Facile Fabrication of Dumbbell-Like β-Bi2O3/Graphene Nanocomposites and Their Highly Efficient Photocatalytic Activity
Abstract
1. Introduction
2. Experimental
2.1. Synthesis of Bi2O3/GN
2.2. Materials’ Characterization
2.3. Test of Photocatalytic Activity
3. Results and Discussion
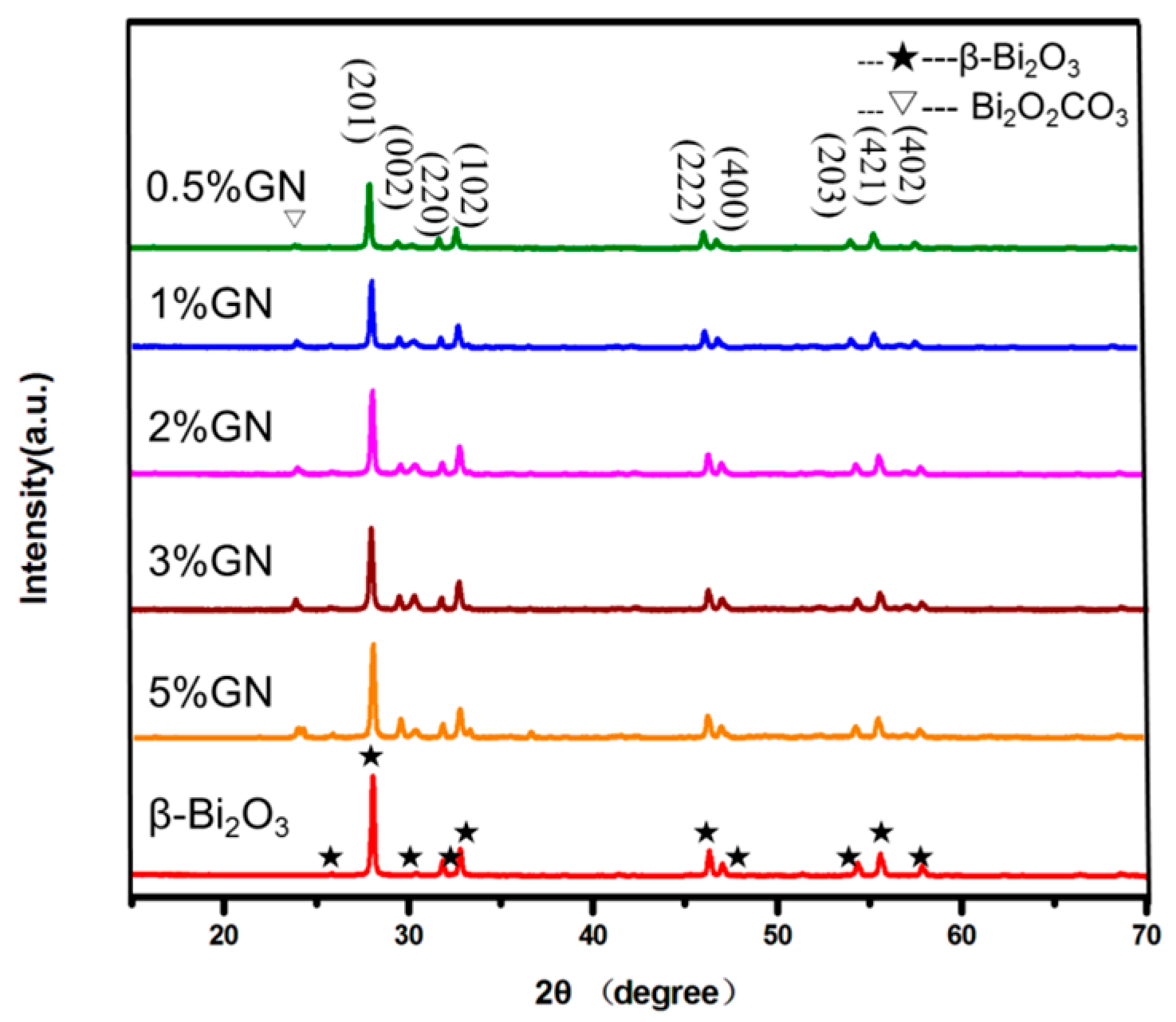
3.1. Crystal Structure Characterization
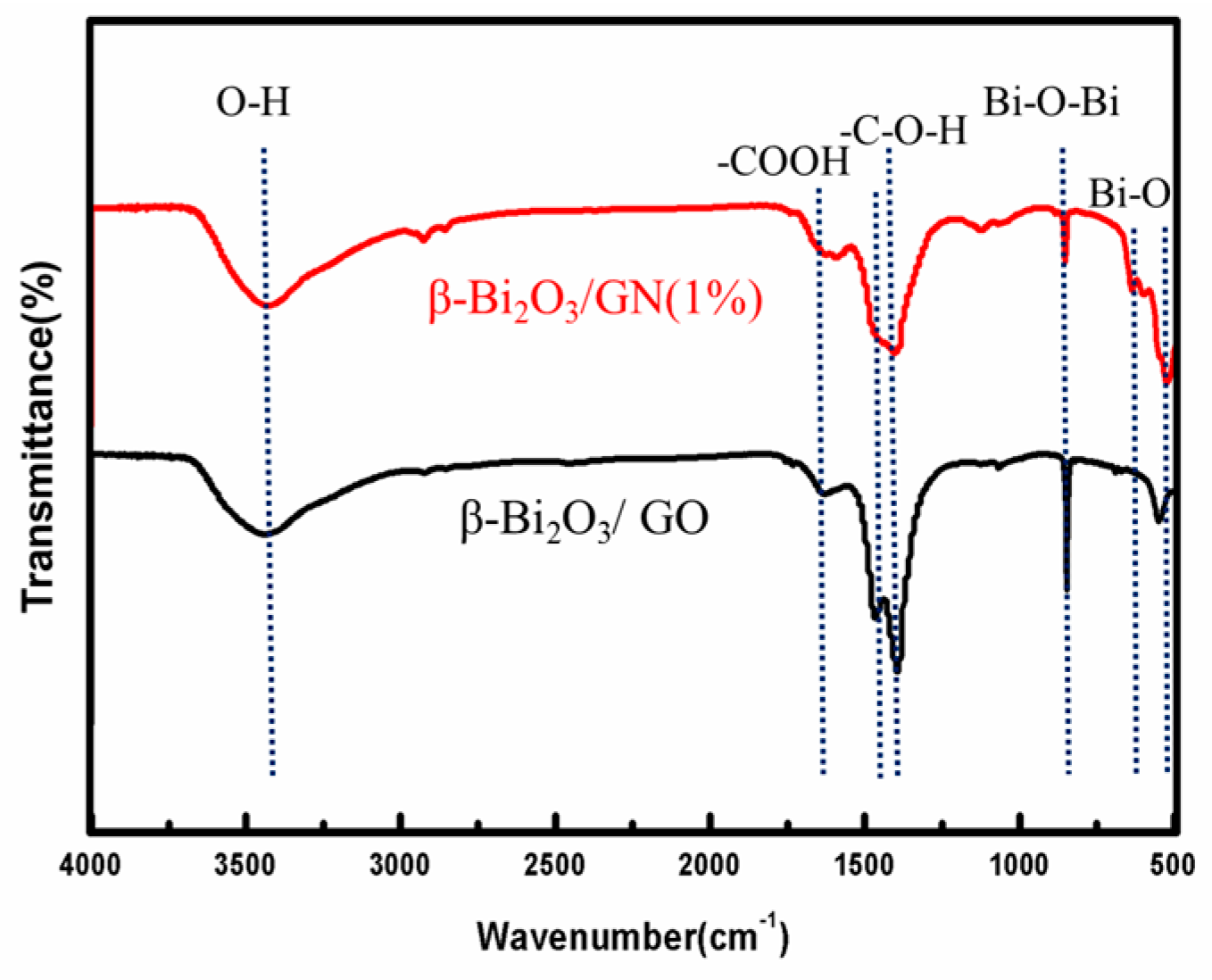
3.2. FTIR Spectra
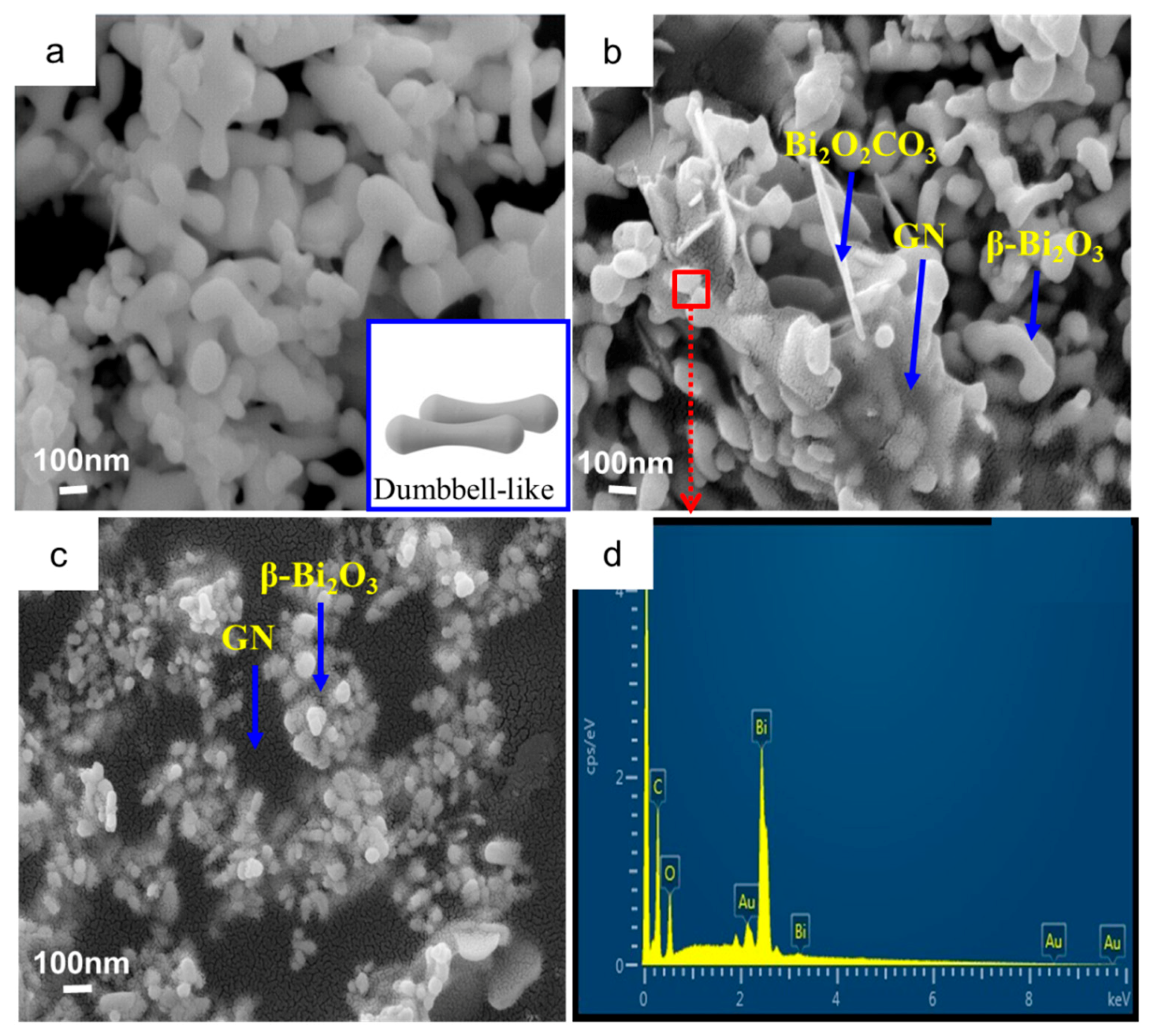
3.3. Surface Morphology Characterization
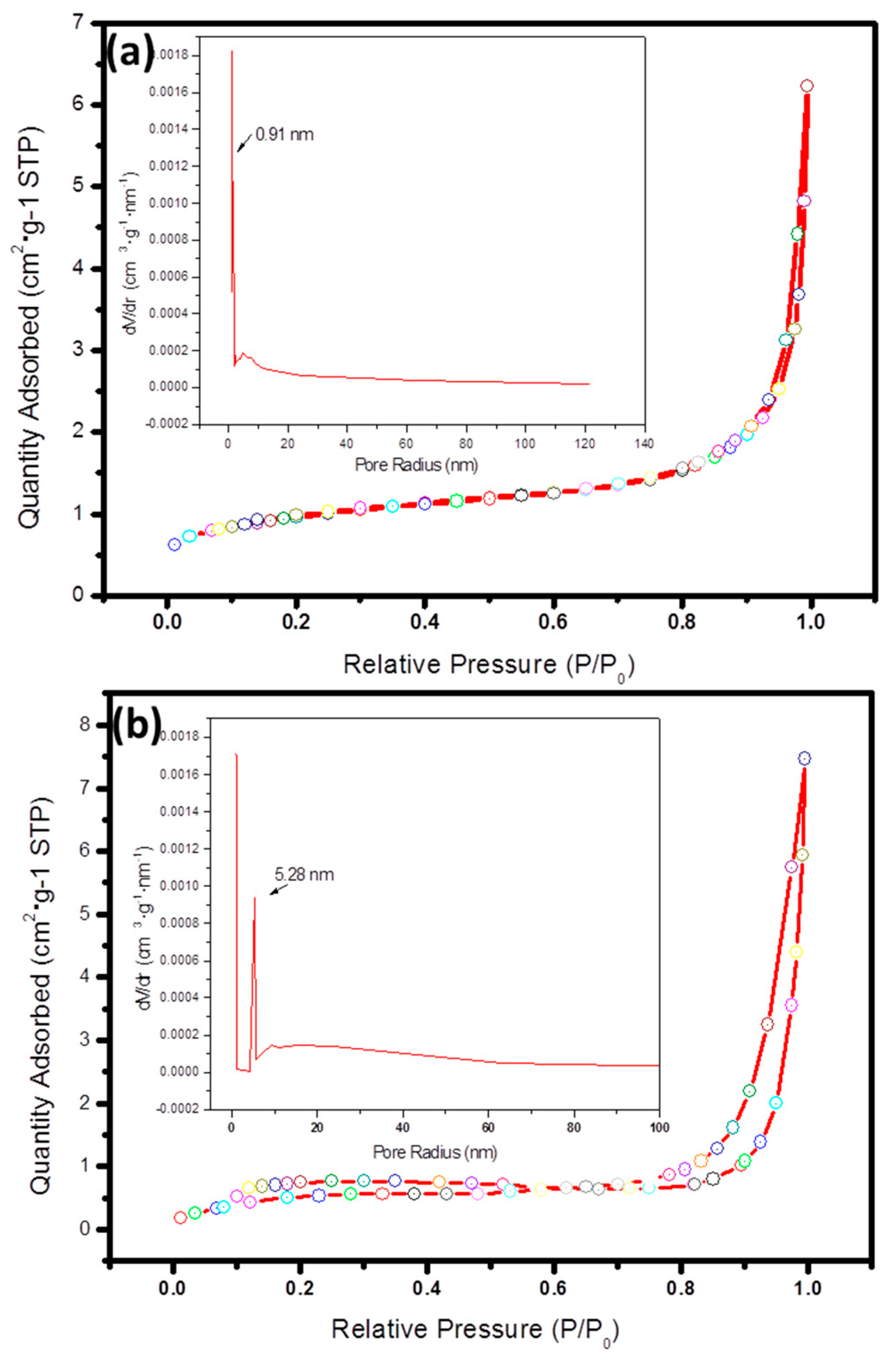
3.4. Surface Areas and Pore Size Distributions
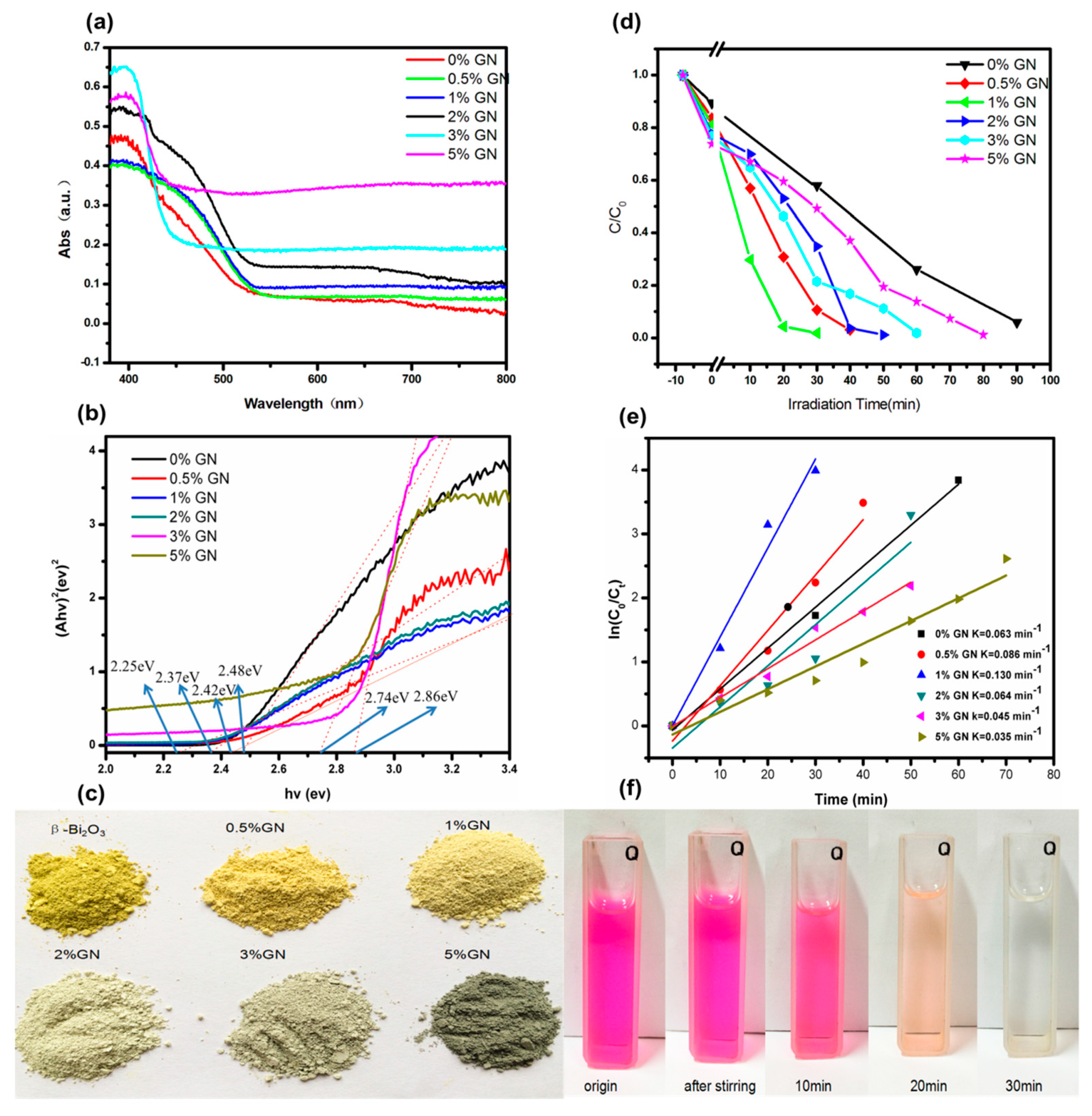
3.5. UV-Vis Diffuse Reflectance and Photocatalytic Activity
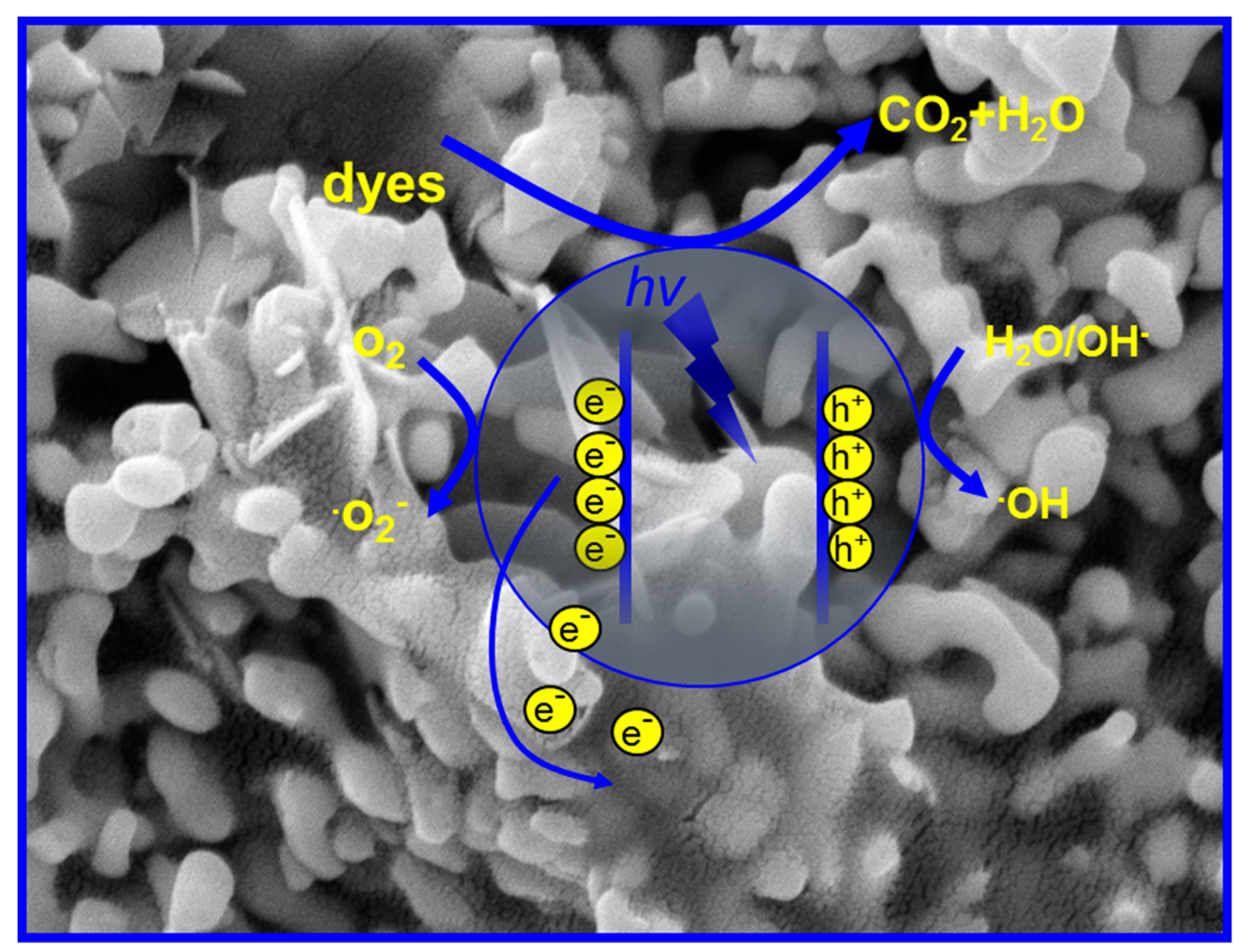
3.6. Possible Mechanisms Speculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maeder, T. Review of Bi2O3 based glasses for electronics and related applications. Int. Mater. Rev. 2013, 58, 3–40. [Google Scholar] [CrossRef]
