Effect of Icariin on Engineered 3D-Printed Porous Scaffolds for Cartilage Repair
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
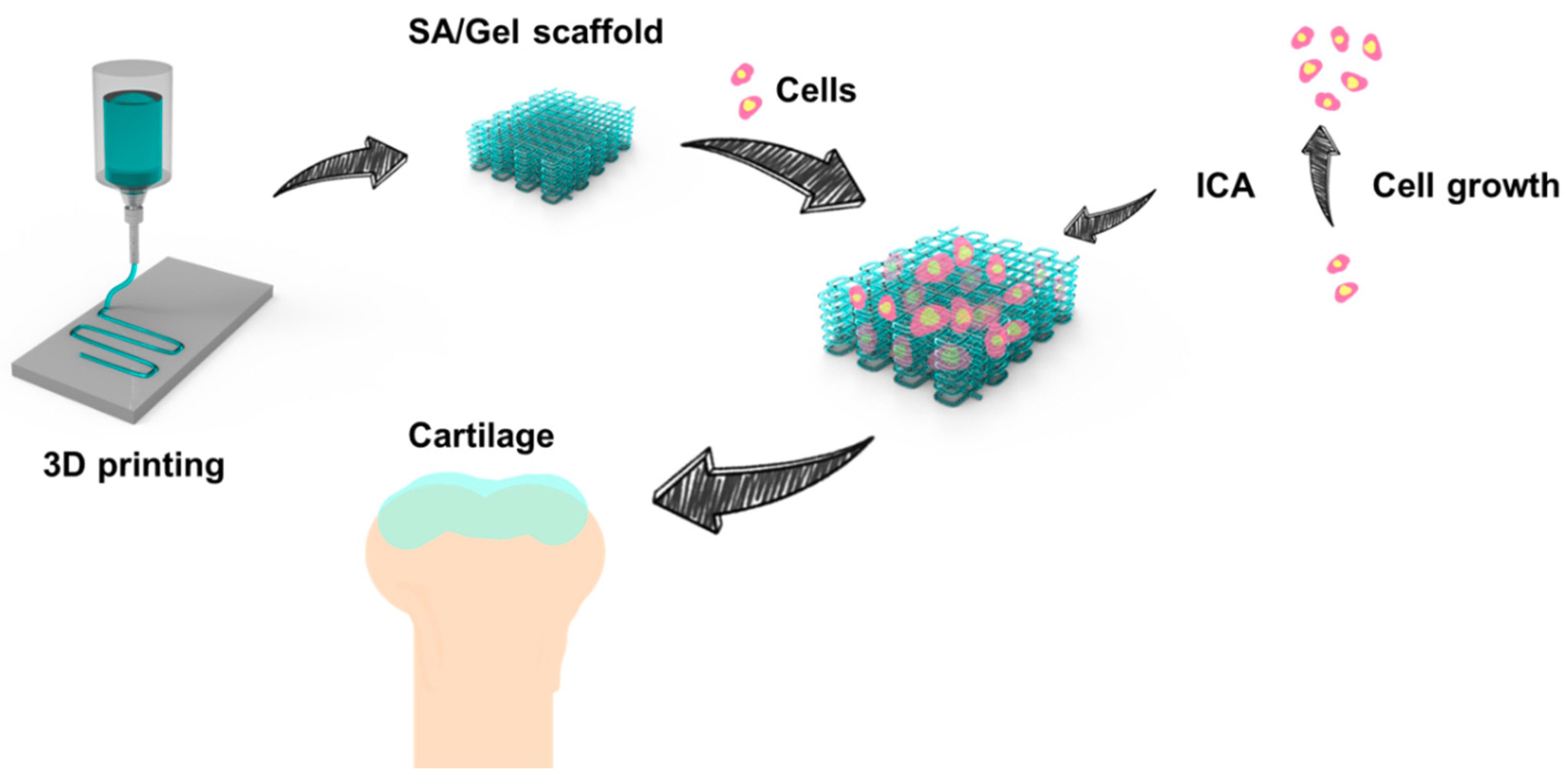
2.2. Fabrication of SA/Gel Scaffolds
2.3. Physical Characterizations of the Composite Scaffolds
2.4. Cytocompatibility Study
2.5. Effect of ICA on Chondrocyte Growth
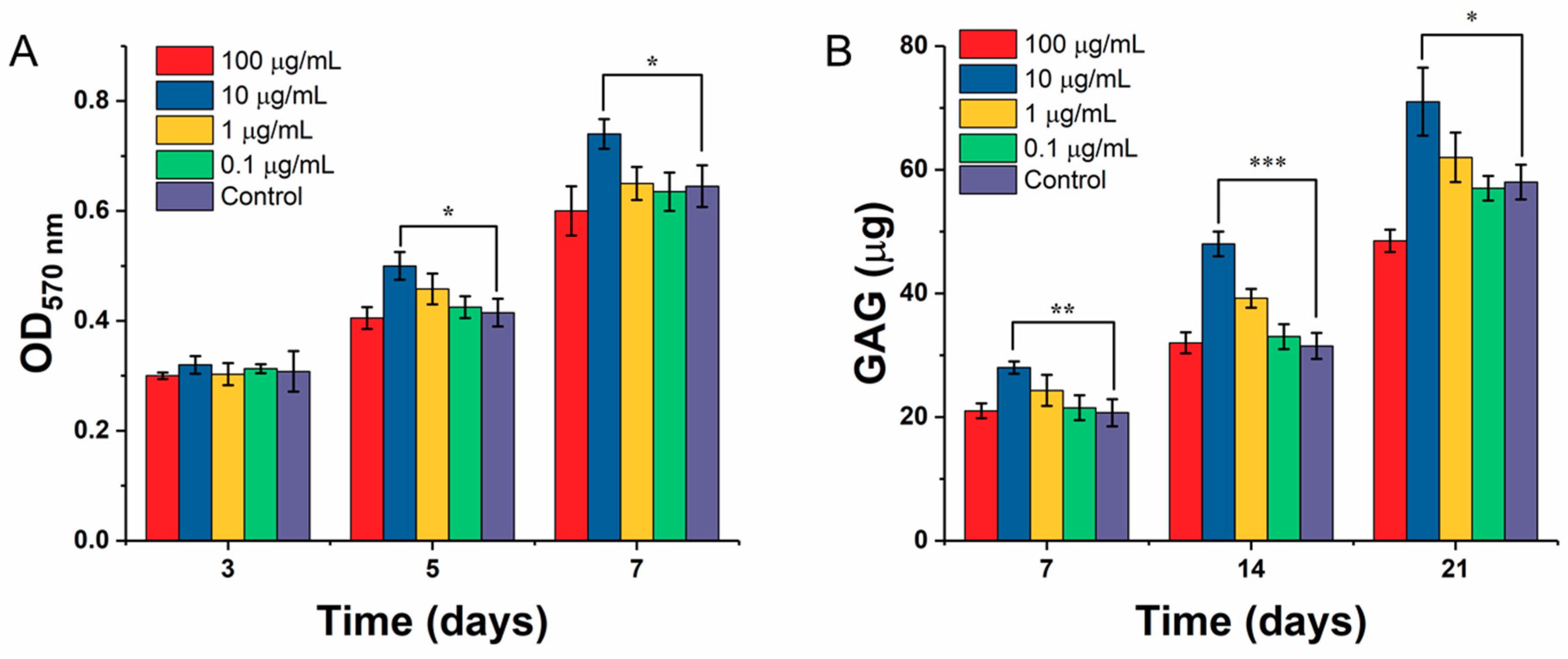
2.5.1. Chondrocyte Proliferation
2.5.2. Determination of Glycosaminoglycan (GAG) by DMMB Assay
2.6. Chondrocyte Differentiation on SA/Gel Scaffolds
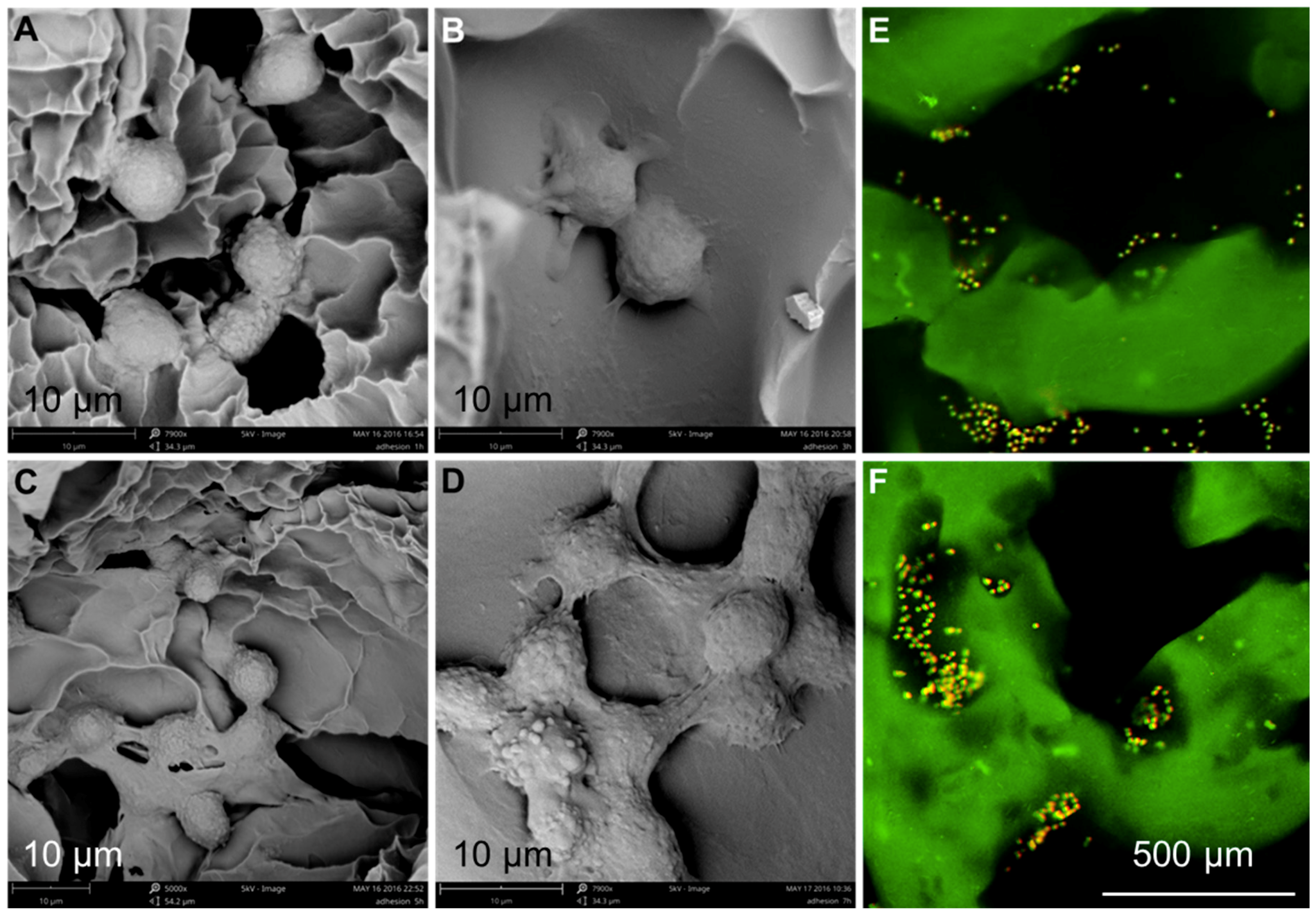
2.6.1. Cell Adhesion and Distribution
2.6.2. Chondrocyte Proliferation
2.6.3. GAG Secretion
2.7. Statistical Analysis
3. Results and Discussions
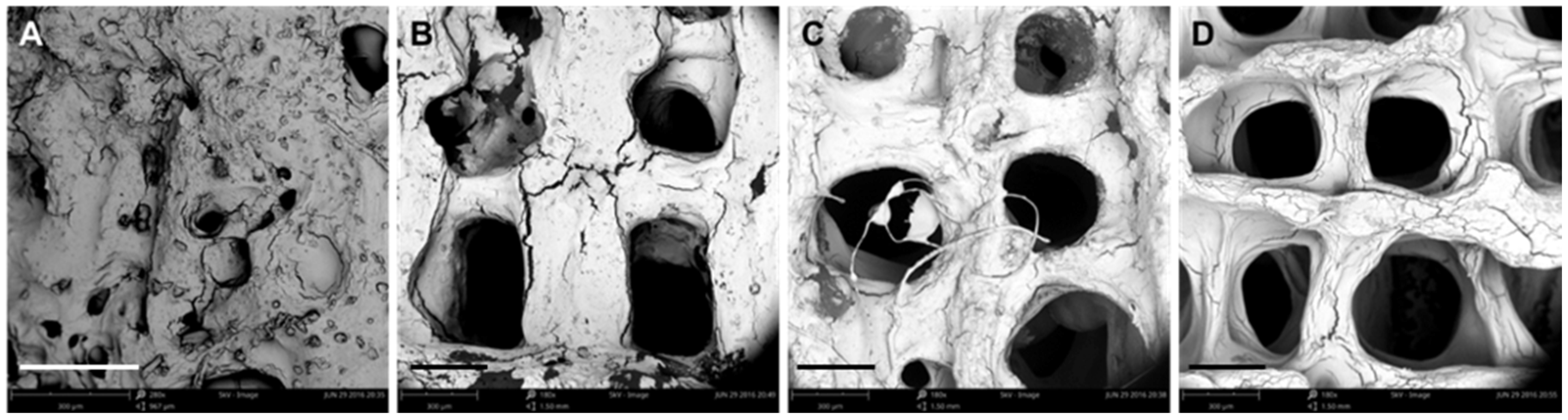
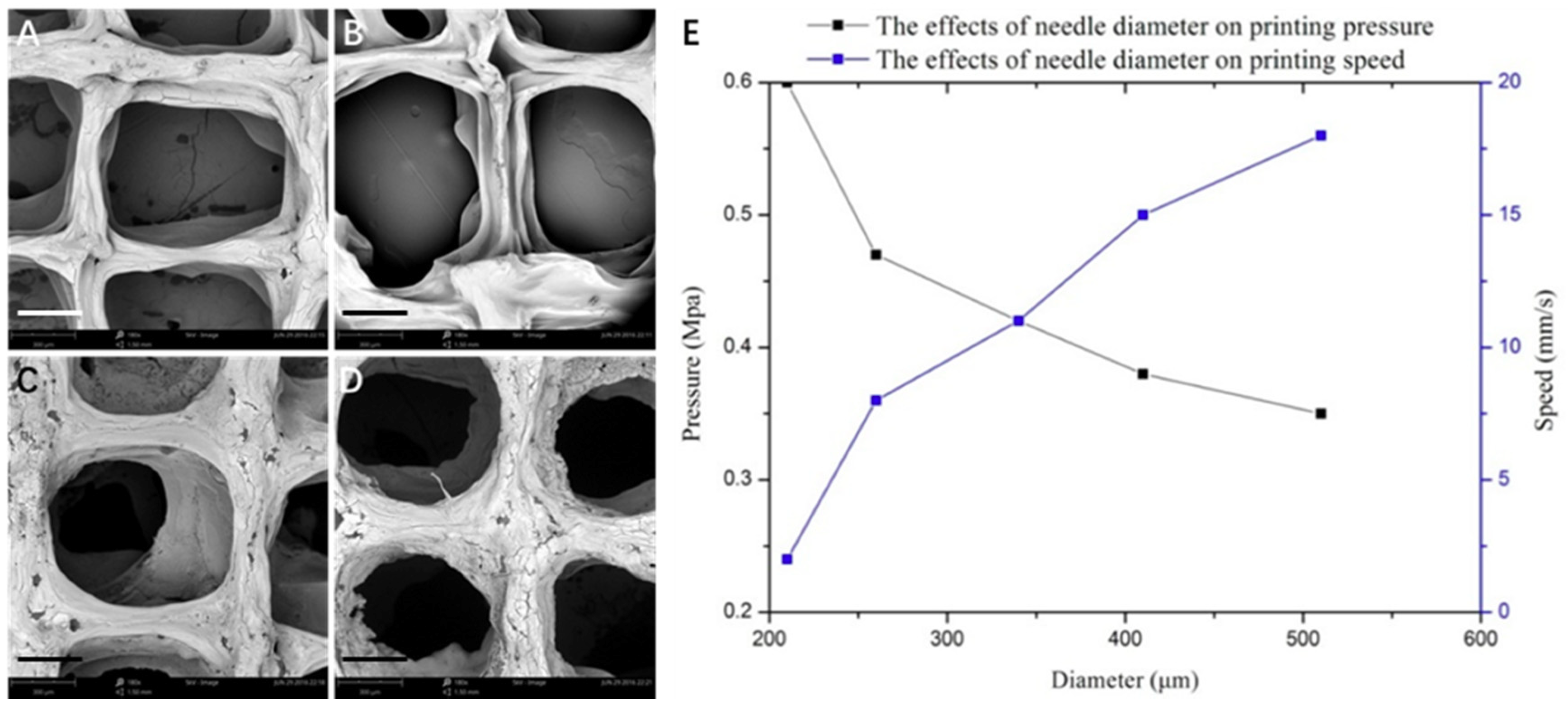
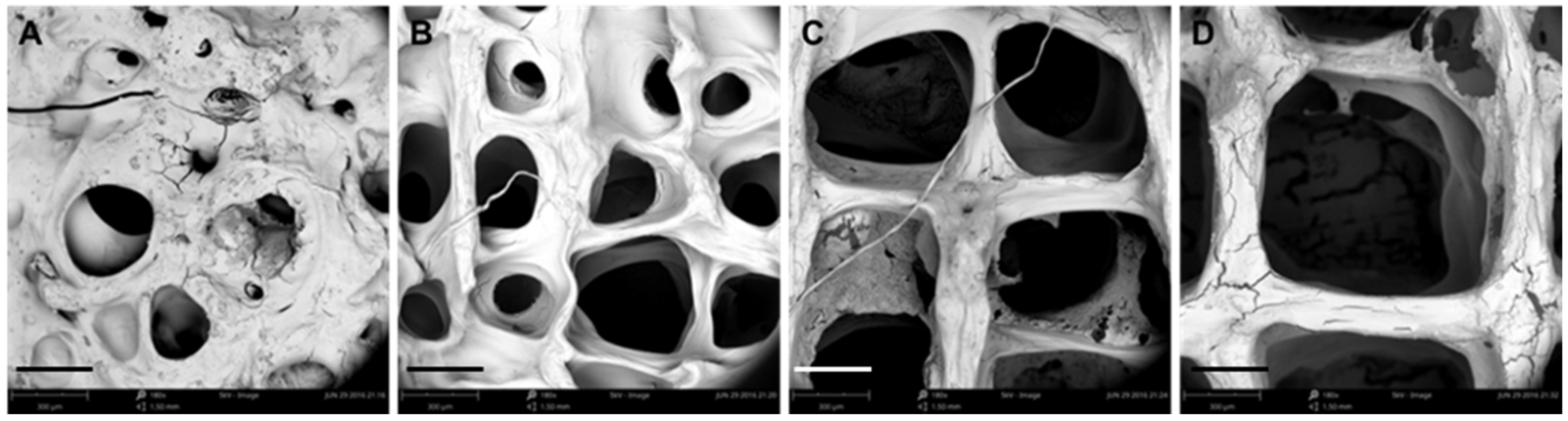
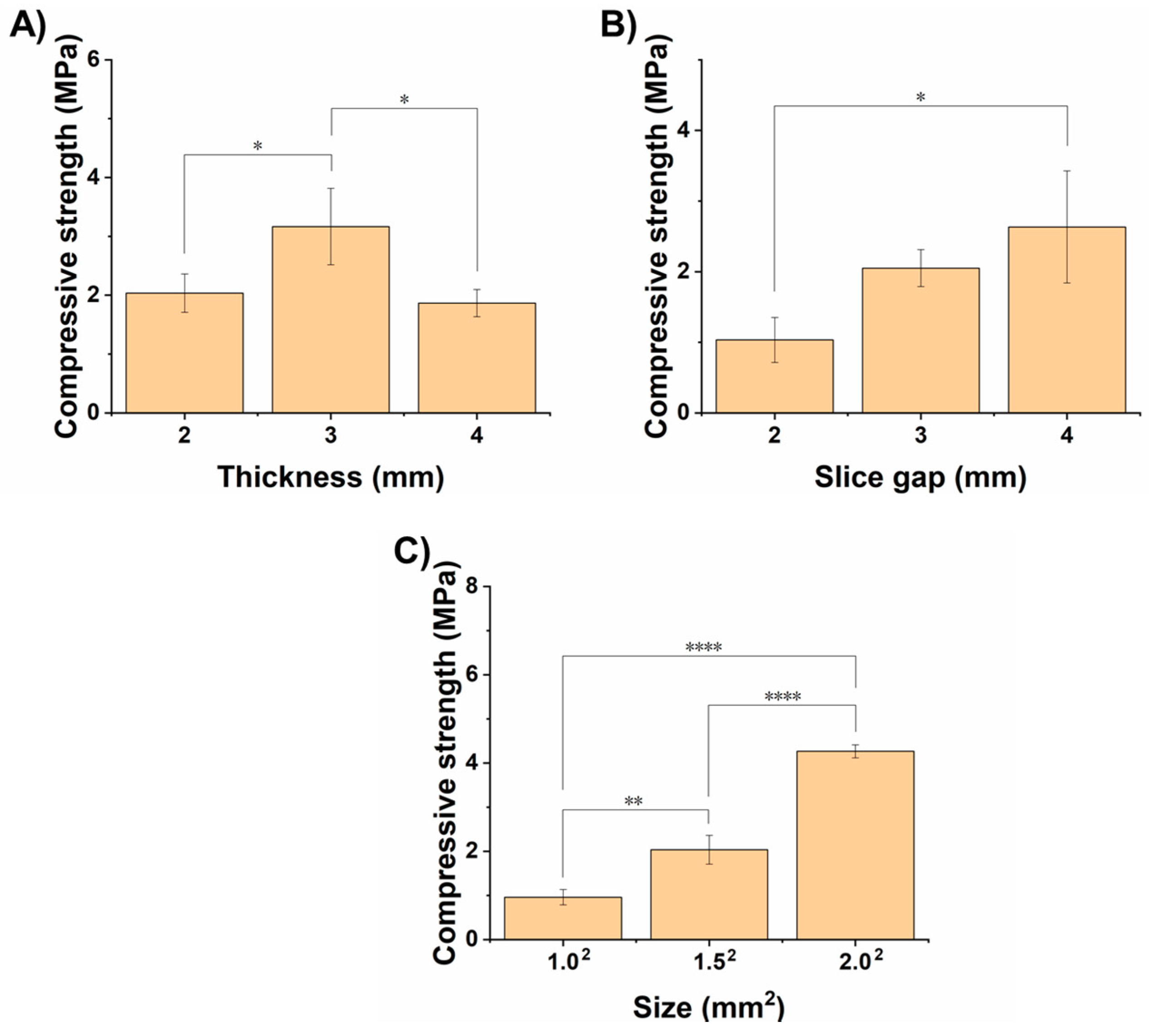
3.1. Optimization of Processing Parameters
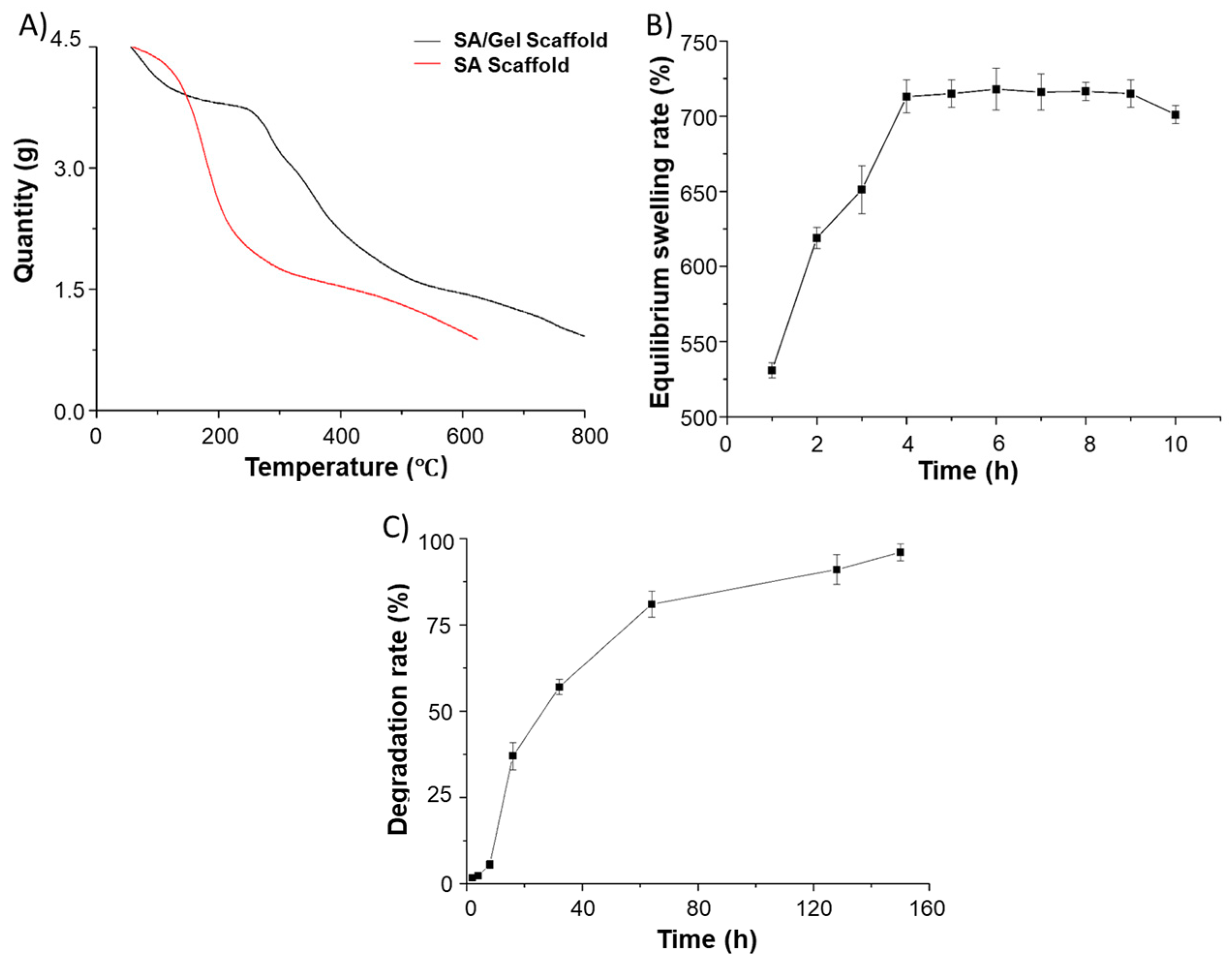
3.2. Physical Characterizations
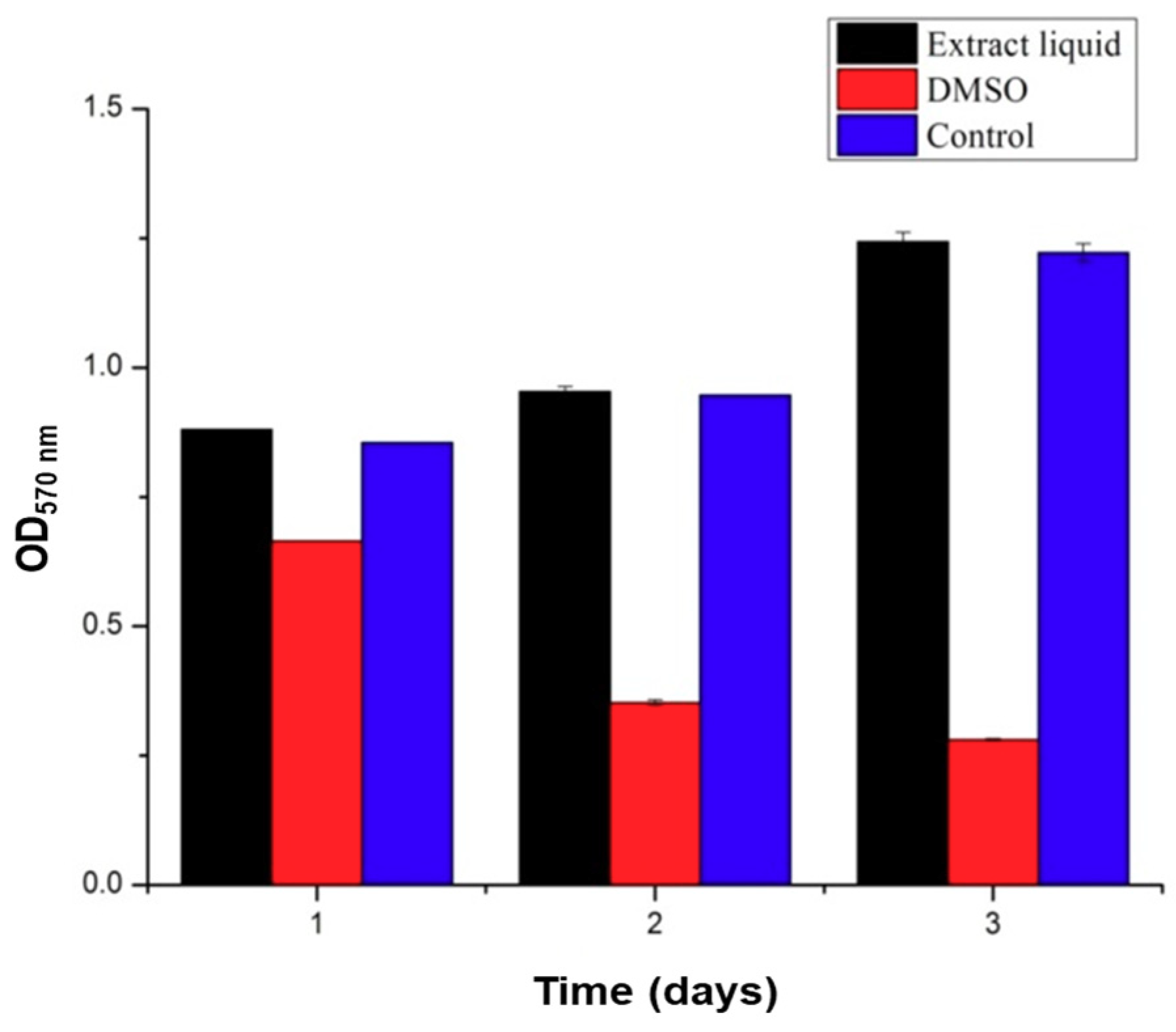
3.3. Cytocompatibility
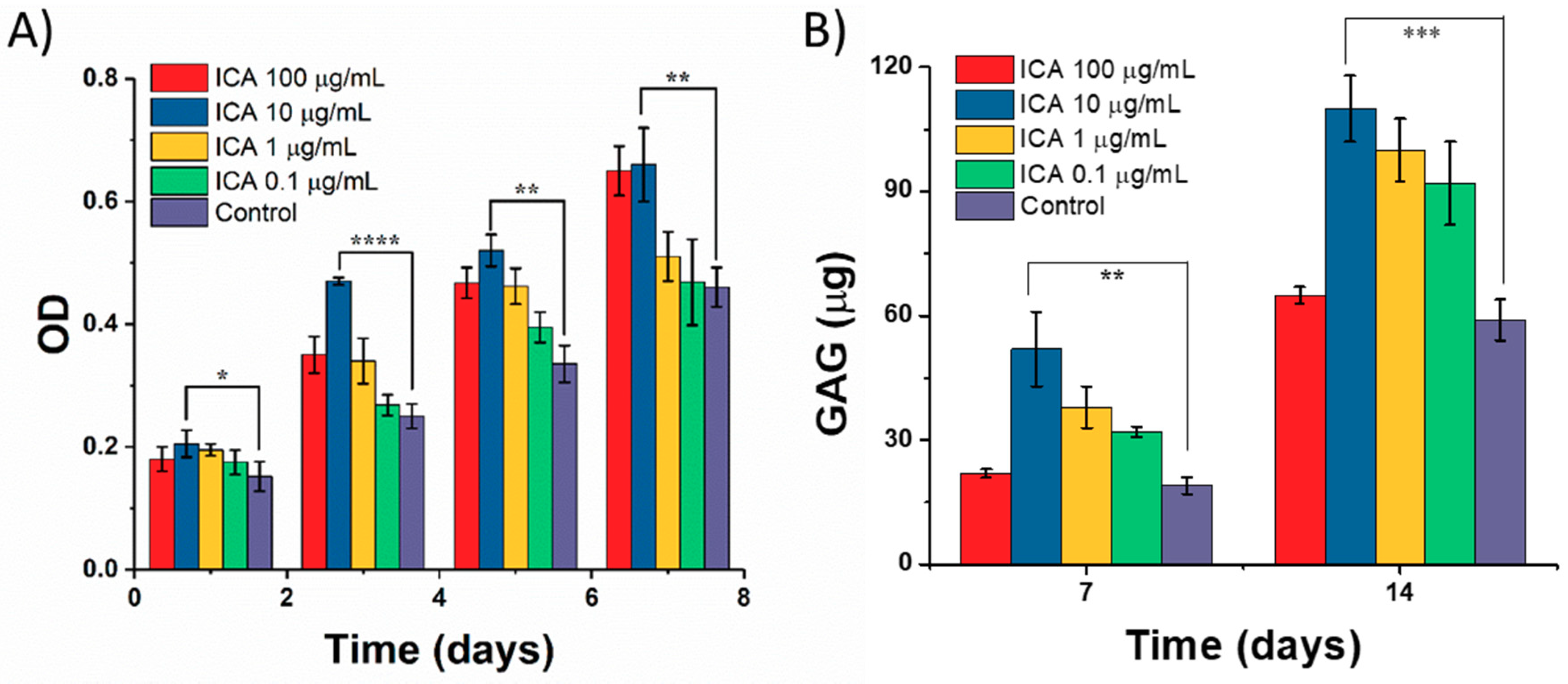
3.4. Effect of ICA on Chondrocyte Growth
3.5. Chondrocyte Growth and Differentiation on 3D-Printed Scaffolds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kankala, R.; Zhu, K.; Sun, X.; Liu, C.; Wang, S.; Chen, A. Cardiac tissue engineering on the nanoscale. ACS Biomater. Sci. Eng. 2018, 4, 800–818. [Google Scholar] [CrossRef]
