Porous Silicon-Based Catalysts for the Dehydration of Glycerol to High Value-Added Products
Abstract
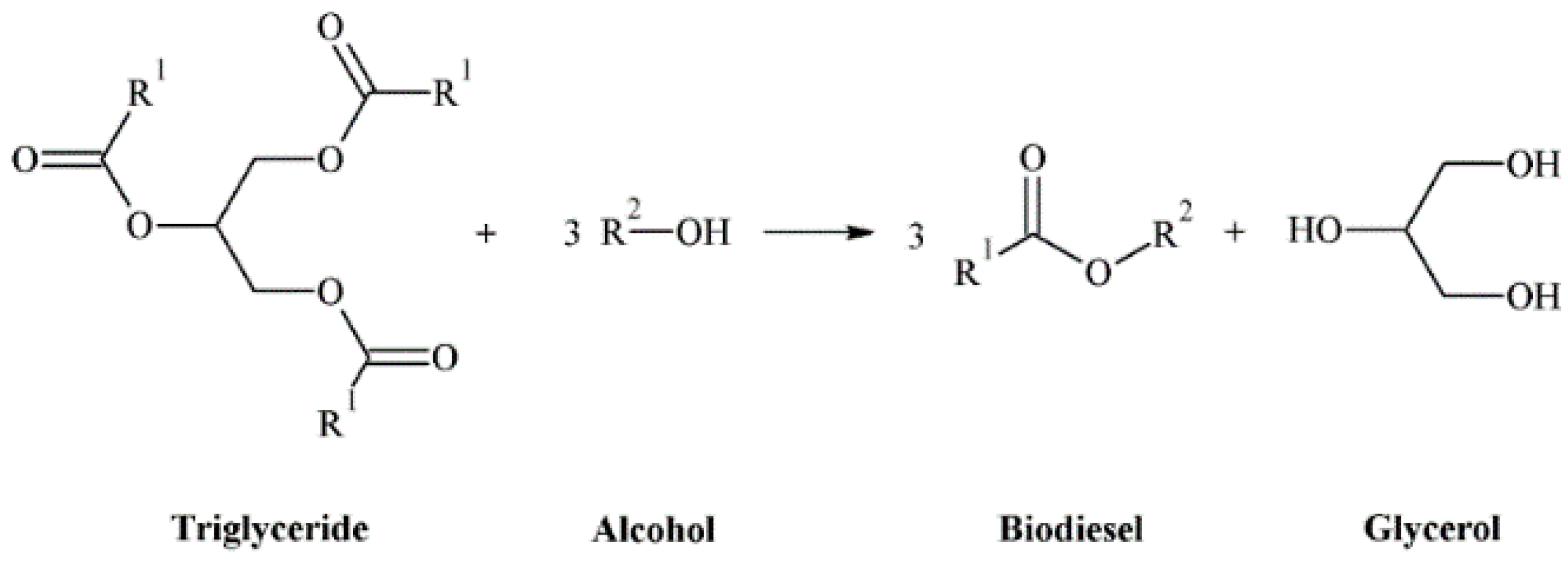
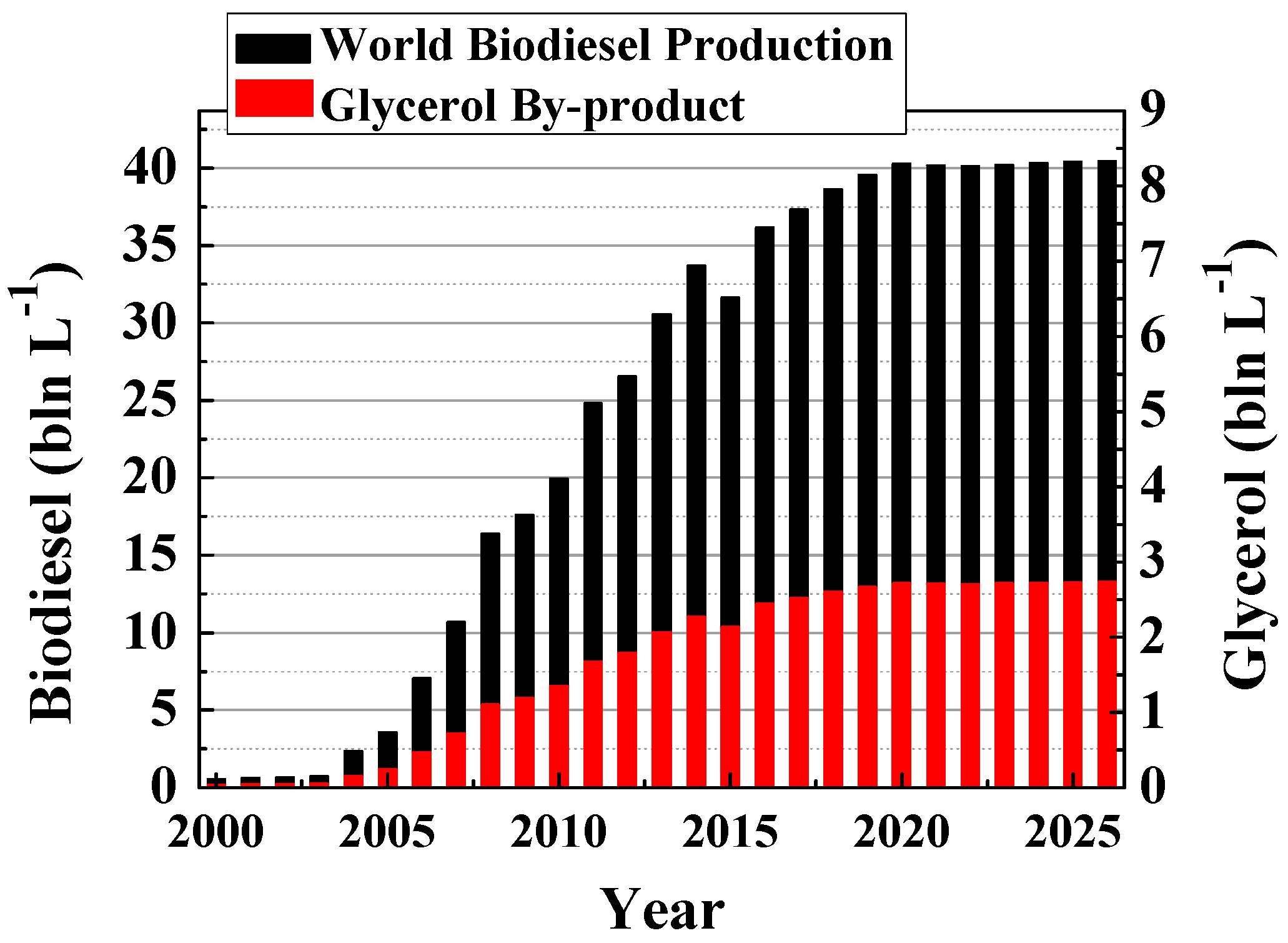
1. Introduction
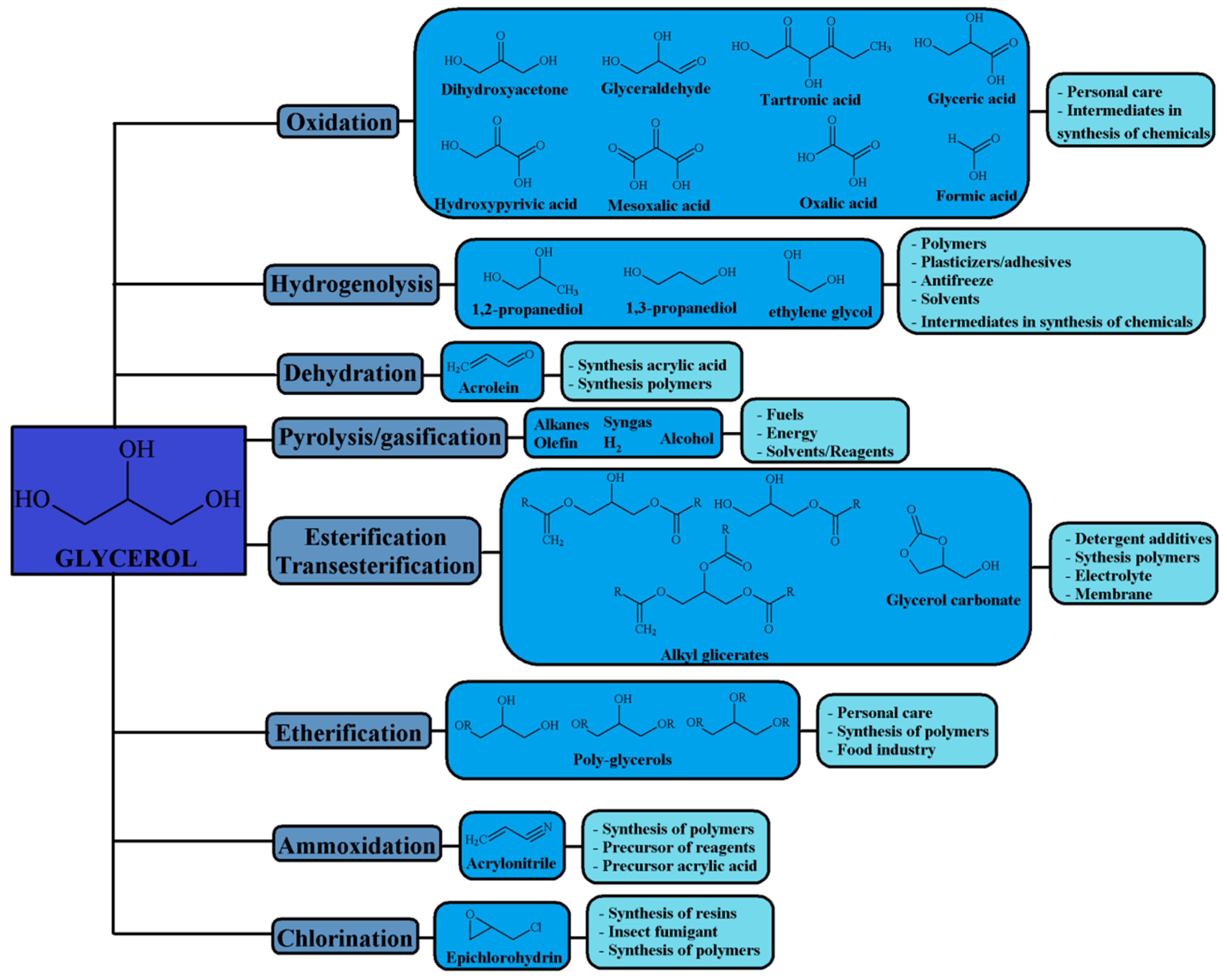
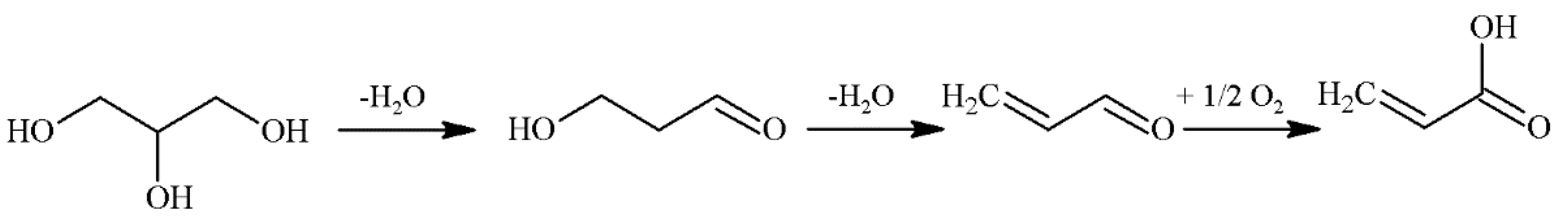
2. Glycerol Valorization
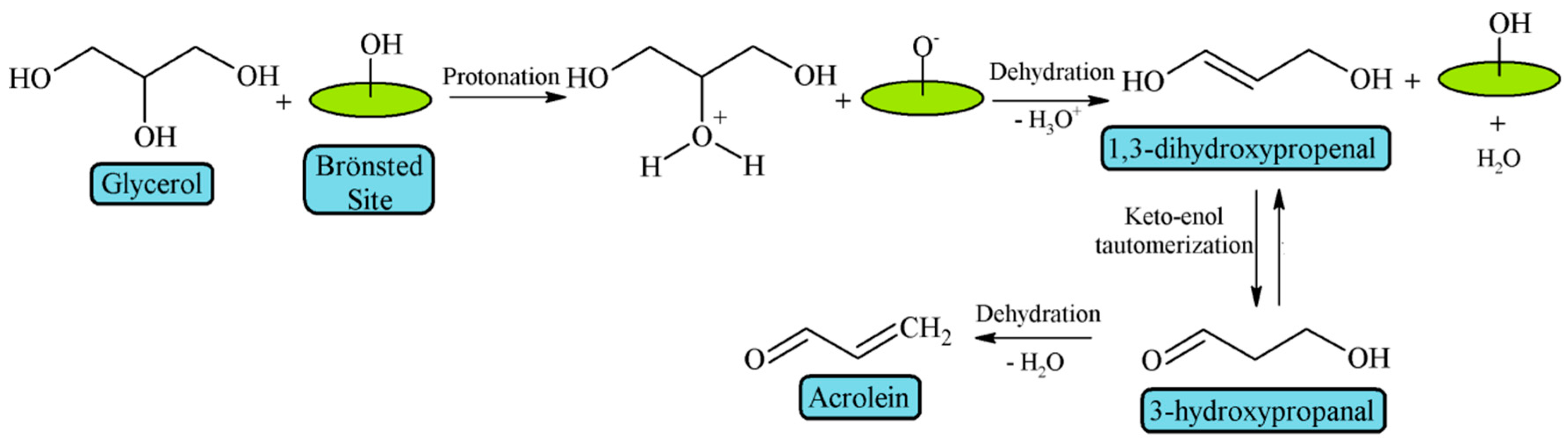
3. Glycerol Dehydration
4. Mesoporous Silica-Based Catalysts Used in the Glycerol Dehydration
4.1. Mesoporous Silica
4.2. Zeolites
4.3. Silicon-Aluminophosphate (SAPO)
4.4. Commercial Silica
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klass, D.L. Biomass for Renewable Energy, Fuels and Chemicals; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar]
- European Parliament and of the Council. Directive 2003/30/EC of the European Parliament and of the Council of 05/08/2003 on the Promotion of the Use of Biofuels or Other Renewable Fuels for Transport; European Parliament and of the Council: Luxembourg, 2003. [Google Scholar]
- Ong, H.; Mahlia, T.; Masjuki, H.; Norhasyima, R. Comparison of palm oil, jatropha curcas and calophyllum inophyllum for biodiesel: A review. Renew. Sustain. Energy Rev. 2011, 15, 3501–3515. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.; Zarei, A.; Noshadi, I. Transesterification of waste cooking oil by heteropoly acid (HPA) catalyst: Optimization and kinetic model. Appl. Energy 2013, 102, 283–292. [Google Scholar] [CrossRef]
- Tan, H.; Aziz, A.; Aroua, M. Glycerol production and its applications as a raw material: A review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Ciriminna, R.; Della Pina, C.; Rossi, M.; Pagliaro, M. Understanding the glycerol market. Eur. J. Lipid Sci. Technol. 2014, 116, 1432–1439. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development/Food and Agriculture Organization of the United Nations (OECD-FAO) Agricultural Outlook 2017–2026. Available online: http://dx.Doi.Org/10.1787/agr_outlook-2017-en (accessed on 21 February 2018).
