Research Status and Prospect on Vanadium-Based Catalysts for NH3-SCR Denitration
Abstract
1. Introduction
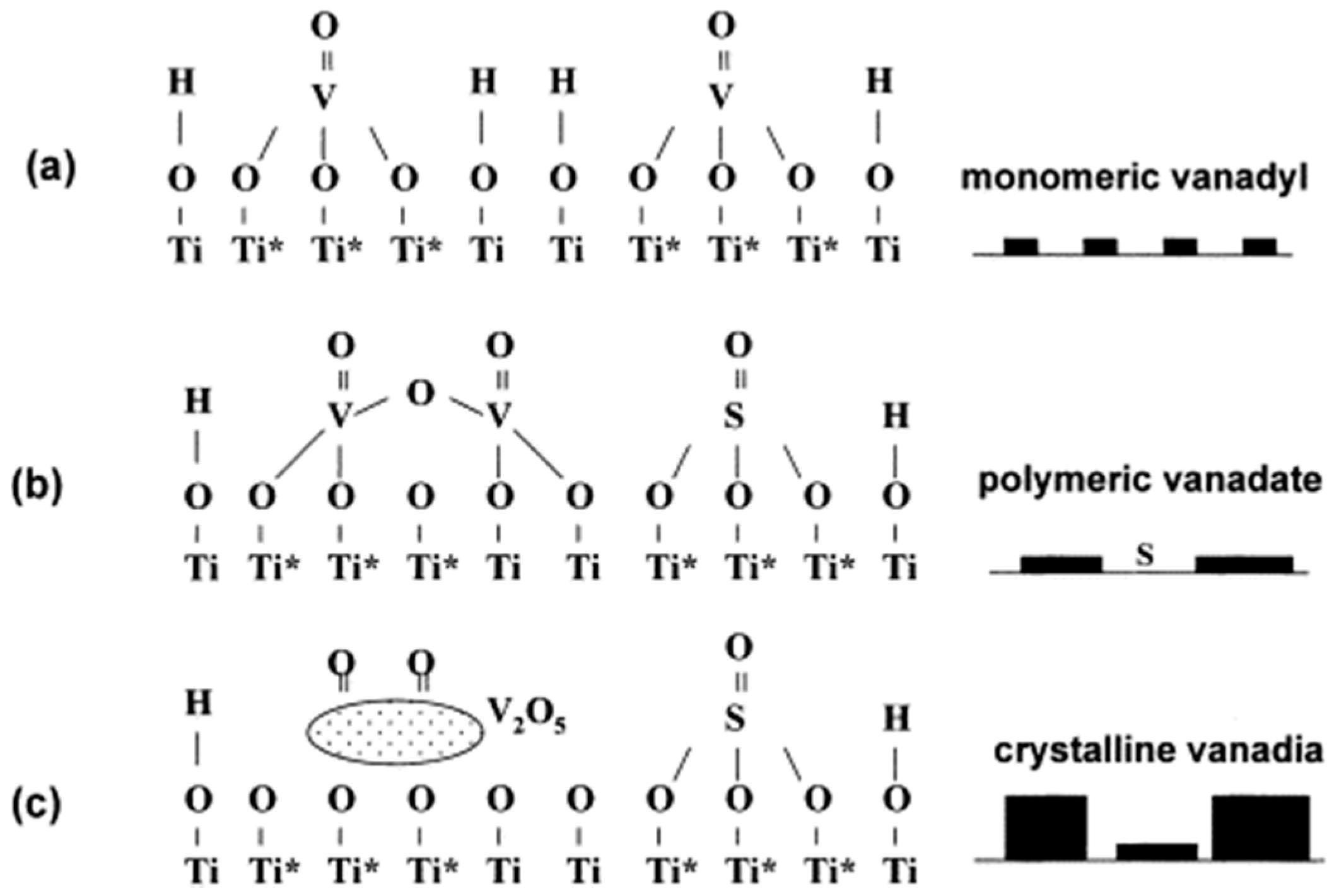
2. Composition of Vanadium Based Catalyst
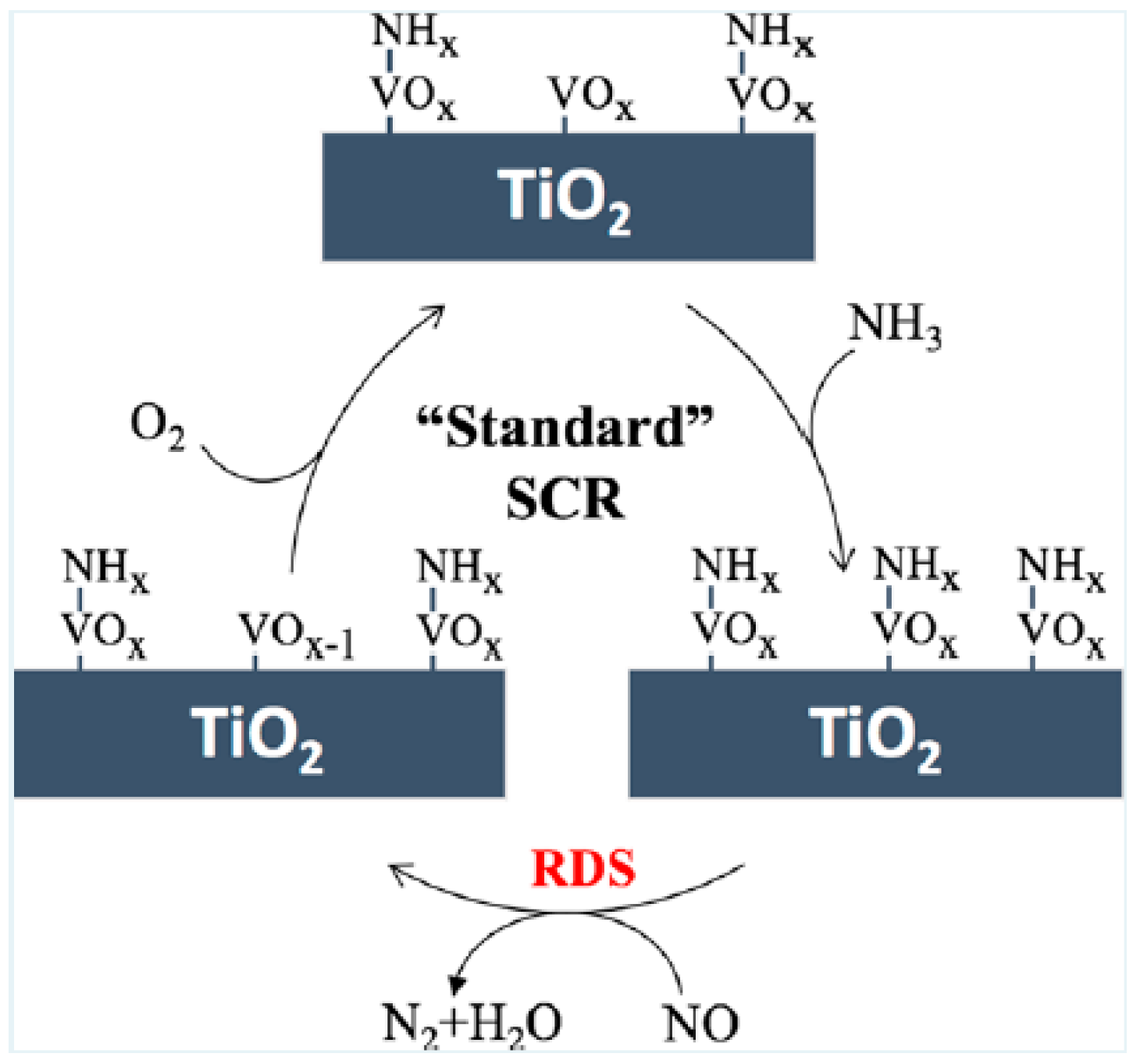
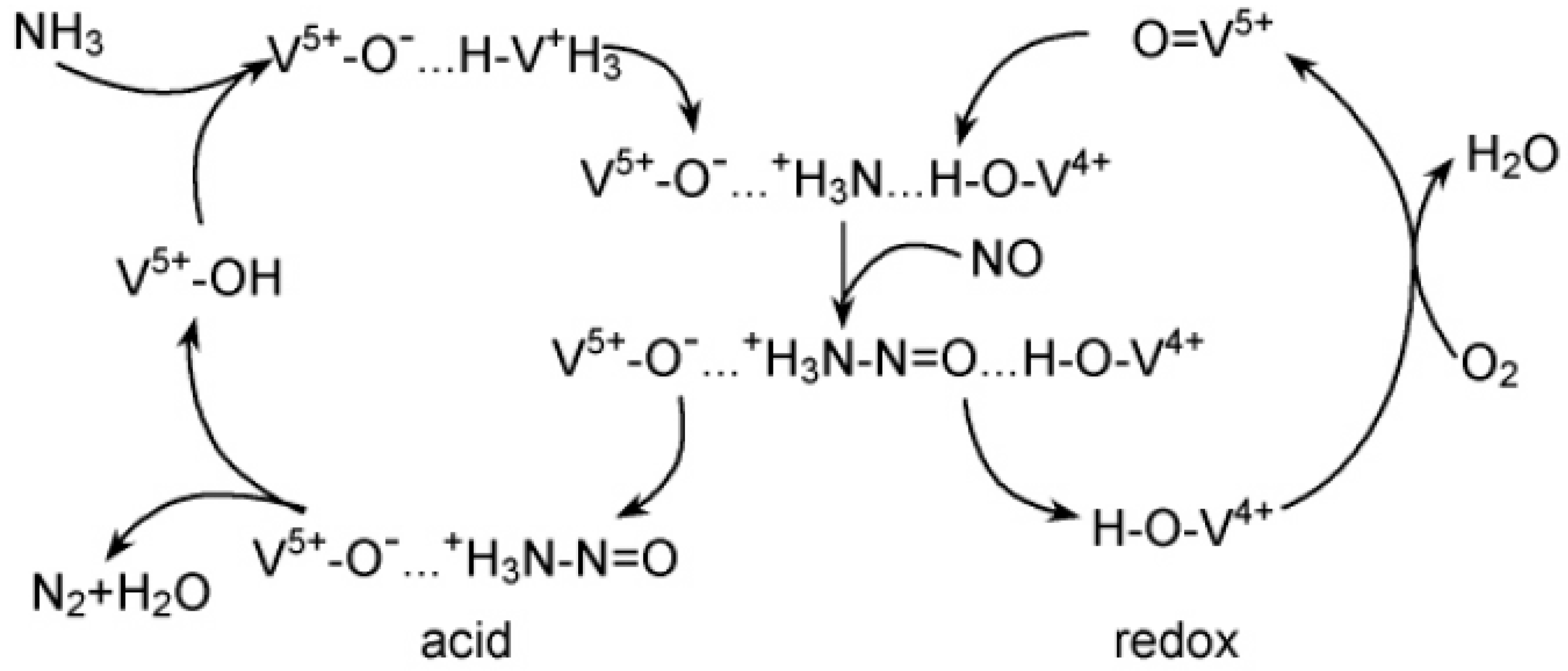
3. NH3-SCR Reaction Mechanism of Vanadium-Based Catalyst
4. Performance Improvement of Vanadium Based Catalysts
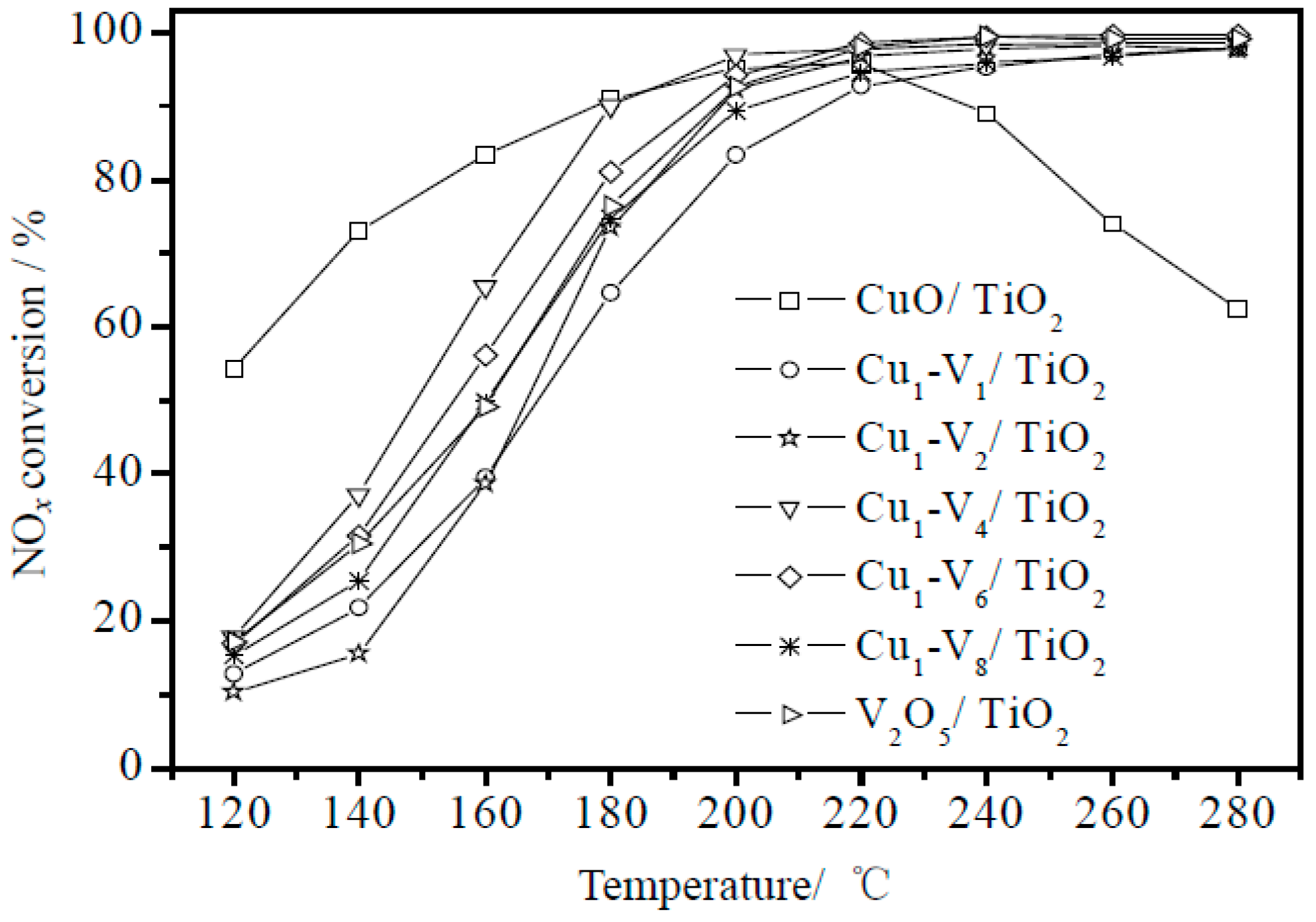
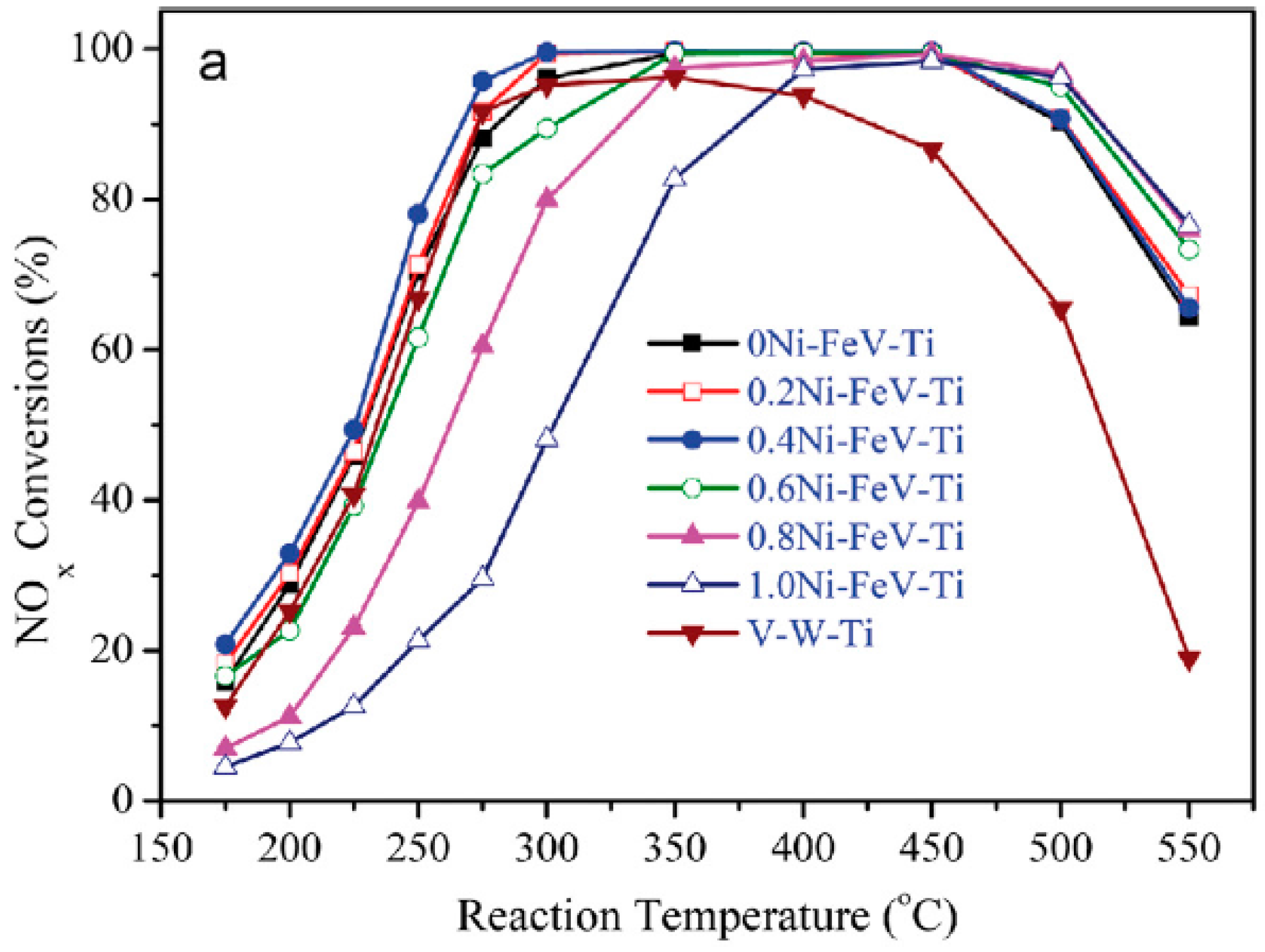
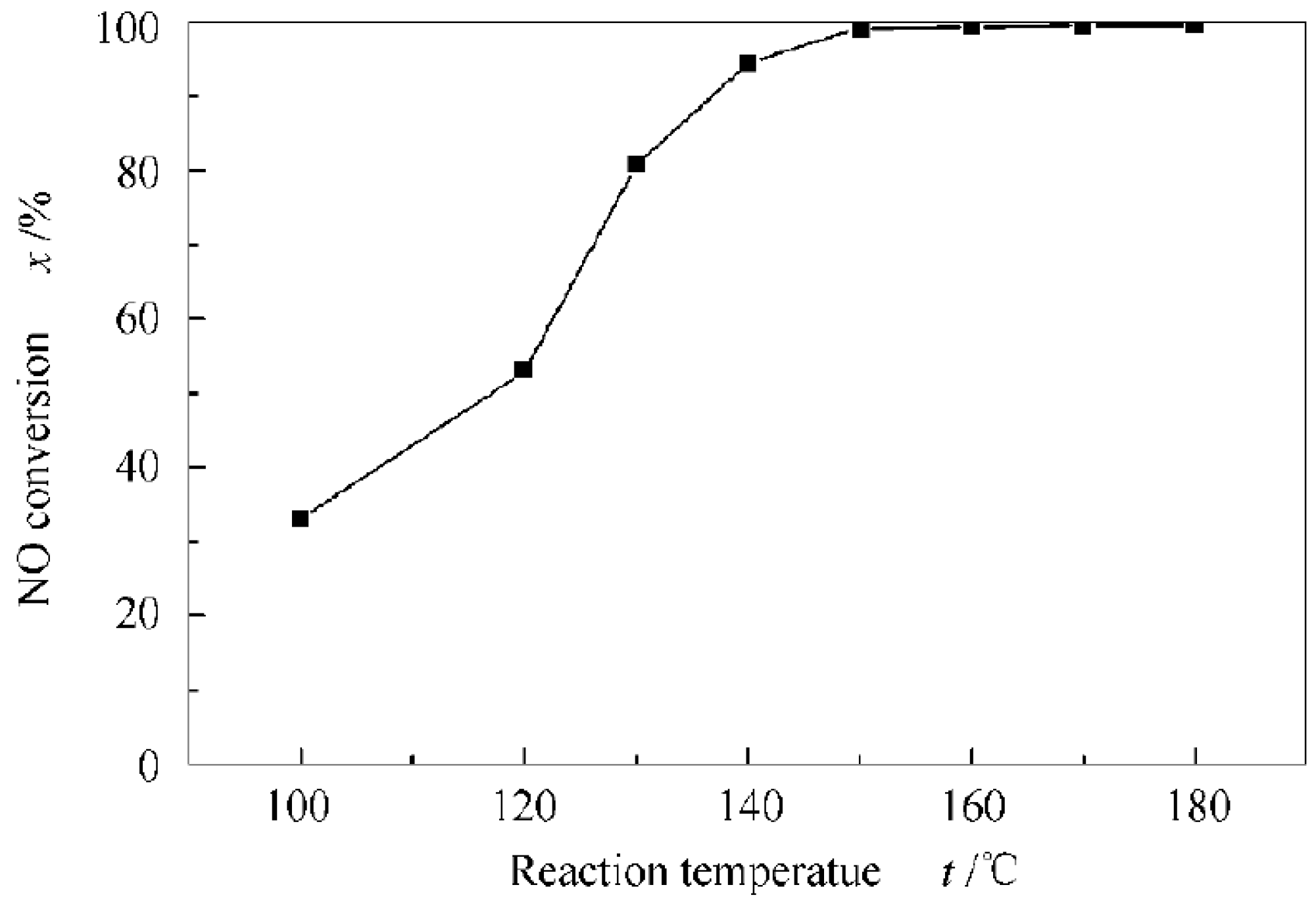
4.1. The Broadening of the Temperature Ranges
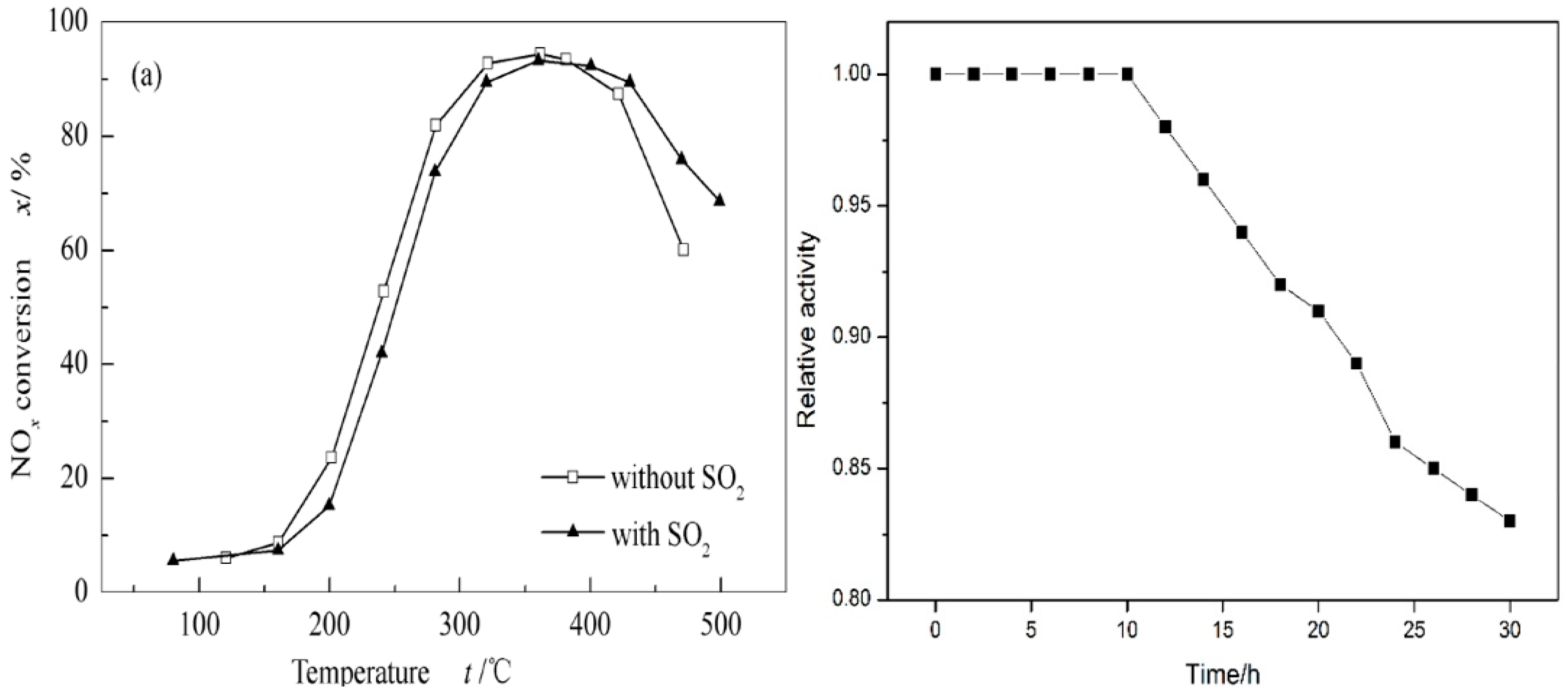
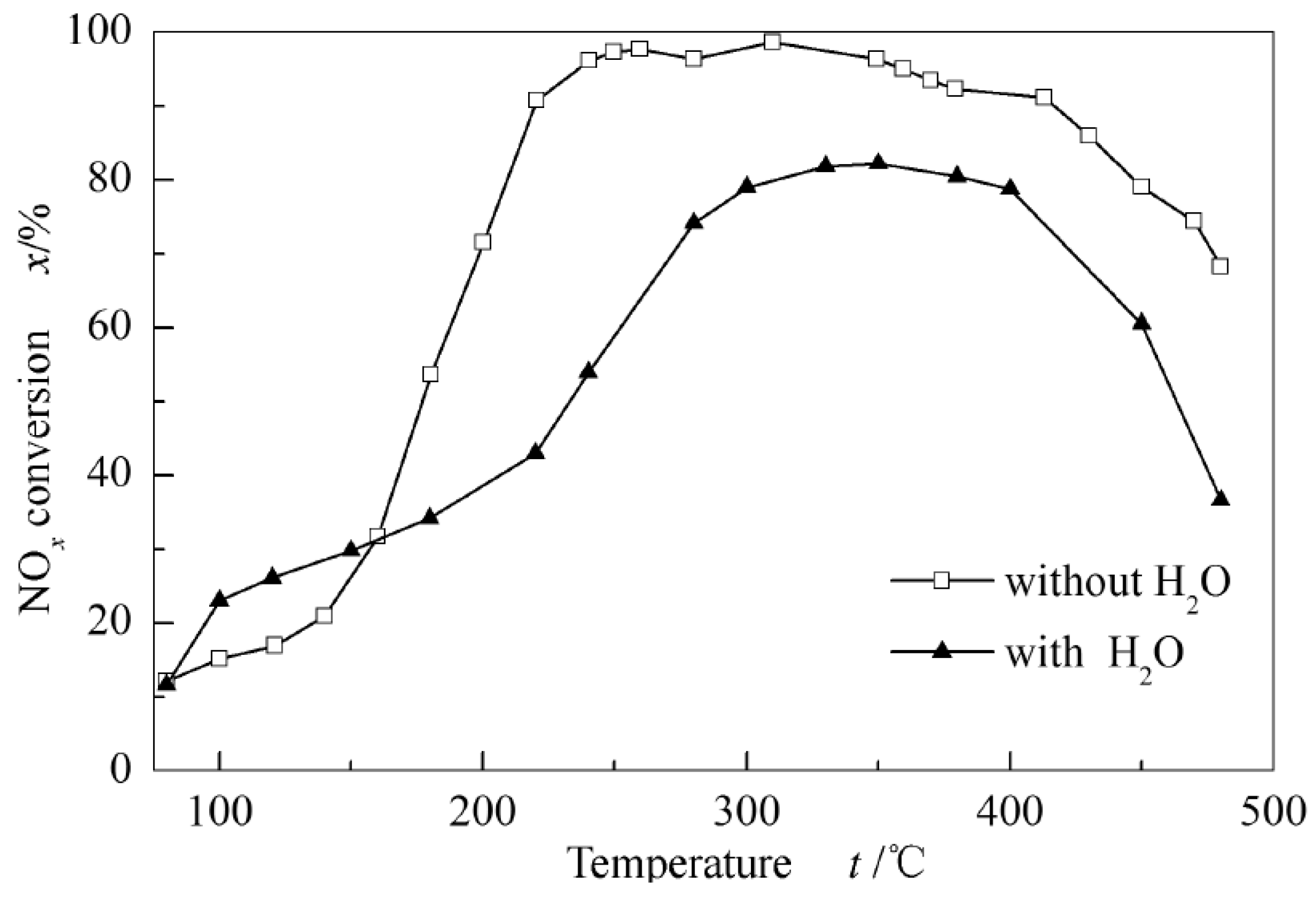
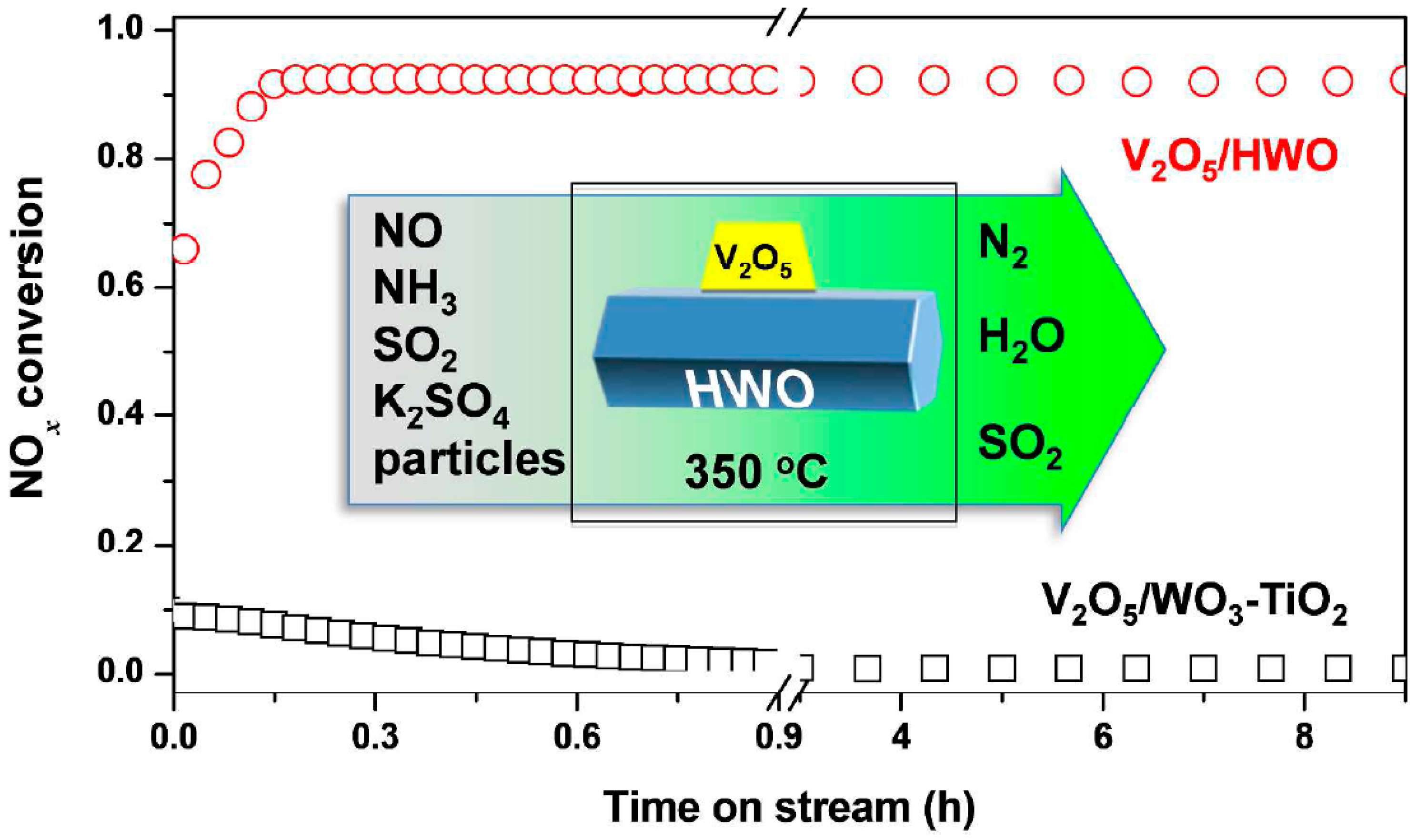
4.2. The Improvement of Erosion Resistance
5. Conclusions and Perspective
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bendrich, M.; Scheuer, A.; Hayes, R.E.; Votsmeier, M. Unified mechanistic model for standard SCR, fast SCR, and NO2 SCR over a copper chabazite catalyst. Appl. Catal. B Environ. 2018, 222, 76–87. [Google Scholar] [CrossRef]
- Zhu, M.H.; Lai, J.K.; Tumuluri, U.; Ford, M.E.; Wu, Z.L.; Wachs, I.E. Reaction pathways and kinetics for selective catalytic reduction (SCR) of acidic NOx emissions from power plants with NH3. ACS Catal. 2017, 7, 8358–8361. [Google Scholar] [CrossRef]
- Liu, F.D.; Shan, W.; Shi, X.; He, H. Vanadium-based catalysts for the selective catalytic reduction of NOx with NH3. Prog. Chem. 2012, 24, 445–455. (In Chinese) [Google Scholar]
- Luo, S.P.; Zhou, W.T.; Xie, A.J.; Wu, F.Q.; Yao, C.; Li, X.Z.; Zuo, S.X.; Liu, T.H. Effect of MnO2 polymorphs structure on the selective catalytic reduction of NOx with NH3 over TiO2-Palygorskite. Chem. Eng. J. 2016, 286, 291–299. (in Chinese). [Google Scholar] [CrossRef]
