Oxidation Behavior of Tyranno ZMI-SiC Fiber/SiC-SiBC Matrix Composite from 800 to 1200 °C
Abstract
:1. Introduction
2. Materials and Methods
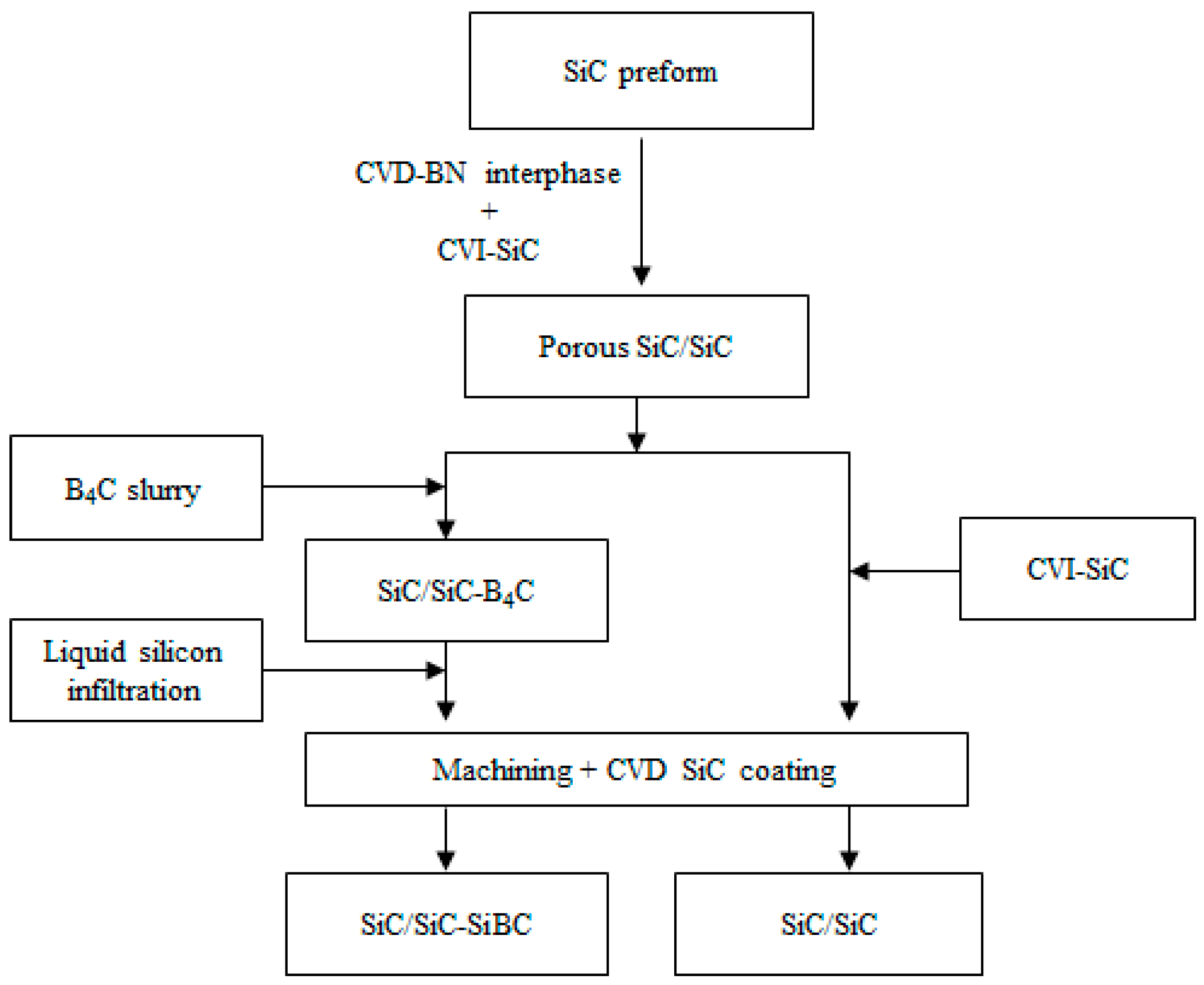
2.1. Materials Preparation
2.2. Characterization
3. Results
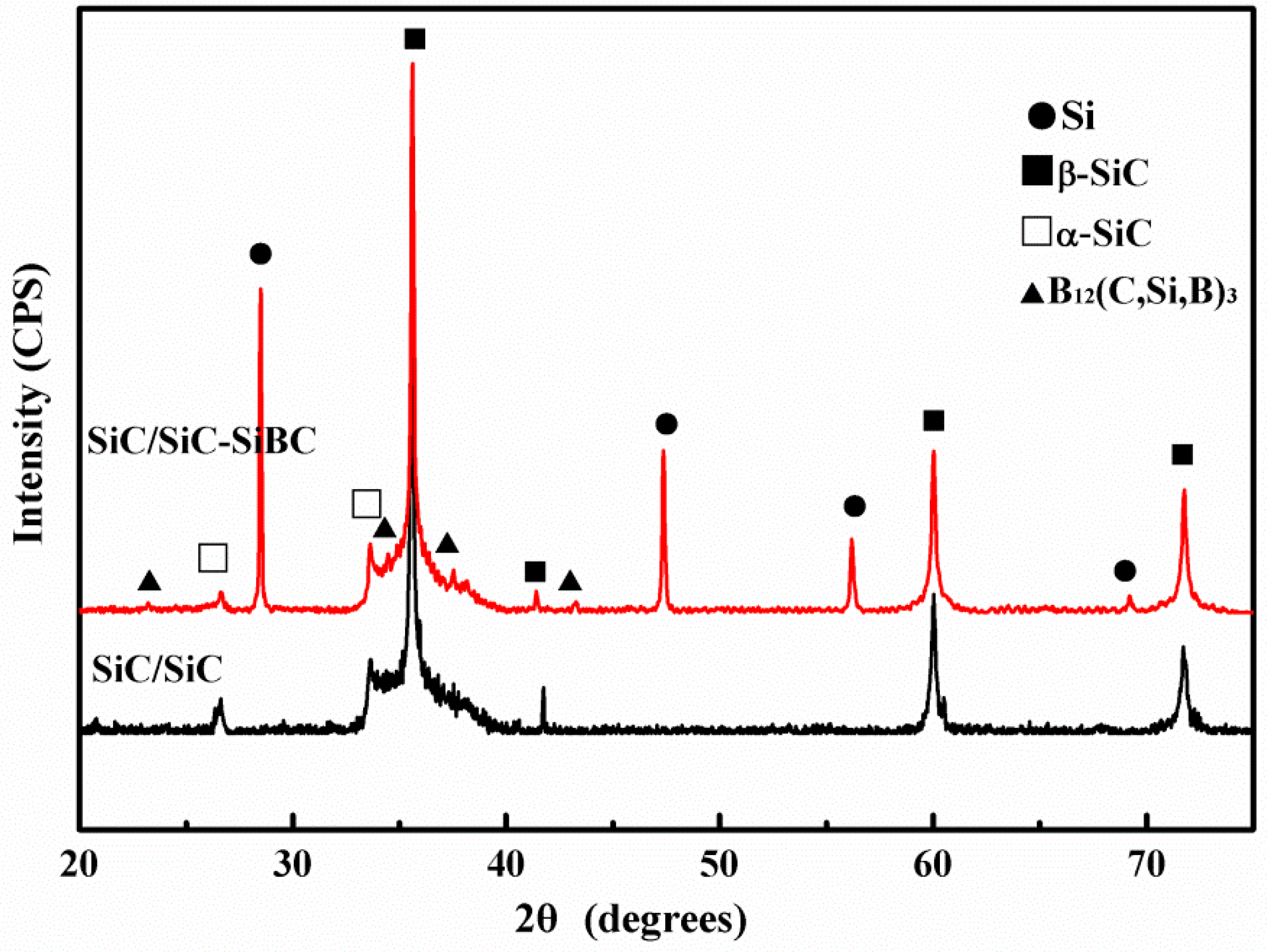
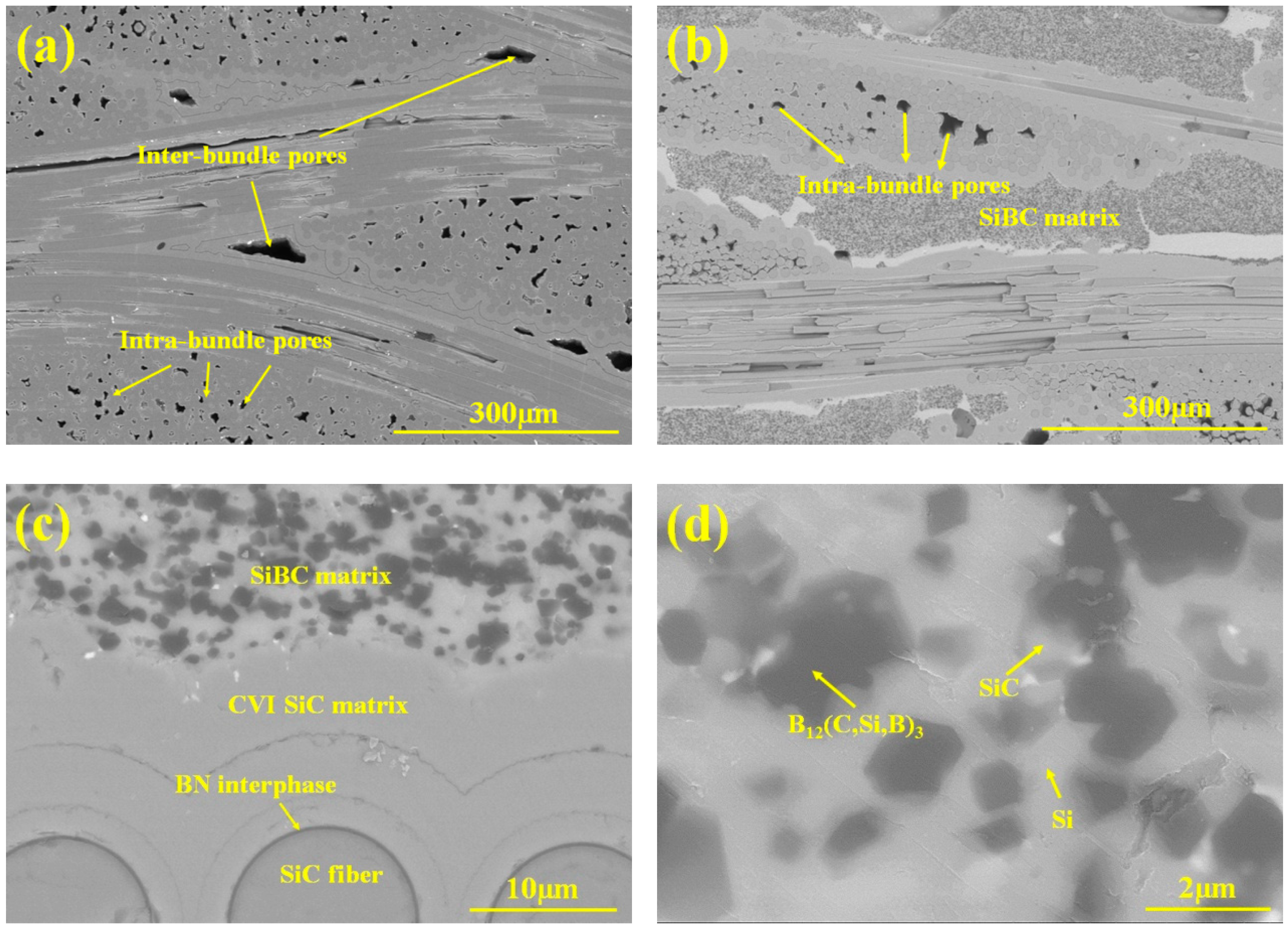
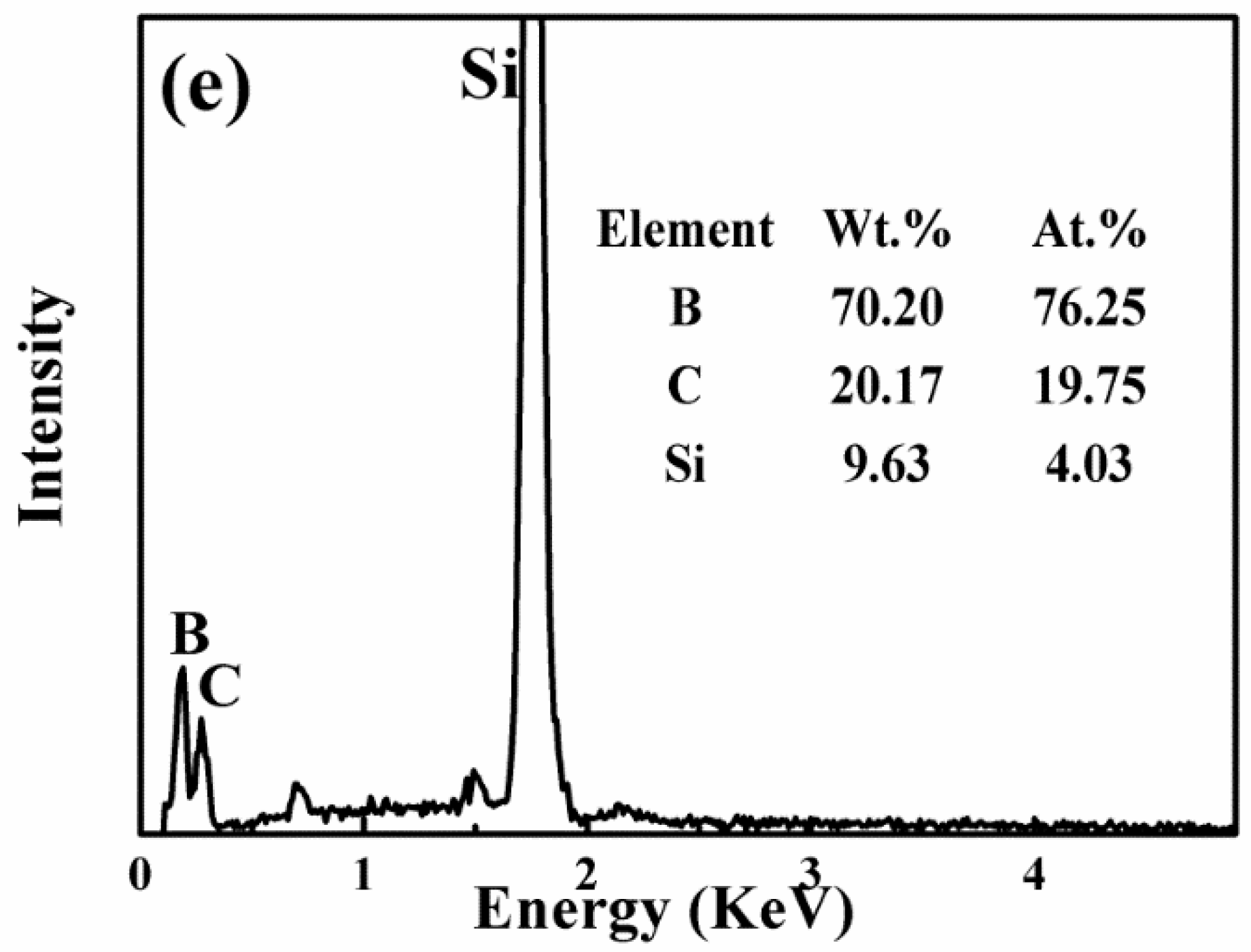
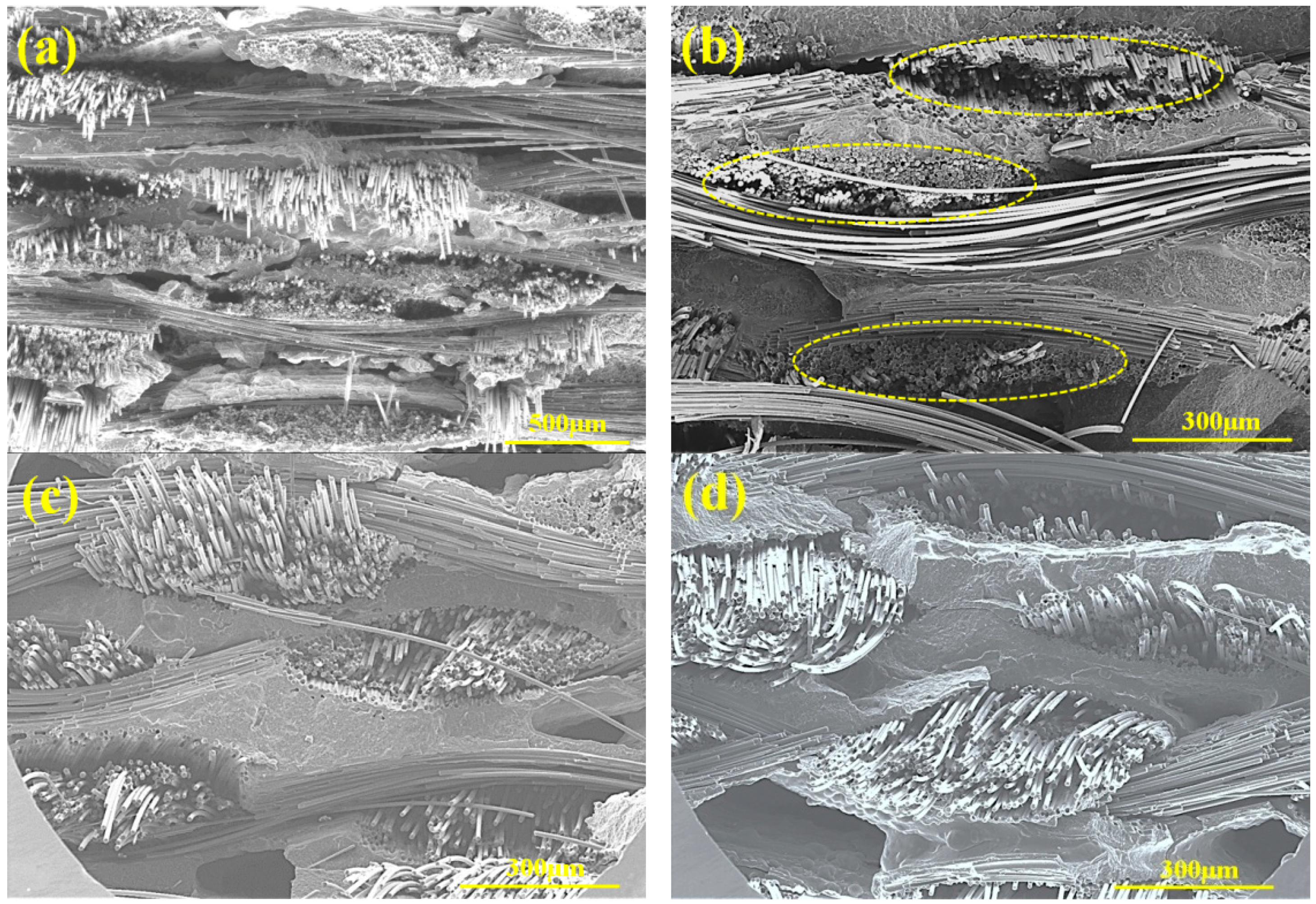
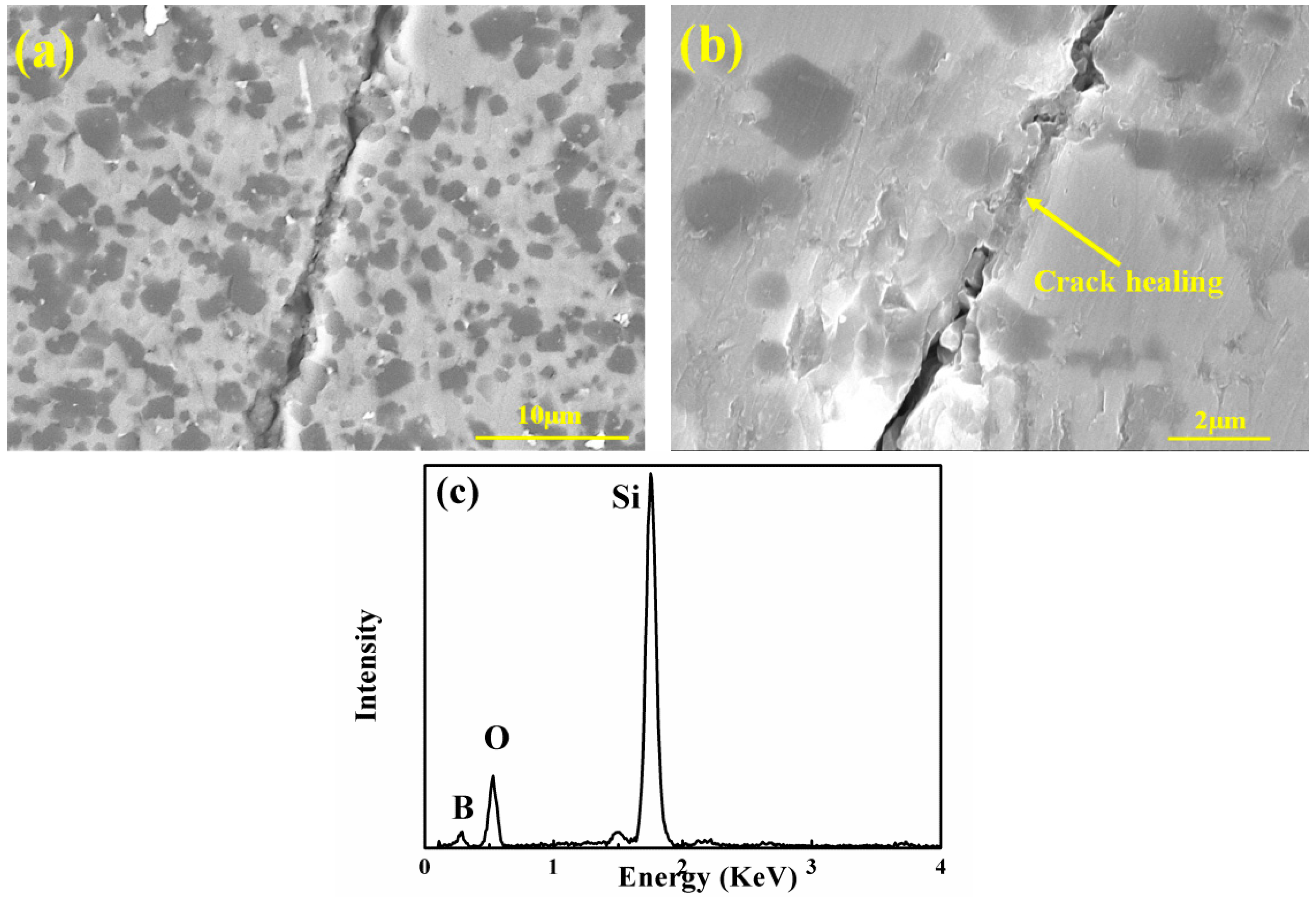
3.1. Microstructure Analysis
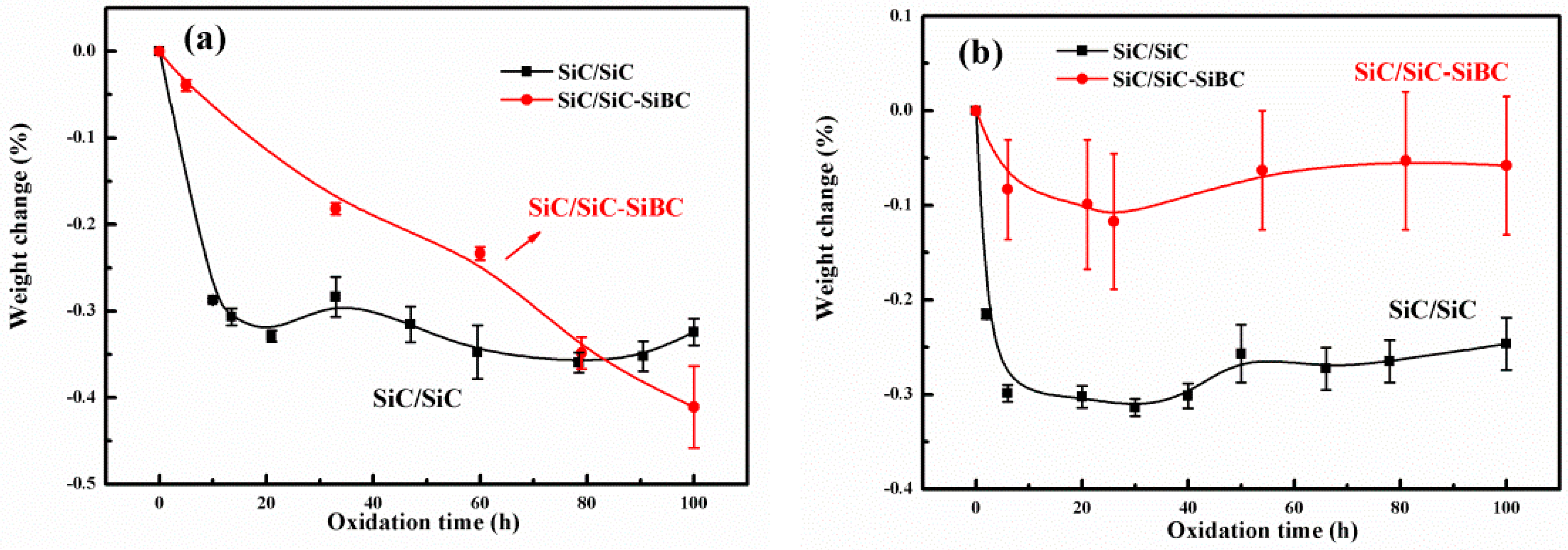
3.2. Weight Variation
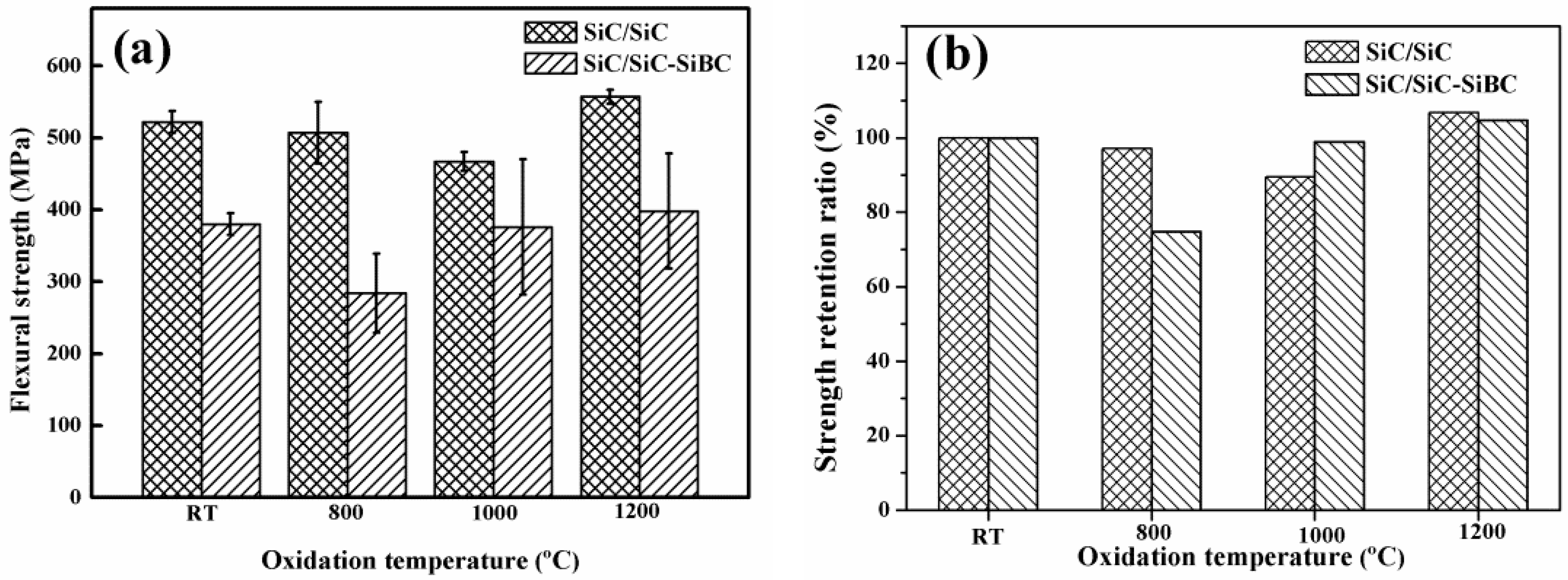
3.3. Residual Flexural Strength
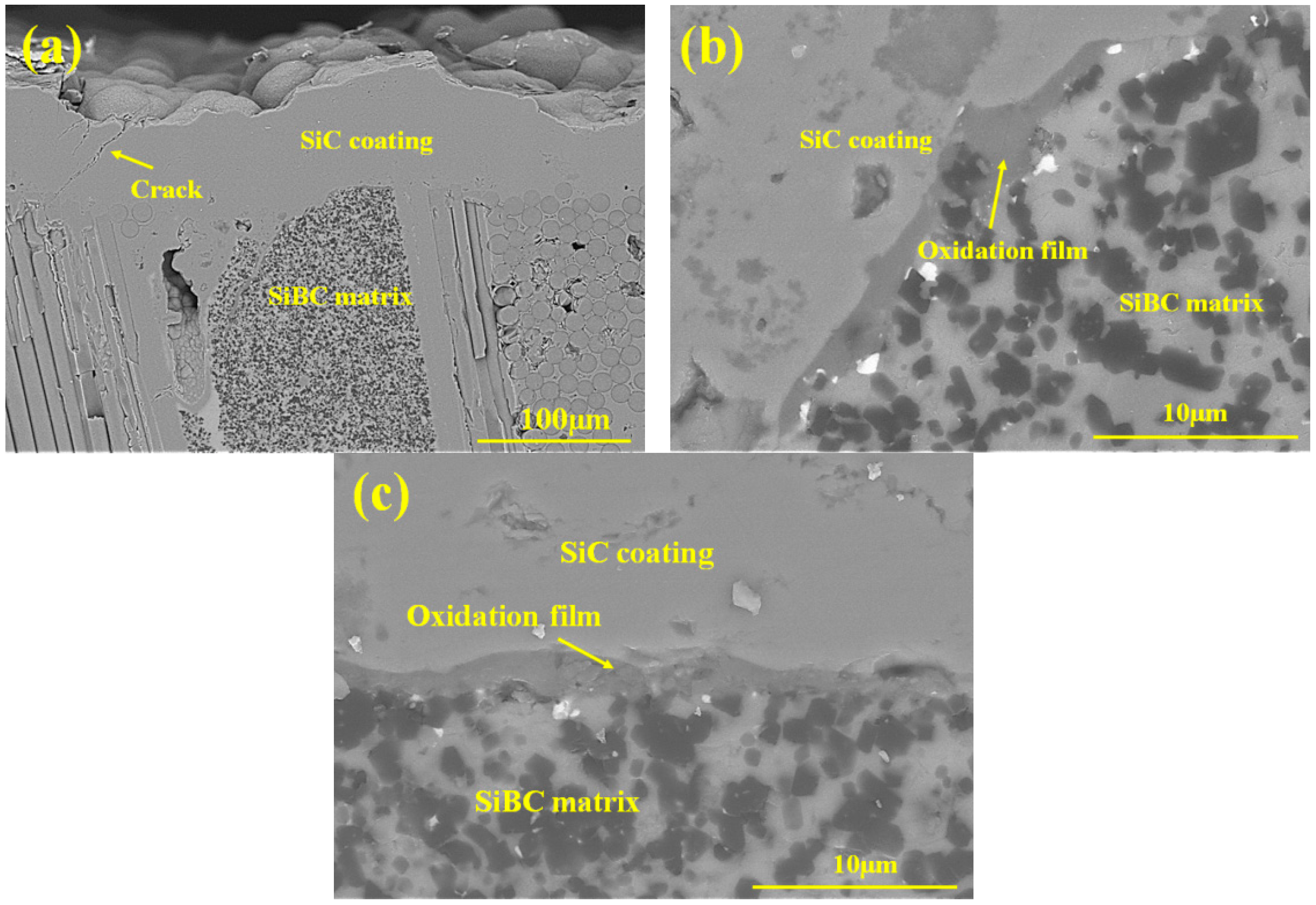