- Katryniok, B.; Paul, S.; Belliere-Baca, V.; Rey, P.; Dumeignil, F. Glycerol dehydration to acrolein in the context of new uses of glycerol. Green Chem. 2010, 12, 2079–2098. [Google Scholar] [CrossRef]
- Tsukuda, E.; Sato, S.; Takahashi, R.; Sodesawa, T. Production of acrolein from glycerol over silica-supported heteropoly acids. Catal. Commun. 2007, 8, 1349–1353. [Google Scholar] [CrossRef]
- Pathak, K.; Reddy, K.; Bakhshi, N.; Dalai, A. Catalytic conversion of glycerol to value added liquid products. Appl. Catal. A Gen. 2010, 372, 224–238. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value-Added Chemicals from Biomass; US Department of Energy (USDOE): Washington, DC, USA, 2004; Volume 1.
- Zhou, C.; Zhao, H.; Tong, D.; Wu, L.; Yu, W. Recent advances in catalytic conversion of glycerol. Catal. Rev. 2013, 55, 369–453. [Google Scholar] [CrossRef]
- Anitha, M.; Kamarudin, S.; Kofli, N. The potential of glycerol as a value-added commodity. Chem. Eng. J. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. From glycerol to value-added products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ye, X.; Bozell, J. A comparative review of petroleum-based and bio-based acrolein production. ChemSusChem 2012, 5, 1162–1180. [Google Scholar] [CrossRef] [PubMed]
- Katryniok, B.; Paul, S.; Capron, M.; Dumeignil, F. Towards the sustainable production of acrolein by glycerol dehydration. ChemSusChem 2009, 2, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, M.; Rossi, M. The Future of Glycerol: New Uses of a Versatile Raw Material; RSC Publishing: Cambridge, UK, 2008. [Google Scholar]
- Chai, S.; Wang, H.; Liang, Y.; Xu, B. Sustainable production of acrolein: Investigation of solid acid-base catalysts for gas-phase dehydration of glycerol. Green Chem. 2007, 9, 1130–1136. [Google Scholar] [CrossRef]
- Tao, L.; Yan, B.; Liang, Y.; Xu, B. Sustainable production of acrolein: Catalytic performance of hydrated tantalum oxides for gas-phase dehydration of glycerol. Green Chem. 2013, 15, 696–705. [Google Scholar] [CrossRef]
- Tao, L.; Chai, S.; Zuo, Y.; Zheng, W.; Liang, Y.; Xu, B. Sustainable production of acrolein: Acidic binary metal oxide catalysts for gas-phase dehydration of glycerol. Catal. Today 2010, 158, 310–316. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, S.; Li, C.; Cui, Q. Selective conversion of glycerol to acrolein over supported nickel sulfate catalysts. J. Catal. 2013, 301, 93–102. [Google Scholar] [CrossRef]
- Cavani, F.; Guidetti, S.; Marinelli, L.; Piccinini, M.; Ghedini, E.; Signoretto, M. The control of selectivity in gas-phase glycerol dehydration to acrolein catalysed by sulfated zirconia. Appl. Catal. B Environ. 2010, 100, 197–204. [Google Scholar] [CrossRef]
- Wang, F.; Dubois, J.; Ueda, W. Catalytic dehydration of glycerol over vanadium phosphate oxides in the presence of molecular oxygen. J. Catal. 2009, 268, 260–267. [Google Scholar] [CrossRef]
- Stosic, D.; Bennici, S.; Sirotin, S.; Calais, C.; Couturier, J.; Dubois, J.; Travert, A.; Auroux, A. Glycerol dehydration over calcium phosphate catalysts: Effect of acidic-basic features on catalytic performance. Appl. Catal. A Gen. 2012, 447, 124–134. [Google Scholar] [CrossRef]
- Suprun, W.; Lutecki, M.; Haber, T.; Papp, H. Acidic catalysts for the dehydration of glycerol: Activity and deactivation. J. Mol. Catal. A Chem. 2009, 309, 71–78. [Google Scholar] [CrossRef]
- Lauriol-Garbey, P.; Postole, G.; Loridant, S.; Auroux, A.; Belliere-Baca, V.; Rey, P.; Millet, J. Acid-base properties of niobium-zirconium mixed oxide catalysts for glycerol dehydration by calorimetric and catalytic investigation. Appl. Catal. B Environ. 2011, 106, 94–102. [Google Scholar] [CrossRef]