- Liu, T.; Wang, T.C.; Wu, R.Q.; Shen, B.X. Research advance review for low-temperature NH3-SCR catalysts. J. Saf. Environ. 2012, 19, 42–44. (in Chinese). [Google Scholar]
- Huang, H.F.; Jin, L.L.; Zhang, H.H.; Yu, H.; Lu, H.F. Preparation and characterization of Cu-V-TiO2 catalysts with strong resistance to SO2 for low-temperature SCR of NOx. J. Chem. Eng. Chin. Univ. 2013, 27, 721–728. (In Chinese) [Google Scholar]
- Marberger, A.; Ferri, D.; Elsener, M.; Sagar, A.; Artner, C.; Schermanz, K.; Krocher, O. Relationship between structures and activities of supported metal vanadates for the selective catalytic reduction of NO by NH3. Appl. Catal. B Environ. 2017, 218, 731–742. [Google Scholar] [CrossRef]
- Cheng, J.; Song, Y.; Ye, Q.; Cheng, S.Y.; Kang, T.F.; Dai, H.X. A mechanistic investigation on the selective catalytic reduction of NO with ammonia over the V-Ce/Ti-PILC catalysts. Mol. Catal. 2018, 445, 111–123. [Google Scholar] [CrossRef]
- Yang, W.W.; Liu, F.D.; Xie, L.J.; Lian, Z.H.; He, H. Effect of V2O5 additive on the SO2 resistance of a Fe2O3/AC catalyst for NH3-SCR of NOx at low temperatures. Ind. Eng. Chem. Res. 2016, 55, 2677–2685. [Google Scholar] [CrossRef]
- Vuong, T.H.; Radnik, J.; Schneider, M.; Atia, H.; Armbruster, U.; Brückner, A. Effect of support synthesis methods on structure and performance of VOxCeO2 catalysts in low-temperature NH3-SCR of NO. Catal. Commun. 2016, 84, 171–174. [Google Scholar] [CrossRef]
- Vuong, T.H.; Radnik, J.; Kondratenko, E.; Schneider, M.; Armbruster, U.; Brückner, A. Structure-reactivity relationships in VOxCexZr1−xO2 catalysts used for low-temperature NH3-SCR of NO. Appl. Catal. B Environ. 2016, 197, 159–167. [Google Scholar] [CrossRef]
