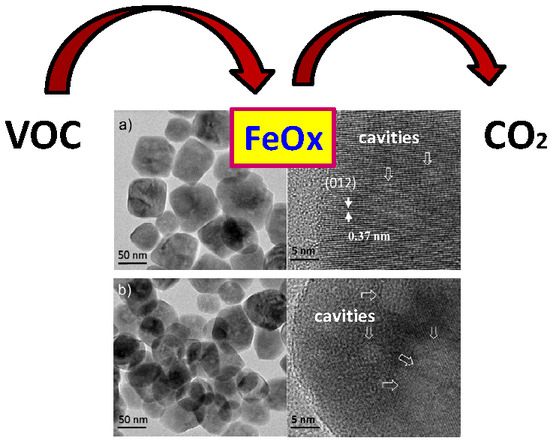
Eco-Friendly Cavity-Containing Iron Oxides Prepared by Mild Routes as Very Efficient Catalysts for the Total Oxidation of VOCs
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Iron Oxide Catalysts
2.2. Physicochemical Characteristics of the Catalysts
2.3. Catalytic Measurements
3. Results
3.1. Catalytic Results
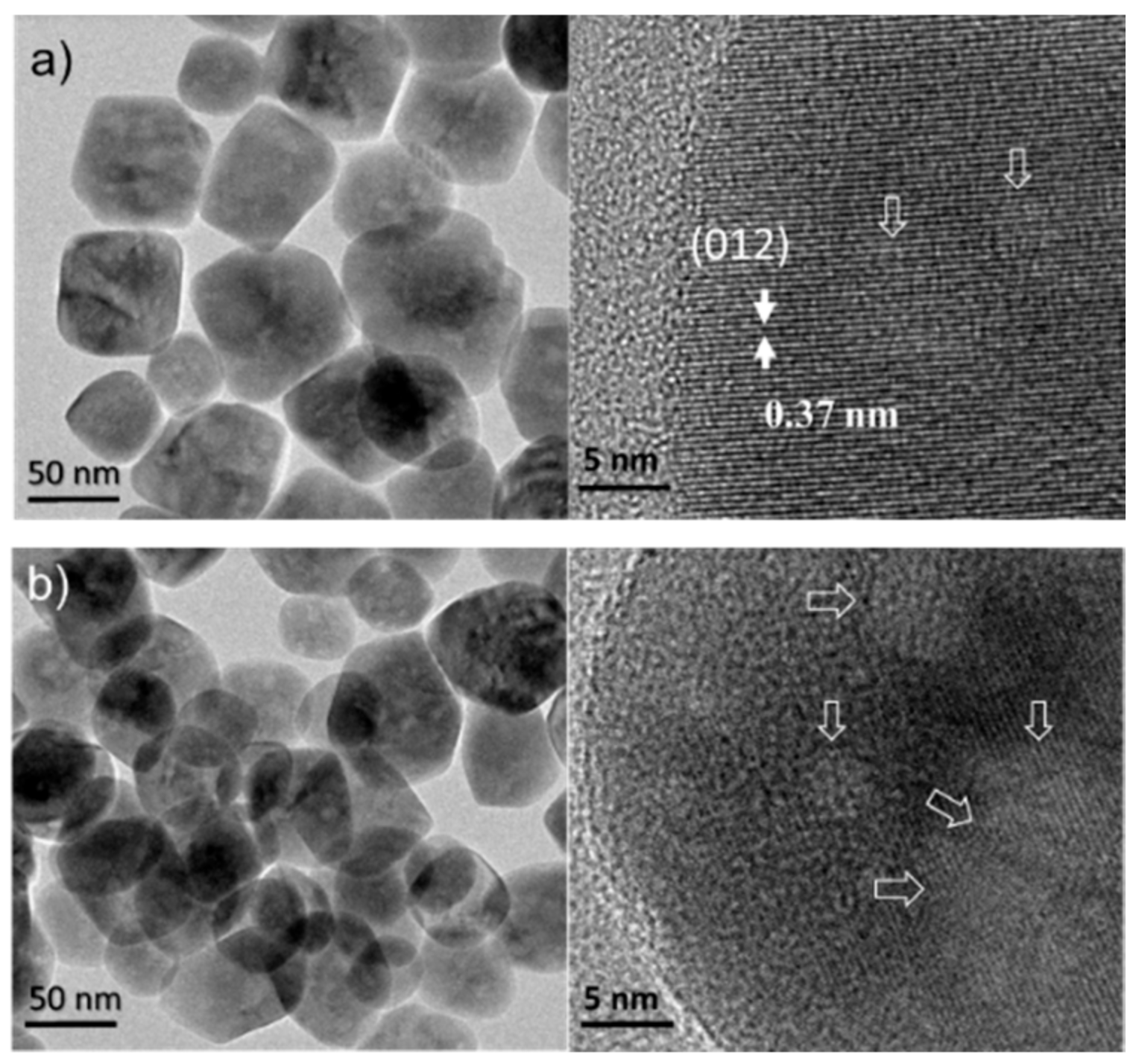
3.2. Characterization Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- García, T.; Solsona, B.; Taylor, S.H. The Catalytic Oxidation of Hydrocarbon Volatile Organic Compounds. In Handbook of Advanced Methods and Processes in Oxidation Catalysis: From Laboratory to Industry; Duprez, D.F., Cavani, F., Eds.; Imperial College Press: London, UK, 2014; Chapter 3; pp. 51–90. ISBN 978-1-84816-750-6. [Google Scholar]
- Gelin, P.; Primet, M. Complete Oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B Environ. 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Sun, H.; Yu, X.; Yang, X.; Ma, X.; Lin, M.; Shao, C.; Zhao, Y.; Wang, F.; Ge, M. Au/Rod-like MnO2 catalyst via thermal decomposition of manganite precursor for the catalytic oxidation of toluene. Catal. Today 2018. [Google Scholar] [CrossRef]
- Li, W.B.; Wang, J.X.; Gong, H. Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today 2009, 148, 81–87. [Google Scholar] [CrossRef]