- Lauriol-Garbay, P.; Millet, J.; Loridant, S.; Belliere-Baca, V.; Rey, P. New efficient and long-life catalyst for gas-phase glycerol dehydration to acrolein. J. Catal. 2011, 280, 68–76. [Google Scholar] [CrossRef]
- Massa, M.; Andersson, A.; Finocchio, E.; Busca, G.; Lenrick, F.; Wallenberg, L. Performance of ZrO2-supported Nb- and W-oxide in the gas-phase dehydration of glycerol to acrolein. J. Catal. 2013, 297, 93–109. [Google Scholar] [CrossRef]
- Chai, S.; Yan, B.; Tao, L.; Liang, Y.; Xu, B. Sustainable production of acrolein: Catalytic gas-phase dehydration of glycerol over dispersed tungsten oxides on alumina, zirconia and silica. Catal. Today 2014, 234, 215–222. [Google Scholar] [CrossRef]
- Massa, M.; Andersson, A.; Finocchio, E.; Busca, G. Gas-phase dehydration of glycerol to acrolein over Al2O3−, SiO2−, and TiO2-supported Nb- and W-oxide catalysts. J. Catal. 2013, 307, 170–184. [Google Scholar] [CrossRef]
- Ulgen, A.; Hoelderich, W. Conversion of glycerol to acrolein in the presence of WO3/ZrO2 catalysts. Catal. Lett. 2009, 131, 122–128. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, K.; Park, E. Gas-phase dehydration of glycerol over ZSM-5 catalysts. Microporous Mesoporous Mater. 2010, 131, 28–36. [Google Scholar] [CrossRef]
- Jia, C.; Liu, Y.; Schmidt, W.; Lu, A.; Schuth, F. Small-sized HZSM-5 zeolite as highly active catalyst for gas phase dehydration of glycerol to acrolein. J. Catal. 2010, 269, 71–79. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, K.; Park, E. A comparative study for gas-phase dehydration of glycerol over H-zeolites. Appl. Catal. A Gen. 2011, 393, 275–287. [Google Scholar] [CrossRef]
- Omata, K.; Izumi, S.; Murayama, T.; Ueda, W. Hydrothermal synthesis of W-Nb complex metal oxides and their application to catalytic dehydration of glycerol to acrolein. Catal. Today 2013, 201, 7–11. [Google Scholar] [CrossRef]
- Oliveira, L.; Portilho, M.; Silva, A.; Taroco, H.; Souza, P. Modified niobia as a bifunctional catalyst for simultaneous dehydration and oxidation of glycerol. Appl. Catal. B Environ. 2012, 117, 29–35. [Google Scholar] [CrossRef]
- Martin, A.; Armbruster, U.; Atia, H. Recent developments in dehydration of glycerol toward acrolein over heteropolyacids. Eur. J. Lipid Sci. Technol. 2012, 114, 10–23. [Google Scholar] [CrossRef]
- Atia, H.; Armbruster, U.; Martin, A. Dehydration of glycerol in gas phase using heteropolyacid catalysts as active compounds. J. Catal. 2008, 258, 71–82. [Google Scholar] [CrossRef]
- Alhanash, A.; Kozhevnikova, E.; Kozhevnikov, I. Gas-phase dehydration of glycerol to acrolein catalysed by caesium heteropoly salt. Appl. Catal. A Gen. 2010, 378, 11–18. [Google Scholar] [CrossRef]
- Kang, T.; Choi, J.; Bang, Y.; Yoo, J.; Song, J.; Joe, W.; Choi, J.; Song, I. Dehydration of glycerin to acrolein over H3PW12O40 heteropolyacid catalyst supported on silica-alumina. J. Mol. Catal. A Chem. 2015, 396, 282–289. [Google Scholar] [CrossRef]
- Laino, T.; Tuma, C.; Curioni, A.; Jochnowitz, E.; Stolz, S. A revisited picture of the mechanism of glycerol dehydration. J. Phys. Chem. A 2011, 115, 3592–3595. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.; Leonowicz, M.; Roth, W.; Vartuli, J.; Beck, J. Ordered mesoporous molecular-sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.; Chmelka, B.; Stucky, G. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, J.; Macedo, M.; Fernandes, A. Sulfonic-functionalized SBA-15 as an active catalyst for the gas-phase dehydration of glycerol. Catal. Commun. 2012, 19, 105–109. [Google Scholar] [CrossRef]
- Dalla Costa, B.O.; Legnoverde, M.; Lago, C.; Decolatti, H.; Querini, C. Sulfonic functionalized SBA-15 catalysts in the gas phase glycerol dehydration. Thermal stability and catalyst deactivation. Microporous Mesoporous Mater. 2016, 230, 66–75. [Google Scholar] [CrossRef]