- Ma, T.; Zhang, Y.; Chen, A.; Ju, J.; Gu, C.; Kankala, R.; Wang, S. Carbon dioxide-assisted bioassembly of cell-loaded scaffolds from polymeric porous microspheres. J. Supercrit. Fluids 2017, 120, 43–51. [Google Scholar] [CrossRef]
- Singh, M.; Sandhu, B.; Scurto, A.; Berkland, C.; Detamore, M.S. Microsphere-based scaffolds for cartilage tissue engineering: Using subcritical co as a sintering agent. Acta Biomater. 2010, 6, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Mikos, A.G.; Bao, Y.; Cima, L.G.; Ingber, D.E.; Vacanti, J.P.; Langer, R. Preparation of poly(glycolic acid) bonded fiber structures for cell attachment and transplantation. J. Biomed. Mater. Res. 1993, 27, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Mooney, D.J.; Mazzoni, C.L.; Breuer, C.; Mcnamara, K.; Hern, D.; Vacanti, J.P.; Langer, R. Stabilized polyglycolic acid fibre-based tubes for tissue engineering. Biomaterials 1996, 17, 115–124. [Google Scholar] [CrossRef]
- Chen, B.; Kankala, R.K.; Chen, A.; Yang, D.; Cheng, X.; Jiang, N.; Zhu, K.; Wang, S. Investigation of silk fibroin nanoparticle-decorated poly(l-lactic acid) composite scaffolds for osteoblast growth and differentiation. Int. J. Nanomed. 