- Everaert, K.; Baeyens, J. Catalytic combustion of volatile organic compounds. J. Hazard. Mater. 2004, 109, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Lan, B.; Lin, T.; Cheng, G.; Ye, F.; Yu, L.; Cheng, X.; Zheng, X. Controlled synthesis of nanostructured manganese oxide: Crystalline evolution and catalytic activities. CrystEngComm 2013, 15, 7010–7018. [Google Scholar] [CrossRef]
- Brunet, J.; Genty, E.; Barroo, C.; Cazier, F.; Poupin, C.; Siffert, S.; Thomas, D.; De Weireld, G.; Visart de Bocarmé, T.; Cousin, R. The CoAlCeO Mixed Oxide: An Alternative to Palladium-Based Catalysts for Total Oxidation of Industrial VOCs. Catalysts 2018, 8, 64. [Google Scholar] [CrossRef]
- Garcia, T.; Agouram, S.; Sánchez-Royo, J.F.; Murillo, R.; Mastral, A.M.; Aranda, A.; Vázquez, I.; Dejoz, A.; Solsona, B. Deep oxidation of volatile organic compounds using ordered cobalt oxides prepared by a nanocasting route. Appl. Catal. A Gen. 2010, 386, 16–27. [Google Scholar] [CrossRef]
- Duran, F.G.; Barbero, B.P.; Cadus, L.E.; Rojas, C.; Centeno, M.A.; Odriozola, J.A. Manganese and iron oxides as combustion catalysts of volatile organic compounds. Appl. Catal. B Environ. 2009, 92, 194–201. [Google Scholar] [CrossRef]
- Barbosa, A.L.; Herguido, J.; Santamaría, J. Methane combustion over unsupported iron oxide catalysts. Catal. Today 2001, 64, 43–50. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Xu, W.; Xu, Z.; Chen, J.; Jia, H.; Chen, J. Hydrolysis driving redox reaction to synthesize Mn-Fe binary oxides as highly active catalysts for the removal of toluene. Chem. Eng. J. 2017, 330, 281–293. [Google Scholar] [CrossRef]
