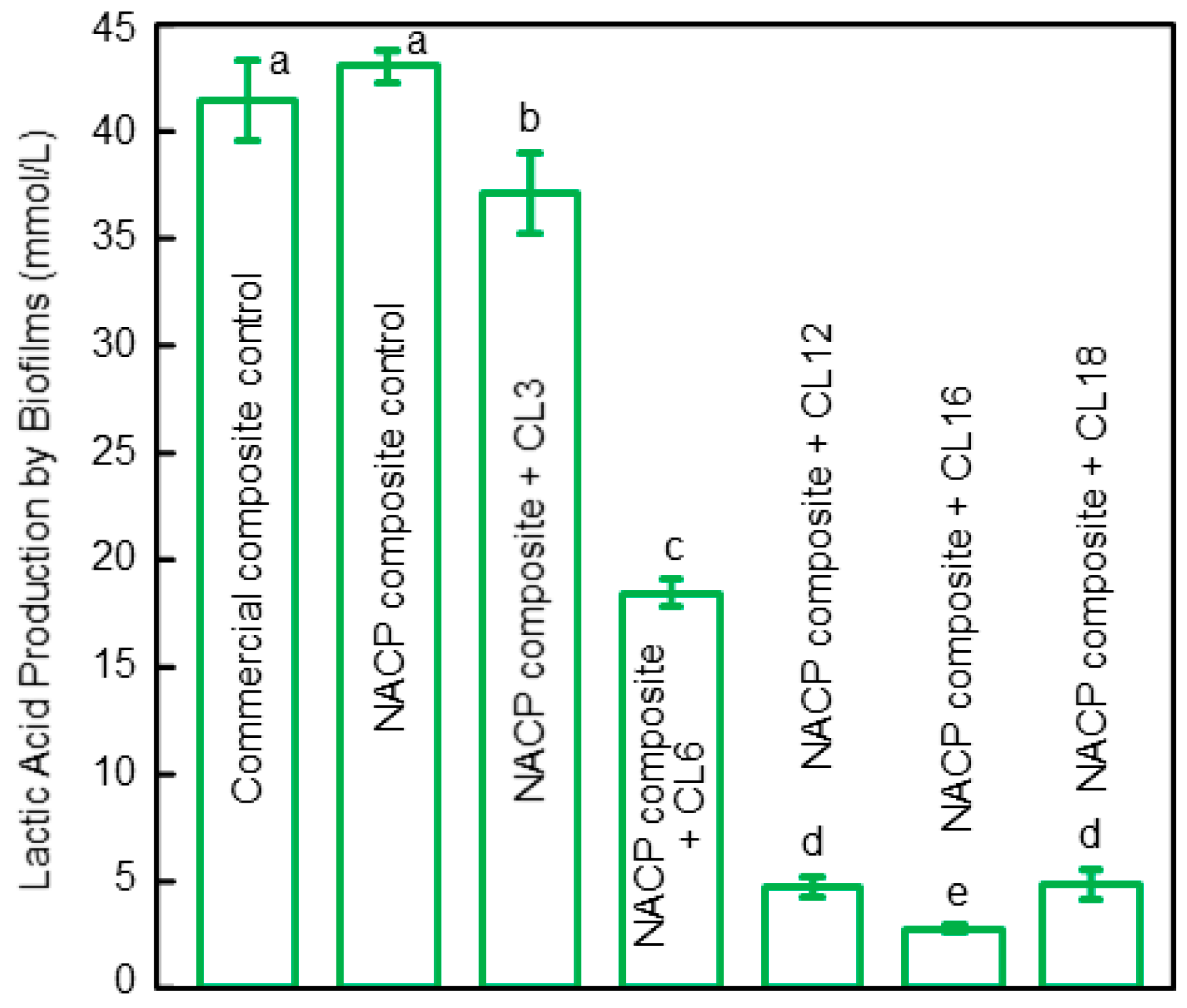
4. Antibacterial Composite for Tooth Root Caries Treatments
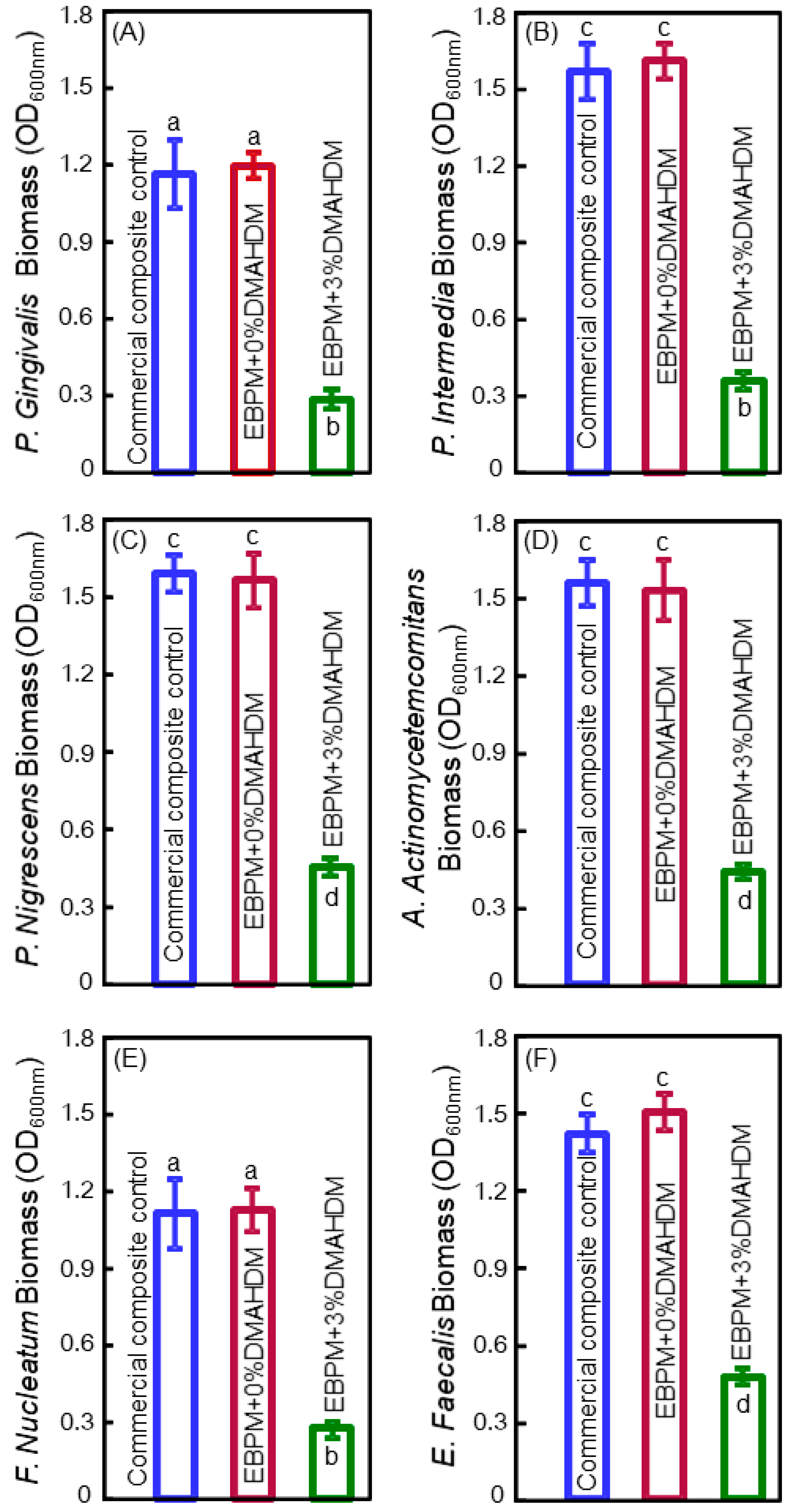
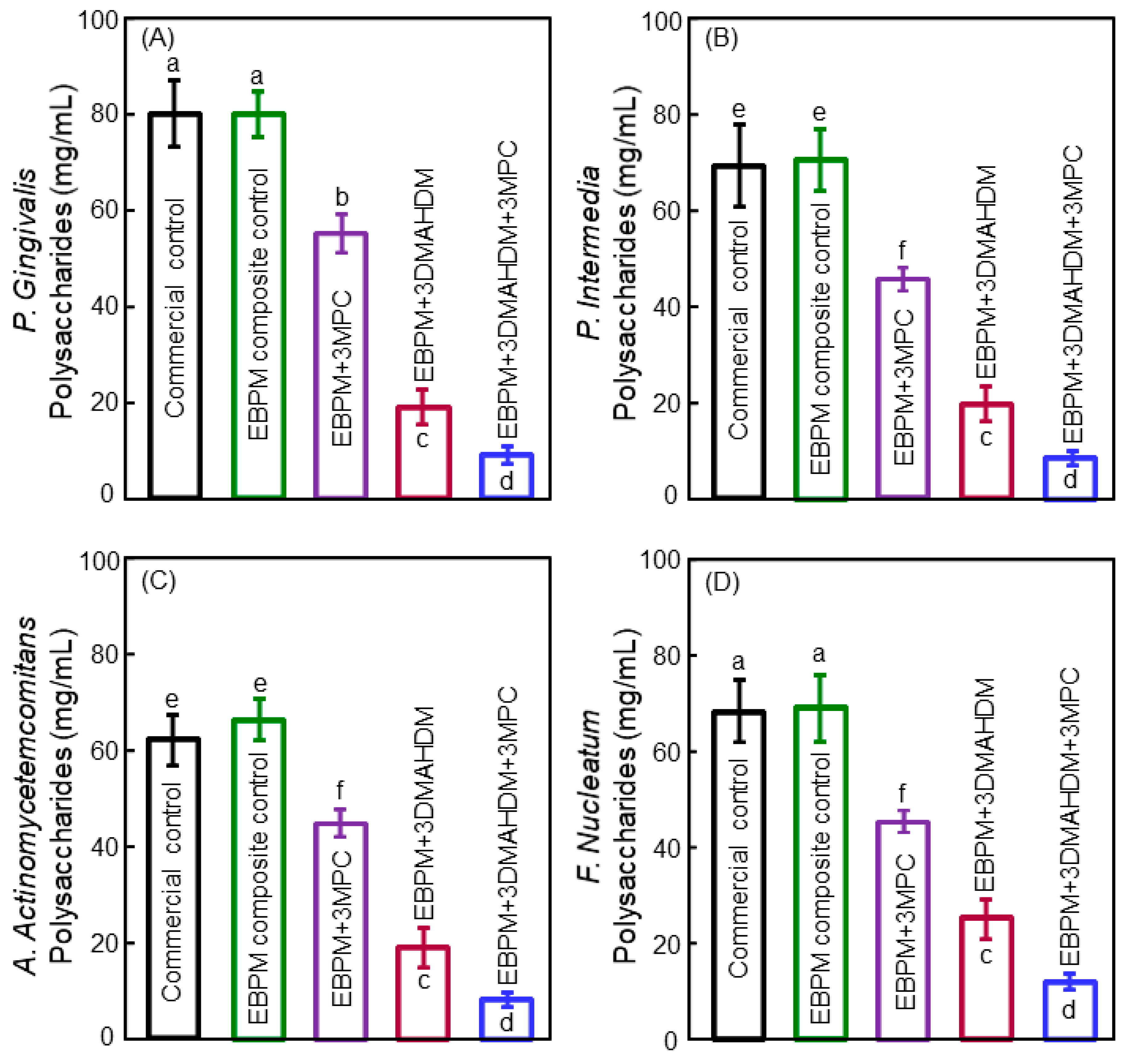
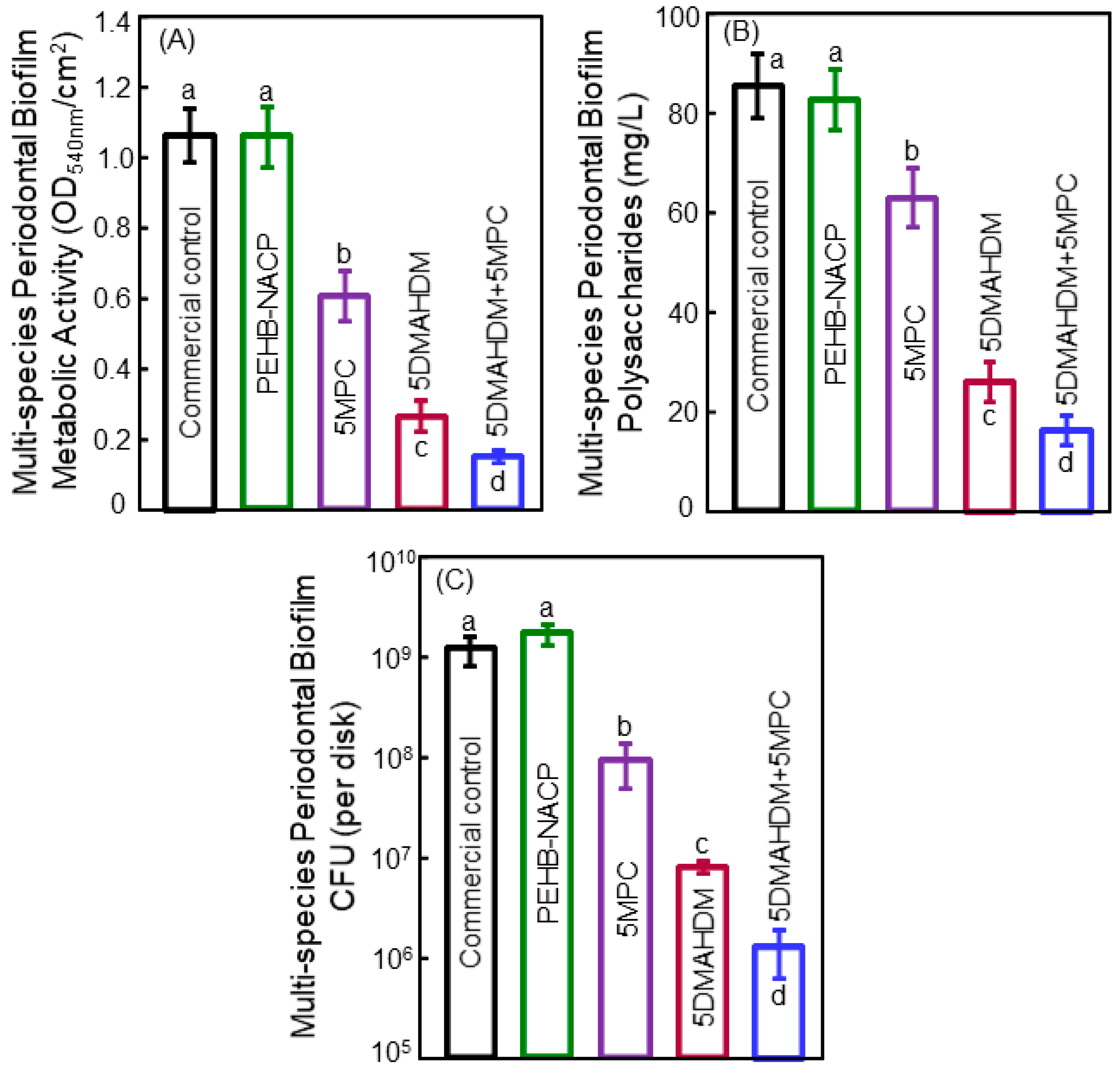
5. Antibacterial Bonding Agents Inhibiting Periodontal Pathogens
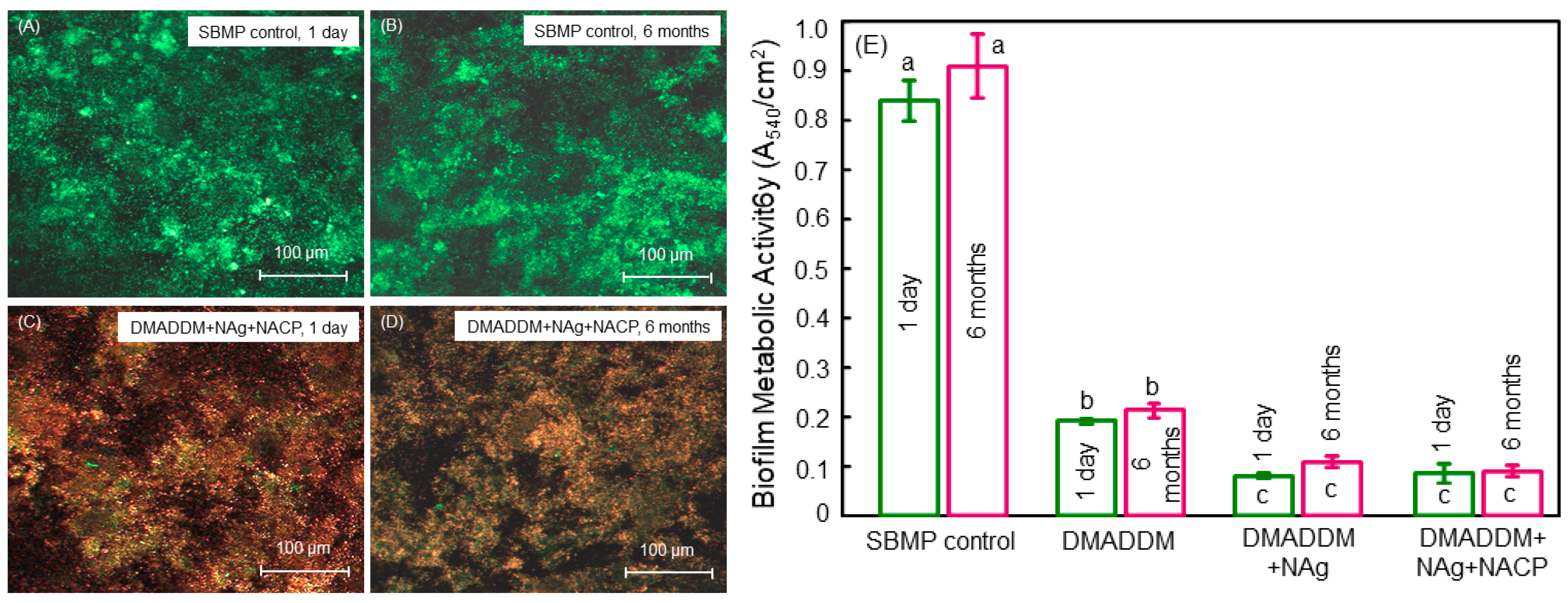
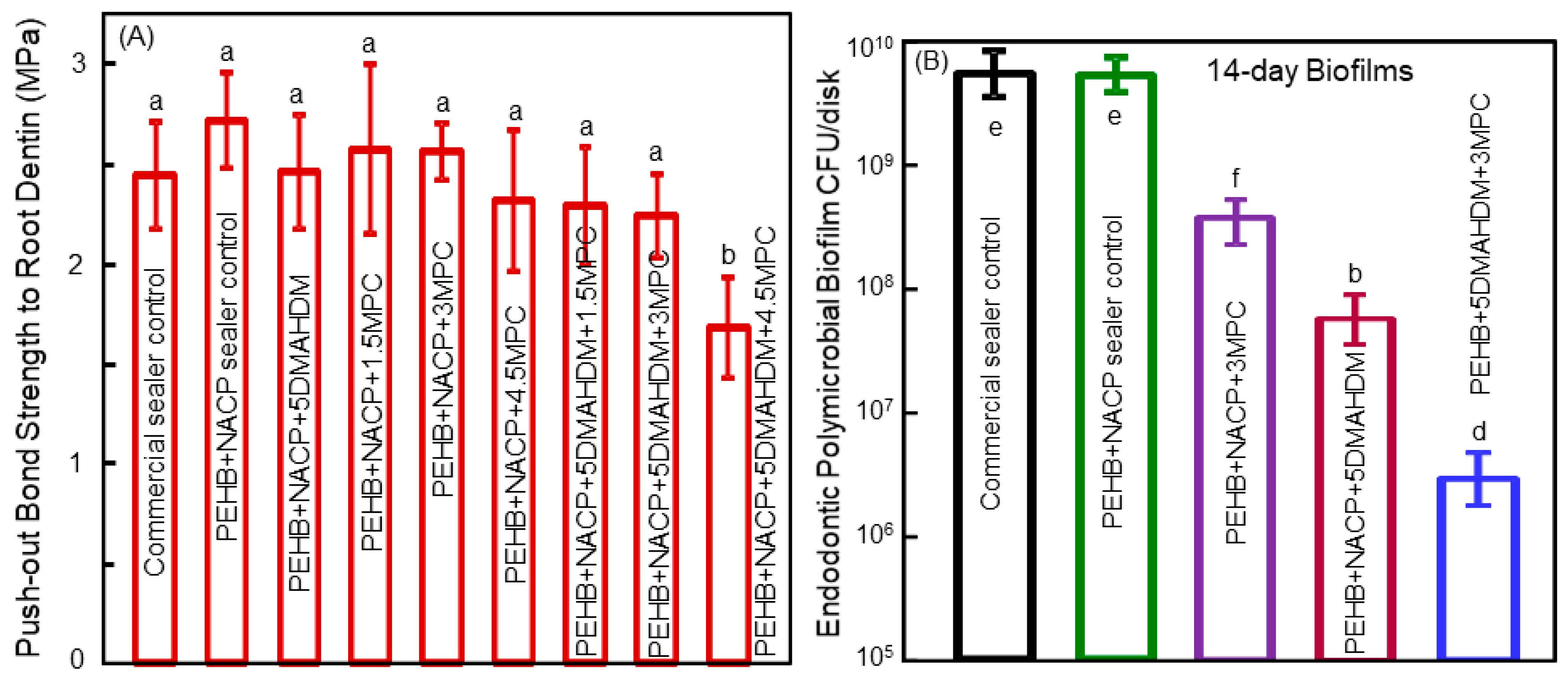
6. Antibacterial Polymeric Endodontic Sealers
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sakaguchi, R.L. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dent. Mater. 2005, 21, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Mjor, I.A.; Toffeneti, F. Secondary caries: a literature review with caries reports. Quintessence Int. 2000, 31, 165–179. [Google Scholar] [PubMed]
- Ferracane, J.L. Resin composite-state of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Frost, P.M. An audit on the placement and replacement of restorations in a general dental practice. Prim. Dent. Care 2002, 9, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Beazoglou, T.; Eklund, S.; Heffley, D.; Meiers, J.; Brown, L.J.; Bailit, H. Economic impact of regulating the use of amalgam restorations. Public Health Rep. 2007, 122, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.H.; Meyerowitz, C. Dental caries in older adults. Dent. Clin. N. Am. 2005, 49, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental adhesion review: aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.; Ye, Q.; Park, J.G.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F.; Eslick, J.; Camarda, K.; Katz, J.L. Adhesive/dentin interface: The weak link in the composite restoration. Annals Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Milward, P.J.; Adusei, G.O.; Lynch, C.D. Improving some selected properties of dental polyacid-modified composite resins. Dent. Mater. 2011, 27, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Camilleri, J. Antimicrobial properties of conventional restorative filling materials and advances in antimicrobial properties of composite resins and glass ionomer cements—A literature review. Dent. Mater. 2015, 31, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N.; Hilton, T.J.; Heintze, S.D.; Hickel, R.; Watts, D.C.; Silikas, N.; Stansbury, J.W.; Cadenaro, M.; Ferracane, J.L. Academy of dental materials guidance—Resin composites: Part I—Mechanical properties. Dent. Mater. 2017, 33, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.S.; Alania, Y.; Natale, L.C.; Rodrigues, M.C.; Watts, D.C.; Braga, R.R. Trends in restorative composites research: what is in the future? Braz. Oral. Res. 2017, 31, 55. