A Study of the Effects of Al, Cr, Hf, and Ti Additions on the Microstructure and Oxidation of Nb-24Ti-18Si Silicide Based Alloys
Abstract
:1. Introduction
2. Experimental
3. Results
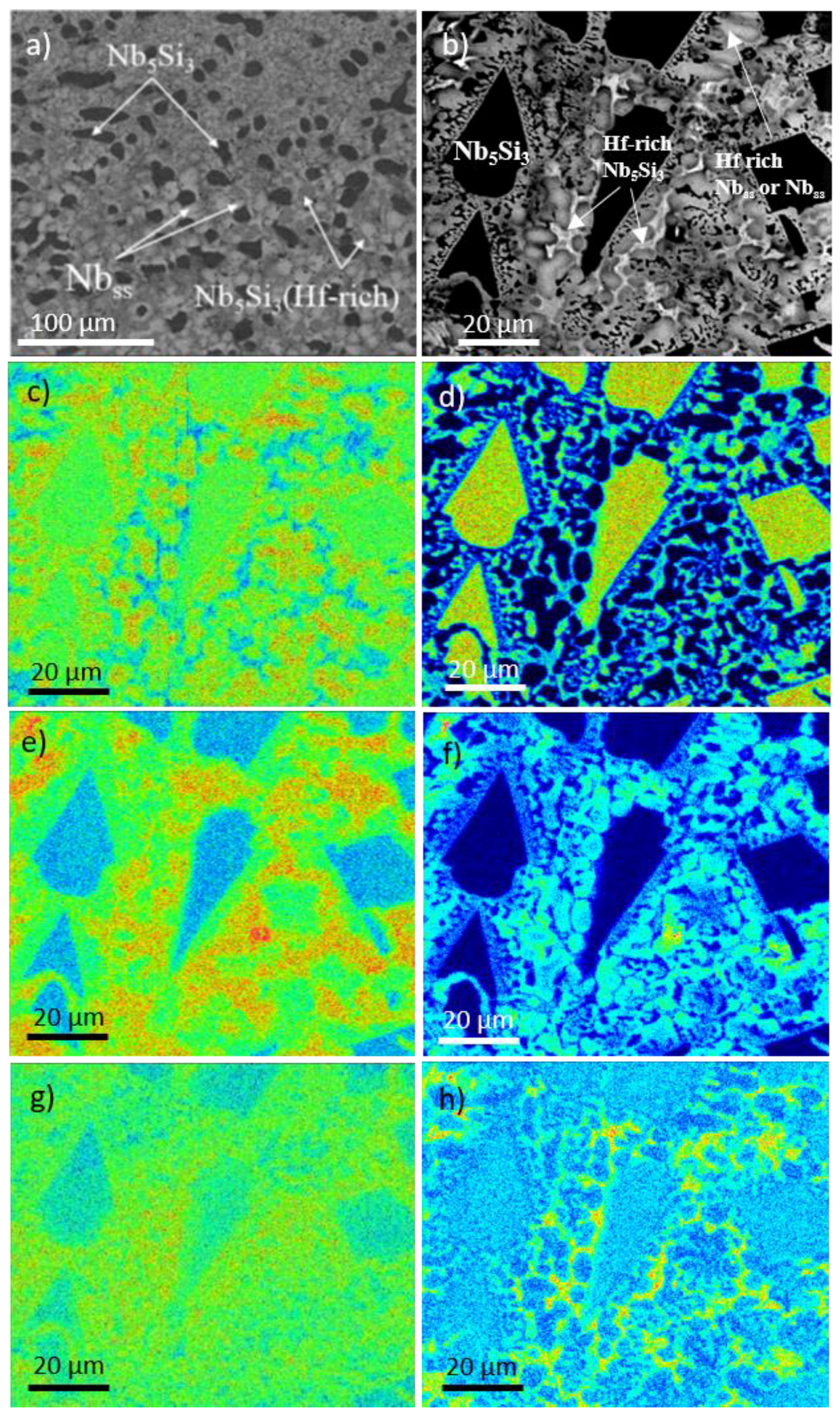
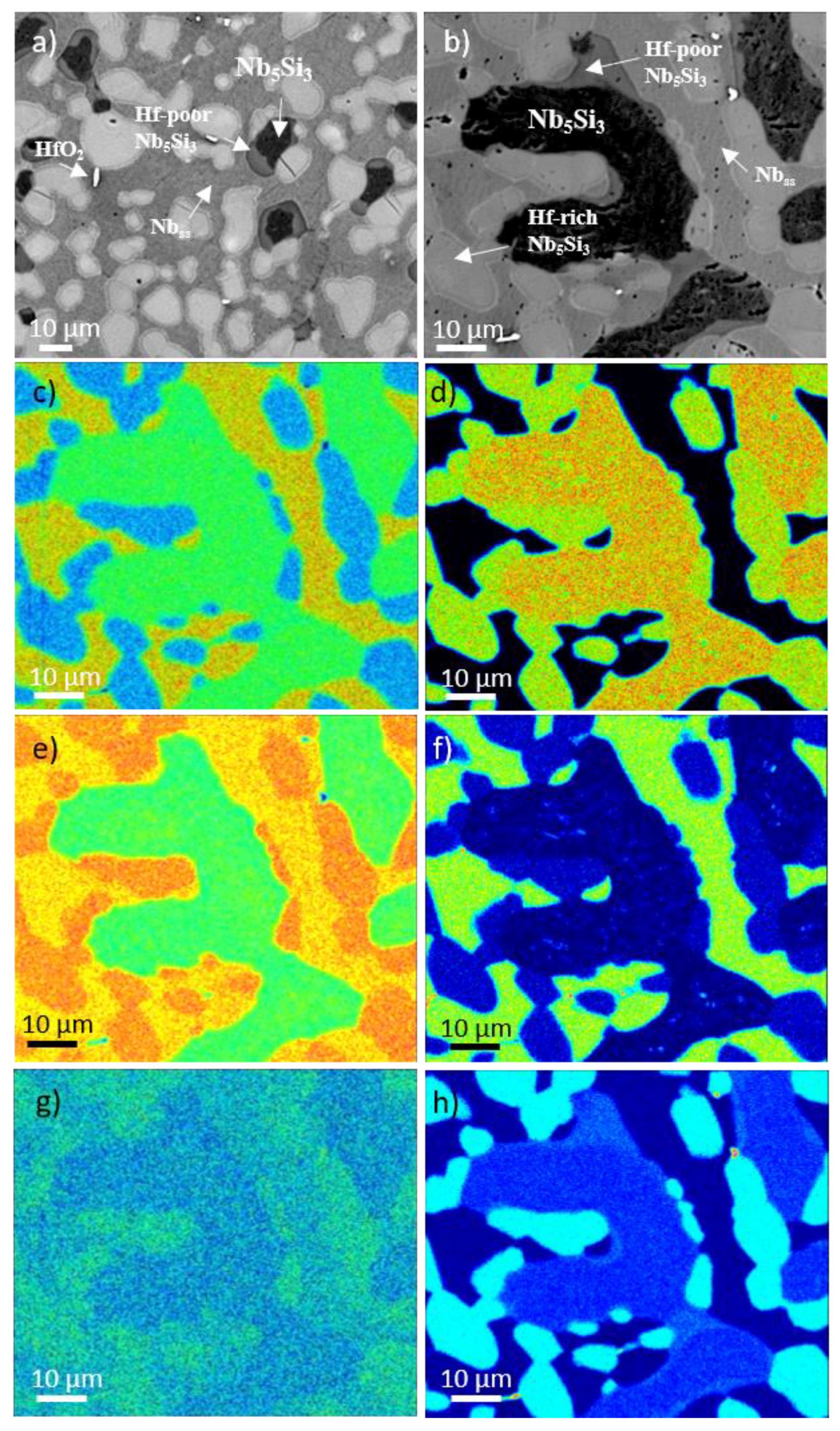
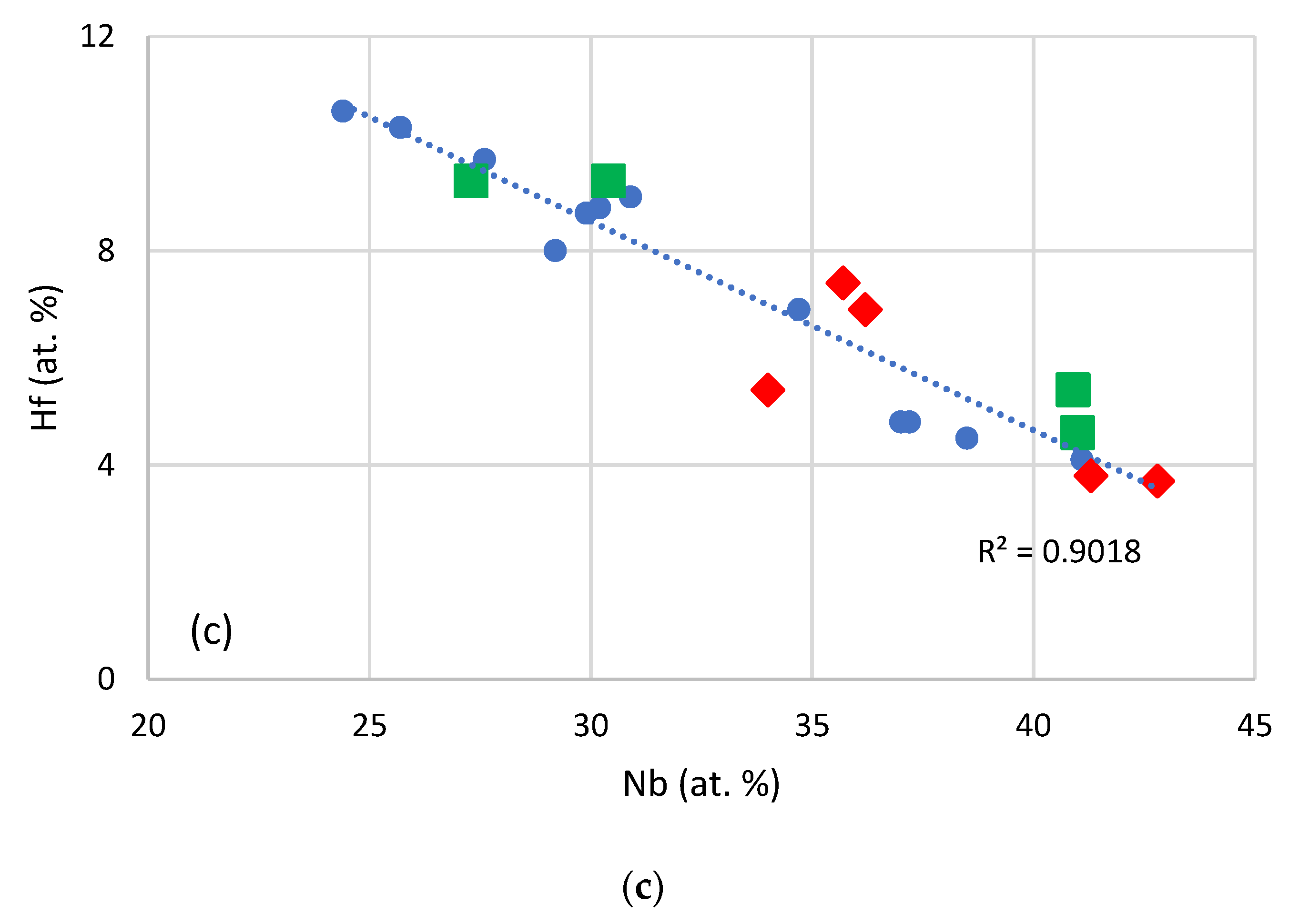
3.1. Cast Alloys
3.2. Heat Treated Alloys
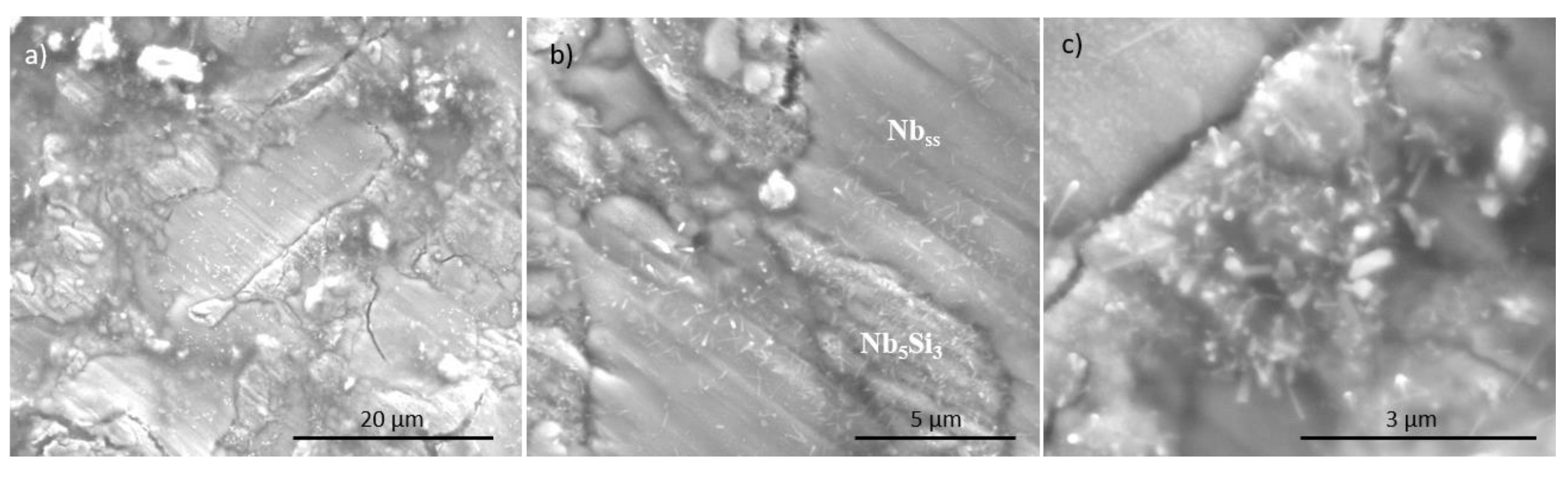
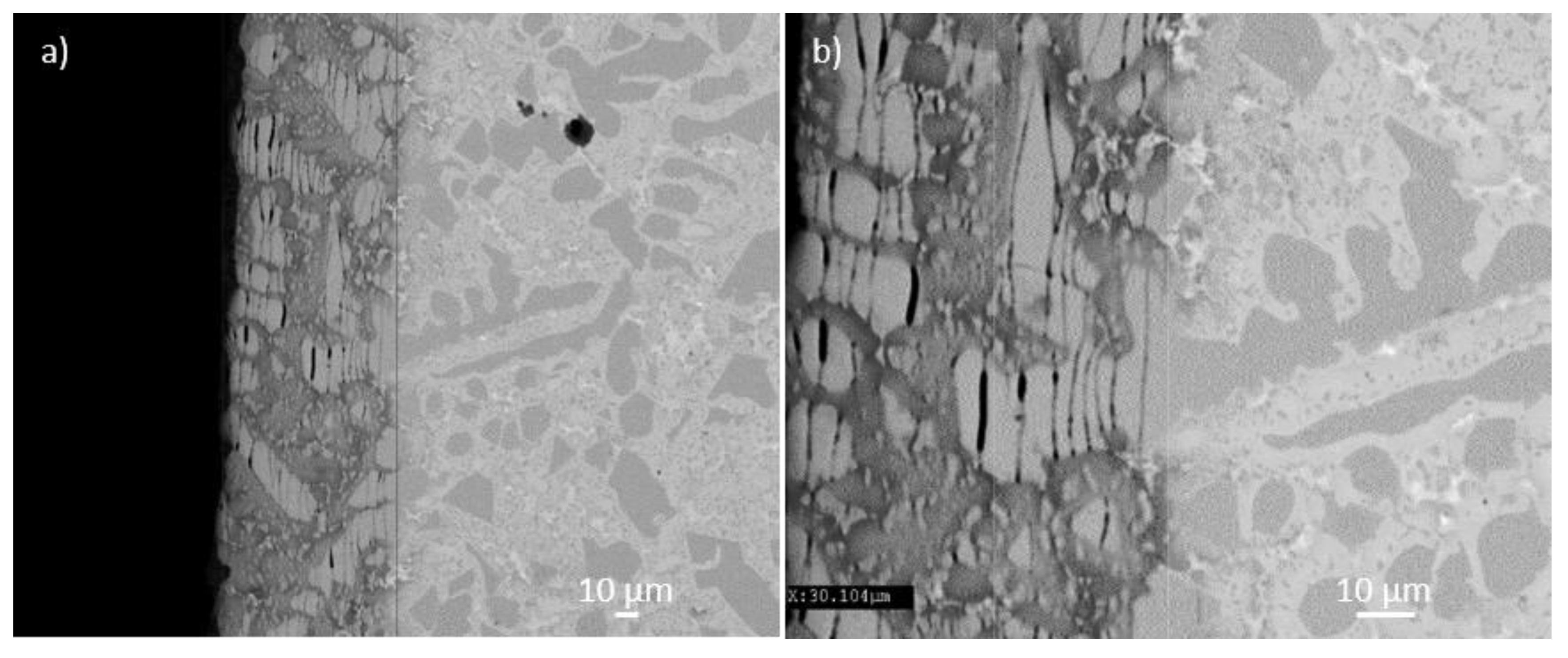
3.3. Oxidation at 800 °C
3.4. Oxidation at 1200 °C
3.5. Hardness and Young’s Modulus
4. Discussion
4.1. Macrosegregation
4.2. Microstructure
4.3. Hardness and Young’s Modulus
4.4. Oxidation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bewlay, B.P.; Jackson, M.R.; Subramanian, P.R.; Zhao, J.-C. A Review in Very-High Temperature Nb-Silicide-Based Composites. Metall. Mater. Trans. A 2003, 34, 2043–2052. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On Niobium silicide based alloys: Alloy design and selection. Materials 2018, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.R.; Bewlay, B.P.; Zhao, J.C. Niobium-Silicide Based Composites Resistant to High Temperature Oxidation. U.S. Patent 6,913,655, B2, 5 July 2005. [Google Scholar]
- Menon, E.S.K.; Mendiratta, M.G.; Dimiduk, D.M. High temperature oxidation mechanisms in Nb-silicide bearing multicomponent alloys. Struct. Intermet. 2001, 591–600. [Google Scholar]
- Geng, J.; Tsakiropoulos, P. A study of the microstructure and oxidation of Nb-Si-Cr-Al-Mo in situ composites alloyed with Ti, Hf and Sn. Intermetallics 2007, 15, 382–395. [Google Scholar] [CrossRef]