- Cabot, A.; Marsal, A.; Arbiol, J. Bi2O3 as a selective sensing material for NO detection. Sens. Actuators B Chem. 2004, 99, 74–89. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.Z.; Xu, H.L.; Sun, S.M.; Shang, M. Bi2O3 hierarchical nanostructures: Controllable synthesis, growth mechanism and their application in photocatalysis. Chem. Eur. J. 2009, 15, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.F.; Huang, B.B.; Lu, J.B.; Wang, Z.Y.; Xu, B.; Dai, Y. Synergistic effect of crystal and electronic structures on the visible-light-driven photocatalytic performances of Bi2O3 polymorphs. Phys. Chem. Chem. Phys. 2010, 12, 15468–15475. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.Y.; Xu, L.L.; Wei, B.; Che, J.X.; Gao, H.; Sun, W.J. Facile synthesis of β-Bi2O3/Bi2O2CO3 nanocomposite with high visible-light photocatalytic activity. Mater. Lett. 2014, 120, 1–4. [Google Scholar] [CrossRef]
- Li, R.H.; Chen, W.X.; Kobay, H.; Ma, C.X. Platinum-nanoparticle-loaded bismuth oxide: An efficient plasmonic photocatalyst active under visible light. Green Chem. 2010, 12, 212–215. [Google Scholar] [CrossRef]
- Anandan, S.; Lee, G.J.; Chen, P.K.; Fan, C.; Wu, J. Removal of Orange II Dye in Water by Visible Light Assisted Photocatalytic Ozonation Using Bi2O3 and Au/ Bi2O3 Nanorods. Ind. Eng. Chem. Res. 2010, 49, 9729–9737. [Google Scholar] [CrossRef]
- Chai, S.Y.; Kim, Y.J.; Jung, M.H.; Chakraborty, A.K.; Jung, D.; Lee, W.I. Heterojunctioned BiOCl/Bi2O3, a new visible light photocatalyst. J. Catal. 2009, 262, 144–149. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Posa, V.R.; Annavaram, V.; Koduru, J.R.; Bobbala, P.; Madhavi, V.; Somala, A.R. Preparation of graphene-TiO2 nanocomposite