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ling, L.; Wang, R.; Burgess, J.O. Formation and characterization of a novel fluoride-releasing dental composite. Dent. Mater. 2006, 22, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Placing dental composites—A stressful experience. Oper. Dent. 2008, 333, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.J.; Silikas, N.; Zhang, Z.T.; Watts, D.C. Hygroscopic dimensional changes of self-adhering and new resin-matrix composites during water sorption/desorption cycles. Dent. Mater. 2011, 27, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Podgórski, M.; Zhang, X.; Sinha, J.; Claudino, M.; Stansbury, J.W.; Bowman, C.N. Dental restorative materials based on thiol-michael photopolymerization. J. Dent. Res. 2018, 97, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Vallittu, P.K.; Boccaccini, A.R.; Hupa, L.; Watts, D.C. Bioactive dental materials—Do they exist and what does bioactivity mean? Dent. Mater. 2018, 34, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Miki-Oka, S.; Mayanagi, G.; Abiko, Y.; Takahashi, N.; Imazato, S. Inhibitory effect of resin composite containing S-PRG filler on Streptococcus mutans glucose metabolism. J. Dent. 2018, 70, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Domb, A.J.; Weiss, E.I. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J. Dent. 2007, 35, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Totiam, P.; Gonzalez-Cabezas, C.; Fontana, M.R.; Zero, D.T. A new in vitro model to study the relationship of gap size and secondary caries. Caries Res. 2007, 41, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Cenci, M.S.; Pereira-Cenci, T.; Cury, J.A.; ten Cate, J.M. Relationship between gap size and dentine secondary caries formation assessed in a microcosm biofilm model. Caries Res. 2009, 43, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S. Review: Antibacterial properties of resin composites and dentin bonding systems. Dent. Mater. 2003, 19, 449–457. [Google Scholar] [CrossRef]
- Imazato, S. Bioactive restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dent. Mater. J. 2009, 28, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Tezvergil-Mutluay, A.; Agee, K.A.; Uchiyama, T.; Imazato, S.; Mutluay, M.M.; Cadenaro, M.; Breschi, L.; Nishitani, Y.; Tay, F.R.; Pashley, D.H. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J. Dent. Res. 2011, 90, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M.; Zeiger, D.N.; Tang, K.; Lin-Gibson, S.; Fowler, B.O.; Lin, N.J. