Determining the Nitrogen Content in (Oxy)Nitride Materials
Abstract
1. Introduction
2. Chemical Methods
2.1. Thermogravimetric Analysis
2.2. Rietveld Analysis
2.3. Kjeldahl Method
2.4. Grekov Method
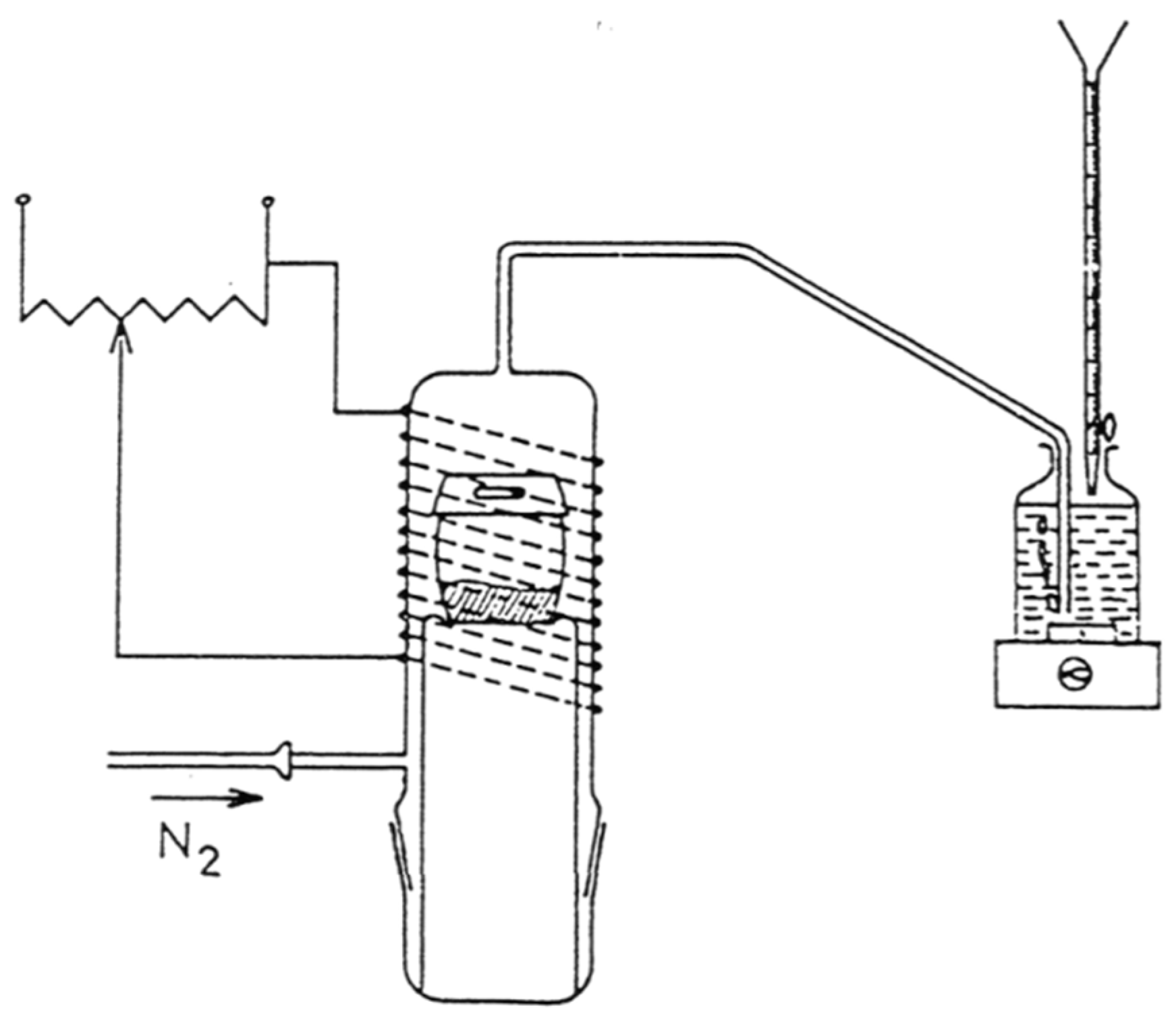
2.5. Dumas Method
2.6. Combustion Analysis
3. Physical Methods
3.1. Electron Probe Microanalysis
3.2. Elastic Recoil Detection Analysis (ERDA)
3.3. Secondary Ion Mass Spectrometry (SIMS)
3.4. Auger Electron Spectroscopy (AES)
3.5. X-Ray Photoelectron Spectroscopy (XPS)
4. Conclusions
Funding
Conflicts of Interest
References
- Xie, R.J.; Hintzen, H.T. Optical properties of (oxy)nitride materials: A review. J. Am. Ceram. Soc. 2013, 96, 665–687. [Google Scholar] [CrossRef]
- Tessier, F.; Maillard, P.; Cheviré, F.; Domen, K.; Kikkawa, S. Optical properties of oxynitride powders. J. Ceram. Soc. Jpn. 2009, 117, 1–5. [Google Scholar] [CrossRef]
- Fuertes, A. Chemistry and applications of oxynitride perovskites. J. Mater. Chem. 2012, 22, 3293–3299. [Google Scholar] [CrossRef]
- Fuertes, A. Synthesis and properties of functional oxynitrides—From photocatalysts to CMR materials. Dalton Trans. 2010, 39, 5942–5948. [Google Scholar] [CrossRef] [PubMed]
- Valour, A.; Cheviré, F.; Tessier, F.; Grasset, F.; Dierre, B.; Jiang, T.; Faulques, E.; Cario, L.; Jobic, S. Preparation of nitrogen doped zinc oxide nanoparticles and thin films by colloidal route and low temperature nitridation process. Solid State Sci. 2016, 54, 30–36. [Google Scholar] [CrossRef]