- Liu, A.; Sun, L.; Li, S.; Han, Y. Effect of Cerium on microstructure and high temperature oxidation resistance of an Nb-Si system in situ composite. J. Rare Earths 2007, 25, 474–479. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Guo, J. Effects of B on the microstructure and oxidation resistance of Nb-Ti-Si based ultra-high temperature alloy. Chin. J. Aeronaut. 2009, 22, 544–550. [Google Scholar]
- Gaillard-Allemand, B.; Vilasi, M.; Belmonte, T.; Steinmetz, J. Silicide coatings for Niobium: Mechanisms of chromium and silicon co-deposition by pack cementation. Mater. Sci. Forum 2001, 369, 727–734. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, C.; Gong, S.; Li, S.; Zhang, Y.; Xu, H. Deposition of Cr-modified silicide coatings on Nb-Si system intermetallics. Intermetallics 2007, 15, 805–809. [Google Scholar] [CrossRef]
- Tian, X.; Guo, X. Structure and oxidation behavior of Si-Y co-deposition coatings on an Nb silicide based ultra-high temperature alloy prepared by pack cementation technique. Surf. Coat. Technol. 2009, 204, 313–318. [Google Scholar] [CrossRef]
- Tabaru, T.; Kim, J.-H.; Shobu, K.; Sakamoto, M.; Hirai, H.; Hanada, S. Development of Mo(Si,Al)2 base oxidation resistant coating on Nb base structural materials. Metall. Mater. Trans. 2005, 36, 617–626. [Google Scholar] [CrossRef]
- Yao, D.; Gong, W.; Zhou, C. Development and oxidation resistance of air plasma sprayed Mo-Si-Al coating on an Nbss/Nb5Si3 in situ composite. Corros. Sci. 2010, 52, 2603–2611. [Google Scholar] [CrossRef]
- Majumdar, S.; Sengupta, P.; Kale, G.B.; Sharma, I.G. Development of multilayer oxidation resistant coatings on niobium and tantalum. Surf. Coat. Technol. 2006, 200, 3713–3718. [Google Scholar] [CrossRef]
- Jackson, M.; Subramanian, P.; Zhao, J.-C.; Bewlay, B.; Darolia, R.; Schafrik, R. Turbine Blade for Extreme Temperature Conditions. U.S. Patent 7,189,459, 13 March 2007. [Google Scholar]
