High Capacity and High Efficiency Maple Tree-Biomass-Derived Hard Carbon as an Anode Material for Sodium-Ion Batteries
Abstract
1. Introduction
2. Results and Discussion
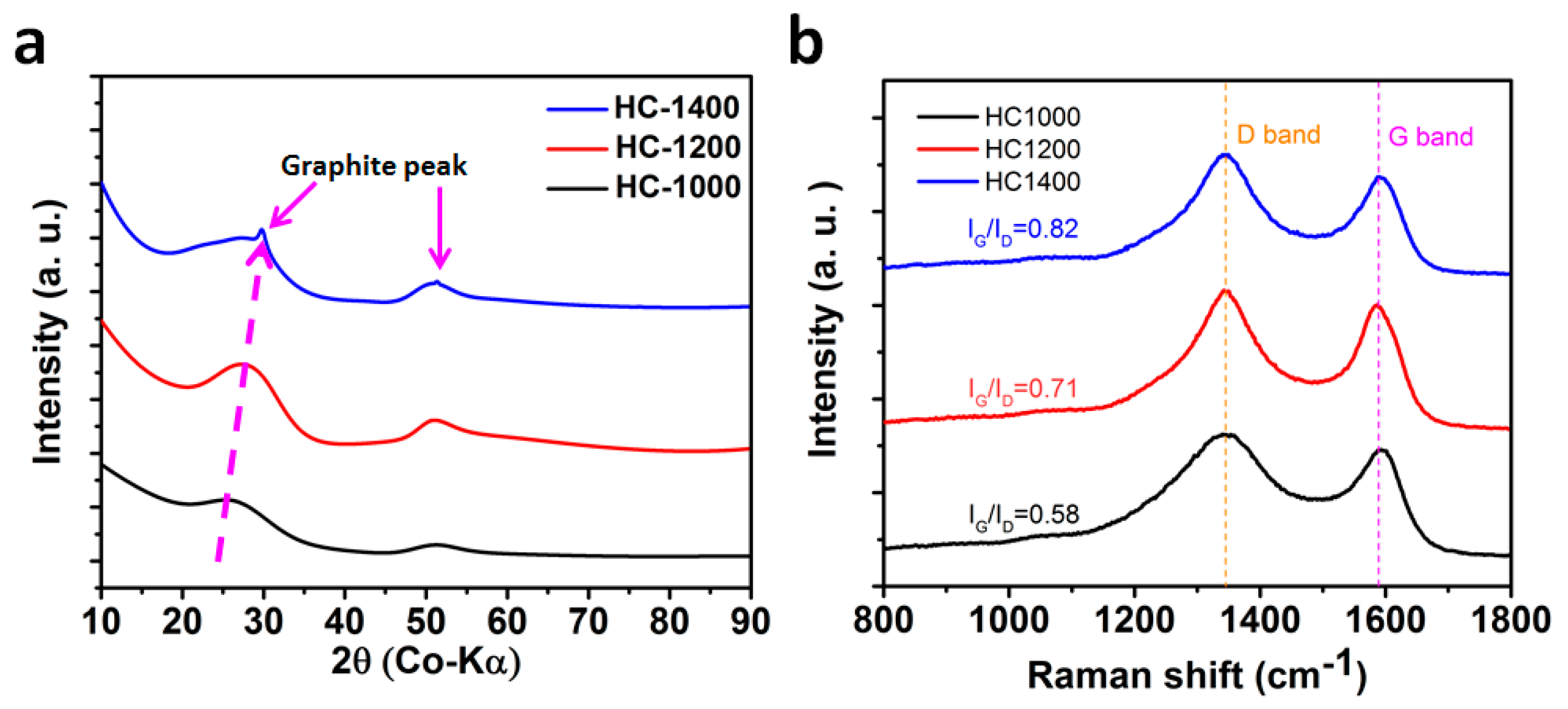