- Chavillon, B.; Cario, L.; Renaud, A.; Tessier, F.; Cheviré, F.; Boujtita, M.; Pellegrin, Y.; Blart, E.; Smeigh, A.; Hammarström, L.; et al. P-type nitrogen-doped ZnO nanoparticles stable under ambient conditions. J. Am. Chem. Soc. 2012, 134, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Polteau, B.; Tessier, F.; Cheviré, F.; Cario, L.; Odobel, F.; Jobic, S. Synthesis of Ni-poor NiO nanoparticles for p-DSSC applications. Solid State Sci. 2016, 54, 37–42. [Google Scholar] [CrossRef]
- Marchand, R.; Laurent, Y.; Guyader, J.; L’haridon, P.; Verdier, P. Nitrides and oxynitrides: Preparation, crystal chemistry and properties. J. Eur. Ceram. Soc. 1991, 8, 197–213. [Google Scholar] [CrossRef]
- Lengauer, W. Nitrides: Transition Metal Solid-State Chemistry. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 197–213. [Google Scholar]
- Ebbinghaus, S.G.; Abicht, H.-P.; Dronskowski, R.; Müller, T.; Reller, A.; Weidenkaff, A. Perovskite-related oxynitrides—Recent developments in synthesis, characterisation and investigations of physical properties. Prog. Solid State Chem. 2009, 37, 173–205. [Google Scholar] [CrossRef]
- Nicolaescu, I.V.; Tardos, G.; Riman, R.E. Thermogravimetric determination of carbon, nitrogen, and oxygen in aluminum nitride. J. Am. Ceram. Soc. 1994, 77, 2265–2272. [Google Scholar] [CrossRef]
- Clarke, S.J.; Hardstone, K.A.; Michie, C.W.; Rosseinsky, M.J. High-Temperature Synthesis and Structures of Perovskite and n = 1 Ruddlesden–Popper Tantalum Oxynitrides. Chem. Mater. 2002, 14, 2664–2669. [Google Scholar] [CrossRef]
- Labconco, C. A Guide to Kjeldahl Nitrogen Determination Methods and Apparatus; Labconco Corporation: Kansas City, MO, USA, 1998. [Google Scholar]
- Kjeldahl Method. Available online: https://en.wikipedia.org/wiki/Kjeldahl_method (accessed on 1 August 2018).