- Jehanno, P.; Kestler, M.; Venskutonis, A.; Boning, M.; Heilmaier, M.; Bewlay, B.; Jackson, M. Assessment of a powder metallurgical route for refractory metal silicide alloys. Metal. Mater. Trans. 2005, 36, 515–523. [Google Scholar] [CrossRef]
- Murakami, T.; Sasaki, S.; Ichikawa, K.; Kitahara, A. Microstructure, mechanical properties and oxidation behaviour of Nb-Si-Al and Nb-Si-N powder compacts prepared by spark plasma sintering. Intermetallics 2001, 9, 621–627. [Google Scholar] [CrossRef]
- Huang, Q.; Guo, X.P.; Kang, Y.W.; Song, J.X.; Qu, S.Y.; Han, Y.F. Microstructures and mechanical properties of directionally solidified multi-element Nb-Si alloy. Prog. Nat. Sci. Mater. Int. 2011, 21, 146–152. [Google Scholar] [CrossRef]
- Sekido, N.; Kimura, Y.; Miura, S.; Mishima, Y. Microstructure development of unidirectionally solidified (Nb)/Nb3Si eutectic alloys. Mater. Sci. Eng. A 2007, 444, 51–57. [Google Scholar] [CrossRef]
- Begley, R.T.; Bechtold, J.H. Effect of alloying on the mechanical properties of Niobium. J. Less-Common Met. 1961, 3, 1–12. [Google Scholar] [CrossRef]
- Argent, B.B.; Phelps, B. The oxidation of Niobium-titanium and Niobium-molybdenum alloys. J. Less-Common Met. 1960, 2, 181–190. [Google Scholar] [CrossRef]
- Davidson, D.L.; Chan, K.S. The failure and fracture resistance of a Nb-Cr-Ti-Al alloy. Metall. Mater. Trans. A 1999, 30, 2007–2018. [Google Scholar] [CrossRef]
- Okamoto, H. Phase Diagrams for Binary Alloys: Desk Handbook, 2nd; ASM International: Geauga County, OH, USA, 2000. [Google Scholar]
- Jackson, M.R.; Bewlay, B.P.; Briant, C.L. Creep Resistant Nb-Silicide Based Two-Phase Composites. U.S. Patent 6,447,623 B1, 10 September 2002. [Google Scholar]