2017, 12, 1877–1890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Duchamp, M.; Oklu, R.; Ellisen, L.W.; Langer, R.; Khademhosseini, A. Bioprinting the cancer microenvironment. ACS Biomater. Sci. Eng. 2016, 2, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Y.; Heinrich, M.A.; De Ferrari, F.; Jang, H.; Bakht, S.M.; Alvarez, M.M.; Yang, J.; Li, Y.; Trujillo-de Santiago, G.; et al. Rapid continuous multimaterial extrusion bioprinting. Adv. Mater. 2017, 29, 1604630. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Zhu, K.; Li, J.; Wang, C.; Wang, S.; Chen, A. Fabrication of arbitrary 3d components in cardiac surgery: From macro-, micro- to nanoscale. Biofabrication 2017, 9, 032002. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.; Chen, A.; Wang, S.; Li, Y.; Liu, Y.; Cheng, X.; Zhao, Z.; Lin, D. Porous nanostructured poly-l-lactide scaffolds prepared by phase inversion using supercritical CO2 as a nonsolvent in the presence of ammonium bicarbonate particles. J. Supercrit. Fluids 2013, 77, 110–116. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhang, Y.S.; Wang, S.B.; Lee, C.H.; Chen, A.Z. Supercritical fluid technology: An emphasis on drug delivery and related biomedical applications. Adv. Healthc. Mater. 2017, 6, 1700433. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, D.; Karbasi, S.; Razavi, S.; Mohammadi, S.; Shokrgozar, M.A.; Bonakdar, S. Electrospun poly(hydroxybutyrate)/chitosan blend fibrous scaffolds for cartilage tissue engineering. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Carfì, P.F.; Palumbo, F.S.; La, C.V.; Bongiovì, F.; Brucato, V.; Pitarresi, G.; Giammona, G. Modulation of physical and biological properties of a composite plla and polyaspartamide derivative obtained via thermally induced phase separation (tips) technique. Mat. Sci. Eng. C Mater. 2016, 67, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Bhamidipati, M.; Sridharan, B.P.; Scurto, A.M.; Detamore, M.S. Subcritical CO2 sintering of microspheres of different polymeric materials to fabricate scaffolds for tissue engineering. Mater. Sci. Eng. C Mater. 2013, 33, 4892–4899. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, L.; Balian, G.; Laurencin, C.T.; Greg, A.D. Demineralized bone matrix gelatin as scaffold for osteochondral tissue engineering. Biomaterials 2006, 27, 2426–2433. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, X.; Li, N.; Li, L.; Zheng, Y.; Miao, X. Microstructure and characteristics of the metal–ceramic composite (mgca-ha/tcp) fabricated by liquid metal infiltration. J. Biomed. Mater. Res. B 2011, 99B, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Shi, P.; Zuo, Y.; Wu, L. Preparation and characterization of n-ha/chitosan scaffold prepared by a new method of emulsion-foaming/freeze-drying process. Mater. Sci. Forum 2007, 544, 789–792. [Google Scholar] [CrossRef]