- Gruner, W.; Hassler, J.; Barth, P.; Behm, J.; Sunderkötter, J. The precise determination of nitrogen in boron nitride. J. Eur. Ceram. Soc. 2009, 29, 2029–2035. [Google Scholar] [CrossRef]
- Guyader, J.; Grekov, F.F.; Marchand, R.; Lang, J. Nouvelles séries de silicoapatites enrichies en azote. Rev. Chim. Minér. 1978, 15, 431–438. [Google Scholar]
- Le Sauze, A.; Guéguen, E.; Marchand, R. Nitrided glass as a reaction medium: Reduction of Ag and Cu within a phosphorus oxynitride glass matrix. J. NonCryst. Solids 1997, 217, 83–91. [Google Scholar] [CrossRef]
- Peltier, V.; Conanec, R.; Marchand, R.; Laurent, Y.; Delsarte, S.; Guéguen, E.; Grange, P. A novel family of mixed gallium aluminum phosphorus oxynitrides: Their synthesis, characterization and utilization in heterogeneous catalysis. Mater. Sci. Eng. B 1997, B47, 177–183. [Google Scholar] [CrossRef]
- Dumas, J.B. Procédés de l’analyse organique. Ann. Chim. Phys. 1831, 247, 198–213. [Google Scholar]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.A.; Parry, E.P.; Davis, J.H. Determination of nitrogen in transition metal nitrides by tube furnace oxidation and gas chromatographic measurement. Anal. Chem. 1967, 39, 1321–1323. [Google Scholar] [CrossRef]
- Gruner, W. Determination of oxygen in oxides by carrier gas hot extraction analysis with simultaneous COx detection. Fresenius J. Anal. Chem. 1999, 7, 597–603. [Google Scholar] [CrossRef]
- Gruner, W. Träger gas-Heißextraktionmethoden. Erzmetall 2002, 3, 151–157. [Google Scholar]
- Data from the Instruction Manual TC600 Nitrogen/Oxygen Determinator (Section 7-3); Leco® Coporation: St. Joseph, MI, USA, 2008.
- Salaville, A.; Marciano, J.; Sakakura, S.; Yoshida, S.; Hayashi, K. Technical Note 35, Horiba Scientific. Available online: www.horiba.com/fileadmin/uploads/Scientific/Documents/Emission/EMGA35.pdf (accessed on 1 August 2018).