- Bewlay, B.P.; Sitzman, S.D.; Brewer, L.N.; Jackson, M.R. Analyses of eutectoid phase transformations in Nb-silicide in situ composites. Microsc. Microanal. 2004, 10, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Zelenitsas, K.; Tsakiropoulos, P. Study of the role of Al and Cr additions in the microstructure of Nb-Ti-Si in situ composites. Intermetallics 2005, 13, 1079–1095. [Google Scholar] [CrossRef]
- Grammenos, I.; Tsakiropoulos, P. Study of the role of Al, Cr and Ti additions in the microstructure of Nb-18Si-5Hf base alloys. Intermetallics 2010, 18, 242–253. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. Alloying and hardness of eutectics with Nbss and Nb5Si3 in Nb-silicide based alloys. Materials 2018, 11, 592. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 6, 1564–1583. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On the macrosegregation of silicon in niobium silicide based alloys. Intermetallics 2014, 55, 95–101. [Google Scholar] [CrossRef]
- Ghosh, G.; Olson, G.B. Integrated design of Nb-based superalloys: Ab initio calculations, computational thermodynamics and kinetics and experimental results. Acta Mater. 2007, 55, 3281–3303. [Google Scholar] [CrossRef]
- Chan, K.S. Alloying effects on fracture mechanisms in Nb-based intermetallic in-situ composites. Mater. Sci. Eng. 2002, 337, 59–66. [Google Scholar] [CrossRef]
- Tian, Y.X.; Guo, J.T.; Zhou, L.Z.; Cheng, G.M.; Ye, H.Q. Microstructure and room temperature fracture toughness of cast Nbss/silicide composites alloyed with Hf. Mater. Lett. 2008, 62, 2657–2660. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On Nb silicide based alloys: Part I-The bcc Nb solid solution. J Alloys Compd. 2017, 708, 961–971. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On the alloying and properties of tetragonal Nb5Si3 in Nb-silicide based alloys. Materials 2018, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, V. Al-Nb-Si (Aluminum-Niobium-Silicon). J. Phase Equilib. Diffus. 2006, 27, 163–165. [Google Scholar]