- Sadeghi, A.R.; Nokhasteh, S.; Molavi, A.M.; Khorsandghayeni, M.; Naderimeshkin, H.; Mahdizadeh, A. Surface modification of electrospun plga scaffold with collagen for bioengineered skin substitutes. Mater. Sci. Eng. C 2016, 66, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, F.; Liu, K.; Shen, H.; Zhu, Y.; Zhang, W.; Liu, W.; Wang, S.; Cao, Y.; Zhou, G. The impact of plga scaffold orientation on invitro cartilage regeneration. Biomaterials 2012, 33, 2926–2935. [Google Scholar] [CrossRef] [PubMed]
- Hai, W.; Gui, W.; Jing, Z.; Zhou, K.; Bo, Y.; Su, X.; Qiu, G.; Yang, G.; Zhang, X.; Gang, Z. Osteogenic effect of controlled released rhbmp-2 in 3d printed porous hydroxyapatite scaffold. Colloid. Surf. B 2016, 141, 491–498. [Google Scholar]
- Lecanda, F.; Avioli, L.V.; Cheng, S.L. Regulation of bone matrix protein expression and induction of differentiation of human osteoblasts and human bone marrow stromal cells by bone morphogenetic protein-2. J. Cell. Biochem. 2015, 67, 386–398. [Google Scholar] [CrossRef]
- Walsh, S.; Jefferiss, C.; Stewart, K.; Jordan, G.R. Expression of the developmental markers stro-1 and alkaline phosphatase in cultures of human marrow stromal cells: Regulation by fibroblast growth factor (fgf)-2 and relationship to the expression of fgf receptors 1–4. Bone 2000, 27, 185–195. [Google Scholar] [CrossRef]