- Xia, Y.; Dai, H.; Jiang, H.; Zhang, L.; Deng, J.; Liu, Y. Three-dimensionally ordered and wormhole-like mesoporous iron oxide catalysts highly active for the oxidation of acetone and methanol. J. Hazard. Mater. 2011, 186, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, J.; Xia, H.; Dai, Q.; Gu, Y.; Lao, Y.; Wang, X. Chlorinated volatile organic compound oxidation over SO42−/Fe2O3 catalysts. J. Catal. 2018, 360, 277–289. [Google Scholar] [CrossRef]
- Francisco, G.E.; Nogueira, J.; Lopes, H.; Adilson, C.S.; Rochel, M.L.; Fabris, J.D.; Oliveira, L.C.A. Catalysts based on clay and iron oxide for oxidation of toluene. Appl. Clay Sci. 2011, 51, 385–389. [Google Scholar] [CrossRef]
- Son, Y.H.; Lee, J.K.; Soong, Y.; Martello, D.; Chyu, M. Structure−Property Correlation in Iron Oxide Nanoparticle−Clay Hybrid Materials. Chem. Mater. 2010, 22, 2226–2232. [Google Scholar] [CrossRef]
- Andersen, S.L.F.; Flores, R.G.; Madeira, V.S.; José, H.J.; Moreira, R.F.P.M. Synthesis and Characterization of Acicular Iron Oxide Particles Obtained from Acid Mine Drainage and Their Catalytic Properties in Toluene Oxidation. Ind. Eng. Chem. Res. 2012, 51, 767–774. [Google Scholar] [CrossRef]
- Kim, I.H.; Park, E.J.; Park, C.H.; Han, S.W.; Seo, H.O.; Kim, Y.D. Activity of catalysts consisting of Fe2O3 nanoparticles decorating entire internal structure of mesoporous Al2O3 bead for toluene total oxidation. Catal. Today 2017, 295, 56–64. [Google Scholar] [CrossRef]