- Ma, T.; Ding, J.; Shao, R.; Yun, Z. Catalytic conversion of glycerol to acrolein over MCM-41 by the grafting of phosphorus species. Can. J. Chem. Eng. 2016, 94, 924–930. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ito, S.; Hara, K.; Fukuoka, A. Conversion of glycerol to acrolein by mesoporous sulfated zirconia-silica catalyst. Chin. J. Catal. 2017, 38, 420–425. [Google Scholar] [CrossRef]
- Ding, J.; Ma, T.; Yun, Z.; Shao, R. Heteropolyacid (H3PW12O40) supported MCM-41: An effective solid acid catalyst for the dehydration of glycerol to acrolein. J. Wuhan Univ. Technol. 2017, 32, 1511–1516. [Google Scholar] [CrossRef]
- Ma, T.; Ding, J.; Shao, R.; Xu, W.; Yun, Z. Dehydration of glycerol to acrolein over wells-dawson and keggin type phosphotungstic acids supported on MCM-41 catalysts. Chem. Eng. J. 2017, 316, 797–806. [Google Scholar] [CrossRef]
- Liu, R.; Wang, T.; Jin, Y. Catalytic dehydration of glycerol to acrolein over HPW supported on Cs+ modified SBA-15. Catal. Today 2014, 233, 127–132. [Google Scholar] [CrossRef]
- Liu, R.; Lyu, S.; Wang, T. Sustainable production of acrolein from biodiesel-derived crude glycerol over H3PW12O40 supported on Cs-modified SBA-15. J. Ind. Eng. Chem. 2016, 37, 354–360. [Google Scholar] [CrossRef]
- Ma, T.; Yun, Z.; Xu, W.; Chen, L.; Li, L.; Ding, J.; Shao, R. Pd-H3PW12O40/Zr-MCM-41: An efficient catalyst for the sustainable dehydration of glycerol to acrolein. Chem. Eng. J. 2016, 294, 343–352. [Google Scholar] [CrossRef]
- Trakarnpruk, W. Platinum/phosphotungstic acid/(Zr)MCM-41 catalysts in glycerol dehydration. Mendeleev Commun. 2014, 24, 167–169. [Google Scholar] [CrossRef]
- Viswanadham, B.; Srikanth, A.; Kumar, V.; Chary, K. Vapor phase dehydration of glycerol to acrolein over SBA-15 supported vanadium substituted phosphomolybdic acid catalyst. J. Nanosci. Nanotechnol. 2015, 15, 5391–5402. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sancho, C.; Moreno-Tost, R.; Merida-Robles, J.; Santamaria-Gonzalez, J.; Jimenez-Lopez, A.; Maireles-Torres, P. Zirconium doped mesoporous silica catalysts for dehydration of glycerol to high added-value products. Appl. Catal. A Gen. 2012, 433, 179–187. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Capron, M.; Lancelot, C.; Belliere-Baca, V.; Rey, P.; Dumeignil, F. A long-life catalyst for glycerol dehydration to acrolein. Green Chem. 2010, 12, 1922–1925. [Google Scholar] [CrossRef]
- Cecilia, J.A.; García-Sancho, C.; Mérida-Robles, J.M.; Santamaría-González, J.; Moreno-Tost, R.; Maireles-Torres, P. V and V–P containing Zr-SBA-15 catalysts for dehydration of glycerol to acrolein. Catal. Today 2015, 254, 43–52. [Google Scholar] [CrossRef]
- García-Sancho, C.; Cecilia, J.A.; Moreno-Ruiz, A.; Mérida-Robles, J.M.; Santamaría-González, J.; Moreno-Tost, R.; Maireles-Torres, P. Influence of the niobium supported species on the catalytic dehydration of glycerol to acrolein. Appl. Catal. B Environ. 2015, 179, 139–149. [Google Scholar] [CrossRef]
- Cecilia, J.; Garcia-Sancho, C.; Merida-Robles, J.; Gonzalez, J.; Moreno-Tost, R.; Maireles-Torres, P. WO3 supported on Zr doped mesoporous SBA-15 silica for glycerol dehydration to acrolein. Appl. Catal. A Gen. 2016, 516, 30–40. [Google Scholar] [CrossRef]
- Garcia-Sancho, C.; Cecilia, J.; Merida-Robles, J.; Gonzalez, J.; Moreno-Tost, R.; Infantes-Molina, A.; Maireles-Torres, P. Effect of the treatment with H3PO4 on the catalytic activity of Nb2O5 supported on Zr-doped mesoporous silica catalyst. Case study: Glycerol dehydration. Appl. Catal. B Environ. 2018, 221, 158–168. [Google Scholar] [CrossRef]
- Cecilia, J.; Garcia-Sancho, C.; Merida-Robles, J.; Santamaria-Gonzalez, J.; Infantes-Molina, A.; Moreno-Tost, R.; Maireles-Torres, P. Aluminum doped mesoporous silica SBA-15 for glycerol dehydration to value-added chemicals. J. Sol-Gel Sci. Technol. 2017, 83, 342–354. [Google Scholar] [CrossRef]