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dent. Mater. 2012, 28, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Y.; Liao, S.; Wen, Z.T.; Fan, Y. Synthesis and characterization of antibacterial dental monomers and composites. J. Biomed. Mater. Res. 2012, 100B, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Zhang, K.; Wu, E.; Xu, S.M.; Zhou, X.D.; Xu, H.H.K. Dental plaque microcosm biofilm behavior on calcium phosphate nanocomposite with quaternary ammonium. Dent. Mater. 2012, 28, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Ebisu, S.; Tay, F.R. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dent. Mater. 2003, 19, 313–319. [Google Scholar] [CrossRef]
- Imazato, S.; Tay, F.R.; Kaneshiro, A.V.; Takahashi, Y.; Ebisu, S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dent. Mater. 2007, 23, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Yudovin-Farber, I.; Bahir, R.; Domb, A.J.; Weiss, E.I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials 2006, 27, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Namba, N.; Yoshida, Y.; Nagaoka, N.; Takashima, S.; Matsuura-Yoshimoto, K.; Maeda, H.; Van Meerbeek, B.; Suzuki, K.; Takashiba, S. Antibacterial effect of bactericide immobilized in resin matrix. Dent. Mater. 2009, 25, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Xu, H.K. Effects of quaternary ammonium chain length on antibacterial bonding agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent. Mater. 2014, 30, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ma, J.; Melo, M.A.S.; Weir, M.D.; Bai, Y.X.; Xu, H.H.K. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J. Dent. 2015, 43, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, K.; Weir, M.D.; Melo, M.A.S.; Zhou, X.D.; Xu, H.H.K. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomedicine 2015, 10, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cheng, L.; Weir, M.D.; Bai, Y.X.; Xu, H.H.K. Effects of quaternary ammonium chain length on antibacterial and remineralizing calcium phosphate nanocomposite. Int. J. Oral Sci. 2016, 8, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kasraei, S.L.; Sami, L.; Hendi, S.; Alikhani, M.-Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial properties of composite resins incorporating silver and zinc oxide nanoparticles on Streptococcus mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Stencel, R.; Kasperski, J.; Pakiela, W.; Mertas, A.; Bobela, E.; Barszcewska-Rybarek, I.; Chladek, G. Properties of experiment dental composite containing antibacterial silver-releasing filler. Materials 2018, 11, 1031. [Google Scholar] [CrossRef] [PubMed]