3.4. Oxidation Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naslain, R. Design, preparation and properties of non-oxide CMCs for application in engines and nuclear reactors: An overview. Compos. Sci. Technol. 2004, 64, 155–170. [Google Scholar] [CrossRef]
- Ohnabe, H.; Masaki, S.; Onozuka, M.; Miyahara, K.; Sasa, T. Potential application of ceramic matrix composites to aero-engine components. Compos. Part A 1999, 30, 489–496. [Google Scholar] [CrossRef]
- Krenkel, W. Carbon fiber reinforced CMC for high-performance structures. Int. J. Appl. Ceram. Technol. 2004, 1, 188–200. [Google Scholar] [CrossRef]
- Naslain, R. SiC-matrix composites: Nonbrittle ceramics for thermostructural application. Int. J. Appl. Ceram. Technol. 2005, 2, 75–84. [Google Scholar] [CrossRef]
- Christin, F. Design, Fabrication, and application of thermostructural composites (TSC) like C/C, C/SiC, and SiC/SiC composites. Adv. Eng. Mater. 2010, 4, 903–912. [Google Scholar] [CrossRef]
- Dong, S.; Katoh, Y.; Kohyama, A. Processing optimization and mechanical evaluation of hot pressed 2D Tyranno-SA/SiC composites. J. Eur. Ceram. Soc. 2003, 23, 1223–1231. [Google Scholar] [CrossRef]
- Rebillat, F.; Guette, A.; Espitalier, L. Oxidation resistance of SiC/SiC micro and minicomposites with a highly crystallised BN interphase. J. Eur. Ceram. Soc. 1998, 18, 1809–1819. [Google Scholar] [CrossRef]
- Morschera, G.N.; Cawleyb, J.D. Intermediate temperature strength degradation in SiC/SiC composites. J. Eur. Ceram. Soc. 2002, 22, 2777–2787. [Google Scholar] [CrossRef]