- Salas, P.; Wang, J.; Armendariz, H.; Angeles-Chavez, C.; Chen, L. Effect of the Si/Zr molar ratio on the synthesis of Zr-based mesoporous molecular sieves. Mater. Chem. Phys. 2009, 114, 139–144. [Google Scholar] [CrossRef]
- Anderson, J.; Fergusson, C.; Rodriguez-Ramos, I.; Guerrero-Ruiz, A. Influence of Si/Zr ratio on the formation of surface acidity in silica-zirconia aerogels. J. Catal. 2000, 192, 344–354. [Google Scholar] [CrossRef]
- Lauriol-Garbey, P.; Loridant, S.; Belliere-Baca, V.; Rey, P.; Millet, J. Gas phase dehydration of glycerol to acrolein over WO3/ZrO2 catalysts: Improvement of selectivity and stability by doping with SiO2. Catal. Commun. 2011, 16, 170–174. [Google Scholar] [CrossRef]
- Katryniok, B.; Paul, S.; Capron, M.; Belliere-Baca, V.; Rey, P.; Dumeignil, F. Regeneration of silica-supported silicotungstic acid as a catalyst for the dehydration of glycerol. ChemSusChem 2012, 5, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Carrico, C.; Cruz, F.; Santos, M.; Pastore, H.; Andrade, H.; Mascarenhas, A. Efficiency of zeolite MCM-22 with different SiO2/Al2O3 molar ratios in gas phase glycerol dehydration to acrolein. Microporous Mesoporous Mater. 2013, 181, 74–82. [Google Scholar] [CrossRef]
- Choi, Y.; Park, H.; Yun, Y.; Yi, J. Effects of catalyst pore structure and acid properties on the dehydration of glycerol. ChemSusChem 2015, 8, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Vignatti, C.; Garetto, T.; Pulcinelli, S.; Santilli, C.; Martins, L. Glycerol dehydration catalyzed by MWW zeolites and the changes in the catalyst deactivation caused by porosity modification. Appl. Catal. A Gen. 2015, 495, 84–91. [Google Scholar] [CrossRef]
- Gu, Y.; Cui, N.; Yu, Q.; Li, C.; Cui, Q. Study on the influence of channel structure properties in the dehydration of glycerol to acrolein over H-zeolite catalysts. Appl. Catal. A Gen. 2012, 429, 9–16. [Google Scholar] [CrossRef]
- Possato, L.; Cassinelli, W.; Garetto, T.; Pulcinelli, S.; Santilli, C.; Martins, L. One-step glycerol oxidehydration to acrylic acid on multifunctional zeolite catalysts. Appl. Catal. A Gen. 2015, 492, 243–251. [Google Scholar] [CrossRef]
- Decolatti, H.P.; Dalla Costa, B.O.; Querini, C.A. Dehydration of glycerol to acrolein using H-ZSM5 zeolite modified by alkali treatment with Naoh. Microporous Mesoporous Mater. 2015, 204, 180–189. [Google Scholar] [CrossRef]
- Possato, L.; Diniz, R.; Garetto, T.; Pulcinelli, S.; Santilli, C.; Martins, L. A comparative study of glycerol dehydration catalyzed by micro/mesoporous MFI zeolites. J. Catal. 2013, 300, 102–112. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Jiang, Y.; Hunger, M.; Huang, J. Cooperativity of Brönsted and Lewis acid sites on zeolite for glycerol dehydration. ACS Catal. 2014, 4, 1144–1147. [Google Scholar] [CrossRef]
- Lari, G.; Chen, Z.; Mondelli, C.; Perez-Ramirez, J. Bifunctional hierarchical zeolite-supported silver catalysts for the conversion of glycerol to allyl alcohol. ChemCatChem 2017, 9, 2195–2202. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Z.; Huang, L.; Zhang, H.; Song, K.; Wang, L.; Shi, Z.; Ma, J.; Zhuang, Y.; Shen, W.; et al. Dehydration of glycerol to acrolein over hierarchical zsm-5 zeolites: Effects of mesoporosity and acidity. ACS Catal. 2015, 5, 2548–2558. [Google Scholar] [CrossRef]
- Beerthuis, R.; Huang, L.; Shiju, N.; Rothenberg, G.; Shen, W.; Xu, H. Facile synthesis of a novel hierarchical ZSM-5 zeolite: A stable acid catalyst for dehydrating glycerol to acrolein. ChemCatChem 2018, 10, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Qin, F.; Huang, Z.; Zhuang, Y.; Ma, J.; Xu, H.; Shen, W. Hierarchical ZSM-5 zeolite synthesized by an ultrasound-assisted method as a long-life catalyst for dehydration of glycerol to acrolein. Ind. Eng. Chem. Res. 2016, 55, 7318–7327. [Google Scholar] [CrossRef]