- Khvostenko, D.; Hilton, T.J.; Ferracane, J.L.; Mitchell, J.C.; Kruzic, J.J. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent. Mater. 2016, 32, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Al-Eesa, N.A.; Wong, F.S.L.; Johal, A.; Hill, R.G. Fluoride containing bioactive glass composite for orthodontic adhesive–ion release properties. Dent. Mater. 2017, 33, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Xu, H.H.K.; Antonucci, J.M.; Lin, N.J.; Lin-Gibson, S.; Xu, S.M.; Zhou, X. Effect of amorphous calcium phosphate and silver nanocomposites on dental plaque microcosm biofilms. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli Hojati, S.; Alaghemand, H.; Hamze, F.; Ahmadian Babaki, F.; Rajad-Nia, R.; Rezvni, M.B.; Kaviani, M.; Atai, M. Antibacterial, physical and mechanical properties of flowable resin composites containing zinc oxide nanoparticles. Dent. Mater. 2013, 29, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Aydin Sevinc, B.; Hanley, L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, K.; Zhou, C.C.; Weir, M.D.; Zhou, X.D.; Xu, H.H. One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms. Int. J. Oral Sci. 2016, 8, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Torii, M.; Tsuchitani, Y.; McCabe, J.F.; Russell, R.R. Incorporation of bacterial inhibitor into resin composite. J. Dent. Res. 1994, 73, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Qiu, S.; Lewis, K.; Klibanov, A.M. Bactericidal properties of flat surfaces and nanoparticles derivatized with alkylated polyethylenimines. Biotechnol. Prog. 2002, 18, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Yudovin-Farber, I.; Perez-Davidi, M.; Domb, A.J.; Weiss, E.I. Polyethyleneimine nanoparticles incorporated into resin composite cause cell death and trigger biofilm stress in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 22038–22043. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Howard, L.; Guo, X.; Chong, V.J.; Gregory, R.L.; Xie, D. A novel antibacterial resin composite for improved dental restoratives. J. Mater. Sci. Mater. Med. 2012, 23, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, K.; Melo, M.A.; Weir, M.D.; Zhou, X.; Xu, H.H. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J. Dent. Res. 2012, 91, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Weng, Y.; Guo, X.; Zhao, J.; Gregory, R.L.; Zheng, C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dent. Mater. 