- Batzner, C.; Beuers, J.; Hoch, M.; Schmid-Fetzer, R. Ternary Alloys; VCH: New York, NY, USA, 1993; pp. 370–374. [Google Scholar]
- Schob, O.; Nowotny, H.; Benesovsky, F. The ternary systems formed by Ti, Zr and Hf with Al and Si. Planseeber Pulvermetall 1962, 10, 65–71. [Google Scholar]
- Bulanova, M.; Tretyachenko, L.; Golovkova, M.; Meleshevich, K. Phase equilibria in the α-Ti-Al-Si region of the Ti-Si-Al system. J. Phase Equilib. Diffus. 2004, 25, 209–229. [Google Scholar] [CrossRef]
- Kim, J.-H.; Tabaru, T.; Hirai, H. Evaluation technique of the hardness and elastic modulus of materials with fine microstructures. Mater. Trans. 2003, 44, 673–676. [Google Scholar] [CrossRef]
- Papadimitriou, I.; Utton, C.; Tsakiropoulos, P. The impact of Ti and temperature on the stability of Nb5Si3 phases: A first-principles study. Sci. Technol. Adv. Mater. 2017, 18, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Knittel, S.; Mathieu, S.; Portebois, L.; Vilasi, M. Effect of tin addition on Nb-Si-based in situ composites. Part II: Oxidation behavior. Intermetallics 2104, 47, 43–52. [Google Scholar] [CrossRef]
- Thandorn, T. Study of the effect of metalloid element addition on the properties of Nb-silicide based alloys. University of Sheffield: Sheffield, UK, Unpublished work. 2010. [Google Scholar]
- Bywater, G. Study of the effect of metalloid element addition on the oxidation of Nb-silicide based alloys. University of Sheffield: Sheffield, UK, Unpublished work. 2013. [Google Scholar]