- Filipuzzi, L.; Camus, G.; Naslain, R. Oxidation mechanisms and kinetics of 1D-SiC/C/SiC composite materials: I, an experimental approach. J. Am. Ceram. Soc. 1994, 77, 459–466. [Google Scholar] [CrossRef]
- Wu, S.; Cheng, L.; Zhang, Q. Thermophysical and mechanical properties of a three-dimensional Hi-Nicalon/SiC composite. Int. J. Appl. Ceram. Technol. 2006, 3, 75–79. [Google Scholar] [CrossRef]
- Lamouroux, F.; Bertrand, S.; Pailler, R.; Naslain, R.; Cataldi, M. Oxidation-resistant carbon-fiber reinforced ceramic-matrix composites. Compos. Sci. Technol. 1999, 59, 1073–1085. [Google Scholar] [CrossRef]
- Brian, W.S.; Ellen, Y.S.; Steven, R.N.; Brennan, J.J. Oxidation of BN-coated SiC fibers in ceramic matrix composites. J. Am. Ceram. Soc. 1996, 79, 539–543. [Google Scholar]
- Viricelle, J.P.; Goursat, P.; Hourlier, D.B. Oxidation behaviour of a multi-layer ceramic-matrix composite (SiC)f/C/(SiBC)m. Compos. Sci. Technol. 2001, 61, 607–614. [Google Scholar] [CrossRef]
- Naslain, R.; Guette, A.; Rebillat, F.; Pailler, R.; langlais, F.; Bourrat, X. Boron-bearing species in ceramic matrix composites for long-term aerospace applications. J. Solid State Chem. 2004, 177, 449–456. [Google Scholar] [CrossRef]
- Shi, F.; Yin, X.; Fan, X.; Cheng, L.; Zhang, L. A new route to fabricate SiB4 modified C/SiC composites. J. Eur. Ceram. Soc. 2010, 30, 1955–1962. [Google Scholar] [CrossRef]