- Gruner, W.; Wollein, B.; Lengauer, W. ε-TaN as a Reference Material for the Determination of High Nitrogen Contents. Microchim. Acta 2004, 146, 1–6. [Google Scholar] [CrossRef]
- Sibilia, J.P. Characterization and Analysis of Materials; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2003. [Google Scholar]
- Ziani, A. Etude De Nouveaux Materiaux: Films Minces Perovskites Oxynitrures, de la Photocatalyse à la Microélectronique. Ph.D. Thesis, Université de Rennes 1, Rennes, France, 2009. [Google Scholar]
- Ziani, A.; Le Paven-Thivet, C.; Le Gendre, L.; Fasquelle, D.; Carru, J.C.; Tessier, F.; Pinel, J. Structural and dielectric properties of oxynitride perovskite LaTiOxNy thin films. Thin Solid Film 2008, 517, 544–549. [Google Scholar] [CrossRef]
- Le Paven-Thivet, C.; Ishikawa, A.; Ziani, A.; Le Gendre, L.; Yoshida, M.; Kubota, J.; Tessier, F.; Domen, K. Photoelectrochemical properties of crystalline perovskite lanthanum titanium oxynitride films under visible light. J. Phys. Chem. 2009, 113, 6156–6162. [Google Scholar] [CrossRef]
- Haye, E.; Bruyere, S.; André, E.; Boulet, P.; Barrat, S.; Capon, F.; Miska, P.; Migot, S.; Carteret, C.; Coustel, R.; et al. LaFeOxNy perovskite thin films: Nitrogen location and its effect on morphological, optical and structural properties. J. Alloys Compd. 2017, 724, 74–83. [Google Scholar] [CrossRef]
- Lengauer, W.; Bohn, M. Thermochemical Basis of the Preparation of Well-Defined Transition Metal Carbide, Nitride and Carbonitride Reference Materials for Electron-Probe Microanalysis (EPMA). Solid State Phenom. 2018, 274, 20–42. [Google Scholar] [CrossRef]
- Haye, E. Substitutions Anioniques et Cationiques de Films Minces D’orthoferrite de Lanthane LaFeO3 Elaborés par Pulvérisation Cathodique Magnétron. Ph.D. Thesis, Université de Lorraine, Nancy, France, 2016. [Google Scholar]
- Pozo-Gonzalo, C.; Kartachova, O.; Torriero, A.A.J.; Howlett, P.C.; Glushenkov, A.M.; Fabijanic, D.M.; Chen, Y.; Poissonnet, S.; Forsyth, M. Nanoporous transition metal oxynitrides as catalysts for the oxygen reduction reaction. Electrochim. Acta 2013, 103, 151–160. [Google Scholar] [CrossRef]
- Lengauer, W.; Bauer, J.; Guillou, A.; Ansel, D.; Bars, J.P.; Bohn, M.; Etchessahar, E.; Debuigne, J.; Ettmayer, P. WDS-EPMA nitrogen profile determination in TiN/Ti diffusion couples using homotypic standard materials. Mikrochim. Acta 1992, 107, 303–310. [Google Scholar] [CrossRef]
- Ucakar, V.; Cheviré, F.; Tessier, F.; Krendelsberger, N.; Marchand, R.; Lengauer, W. Defect and Diffusion Forum. Trans. Tech. Publ. 2001, 194–199, 1607–1612. [Google Scholar]
- Messier, D.R.; Gleisner, R.P.; Rich, R.E. Yttrium-Silicon-Aluminum Oxynitride Glass Fibers. J. Am. Ceram. Soc. 1989, 72, 2183–2186. [Google Scholar] [CrossRef]
- Logvinovich, D.; Börger, A.; Döbeli, M.; Ebbinghaus, S.G.; Reller, A.; Weidenkaff, A. Synthesis and physical chemical properties of Ca-substituted LaTiO2N. Prog. Solid State Chem. 2007, 35, 281–290. [Google Scholar] [CrossRef]
- Jensen, J.; Possnert, G.; Zhang, Y. Temperature effect on low-k dielectric thin films studied by ERDA. J. Phys. Conf. Ser. 2008, 100, 012041. [Google Scholar] [CrossRef]
- Criado, D.; Pereyra, I.; Alayo, M.I. Study of nitrogen-rich silicon oxynitride films obtained by PECVD. Mater. Charact. 2003, 50, 167–171. [Google Scholar] [CrossRef]