- Zhao, J. Study of the effect of refractory metal addition on the properties of Nb-silicide based alloys. University of Sheffield: Sheffield, UK, Unpublished work. 2017. [Google Scholar]
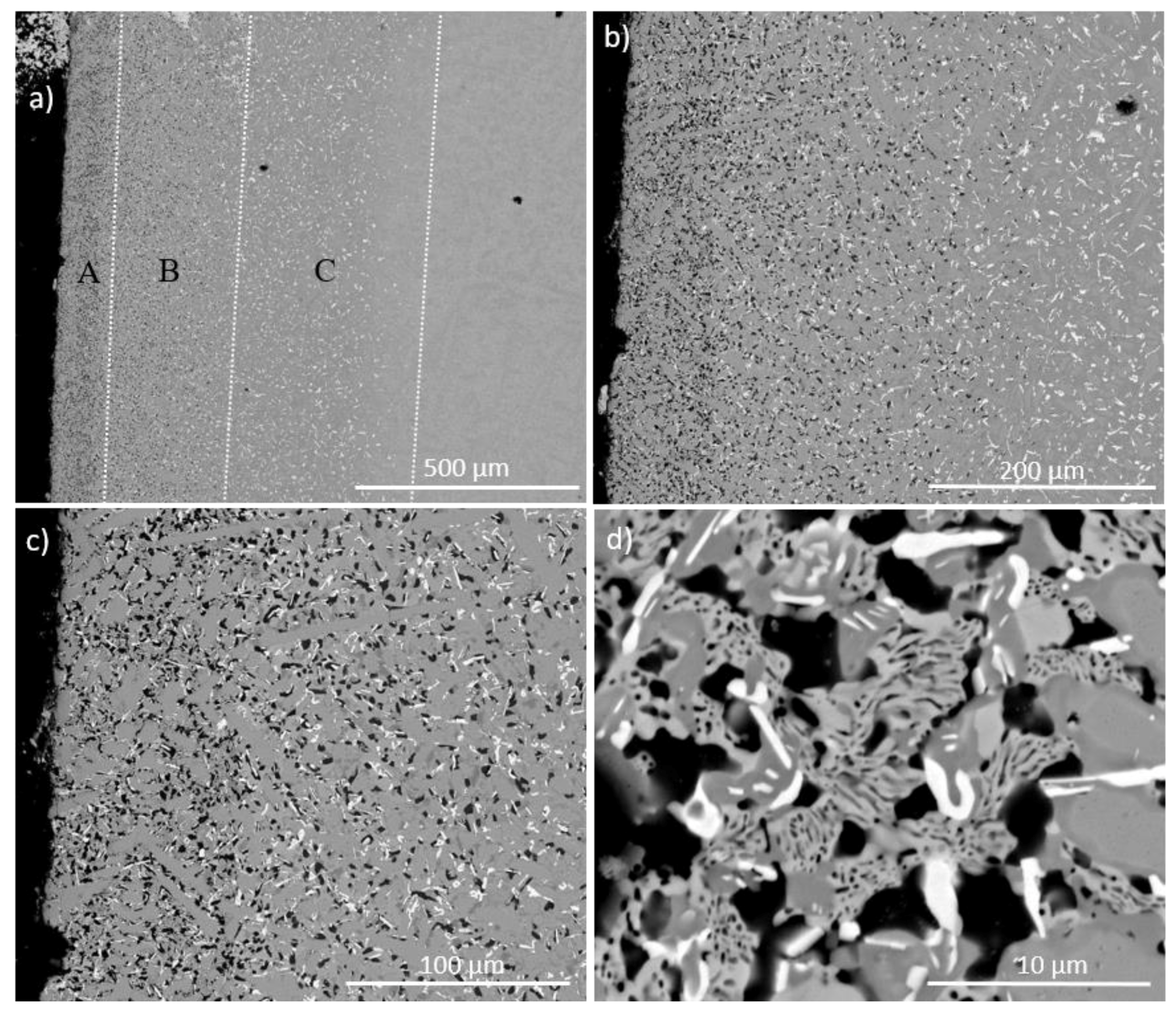
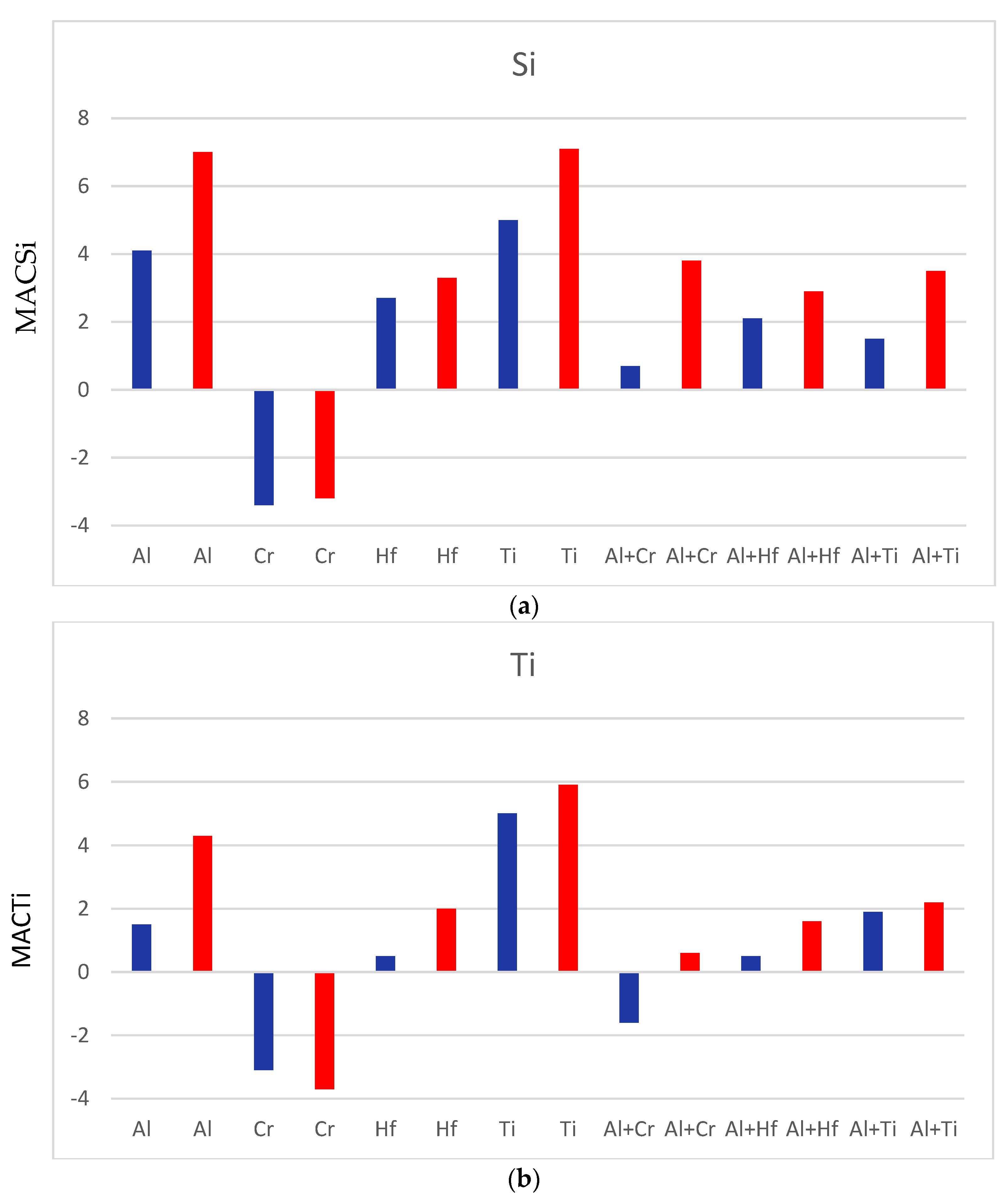
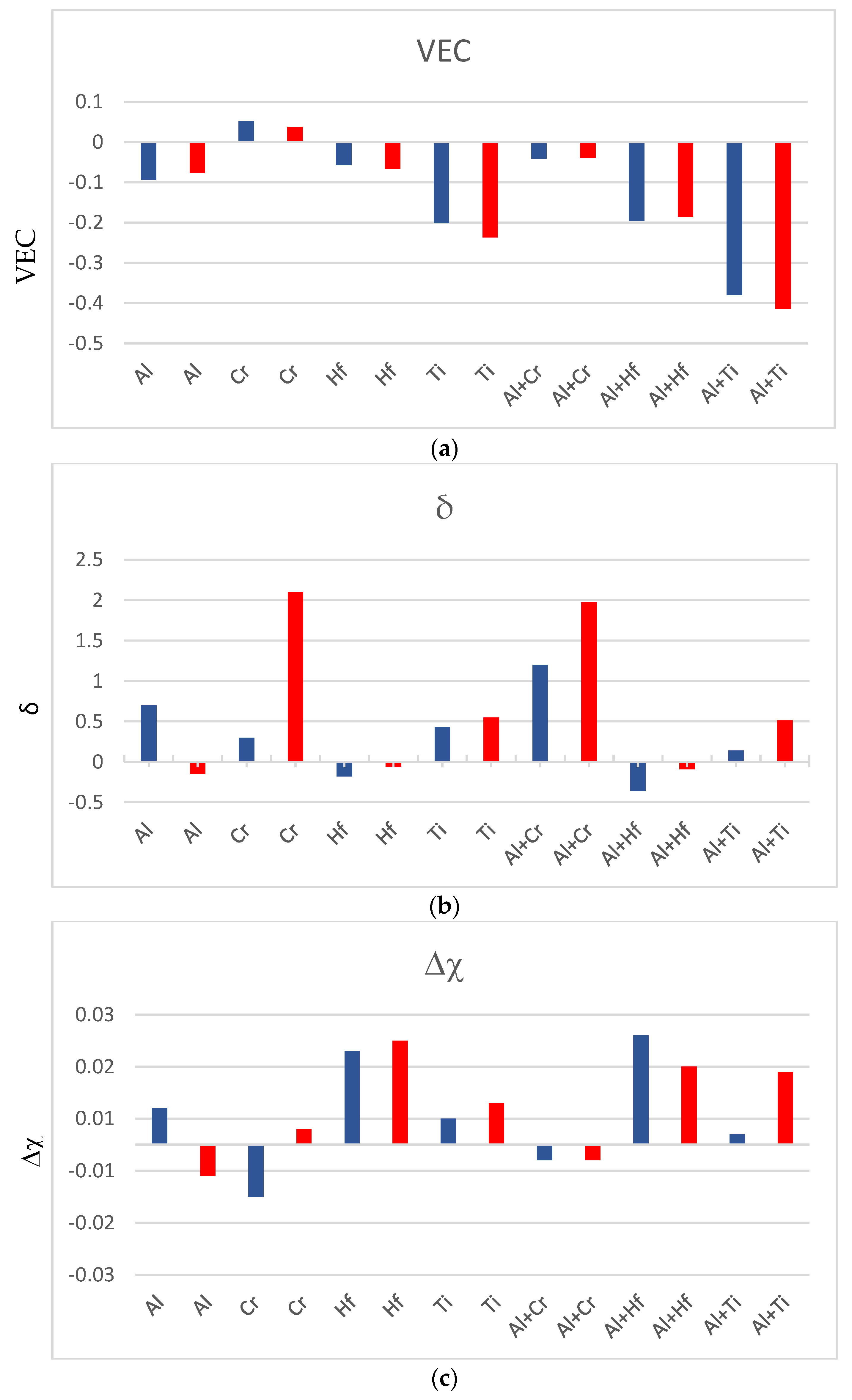
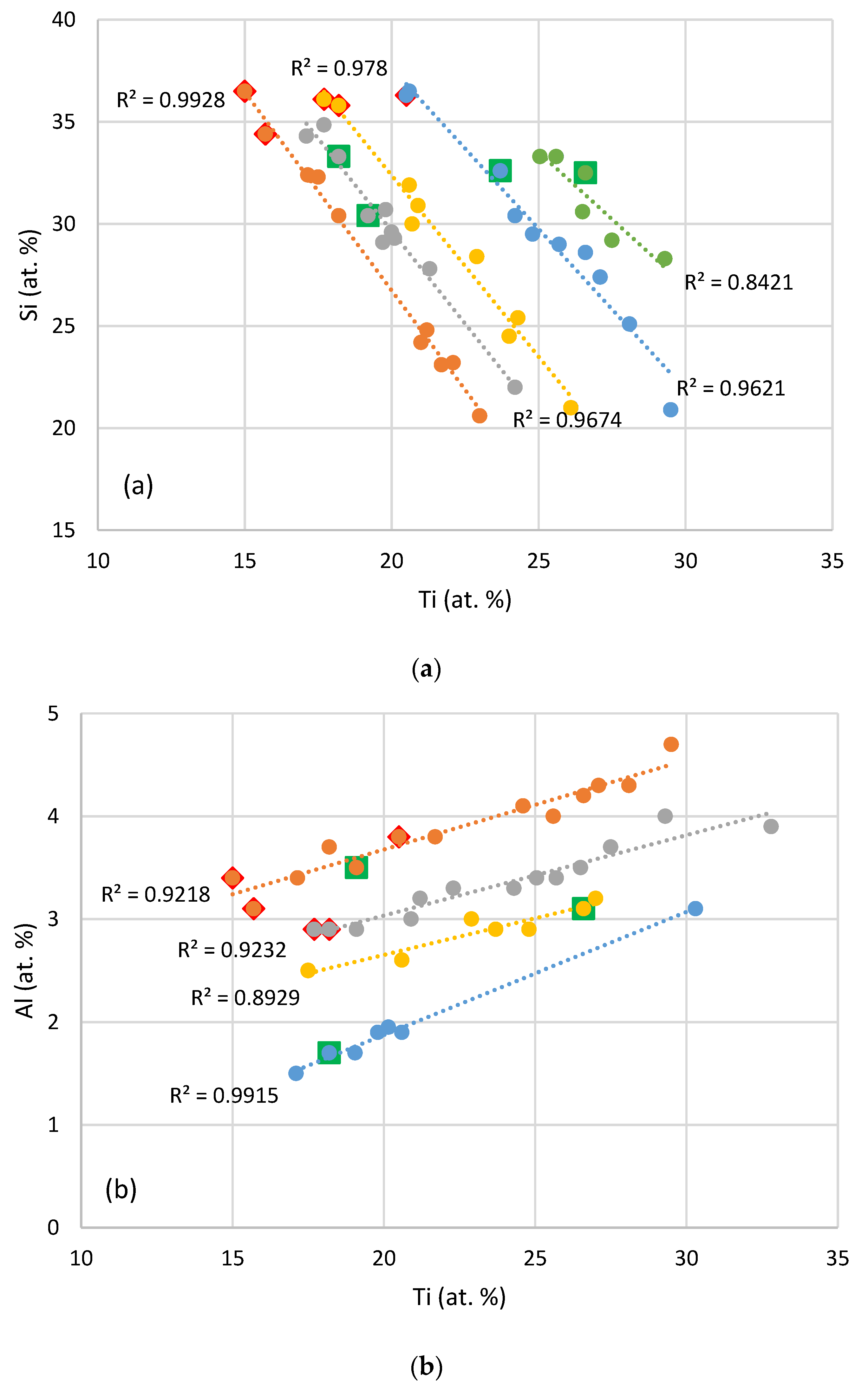

| Alloy & Phase | Nb | Si | Ti | Al | Hf |
|---|---|---|---|---|---|
| As Cast | |||||
| Average alloy | 46.2 ± 2.1 42.1–49.8 | 21.3 ± 1.8 18.8–26.2 | 22.3 ± 1.2 20.1–25.3 | 4.5 ± 0.4 3.5–5.2 | 5.4 ± 0.4 4.5–6.0 |
| Nbss | 64.8 ± 2.9 58.9–68.6 | 3.8 ± 1.2 2.5–5.4 | 21.9 ± 1.6 19.5–25.7 | 6.2 ± 0.2 6.0–6.7 | 3.3 ± 0.3 2.9–4.0 |
| Nb5Si3 | 41.3 ± 1.2 40.5–44.5 | 36.5 ± 1.4 34.1–38.5 | 15.0 ± 0.3 14.0–15.5 | 3.4 ± 0.3 3.0–3.9 | 3.8 ± 0.3 3.6–4.4 |
| Hf rich Nb5Si3 | 36.2 ± 2.2 31.5–38.1 | 36.1 ± 1.5 32.7–37.5 | 17.7 ± 3.0 15.9–24.7 | 2.9 ± 0.4 2.5–3.8 | 6.9 ± 0.5 6.5–7.8 |
| Nbss + βNb5Si3 eutectic | 52.6 ± 2.4 49.3–57.8 | 15.5 ± 1.2 12.1–17.9 | 22.1 ± 2.1 18.5–26.1 | 5.1 ± 0.4 4.5–5.7 | 4.6 ± 0.3 3.9–5.2 |
| Heat Treated | |||||
| Average alloy | 48.0 ± 1.9 44.5–51.6 | 20.5 ± 2.6 16.3–25.4 | 21.3 ± 1.2 19.2–25.1 | 4.3 ± 0.4 3.4–5.1 | 5.7 ± 0.3 5.1–6.4 |
| Nbss | 68.2 ± 2.5 65.8–75.5 | 0.9 ± 0.3 0.4–1.5 | 22.6 ± 2.5 14.7–24.1 | 6.1 ± 0.5 4.1–7.0 | 2.1 ± 0.3 1.4–2.6 |
| Hf rich Nb5Si3 (bulk *) | 34.0 ± 1.1 33.2–34.8 | 36.3 ± 1.4 35.2–37.3 | 20.5 ± 0.6 20.1–20.9 | 3.8 ± 0.1 3.8–3.9 | 5.4 ± 0.1 5.3–5.5 |
| Nb5Si3 | 42.8 ± 1.0 41.3–43.9 | 34.4 ± 0.6 33.5–35.1 | 15.7 ± 1.2 14.4–17.5 | 3.1 ± 0.4 2.5–3.6 | 3.7 ± 0.3 3.3–4.2 |
| Hf rich Nb5Si3 (bottom *) | 35.7 ± 3.1 27.9–38.6 | 35.8 ± 2.1 30.7–38.0 | 18.2 ± 3.1 14.5–24.6 | 2.9 ± 0.6 2.0–4.1 | 7.4 ± 0.7 6.3–8.6 |