- Cao, X.; Yin, X.; Fan, X.; Cheng, L.; Zhang, L. Effect of PyC interphase thickness on mechanical behaviors of SiBC matrix modified C/SiC composites fabricated by reactive melt infiltration. Carbon 2014, 77, 886–895. [Google Scholar] [CrossRef]
- Cao, X.; Yin, X.; Ma, X.; Fan, X.; Cheng, L.; Zhang, L. Oxidation behavior of SiBC matrix modified C/SiC composites with different PyC interphase thicknesses. Ceram. Int. 2015, 41, 1695–1700. [Google Scholar] [CrossRef]
- Hayun, S.; Weizmann, A.; Dariel, M.; Frage, N. Microstructural evolution during the infiltration of boron carbide with molten silicon. J. Eur. Ceram. Soc. 2010, 30, 1007–1014. [Google Scholar] [CrossRef]
- Morscher, G.; Yun, H.; DiCarlo, J.; Thomas-Ogbuji, L. Effect of a boron nitride interphase that debonds between the interphase and the matrix in SiC/SiC composites. J. Am. Ceram. Soc. 2004, 87, 104–112. [Google Scholar] [CrossRef]
- Mo, R.; Yin, X.; Ye, F.; Liu, X.; Cheng, L.; Zhang, L. Mechanical and microwave absorbing properties of Tyranno® ZMI fiber annealed at elevated temperatures. Ceram. Int. 2017, 43, 8922–8931. [Google Scholar] [CrossRef]
- Fan, X.; Yin, X.; Chen, L.; Zhang, L.; Cheng, L. Mechanical behavior and electromagnetic interference shielding properties of C/SiC-Ti3Si(Al)C2. J. Am. Ceram. Soc. 2016, 99, 1717–1724. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Fan, X.; Yin, X.; Cao, X.; Zhang, J. Oxidation Behavior of Tyranno ZMI-SiC Fiber/SiC-SiBC Matrix Composite from 800 to 1200 °C. Materials 2018, 11, 1367. https://doi.org/10.3390/ma11081367
Zhao D, Fan X, Yin X, Cao X, Zhang J. Oxidation Behavior of Tyranno ZMI-SiC Fiber/SiC-SiBC Matrix Composite from 800 to 1200 °C. Materials. 2018; 11(8):1367. https://doi.org/10.3390/ma11081367
Chicago/Turabian StyleZhao, Donglin, Xiaomeng Fan, Xiaowei Yin, Xiaoyu Cao, and Jing Zhang. 2018. "Oxidation Behavior of Tyranno ZMI-SiC Fiber/SiC-SiBC Matrix Composite from 800 to 1200 °C" Materials 11, no. 8: 1367. https://doi.org/10.3390/ma11081367
APA StyleZhao, D., Fan, X., Yin, X., Cao, X., & Zhang, J. (2018). Oxidation Behavior of Tyranno ZMI-SiC Fiber/SiC-SiBC Matrix Composite from 800 to 1200 °C. Materials, 11(8), 1367. https://doi.org/10.3390/ma11081367