- Solsona, B.; García, T.; Sanchis, R.; Soriano, M.D.; Moreno, M.; Rodríguez-Castellón, E.; Agouram, S.; Dejoz, A.; López Nieto, J.M. Total oxidation of VOCs on mesoporous iron oxide catalysts: Soft chemistry route versus hard template method. Chem. Eng. J. 2016, 290, 273–281. [Google Scholar] [CrossRef]
- Sanchis, R.; Cecilia, J.A.; Soriano, M.D.; Vázquez, M.I.; Dejoz, A.; López Nieto, J.M.; Rodríguez-Castellón, E.; Solsona, B. Porous clays heterostructures as supports of iron oxide for environmental catalysis. Chem. Eng. J. 2018, 334, 1159–1168. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Chang, C.Y.; Hsieh, Y.H.; Yao, K.S.; Cheng, T.C.; Cheng, C.Y. Catalytic destruction and removal of toluene by microwave/Fe3O4 system. Adv. Mater. Res. 2008, 47–50, 335–338. [Google Scholar] [CrossRef]
- Sihaib, Z.; Puleo, F.; Garcia-Vargas, J.M.; Retailleau, L.; Descorme, C.; Liotta, L.F.; Valverde, J.L.; Gil, S.; Giroir-Fendler, A. Manganese oxide-based catalysts for toluene oxidation. Appl. Catal. B Environ. 2017, 209, 689–700. [Google Scholar] [CrossRef]
- Ruiz-Heredia, Y.; Álvarez-Serrano, I.; López, M.L.; Pico, C.; Veiga, M.L. Characterization of nanoparticulated phases in the manganese oxo/hydroxide system obtained in supercritical water: Optimized conditions for selected compositions. J. Supercrit. Fluids 2013, 78, 21–27. [Google Scholar] [CrossRef]
- Alonso-Domínguez, D.; Álvarez-Serrano, I.; Pico, M.P.; López, M.L.; Urones-Garrote, E.; Pico, C.; Veiga, M.L. Nanoparticulated spinel-type iron oxides obtained in supercritical water and their electrochemical performance as anodes for Li ion batteries. J. Alloys Compd. 2013, 695, 3239–3242. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Lin, T.C.; Seshadri, G.; Kelber, J.A. A consistent method for quantitative XPS peak analysis of thin oxide films on clean polycrystalline iron surfaces. Appl. Surf. Sci. 1997, 119, 83–92. [Google Scholar] [CrossRef]
- Fujii, T.; de Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In Situ XPS Analysis of Various Iron Oxide Films Grown by NO2 -assisted Molecular-beam Epitaxy. Phys. Rev. B 1999, 59, 3195–3202. [Google Scholar] [CrossRef]
- Kendelewicz, T.; Liu, P.; Doyle, C.S.; Brown, G.E., Jr.; Nelson, E.J.; Chambers, S.A. Reaction of water with the (100) and (111) surfaces of Fe3O4. Surf. Sci. 2000, 453, 32–46. [Google Scholar] [CrossRef]
- Ruby, C.; Humbert, B.; Fusy, J. Surface and Interface Properties of Epitaxial Iron Oxide Thin Films Deposited on MgO(001) Studied by XPS and Raman Spectroscopy. Surf. Interface Anal. 2000, 29, 377–380. [Google Scholar] [CrossRef]
- Lu, L.; Ai, Z.; Li, J.; Zheng, Z.; Li, Q.; Zhang, L. Synthesis and characterization of Fe–Fe2O3 core–shell nanowires and nanoneckaces. Cryst. Growth Des. 2007, 7, 459–464. [Google Scholar] [CrossRef]
- Galtayries, A.; Sporken, R.; Riga, J.; Blanchard, G.; Caudano, R. XPS comparative study of ceria/zirconia mixed oxides: Powders and thin film characterization. J. Electron. Spectrosc. Relat. Phenom. 1998, 88, 951–956. [Google Scholar] [CrossRef]
- Barr, T.L. An ESCA study of the termination of the passivation of elemental metals. J. Phys. Chem. 1978, 82, 1801–1810. [Google Scholar] [CrossRef]