- He, Y.Y.; Ford, M.E.; Zhu, M.H.; Liu, Q.C.; Tumuluri, U.; Wu, Z.L.; Wachs, I.E. Influence of catalyst synthesis method on selective catalytic reduction (SCR) of NO by NH3 with V2O5-WO3 TiO2 catalysts. Appl. Catal. B Environ. 2016, 193, 141–150. [Google Scholar] [CrossRef]
- Tao, P.; Sun, M.H.; Qu, S.C.; Song, C.W.; Li, C.; Yin, Y.Y.; Cheng, M.R. Effects of V2O5 and WO3 loadings on the catalytic performance of V2O5-WO3TiO2 catalyst for SCR of NO with NH3. Glob. NEST 2017, 19, 160–166. [Google Scholar]
- Marberger, A.; Elsener, M.; Ferri, D.; Krocher, O. VOx surface coverage optimization of V2O5/WO3-TiO2 SCR catalysts by variation of the v loading and by aging. Catalysts 2015, 5, 1704–1720. [Google Scholar] [CrossRef]
- Rasmussen, S.B.; Portela, R.; Bazin, P.; Avila, P.; Banares, M.A.; Daturi, M. Transient operando study on the NH3/NH4+ interplay in V-SCR monolithic catalysts. Appl. Catal. B Environ. 2018, 224, 109–115. [Google Scholar] [CrossRef]
- Giakoumelou, I.; Fountzoula, C.; Kordulis, C.; Boghosian, S. Molecular structure and catalytic activity of V2O5 /TiO2, catalysts for the SCR of NO by NH3: In situ Raman spectra in the presence of O2, NH3, NO, H2, H2O, and SO2. J. Catal. 2006, 239, 1–12. [Google Scholar] [CrossRef]
- Huang, H.F.; Chen, Y.J.; Yang, R.; Zhu, Q.L.; Lu, H.F. Fe-V/TiO2 catalysts for selective catalytic reduction of NOx with NH3 in diesel exhaust. J. Fuel Chem. Technol. 2014, 42, 751–757. (In Chinese) [Google Scholar]
- Gillot, S.; Tricot, G.; Vezin, H.; Dacquin, J.-P.; Dujardin, C.; Granger, P. Development of stable and efficient CeVO4 systems for the selective reduction of NOx by ammonia: Structure-activity relationship. Appl. Catal. B Environ. 2017, 218, 338–348. [Google Scholar] [CrossRef]
- Zhou, X.M.; Huang, X.Y.; Xie, A.J.; Luo, S.P.; Yao, C.; Li, X.Z.; Zuo, S.X. V2O5-decorated Mn-Fe/attapulgite catalyst with high SO2 tolerance for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 326, 1074–1085. [Google Scholar] [CrossRef]