2011, 27, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Weir, M.D.; Antonucci, J.M.; Antonucci, J.M.; Schumacher, G.E.; Zhou, X.D.; Xu, H.H. Evaluation of three-dimensional biofilms on antibacterial bonding agents containing novel quaternary ammonium methacrylates. Int. J. Oral Sci. 2014, 6, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjaderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Kuramoto, A.; Takahashi, Y.; Ebisu, S.; Peters, M.C. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dent. Mater. 2006, 22, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, J.; Chai, Z.; Zhang, L.; Xiao, Y.; Fang, M.; Ma, S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J. Dent. 2009, 37, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, N.; Yiu, C.K.; King, N.M.; Tay, F.R. Effect of chlorhexidine incorporation into a self-etching primer on dentine bond strength of a luting cement. J. Dent. 2010, 38, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cheng, L.; Wu, E.J.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Effect of water-aging on dentin bond strength and anti-biofilm activity of bonding agent containing antibacterial monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Burrow, M.F.; Tyas, M.J. Clinical evaluation of three adhesive systems for the restoration of non-carious cervical lesions. Oper. Dent. 2007, 32, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Manuja, N.; NAgpal, R.; Pandit, I.K. Dental adhesion: mechanism, techniques and durability. J. Clin. Pediatr. Dent. 2012, 36, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Kitasako, Y.; Burrow, M.F.; Katahira, N.; Nikaido, T.; Tagami, J. Shear bond strengths of three resin cements to dentine over 3 years in vitro. J. Dent. 2001, 29, 139–144. [Google Scholar] [CrossRef]
- De Munck, J.; Van Landuyt, K.; Peumans, M.; Poitevin, A.; Lambrechts, P.; Braem, M.; Van Meerbeek, B. A critical review of the durability of adhesion to tooth tissue: methods and results. J. Dent. Res. 2005, 84, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Koshiro, K.; Inoue, S.; Sano, H.; De Munck, J.; Van Meerbeek, B. In vivo degradation of resin–dentin bonds produced by a self-etch and an etch-and-rinse adhesive. Eur. J. Oral Sci. 2005, 113, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Water treeing—A potential mechanism for degradation of dentin adhesives. Am. J. Dent. 2003, 16, 6–12. [Google Scholar] [PubMed]