- Dos Santos, M.; Andrade, H.; Mascarenhas, A. Reduced coke formation during the gas phase oxidative dehydration of glycerol over ferrierite zeolites synthesized in fluoride medium. Microporous Mesoporous Mater. 2016, 223, 105–113. [Google Scholar] [CrossRef]
- Viswanadham, B.; Nagaraju, N.; Rohitha, C.; Vishwanathan, V.; Chary, K. Synthesis, characterization and catalytic dehydration of glycerol to acrolein over phosphotungstic acid supported Y-zeolite catalysts. Catal. Lett. 2018, 148, 397–406. [Google Scholar] [CrossRef]
- Silva, T.; dos Santos, M.; Santiago, A.; Santana, D.; Cruz, F.; Andrade, H.; Mascarenhas, A. Gas phase glycerol oxidative dehydration over bifunctional V/H-zeolite catalysts with different zeolite topologies. Catal. Today 2017, 289, 38–46. [Google Scholar] [CrossRef]
- Pestana, C.; Guerra, A.; Ferreira, G.; Turci, C.; Mota, C. Oxidative dehydration of glycerol to acrylic acid over vanadium-impregnated zeolite beta. J. Braz. Chem. Soc. 2013, 24, 100–105. [Google Scholar] [CrossRef]
- Wilson, S.; Lok, B.; Messina, C.; Cannan, T.; Flanigen, E. Aluminophosphate molecular-sieves. A new class of microporous crystalline inorganic solids. J. Am. Chem. Soc. 1982, 104, 1146–1147. [Google Scholar] [CrossRef]
- Sastre, G.; Lewis, D.; Catlow, C. Mechanisms of silicon incorporation in aluminophosphate molecular sieves. J. Mol. Catal. A Chem. 1997, 119, 349–356. [Google Scholar] [CrossRef]
- Lourenco, J.; Fernandes, A.; Bertolo, R.; Ribeiro, M. Gas-phase dehydration of glycerol over thermallystable sapo-40 catalyst. RSC Adv. 2015, 5, 10667–10674. [Google Scholar] [CrossRef]
- Fernandes, A.; Filipa Ribeiro, M.; Lourenço, J.P. Gas-phase dehydration of glycerol over hierarchical silicoaluminophosphate sapo-40. Catal. Commun. 2017, 95, 16–20. [Google Scholar] [CrossRef]
- Kozhevnikov, I. Catalysis by heteropolyacids and multicomponent polyoxometalates in liquid-phase reactions. Chem. Rev. 1998, 98, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Dablemont, C.; Basset, J.; Lefebvre, F. Comparison of H3PW12O40 and H4SiW12O40 heteropolyacids supported on silica by h-1 mas nmr. C. R. Chim. 2005, 8, 1969–1974. [Google Scholar] [CrossRef]
- Kim, Y.; You, S.; Jung, K.; Park, E. Effect of al content on the gas-phase dehydration of glycerol over silica-alumina-supported silicotungstic acid catalysts. Bull. Korean Chem. Soc. 2012, 33, 2369–2377. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, K.; Park, E. Gas-phase dehydration of glycerol over silica-alumina catalysts. Appl. Catal. B Environ. 2011, 107, 177–187. [Google Scholar] [CrossRef]
- Chai, S.; Wang, H.; Liang, Y.; Xu, B. Sustainable production of acrolein: Gas-phase dehydration of glycerol over 12-tungstophosphoric acid supported on ZrO2 and SiO2. Green Chem. 2008, 10, 1087–1093. [Google Scholar] [CrossRef]
- Shiju, N.; Brown, D.; Wilson, K.; Rothenberg, G. Glycerol valorization: Dehydration to acrolein over silica-supported niobia catalysts. Top. Catal. 2010, 53, 1217–1223. [Google Scholar] [CrossRef]
- Foo, G.; Wei, D.; Sholl, D.; Sievers, C. Role of Lewis and Brönsted acid sites in the dehydration of glycerol over niobia. ACS Catal. 2014, 4, 3180–3192. [Google Scholar] [CrossRef]
- Baertsch, C.; Komala, K.; Chua, Y.; Iglesia, E. Genesis of Brönsted acid sites during dehydration of 2-butanol on tungsten oxide catalysts. J. Catal. 2002, 205, 44–57. [Google Scholar] [CrossRef]
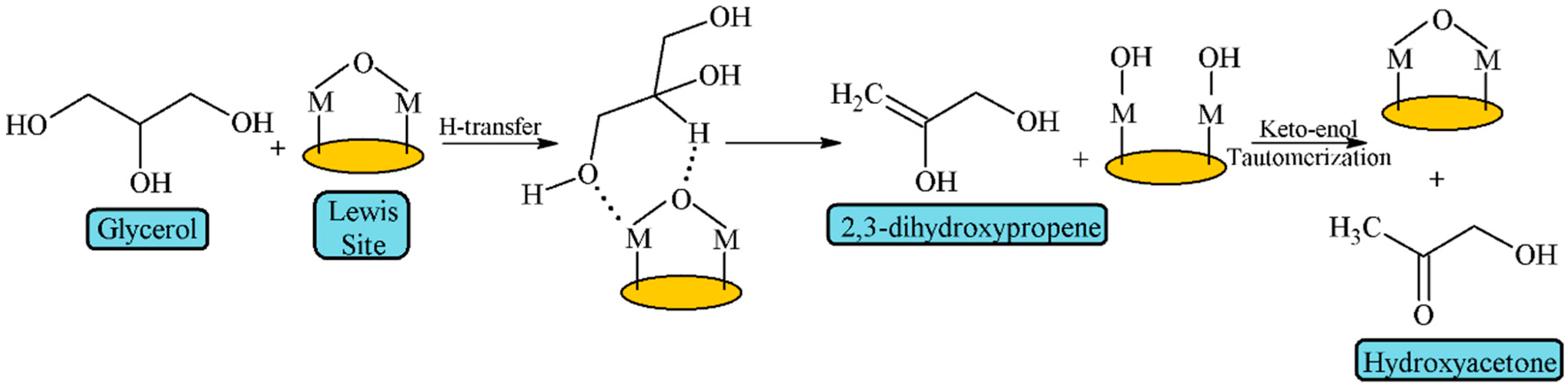
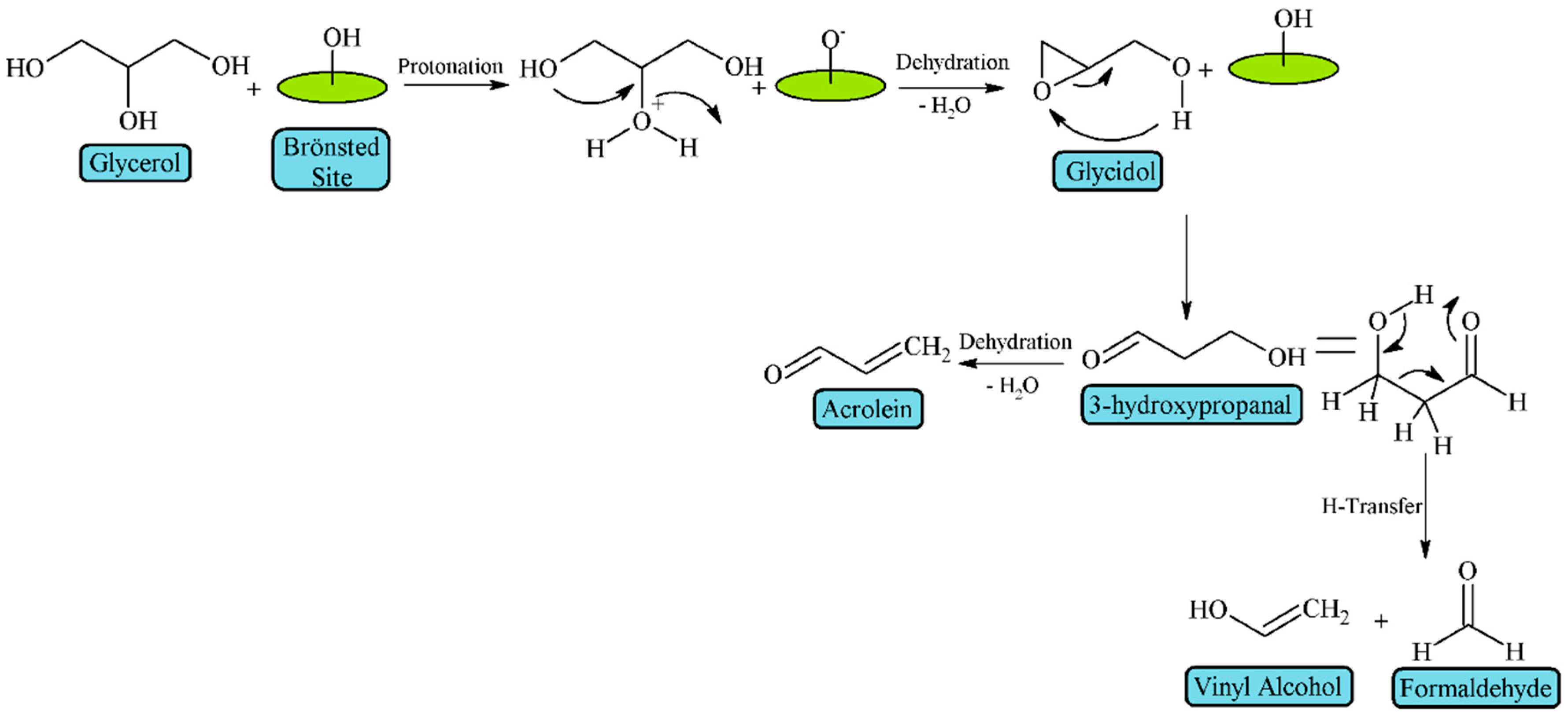
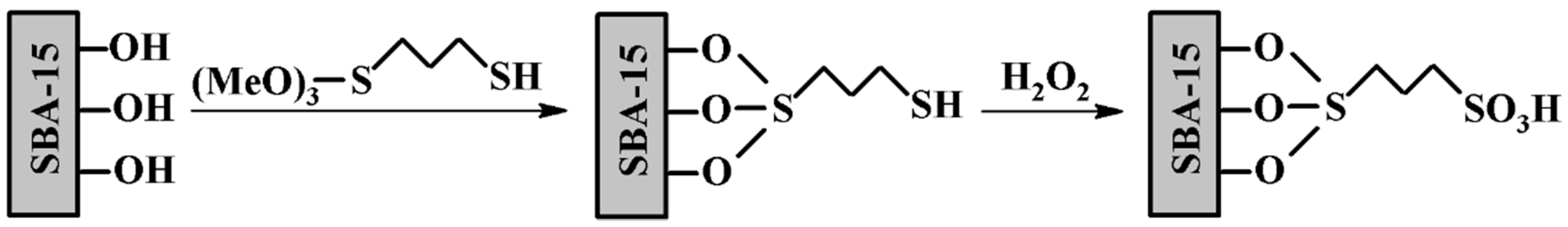
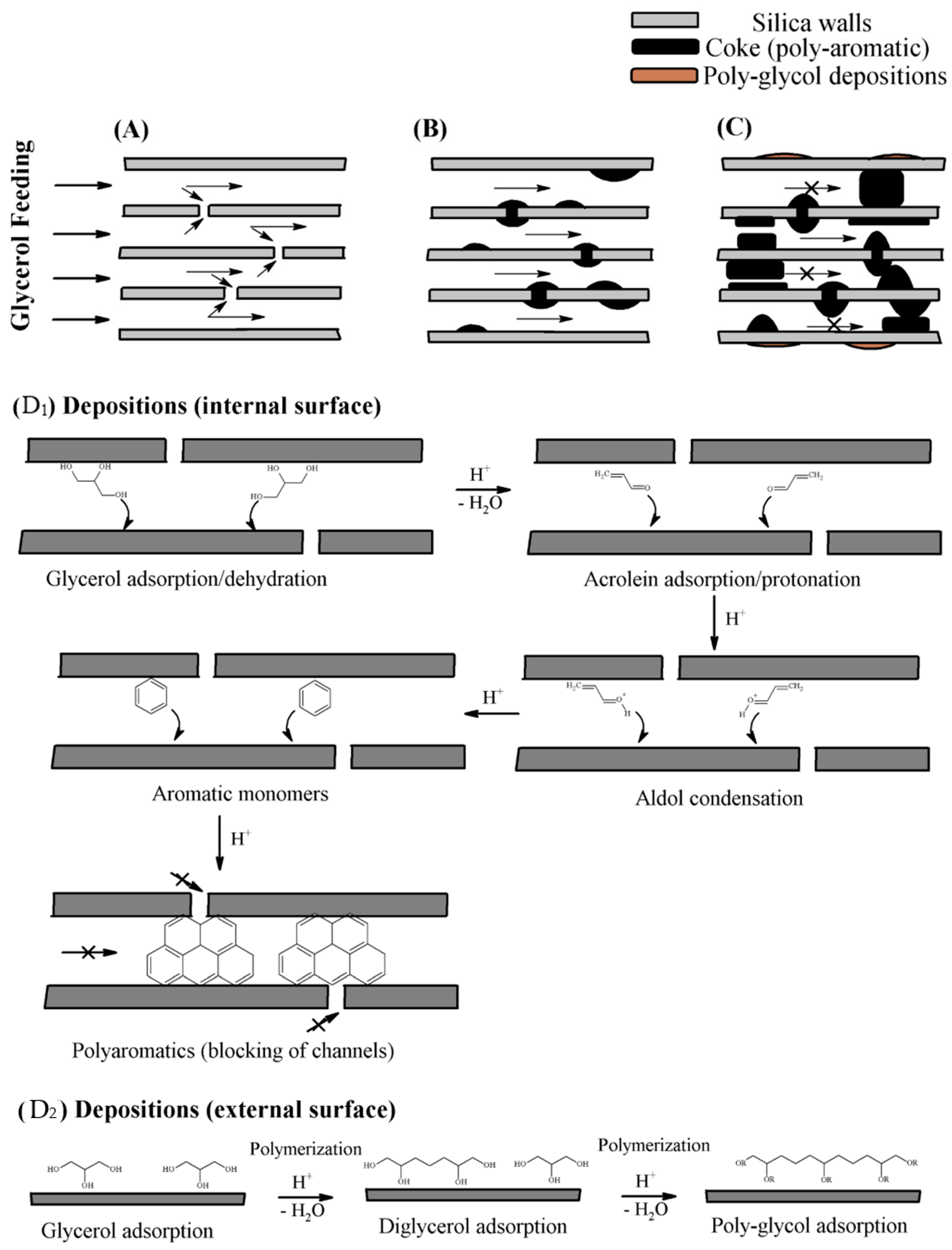
| Feedstock | Types | Remarks |
|---|---|---|
| First generation |
|
|
| Second generation |
|
|
| Active Phase | T (°C) | TOS (h) | Cgly (%) | Yacrol (%) | Mmolacrol h−1 gcat−1 | Ref. |