- Choo, S.T.; Lee, Y.G.; Nam, I.S.; Ham, S.W.; Lee, J.B. Characteristics of V2O5, supported on sulfated TiO2, for selective catalytic reduction of NO by NH3. Appl. Catal. A Gen. 2000, 200, 177–188. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, D.W.; Lee, S.; Ha, H.P. Exploration of surface properties of Sb-promoted copper vanadate catalysts for selective catalytic reduction of NOX by NH3. Appl. Catal. B Environ. 2018, 236, 314–325. [Google Scholar] [CrossRef]
- Topsoe, N.Y.; Dumesic, J.A.; Topsoe, H. Vanadia-titania catalysts for selective catalytic reduction of nitric-oxide by ammonia: I.I. studies of active sites and formulation of catalytic cycles. J. Catal. 1995, 151, 241–252. [Google Scholar] [CrossRef]
- Nova, I.; Tronconi, E. Kinetic study of the NO/NO2-NH3 SCR reactions over a V2O5-WO3/TiO2 commercial catalyst for the after treatment of diesel engines exhausts. Engine Powertrain Control Simul. Model. 2009, 32, 183–190. [Google Scholar] [CrossRef]
- Yun, D.M.; Wang, Y.; Herrera, J.E. Ethanol partial oxidation over VOx/TiO2 catalysts: The role of Titania surface oxygen on vanadia reoxidation in the mars–van krevelen mechanism. ACS Catal. 2018, 8, 4681–4693. [Google Scholar] [CrossRef]
- Youn, S.; Song, I.; Lee, H.; Cho, S.J.; Kim, D.H. Effect of pore structure of TiO2 on the SO2 poisoning over V2O5/TiO2 catalysts for selective catalytic reduction of NOx with NH3. Catal. Today 2018, 303, 19–24. [Google Scholar] [CrossRef]
- Zhuang, F.L.; Liu, S.H.; Lin, W.S.; Chen, Y.; Chen, C.W. Research progress in low vanadium and non-vanadium oxide catalysts for the selective catalytic reduction by NH3. Environ. Eng. 2016, 34, 98–102. (In Chinese) [Google Scholar]