- Lisignoli, G.; Zini, N.; Remiddi, G.; Piacentini, A.; Puggioli, A.; Trimarchi, C.; Fini, M.; Maraldi, N.M.; Facchini, A. Basic fibroblast growth factor enhances in vitro mineralization of rat bone marrow stromal cells grown on non-woven hyaluronic acid based polymer scaffold. Biomaterials 2001, 22, 2095–2105. [Google Scholar] [CrossRef]
- Wildemann, B.; Kadowromacker, A.; Haas, N.P.; Schmidmaier, G. Quantification of various growth factors in different demineralized bone matrix preparations. J. Biomed. Mater. Res. A 2010, 81A, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Alt, V.; Heissel, A. Economic considerations for the use of recombinant human bone morphogenetic protein-2 in open tibial fractures in europe: The german model. Curr. Med. Res. Opin. 2006, 22, S19–S22. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Peel, S.A.; Sandor, G.K.; Clokie, C.M. The osteoinductive activity of bone morphogenetic protein (bmp) purified by repeated extracts of bovine bone. Growth Factors 2004, 22, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Montesano, R. Bone morphogenetic protein-4 abrogates lumen formation by mammary epithelial cells and promotes invasive growth. Biochem. Biophys. Res. Commun. 2007, 353, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Miriyala, S.; Nieto, M.C.G.; Mingone, C.; Smith, D.; Dikalov, S.; Harrison, D.G.; Jo, H. Bone morphogenic protein-4 induces hypertension in mice role of noggin, vascular nadph oxidases, and impaired vasorelaxation. Circulation 2006, 113, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Makizumi, R.; Ravikumar, T.S.; Dong, H.; Yang, W.; Yang, W.L. Bone morphogenetic protein-4 is overexpressed in colonic adenocarcinomas and promotes migration and invasion of hct116 cells. Exp. Cell Res. 2007, 313, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xia, L.; Zhou, Y.; Xu, Y.; Jiang, X. Icariin induces osteogenic differentiation of bone mesenchymal stem cells in a mapk-dependent manner. Cell Proliferat. 2015, 48, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Han, J.; Han, G.; French, A.D.; Qi, Y.; Wu, Q. Cellulose nanofibers reinforced sodium alginate-polyvinyl alcohol hydrogels: Core-shell structure formation and property characterization. Carbohydr. Polym. 2016, 147, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Y.; He, L.; Wang, Q.; Wang, L.; Yuan, T.; Xiao, Y.; Fan, Y.; Zhang, X. Icariin conjugated hyaluronic acid/collagen hydrogel for osteochondral interface restoration. Acta Biomater. 2018, 74, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Hagmann, S.; Frank, S.; Gotterbarm, T.; Dreher, T.; Eckstein, V.; Moradi, B. Fluorescence activated enrichment of cd146+ cells during expansion of human bone-marrow derived mesenchymal stromal cells augments proliferation and gag/DNA content in chondrogenic media. BMC Musculoskel. Dis. 2014, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Caron, M.M.J.; Emans, P.J.; Coolsen, M.M.E.; Voss, L.; Surtel, D.A.M.; Cremers, A.; van Rhijn, L.W.; Welting, T.J.M. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2d and 3d cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Yaylaci, S.U.; Sen, M.; Bulut, O.; Arslan, E.; Guler, M.O.; Tekinay, A.B. Chondrogenic differentiation of mesenchymal stem cells on glycosaminoglycan-mimetic peptide nanofibers. ACS Biomater. Sci. Eng. 2016, 2, 871–878. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kankala, R.K.; Lu, F.-J.; Liu, C.-G.; Zhang, S.-S.; Chen, A.-Z.; Wang, S.-B. Effect of Icariin on Engineered 3D-Printed Porous Scaffolds for Cartilage Repair. Materials 2018, 11, 1390. https://doi.org/10.3390/ma11081390
Kankala RK, Lu F-J, Liu C-G, Zhang S-S, Chen A-Z, Wang S-B. Effect of Icariin on Engineered 3D-Printed Porous Scaffolds for Cartilage Repair. Materials. 2018; 11(8):1390. https://doi.org/10.3390/ma11081390
Chicago/Turabian StyleKankala, Ranjith Kumar, Feng-Jun Lu, Chen-Guang Liu, Shan-Shan Zhang, Ai-Zheng Chen, and Shi-Bin Wang. 2018. "Effect of Icariin on Engineered 3D-Printed Porous Scaffolds for Cartilage Repair" Materials 11, no. 8: 1390. https://doi.org/10.3390/ma11081390
APA StyleKankala, R. K., Lu, F.-J., Liu, C.-G., Zhang, S.-S., Chen, A.-Z., & Wang, S.-B. (2018). Effect of Icariin on Engineered 3D-Printed Porous Scaffolds for Cartilage Repair. Materials, 11(8), 1390. https://doi.org/10.3390/ma11081390