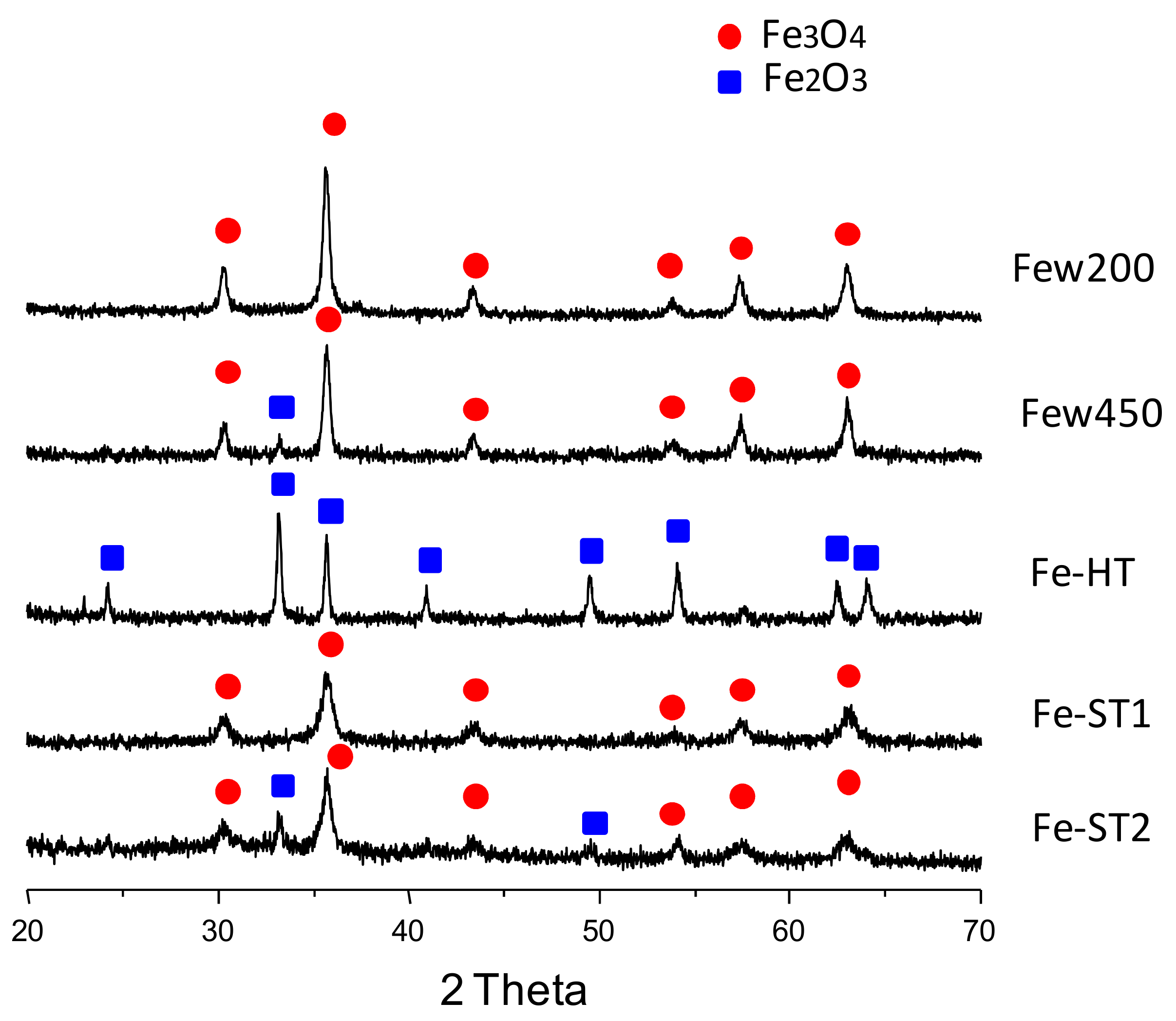



| Catalyst | Synthesis Method | SBET (m2 g−1) | Morphology and Size a | Fe-Oxide Phases b |
|---|---|---|---|---|
| Few200 | In supercritical water. Continuous regime. T = 200 °C (subcritical value) | 21 | Spherical φ ~7–45 nm | Fe3O4 spinel |
| Few450 | In supercritical water. Continuous regime. T = 450 °C (supercritical value) | 30 | Spherical φ ~10–70 nm | Fe3O4 >> Fe2O3 |
| Fe-HT | Hydrothermal in subcritical conditions. Batch regime (sealed autoclave) | 20 | Spherical with cavities φ ~30–100 nm | Fe2O3 corundum |
| Fe-ST1 | Solvothermal in subcritical conditions. Ethylene glycol Batch regime (sealed autoclave) | 18 | Blackberry aggregates 200 units (~6 nm) | Fe3O4 spinel |
| Fe-ST2 | Solvothermal in subcritical conditions. Polyethylene glycol in ethylene glycol. Batch regime (sealed autoclave) | 55 | Flower-like aggregates (~10 μm) | Fe3O4 >> Fe2O3 |
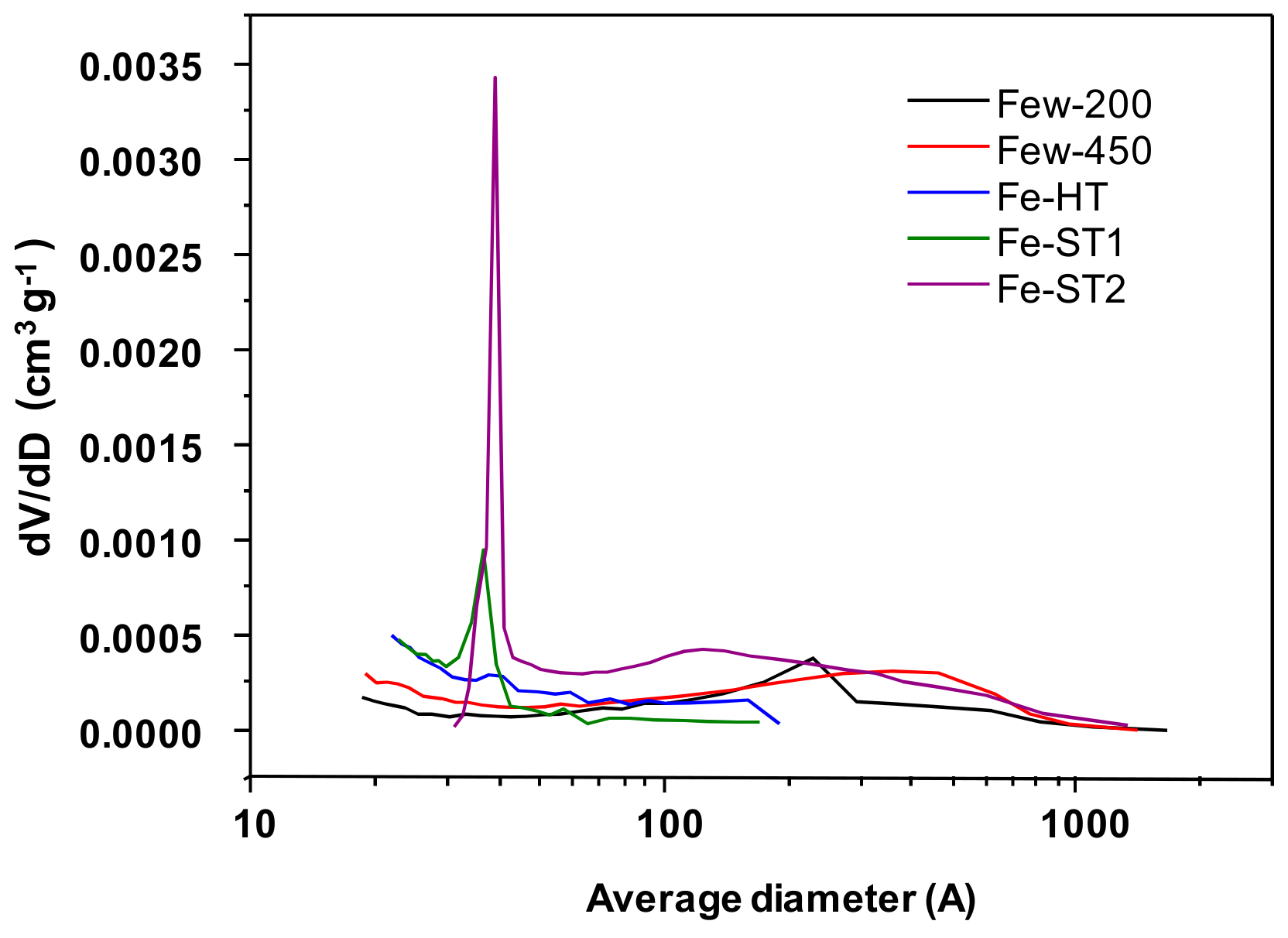
| Sample | SBET (m2 g−1) | VMeso (cm3 g−1) | DMeso (Å) | D4V/A-Meso (Å) |
|---|---|---|---|---|
| Few200 | 21 | 0.14 | 231.1 | 236.5 |
| Few450 | 30 | 0.21 | 362.1 | 248.3 |
| Fe-HT | 20 | 0.03 | 39.5 | 61.4 |
| Fe-ST1 | 18 | 0.02 | 36.5 | 42.5 |
| Fe-ST2 | 55 | 0.26 | 38.5 | 204.2 |
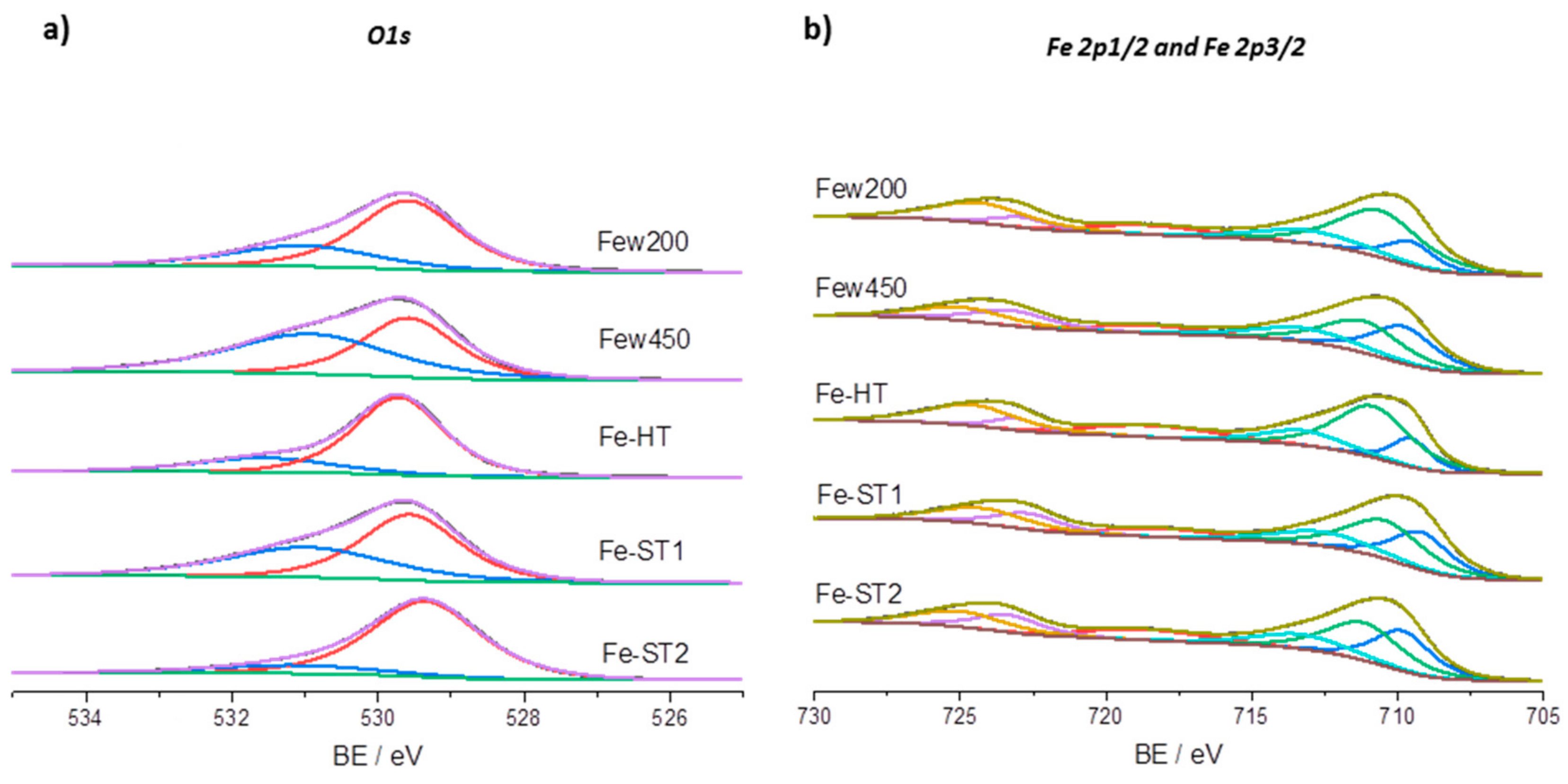
| Catalyst | Oxygen Signals Detected O1s | Iron Signals Detected (eV) | ||||
|---|---|---|---|---|---|---|
| Oα (eV) | Oβ (eV) | Oα/O | 2p3/2 | Satellite | Fe2+/(Fe2+ + Fe3+) | |
| Few200 | 529.5 | 531.0 | 54 | 710.6 | 718.7 | 34 |