- Dunn, J.P.; Stenger, H.G., Jr.; Wachs, I.E. Oxidation of sulfur dioxide over supported vanadia catalysts: molecular structure–reactivity relationships and reaction kinetics. Catal. Today 1999, 51, 301–308. [Google Scholar] [CrossRef]
- Pappas, D.K.; Boningari, T.; Boolchand, P.; Smirniotis, P.G. Novel manganese oxide confined interweaved titania nanotubes for the low-temperature selective catalytic reduction (SCR) of NOx by NH3. J. Catal. 2016, 334, 1–13. [Google Scholar] [CrossRef]
- Wachs, I.E. Raman and IR studies of surface metal oxide species on oxide supports: Supported metal oxide catalysts. Catal. Today 1996, 27, 437–455. [Google Scholar] [CrossRef]
- Nielsen, U.G.; Topsoe, N.Y.; Brorson, M.; Skibated, J.; Jakobsen, H.J. The complete 51V MAS NMR spectrum of surface vanadia nanoparticles on anatase (TiO2): Vanadia surface structure of a DeNOx catalyst. J. Am. Chem. Soc. 2004, 126, 4926–4933. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Huerta, M.V.; Coronado, J.M.; Fernandez-Garcia, M.; Iglesias-Juez, A.; Deo, J.; Fierro, J.L.G.; Banares, M.A. Nature of the vanadia-ceria interface in V5+/CeO2 catalysts and its relevance for the solid-state reaction toward CeVO4 and catalytic properties. J. Catal. 2004, 225, 240–248. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, C.; Li, J. Structure–activity relationship of VOx/CeO2 nanorod for NO removal with ammonia. Appl. Catal. B Environ. 2014, 144, 538–546. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Ge, M. Promotional effect of Ce-doped V2O5-WO3/TiO2 with low vanadium loadings for selective catalytic reduction of NOx by NH3. J. Phys. Chem. C 2009, 113, 21177–21184. [Google Scholar] [CrossRef]