- Pashley, D.H.; Tay, F.R.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvalho, R.M.; Ito, S. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 2004, 83, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Ohno, H.; Sano, H.; Tay, F.R.; Kaga, M.; Kudou, Y.; Oguchi, H.; Araki, Y.; Kubota, M. Micromorphological changes in resin–dentin bonds after 1 year of water storage. J. Biomed. Mater. Res. 2002, 63, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Spencer, P. Hybridization efficiency of the adhesive/dentin interface with wet bonding. J. Dent. Res. 2003, 82, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, Y.; Yoshiyama, M.; Wadgaonkar, B.; Breschi, L.; Mannello, F.; Mazzoni, A.; Carvalho, R.M.; Tjäderhane, L.; Tay, F.R.; Pashley, D.H. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. Eur. J. Oral Sci. 2006, 114, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Gendron, R.; Grenier, D.; Sorsa, T.; Mayrand, D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clin. Diagn. Lab. Immunol. 1999, 6, 437–439. [Google Scholar] [PubMed]
- Breschi, L.; Mazzoni, A.; Nato, F.; Carrilho, M.; Visintini, E.; Tjaderhane, L.; Ruggeri, A.J.R.; Tay, F.R.; Dorigo Ede, S.; Pashley, D.H. Chlorhexidine stabilizes the adhesive interface: A 2-year in vitro study. Dent. Mater. 2010, 26, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Yamauti, M.; Osorio, E.; Ruiz-Requena, M.E.; Pashley, D.; Tay, F.; Toledano, M. Effect of dentin etching and chlorhexidine application on metalloproteinase-mediated collagen degradation. Eur. J. Oral Sci. 2011, 119, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.D.; Frazier, K.B.; McConnell, R.J.; Blum, I.R.; Wilson, N.H. Minimally invasive management of dental caries: contemporary teaching of posterior resin-based composite placement in U.S. and Canadian dental schools. J. Am. Dent. Assoc. 2011, 142, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, A.D.; Reis, A.; Bortoli, G.; Patzlaft, R.; Kenshima, S.; Rodrigues Filho, L.E.; Accorinte, M.d.e.L.; van Dijken, J.W. Influence of adhesive systems on interfacial dentin gap formation in vitro. Oper. Dent. 2006, 31, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Fure, S. Ten-year incidence of tooth loss and dental caries in elderly Swedish individuals. Caries Res. 2003, 37, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Ravald, N.; Johansson, C.S. Tooth loss in periodontally treated patients. A long-term study of periodontal disease and root caries. J. Clin. Periodontol. 2012, 39, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Griffen, A.L.; Moeschberger, M.L.; Leys, E.J. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J. Clin. Microbiol. 