- Lu, Y. Nouveaux Matériaux Pour Antennes Miniatures Agiles en Fréquence: Synthèse et Caractérisation Diélectrique de Films Minces Oxynitrures. Ph.D. Thesis, Université de Rennes 1, Rennes, France, 2012. [Google Scholar]
- Chappé, J.M.; Martin, N.; Lintymer, J.; Sthal, F.; Terwagne, G.; Takadoum, J. Titanium oxynitride thin films sputter deposited by the reactive gas pulsing process. Appl. Surf. Sci. 2007, 253, 5312–5316. [Google Scholar] [CrossRef]
- Oka, D.; Hirose, Y.; Kamisaka, H.; Kukumura, T.; Sasa, K.; Ishii, S.; Matsuzaki, H.; Sato, Y.; Ikuhara, Y.; Hasegawa, T. Possible ferroelectricity in perovskite oxynitride SrTaO2N epitaxial thin films. Sci. Rep. 2014, 4, 4987. [Google Scholar] [CrossRef]
- Gui, D.; Kang, J.; Yu, H.; Lim, H.F. SIMS study on N diffusion in hafnium oxynitride. Appl. Surf. Sci. 2004, 231–232, 590–593. [Google Scholar] [CrossRef]
- Pichler, M.; Si, W.; Haydous, F.; Tellez, H.; Druce, J.; Fabbri, E.; El Kazzi, M.; Döbeli, M.; Ninova, S.; Aschauer, U.; Wokaun, A.; et al. LaTiOxNy Thin Film Model Systems for Photocatalytic Water Splitting: Physicochemical Evolution of the Solid–Liquid Interface and the Role of the Crystallographic Orientation. Adv. Funct. Mater. 2017, 27, 1605690. [Google Scholar] [CrossRef]
- Haye, E.; Capon, F.; Barrat, S.; Mangin, D.; Pierson, J.F. Phenomenological study of iron and lanthanum magnetron co-sputtering using two reactive gases. Surf. Coat. Technol. 2016, 298, 39–44. [Google Scholar] [CrossRef]
- Mathieu, H.J. Auger electron spectroscopy. In Surface Analysis. The Principal Techniques, 2nd ed.; Vickerman, J.C., Gilmore, I.S., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2009. [Google Scholar]
- Jung, H.; Im, K.; Hwang, H. Electrical characteristics of an ultrathin (1.6 nm) gate dielectric. Appl. Phys. Lett. 2000, 76, 3630–3631. [Google Scholar] [CrossRef]
- Pantel, R.; Lecy, D.; Nicolas, D. Auger electron spectroscopy technique for nitrogen depth profiling in titanium compounds. J. Vac. Sci. Technol. A 1988, 6, 2953–2956. [Google Scholar] [CrossRef]
- Lamour, P.; Fioux, P.; Ponche, A.; Nardin, M.; Vallat, M.F.; Dugay, P.; Brun, J.P.; Moreaud, N.; Pinvidic, J.M. Direct measurement of the nitrogen content by XPS in self-passivated TaNx thin films. Surf. Interface Anal. 2008, 40, 1430–1437. [Google Scholar] [CrossRef]

| Techniques | Thin Film | Characteristics |
|---|---|---|
| TGA | × |
|
| Rietveld analysis | × |
|
| Kjeldahl method | × |
|
| Grekov method | × |
|
| Combustion analysis | × |
|
| Techniques | Thin Films | Characteristics |
|---|---|---|
| EDS | × |
|
| WDS | √ |
|
| ERDA | √ |
|
| RBS | √ |
|
| NRA | √ |
|
| SIMS | √ |
|
| AES | √ |
|
| XPS | √ |
|
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tessier, F. Determining the Nitrogen Content in (Oxy)Nitride Materials. Materials 2018, 11, 1331. https://doi.org/10.3390/ma11081331
Tessier F. Determining the Nitrogen Content in (Oxy)Nitride Materials. Materials. 2018; 11(8):1331. https://doi.org/10.3390/ma11081331
Chicago/Turabian StyleTessier, Franck. 2018. "Determining the Nitrogen Content in (Oxy)Nitride Materials" Materials 11, no. 8: 1331. https://doi.org/10.3390/ma11081331
APA StyleTessier, F. (2018). Determining the Nitrogen Content in (Oxy)Nitride Materials. Materials, 11(8), 1331. https://doi.org/10.3390/ma11081331