- Haggblad, R.; Massa, M.; Andersson, A. Stability and performance of supported Fe–V-oxide catalysts in methanol oxidation. J. Catal. 2009, 266, 218–227. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Ma, L.; Peng, Y.; Qu, Z.; Yan, N.; Chen, J.; Chang, H.; Li, J. Substitution of WO3 in V2O5/WO3–TiO2 by Fe2O3 for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2013, 3, 161–168. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Lian, Z.; Shan, W.; Xie, L.; Asakura, K.; Yang, W.; Deng, H. Highly dispersed iron vanadate catalyst supported on TiO2 for the selective catalytic reduction of NOx with NH3. J. Catal. 2013, 307, 340–351. [Google Scholar] [CrossRef]
- Marberger, A.; Elsener, M.; Ferri, D.; Sagar, A.; Schermanz, K.; Krocher, O. Generation of NH3 selective catalytic reduction active catalysts from decomposition of supported FeVO4. ACS Catal. 2015, 5, 4180–4188. [Google Scholar] [CrossRef]
- Casanova, M.; Nodari, L.; Sagar, A.; Schermanz, K.; Trovarelli, A. Preparation, characterization and NH3–SCR activity of FeVO4 supported on TiO2–WO3–SiO2. Appl. Catal. B Environ. 2015, 176, 699–708. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, X.; Lei, Z.; Gao, L.; Sha, X.; Ma, Z.; He, H.; Wang, Y.; Jia, Y.; Li, Y. Study on the mechanism of a manganese-based catalyst for catalytic NOx flue gas denitration. AIP Adv. 2018, 8, 045004. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Zhu, T.; Su, H.; Zhu, J. Selective catalytic reduction of NOx by NH3 over Mn-promoted V2O5/TiO2 catalyst. Ind. Eng. Chem. Res. 2014, 53, 12964–12970. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Li, H.; Hu, H.; Han, J.; Shi, L.; Zhang, D. Highly dispersed V2O5/TiO2, modified with transition metals (Cu, Fe, Mn, Co) as efficient catalysts for the selective reduction of NO with NH3. Chin. J. Catal. 2015, 36, 1886–1899. [Google Scholar] [CrossRef]