2005, 43, 3944–3955. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.; Hajishengallis, G.; Curtis, M. Porphyromonas gingivalis as a potential community activist for disease. J. Dent. Res. 2012, 91, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Fteita, D.; Könönen, E.; Söderling, E.; Gürsoy, U.K. Effect of estradiol on planktonic growth, coaggregation, and biofilm formation of the Prevotella intermedia group bacteria. Anaerobe 2014, 27, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Charalampakis, G.; Leonhardt, Å.; Rabe, P.; Dahlén, G. Clinical and microbiological characteristics of peri-implantitis cases: a retrospective multicentre study. Clin. Oral Implants Res. 2012, 23, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Signat, B.; Roques, C.; Poulet, P.; Duffaut, D. Role of Fusobacterium nucleatum in periodontal health and disease. Curr. Issues Mol. Biol. 2011, 13, 25–36. [Google Scholar] [PubMed]
- Saito, A.; Inagaki, S.; Kimizuka, R.; Okuda, K.; Hosaka, Y.; Nakagawa, T.; Ishihara, K. Fusobacterium nucleatum enhances invasion of human gingival epithelial and aortic endothelial cells by Porphyromonas gingivalis. FEMS Immunol. Med. Microbiol. 2008, 54, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Souto, R.; Colombo, A.P.V. Prevalence of Enterococcus faecalis in subgingival biofilm and saliva of subjects with chronic periodontal infection. Arch. Oral Biol. 2008, 53, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Melo, M.A.S.; Weir, M.D.; Xie, X.J.; Reynolds, M.A.; Xu, H.H.K. Novel bioactive nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Dent. Mater. 2016, 32, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, X.J.; Imazato, S.; Weir, M.D.; Reynolds, M.; Xu, H.H.K. Protein-repellent and antibacterial nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.Y.; Weir, M.D.; Zhang, K.; Zhou, Y.M.; Xu, H.H.K.; Reynolds, M.A. Novel multifunctional dental bonding agent for class-V restorations to inhibit periodontal biofilms. RSC Adv. 2017, 7, 29004–29014. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Kolenbrander, P.E. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J. Bacteriol. 2009, 191, 6804–6811. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, J.K.; Um, H.S.; Chang, B.S.; Lee, S.Y. A periodontitis-associated multispecies model of an oral biofilm. J. Periodontal Implant Sci. 2014, 44, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Nair, P. On the causes of persistent apical periodontitis: a review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Araújo, M.C.; Garcia, P.F.; Fraga, R.C.; Dantas, C.J.S. Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. J. Endod. 1997, 23, 499–502. [Google Scholar] [CrossRef]