| Prior eutectic | 48.7 ± 1.4 46.2–51.7 | 17.7 ± 1.2 15.2–19.4 | 23.4 ± 0.6 22.4–23.9 | 4.7 ± 0.3 4.4–5.1 | 5.5 ± 0.2 5.2–5.9 |
| Alloy & Phase | Nb | Si | Ti | Cr | Al | Hf |
|---|---|---|---|---|---|---|
| As Cast | ||||||
| Average alloy | 41.2 ± 0.6 40.4–42.4 | 20.1 ± 1.0 17.7–21.7 | 23.5 ± 0.5 22.6–24.5 | 4.5 ± 0.3 4.0–5.0 | 5.5 ± 0.2 5.0–5.9 | 5.2 ± 0.2 5.0–5.6 |
| Nbss | 53.7 ± 3.2 48.7–57.0 | 1.7 ± 0.4 1.3–2.5 | 26.5 ± 1. 924.7–29.5 | 8.0 ± 1.1 6.7–9.3 | 6.2 ± 0.2 6.2–6.8 | 3.6 ± 0.7 3.0–4.5 |
| Hf rich Nbss | 45.9 ± 4.0 40.6–51.8 | 2.0 ± 0.7 1.3–3.5 | 30.7 ± 1.7 28.1–33.4 | 9.4 ± 1.0 7.5–11.1 | 6.5 ± 0.2 6.2–6.8 | 5.5 ± 0.7 4.7–6.4 |
| Nb5Si3 | 41.0 ± 1.4 38.8–42.6 | 30.4 ± 1.2 28.2–31.5 | 19.2 ± 1.4 17.8–20.8 | 1.3 ± 0.5 0.9–2.3 | 3.5 ± 0.5 3.0–4.2 | 4.6 ± 0.1 4.4–4.7 |
| Hf rich Nb5Si3 | 30.4 ± 0.6 30.1–31.3 | 32.6 ± 0. 931.2–33.3 | 23.7 ± 0.1 23.5–23.7 | 1.1 ± 0.4 0.9–1.8 | 2.9 ± 0.1 2.8–3.0 | 9.3 ± 0.3 8.9–9.5 |
| Nbss + βNb5Si3 eutectic | 46.8 ± 2.2 44.1–49.2 | 13.3 ± 1.5 12.0–14.9 | 24.2 ± 1.1 23.2–25.5 | 5.3 ± 0.6 4.8–6.1 | 5.6 ± 0.2 5.2–6.0 | 4.8 ± 0.5 4.6–4.9 |
| Heat Treated | ||||||
| Average alloy | 43.1 ± 1.0 42.2–44.7 | 19.3 ± 1.8 15.3–21.2 | 24.1 ± 0.8 22.9–25.1 | 4.1 ± 0.5 3.5–5.0 | 3.8 ± 0. 92.5–4.9 | 5.7 ± 0.3 5.3–6.0 |
| Nbss | 53.7 ± 0.8 53.1–54.9 | 0.5 ± 0.1 0.5–0.8 | 27.0 ± 0.1 26.9–27.2 | 9.4 ± 0.3 9.0–9.8 | 7.1 ± 0.7 6.1–7.7 | 2.2 2.1–2.2 |
| Nb5Si3 | 40.9 ± 0.3 40.6–41.2 | 33.3 ± 0.2 33.1–33.6 | 18.2 ± 0.2 17.9–18.5 | 0.5 0.4–0.5 | 1.7 ± 0.1 1.6–1.8 | 5.4 ± 0.2 5.2–5.7 |
| Hf rich * Nb5Si3 * | 27.3 ± 0.3 27.1–27.9 | 32.5 ± 0.1 32.3–32.7 | 26.6 ± 0.1 26.3–26.7 | 1.2 1.2–1.3 | 3.1 ± 0.2 2.7–3.3 | 9.3 ± 0.1 9.0–9.5 |