- Shan, W.P.; Liu, F.D.; He, H.; Shi, X.Y.; Zhang, C.B. A superior Ce-W-Ti mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2012, 115, 100–106. [Google Scholar] [CrossRef]
- De, S.; Zhang, J.G.; Luque, R.; Yan, N. Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- Wu, G.; Feng, X.; Zhang, H.; Zhang, Y.; Wang, J.; Chen, Y.; Dan, Y. The promotional role of Ni in FeVO4 /TiO2 monolith catalyst for selective catalytic reduction of NOx with NH3. Appl. Surf. Sci. 2018, 427, 24–36. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.Y.; Li, R.Y.; Zhao, Q.D.; Ke, J.; Xiao, H.N.; Wang, L.D.; Liu, S.M.; Tade, M.; Wang, S.B. Facile synthesis of tube-shaped Mn-Ni-Ti solid solution and preferable Langmuir-Hinshelwood mechanism for selective catalytic reduction of NOx by NH3. Appl. Catal. A Gen. 2018, 549, 289–301. [Google Scholar] [CrossRef]
- Sun, P.; Guo, R.T.; Liu, S.M.; Wang, S.X.; Pan, W.G.; Li, M.Y. The enhanced performance of MnOx catalyst for NH3-SCR reaction by the modification with Eu. Appl. Catal. A Gen. 2017, 531, 129–138. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Tong, H.; Tong, Z.Q.; Huang, Y.; Luo, J. Catalytic reduction of NO over Mn-V-Ce/TiO2 catalysts at low reaction temperature. J. Fuel Chem. Technol. 2010, 38, 343–351. (In Chinese) [Google Scholar]
- Li, C.X.; Shen, M.Q.; Yu, T.; Wang, J.Q.; Wang, J.; Zhai, Y.P. The mechanism of ammonium bisulfate formation and decomposition over V/WTi catalysts for NH3-selective catalytic reduction at various temperatures. Phys. Chem. Chem. Phys. 2017, 19, 15194–15206. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.W.; Park, K.H.; Hong, S.C. Enhancement of SCR activity and SO2 resistance on VOx/TiO2 catalyst by addition of molybdenum. Chem. Eng. J. 2016, 284, 315–324. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.H.; Ge, M. The poisoning effect of alkali metals doping over nano V2O5–WO3/TiO2 catalysts on selective catalytic reduction of NOx by NH3. Chem. Eng. J. 2011, 170, 531–537. [Google Scholar] [CrossRef]