- Stuart, C.D.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, X.J.; Li, C.Y.; Liu, H.B.; Zhang, K.; Zhou, Y.M.; Chang, X.F.; Xu, H.H.K. Novel bioactive root canal sealer to inhibit endodontic multispecies biofilms with remineralizing calcium phosphate ions. J. Dent. 2017, 60, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, H.; Weir, M.D.; Reynolds, M.A.; Zhang, K.; Xu, H.H. Three-dimensional biofilm properties on dental bonding agent with varying quaternary ammonium charge densities. J. Dent. 2016, 53, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.S.; Thompson, L.S.; James, S.; Charlton, T.; Tolker-Nielsen, T.; Koch, B.; Givskov, M.; Kjelleberg, S. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol. 2003, 185, 4585–4592. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Giannobile, W.V. Novel biomaterials and technologies for the dental, oral, and craniofacial structures. J. Dent. Res. 2014, 93, 1185–1186. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.; Ferracane, J.L. Enhancing the value of dental biomaterials research: Reducing the noise. J. Dent. Res. 2018, 97, 481–482. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Baras, B.; Lynch, C.D.; Weir, M.D.; Melo, M.A.S.; Li, Y.; Reynolds, M.A.; Bai, Y.; Wang, L.; Wang, S.; et al. Developing a New Generation of Therapeutic Dental Polymers to Inhibit Oral Biofilms and Protect Teeth. Materials 2018, 11, 1747. https://doi.org/10.3390/ma11091747
Zhang K, Baras B, Lynch CD, Weir MD, Melo MAS, Li Y, Reynolds MA, Bai Y, Wang L, Wang S, et al. Developing a New Generation of Therapeutic Dental Polymers to Inhibit Oral Biofilms and Protect Teeth. Materials. 2018; 11(9):1747. https://doi.org/10.3390/ma11091747
Chicago/Turabian StyleZhang, Ke, Bashayer Baras, Christopher D. Lynch, Michael D. Weir, Mary Anne S. Melo, Yuncong Li, Mark A. Reynolds, Yuxing Bai, Lin Wang, Suping Wang, and et al. 2018. "Developing a New Generation of Therapeutic Dental Polymers to Inhibit Oral Biofilms and Protect Teeth" Materials 11, no. 9: 1747. https://doi.org/10.3390/ma11091747
APA StyleZhang, K., Baras, B., Lynch, C. D., Weir, M. D., Melo, M. A. S., Li, Y., Reynolds, M. A., Bai, Y., Wang, L., Wang, S., & Xu, H. H. K. (2018). Developing a New Generation of Therapeutic Dental Polymers to Inhibit Oral Biofilms and Protect Teeth. Materials, 11(9), 1747. https://doi.org/10.3390/ma11091747