and photocatalytic degradation of Rhodamine B under solar light irradiation. J. Exp. Nano Sci. 2016, 11, 722–736. [Google Scholar] [CrossRef]
- Rania, S.; Aggarwal, M.; Kumarc, M.; Sharma, S.; Kumard, D. Removal of methylene blue and rhodamine B from water byzirconium oxide/graphene. Water Sci. 2016, 30, 51–60. [Google Scholar] [CrossRef]
- Suresh, M.; Sivasamy, A. Bismuth oxide nanoparticles decorated raphene layers for the degradation of Methylene blue dye under visible light irradiations and antimicrobial activities. J. Environ. Chem. Eng. 2018, 6, 3745–3756. [Google Scholar] [CrossRef]
- Tirtha, S.T.; Troppenz, G.V.; Wendt, R.R.; Wollgarten, M. Graphene Oxide/α-Bi2O3 Composites for Visible-Light Photocatalysis Chemical Catalysis and Solar Energy Conversion. ChemSusChem 2014, 7, 854–865. [Google Scholar] [CrossRef]
- Maruthamania, D.; Vadivela, S.; Kumaravela, M.; Saravanakumarb, B.; Paulc, B.; Sankar, D.S.; Habibi-Yangjehd, A.; Manikandane, A.; Ramadoss, G. Fine cutting edge shaped Bi2O3 rods/reduced graphene oxide (RGO)composite for supercapacitor and visible-light photocatalytic applications. J. Colloid Interface Sci. 2017, 498, 449–459. [Google Scholar] [CrossRef]
- Cao, S.; Chen, C.; Xie, X. Hypothermia-controlled Co-precipitation route to deposit well-dispersed β-Bi2O3 nanospheres on polymorphic graphene flakes. Vacuum 2014, 102, 1–4. [Google Scholar] [CrossRef]
- Hu, X.F.; Mohamood, T.; Ma, W.H.; Chen, C.C.; Zhao, J.C. Oxidative Decomposition of Rhodamine B Dye in the Presence of VO2+ and/or Pt(IV) under Visible Light Irradiation: N-Deethylation, Chromophore Cleavage, and Mineralization. J. Phys. Chem. B 2006, 110, 26012–26018. [Google Scholar] [CrossRef] [PubMed]