- Hu, W.S.; Zhang, Y.H.; Liu, S.J.; Zheng, C.H.; Gao, X.; Nova, I.; Tronconi, E. Improvement in activity and alkali resistance of a novel V-Ce(SO4)2/Ti catalyst for selective catalytic reduction of NO with NH3. Appl. Catal. B Environ. 2017, 206, 449–460. [Google Scholar] [CrossRef]
- Gao, S.; Wang, P.L.; Yu, F.X.; Wang, H.Q.; Wu, Z.B. Dual resistance to alkali metals and SO2: Vanadium and cerium supported on sulfated zirconia as an efficient catalyst for NH3-SCR. Catal. Sci. Technol. 2016, 6, 8148–8156. [Google Scholar] [CrossRef]
- Huang, Z.W.; Li, H.; Gao, J.Y.; Gu, X.; Zheng, L.; Hu, P.P.; Xin, Y.; Chen, J.X.; Chen, Y.X.; Zhang, Z.L.; et al. Alkali- and sulfur-resistant tungsten-based catalysts for NOx emissions control. Environ. Sci. Technol. 2015, 49, 14460–14465. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, X.; Chen, P.; Zhu, B. Research Status and Prospect on Vanadium-Based Catalysts for NH3-SCR Denitration. Materials 2018, 11, 1632. https://doi.org/10.3390/ma11091632
Zhang J, Li X, Chen P, Zhu B. Research Status and Prospect on Vanadium-Based Catalysts for NH3-SCR Denitration. Materials. 2018; 11(9):1632. https://doi.org/10.3390/ma11091632
Chicago/Turabian StyleZhang, Jie, Xiangcheng Li, Pingan Chen, and Boquan Zhu. 2018. "Research Status and Prospect on Vanadium-Based Catalysts for NH3-SCR Denitration" Materials 11, no. 9: 1632. https://doi.org/10.3390/ma11091632
APA StyleZhang, J., Li, X., Chen, P., & Zhu, B. (2018). Research Status and Prospect on Vanadium-Based Catalysts for NH3-SCR Denitration. Materials, 11(9), 1632. https://doi.org/10.3390/ma11091632