| Hf poor * Nb5Si3 * | 41.3 ± 0.1 41.2–41.5 | 32.0 ± 0.3 31.4–32.4 | 18.8 ± 0.1 18.7–18.8 | 0.6 0.5–0.7 | 2.7 ± 0.3 2.4–3.2 | 4.6 ± 0.1 4.5–4.7 |
| Alloy | Phase | Micro-Hardness (Vickers) | Nano-Hardness (Vickers) | Nano-Hardness (GPa) | Young’s Modulus (GPa) |
|---|---|---|---|---|---|
| KZ7-HT | Nbss | 395.0 ± 19.0 | 502.0 ± 16.9 | 4.95 | 138.0 ± 12.0 |
| KZ5-HT | Nbss | 466.0 ± 20.0 | 645.0 ± 31.2 | 6.5 | 131.0 ± 18.0 |
| JN1-HT | Nbss | 450.0 * | 597.0 ± 35.8 | 5.85 | 137.6 ± 10.4 |
| KZ7-HT | αNb5Si3 | 1136.0 ± 60.0 | 1907.0 ± 106.0 | 18.7 | 283.8 ± 25.2 |
| KZ5-HT | Nb5Si3 | 1131.0 ± 54.0 | 1685.0 ± 142.0 | 16.5 | 238.5 ± 23.6 |
| JN1-HT | βNb5Si3 | 1124.0 * | 1775.0 ± 153.0 | 17.4 | 241.4 ± 24.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, J.; Ghadyani, M.; Utton, C.; Tsakiropoulos, P. A Study of the Effects of Al, Cr, Hf, and Ti Additions on the Microstructure and Oxidation of Nb-24Ti-18Si Silicide Based Alloys. Materials 2018, 11, 1579. https://doi.org/10.3390/ma11091579
Nelson J, Ghadyani M, Utton C, Tsakiropoulos P. A Study of the Effects of Al, Cr, Hf, and Ti Additions on the Microstructure and Oxidation of Nb-24Ti-18Si Silicide Based Alloys. Materials. 2018; 11(9):1579. https://doi.org/10.3390/ma11091579
Chicago/Turabian StyleNelson, Jack, Mohammad Ghadyani, Claire Utton, and Panos Tsakiropoulos. 2018. "A Study of the Effects of Al, Cr, Hf, and Ti Additions on the Microstructure and Oxidation of Nb-24Ti-18Si Silicide Based Alloys" Materials 11, no. 9: 1579. https://doi.org/10.3390/ma11091579
APA StyleNelson, J., Ghadyani, M., Utton, C., & Tsakiropoulos, P. (2018). A Study of the Effects of Al, Cr, Hf, and Ti Additions on the Microstructure and Oxidation of Nb-24Ti-18Si Silicide Based Alloys. Materials, 11(9), 1579. https://doi.org/10.3390/ma11091579