- Patrick, W.; Stephan, D. Photodegradation of rhodamine B in aqueous solution via SiO2@TiO2 nano-spheres. J. Photochem. Photobiol. A 2007, 185, 19–25. [Google Scholar] [CrossRef]
- Daniela, C.M.; Dmitry, V.K.; Jacob, M.B.; Alexander, S.; Sun, Z.Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Adivel, S.; Keerthi, V.M.; Muthukrishnaraj, A.; Balasubramanian, N. Solvothermal synthesis of Sm-doped BiOBr/RGO composite as an efficient photocatalytic material, for methyl orange degradation. Mater. Lett. 2014, 128, 287–290. [Google Scholar] [CrossRef]
- Lu, Y.G.; Yang, Y.C.; Ye, Z.X.; Liu, S.Y. Preparation and Visible Light Responsive Photocatalytic Activity of Nitrogen-doped Bi2O3 Phocatalyst. J. Inorg. Mater. 2012, 6, 643–648. [Google Scholar] [CrossRef]
- Chi, S.; Kim, B. Nuclear Graphites (I): Oxidation Behaviors. Carbon Lett. 2009, 10, 239–249. [Google Scholar] [CrossRef]
- Chen, C.; Long, M.; Xia, M.; Cai, W. Reduction of Graphene Oxide by An in-Situ Photoelectrochemical Method in a Dye-Sensitized Solar Cell Assembly. Nanoscale Res. Lett. 2012, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, B.; Yu, J.; Ran, J.; Zhang, B.; Yan, H.; Gong, JR. Highly Efficient Visible-Light-Driven Photocatalytic Hydrogen Production of CdS Cluster Decorated Graphene Nanosheets. J. Am. Chem. Soc. 2011, 133, 10878–10884. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ma, T.; Liu, S. Enhanced photocatalytic activity of mesoporous TiO2 aggregates by embedding carbon nanotubes as electron-transfer channel. Phys. Chem. Chem. Phys. 2011, 13, 3491–3501. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Kim, S.; Kang, J.K.; Choi, W.Y. Graphene oxide embedded into TiO2 nanofiber: Effective hybrid photocatalyst for solar conversion. J. Catal. 2014, 309, 49–57. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Xie, T.; Liu, C.; Xu, L. Facile Fabrication of Dumbbell-Like β-Bi2O3/Graphene Nanocomposites and Their Highly Efficient Photocatalytic Activity. Materials 2018, 11, 1359. https://doi.org/10.3390/ma11081359
Yang J, Xie T, Liu C, Xu L. Facile Fabrication of Dumbbell-Like β-Bi2O3/Graphene Nanocomposites and Their Highly Efficient Photocatalytic Activity. Materials. 2018; 11(8):1359. https://doi.org/10.3390/ma11081359
Chicago/Turabian StyleYang, Jun, Taiping Xie, Chenglun Liu, and Longjun Xu. 2018. "Facile Fabrication of Dumbbell-Like β-Bi2O3/Graphene Nanocomposites and Their Highly Efficient Photocatalytic Activity" Materials 11, no. 8: 1359. https://doi.org/10.3390/ma11081359
APA StyleYang, J., Xie, T., Liu, C., & Xu, L. (2018). Facile Fabrication of Dumbbell-Like β-Bi2O3/Graphene Nanocomposites and Their Highly Efficient Photocatalytic Activity. Materials, 11(8), 1359. https://doi.org/10.3390/ma11081359