|---|---|---|---|---|---|---|
| SBA-15-SO3H | 300 | 3 | 100 | 93 | 10.5 | [44] |
| SBA-15-SO3H | 300 | 6 | 45 | 35 | 13.9 | [45] |
| SBA-15-PO3H | 320 | 2 | 97 | 81 | 3.71 | [46] |
| Meso SiO2-ZrO2/SO42− | 250 | 2 | 99 | 81 | 10.9 | [47] |
| H3PW12O40/MCM-41 | 320 | 2 | 85 | 68 | 3.72 | [48] |
| H3PW12O40/MCM-41 | 320 | 5 | 87 | 44 | 3.59 | [49] |
| H6P2W18O62/MCM-41 | 320 | 5 | 82 | 51 | 4.20 | |
| Cs2.5H0.5PW12O40/MCM-41 | 300 | 2 | 100 | 86 | 8.47 | [50] |
| Cs2.5H0.5PW12O40/MCM-41 | 300 | 30 | 100 | 88 | 24.08 | [51] |
| MCM41-Zr | 320 | 5 | 74 | 19 | 0.87 | [52] |
| Pd-HPW/Zr-MCM-41 | 320 | 5 | 94 | 80 | 3.64 | |
| Pt-HPW/Zr-MCM-41 | 320 | 5 | 83 | 62 | 2.82 | |
| MCM41-Zr | 300 | 5 | 66 | 18 | 5.31 | [53] |
| Pt-HPW/Zr-MCM-41 | 350 | 5 | 82 | 70 | 20.31 | |
| H4PMo11VO40/SBA-15 | 225 | 4 | 100 | 74 | 1.69 | [54] |
| MCM41-Zr | 315 | 5 | 97 | 38 | 5.28 | [55] |
| MCM41-Zr | 250 | 0–5 | 96 | 71 | 4.86 | [56] |
| V2O5/MCM41-Zr | 325 | 2 | 90 | 25 | 4.41 | [57] |
| V2O5-P/MCM41-Zr | 325 | 2 | 90 | 41 | 6.71 | |
| Nb2O5/MCM-41-Zr | 325 | 2 | 77 | 45 | 7.43 | [58] |
| WO3/MCM-41-Zr | 325 | 2 | 97 | 41 | 6.71 | [59] |
| WO3-P/MCM-41-Zr | 325 | 2 | 78 | 51 | 8.36 | |
| Nb2O5-P/MCM-41-Zr | 325 | 2 | 100 | 56 | 9.18 | [60] |
| Al-SBA-15 | 325 | 2 | 94 | 35 | 5.74 | [61] |
| Catalyst | T (°C) | TOS (h) | Cgly (%) | Yacrol (%) | Mmolacrol h−1 gcat−1 | Ref. |
|---|---|---|---|---|---|---|
| SAPO-11 | 350 | 2.5 | 100 | 73 | 2.4 | [84] |
| 120 | 26 | 19 | 0.6 | |||
| SAPO-34 | 350 | 2.5 | 90 | 69 | 2.3 | |
| 120 | 16 | 12 | 0.4 | |||
| SAPO-40 | 350 | 2.5 | 100 | 75 | 2.5 | |
| 120 | 54 | 39 | 4.0 | |||
| SAPO-34 | 315 | 9 | 32 | 15 | 0.1 | [18] |
| SAPO-11 | 280 | 1 | 88 | 55 | 0.9 | [25] |
| SAPO-34 | 280 | 1 | 59 | 42 | 0.7 | |
| Meso-SAPO-40 | 320 | 2.5 | 100 | 81 | 2.7 | [85] |
| 320 | 120 | 87 | 68 | 2.3 |
| Active Phase | T (°C) | TOS (h) | Cgly (%) | Yacrol (%) | Mmolacrol h−1 gcat−1 | Ref. |
|---|---|---|---|---|---|---|
| SiO2-H3PO4 | 325 | 5 | 70 | 38 | 2.0 | [9] |
| SiO2-H3BO3 | 325 | 5 | 15 | 0.3 | 0.02 | |
| SiO2-PW | 325 | 5 | 100 | 65 | 3.9 | |
| SiO2-PMo | 325 | 5 | 98 | 33 | 2.0 | |
| SiO2-SiW | 325 | 5 | 100 | 74 | 4.4 | |
| SiO2-SiW | 315 | 2 | 23 | 6 | 13.1 | [88] |
| Si0.9Al0.1Ox-SiW | 315 | 2 | 97 | 54 | 127.3 | |
| Al2O3-SiW | 315 | 2 | 87 | 33 | 26.1 | |
| SiO2-PW | 275 | 10 | 16 | 4 | 78.3 | [40] |
| Si0.85Al0.15Ox-PW | 275 | 10 | 43 | 20 | 83.6 | |
| Al2O3-PW | 275 | 10 | 13 | 3 | 13.0 | |
| Si0.8Al0.2Ox | 315 | 2 | 43 | 21 | 50.7 | [89] |
| SiO2-PW | 315 | 10 | 20 | 11 | 3.3 | [90] |
| ZrO2-PW | 315 | 10 | 70 | 49 | 8.1 | |
| 0.5Nb2O50.5WO3/SiO2 | 305 | 1 | 99 | 47 | 0.4 | [30] |
| SiO2/WO3/ZrO2 | 300 | 8 | 100 | 65 | 0.5 | [64] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecilia, J.A.; García-Sancho, C.; Jiménez-Gómez, C.P.; Moreno-Tost, R.; Maireles-Torres, P. Porous Silicon-Based Catalysts for the Dehydration of Glycerol to High Value-Added Products. Materials 2018, 11, 1569. https://doi.org/10.3390/ma11091569
Cecilia JA, García-Sancho C, Jiménez-Gómez CP, Moreno-Tost R, Maireles-Torres P. Porous Silicon-Based Catalysts for the Dehydration of Glycerol to High Value-Added Products. Materials. 2018; 11(9):1569. https://doi.org/10.3390/ma11091569
Chicago/Turabian StyleCecilia, Juan Antonio, Cristina García-Sancho, Carmen Pilar Jiménez-Gómez, Ramón Moreno-Tost, and Pedro Maireles-Torres. 2018. "Porous Silicon-Based Catalysts for the Dehydration of Glycerol to High Value-Added Products" Materials 11, no. 9: 1569. https://doi.org/10.3390/ma11091569
APA StyleCecilia, J. A., García-Sancho, C., Jiménez-Gómez, C. P., Moreno-Tost, R., & Maireles-Torres, P. (2018). Porous Silicon-Based Catalysts for the Dehydration of Glycerol to High Value-Added Products. Materials, 11(9), 1569. https://doi.org/10.3390/ma11091569