| Few450 | 529.6 | 530.9 | 45 | 710.7 | 718.8 | 49 |
| Fe-HT | 529.8 | 531.6 | 73 | 710.7 | 718.8 | 22 |
| Fe-ST1 | 529.6 | 531.0 | 62 | 710.7 | 718.8 | 44 |
| Fe-ST2 | 529.3 | 531.1 | 79 | 710.7 | 718.9 | 45 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchis, R.; Alonso-Domínguez, D.; Dejoz, A.; Pico, M.P.; Álvarez-Serrano, I.; García, T.; López, M.L.; Solsona, B. Eco-Friendly Cavity-Containing Iron Oxides Prepared by Mild Routes as Very Efficient Catalysts for the Total Oxidation of VOCs. Materials 2018, 11, 1387. https://doi.org/10.3390/ma11081387
Sanchis R, Alonso-Domínguez D, Dejoz A, Pico MP, Álvarez-Serrano I, García T, López ML, Solsona B. Eco-Friendly Cavity-Containing Iron Oxides Prepared by Mild Routes as Very Efficient Catalysts for the Total Oxidation of VOCs. Materials. 2018; 11(8):1387. https://doi.org/10.3390/ma11081387
Chicago/Turabian StyleSanchis, Rut, Daniel Alonso-Domínguez, Ana Dejoz, María Pilar Pico, Inmaculada Álvarez-Serrano, Tomás García, María Luisa López, and Benjamín Solsona. 2018. "Eco-Friendly Cavity-Containing Iron Oxides Prepared by Mild Routes as Very Efficient Catalysts for the Total Oxidation of VOCs" Materials 11, no. 8: 1387. https://doi.org/10.3390/ma11081387
APA StyleSanchis, R., Alonso-Domínguez, D., Dejoz, A., Pico, M. P., Álvarez-Serrano, I., García, T., López, M. L., & Solsona, B. (2018). Eco-Friendly Cavity-Containing Iron Oxides Prepared by Mild Routes as Very Efficient Catalysts for the Total Oxidation of VOCs. Materials, 11(8), 1387. https://doi.org/10.3390/ma11081387