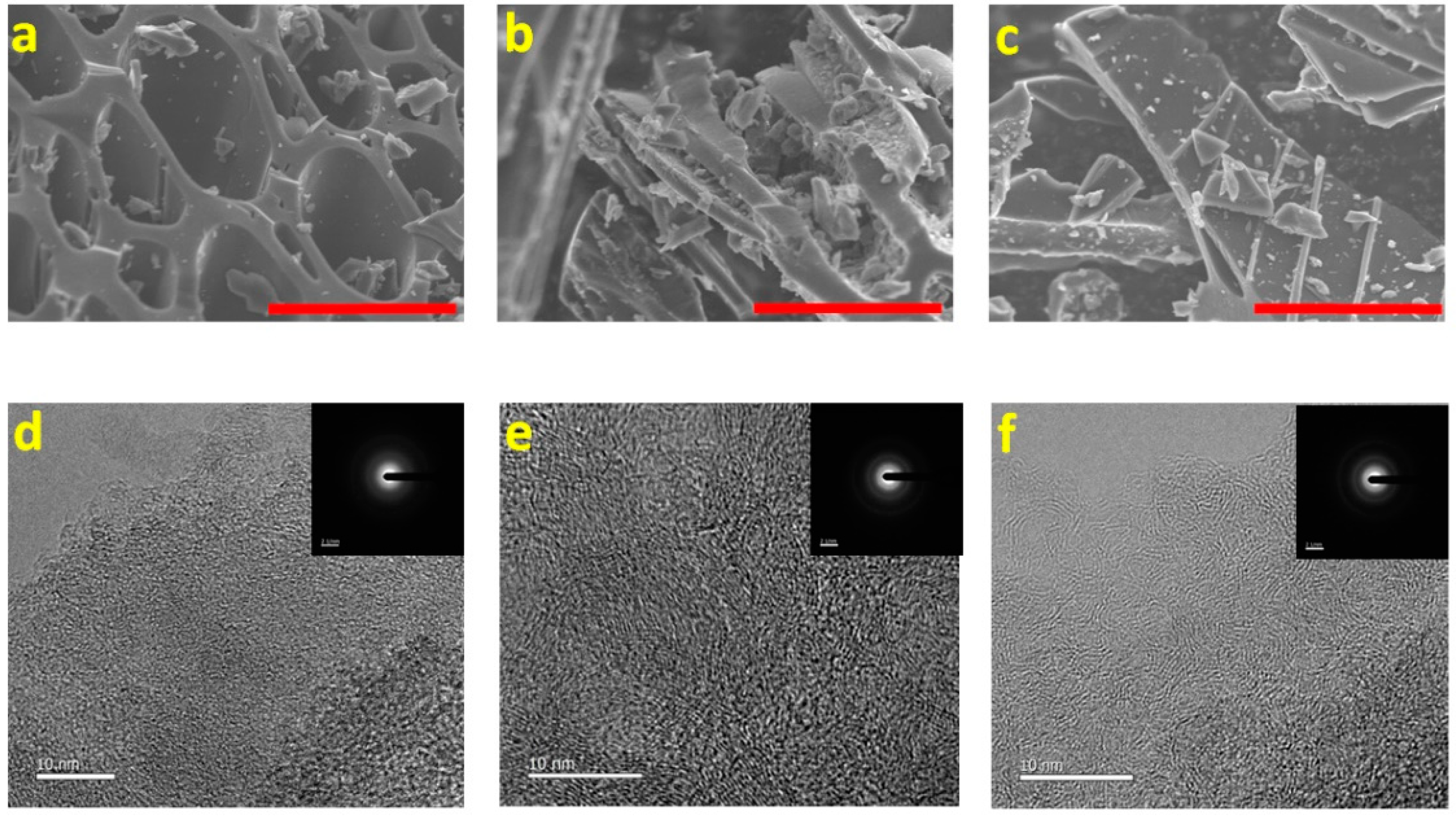
2.1. Structure and Morphology
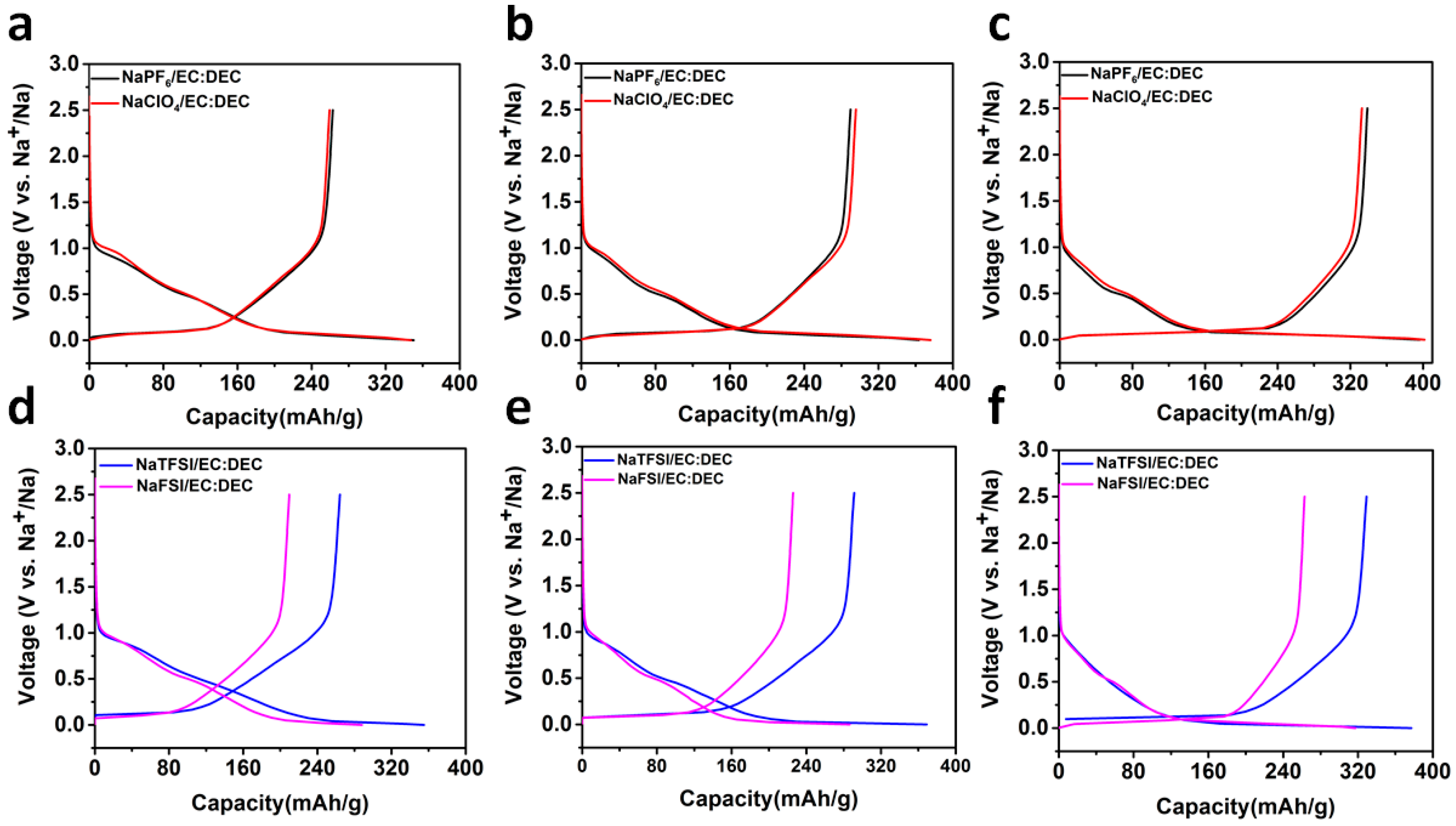
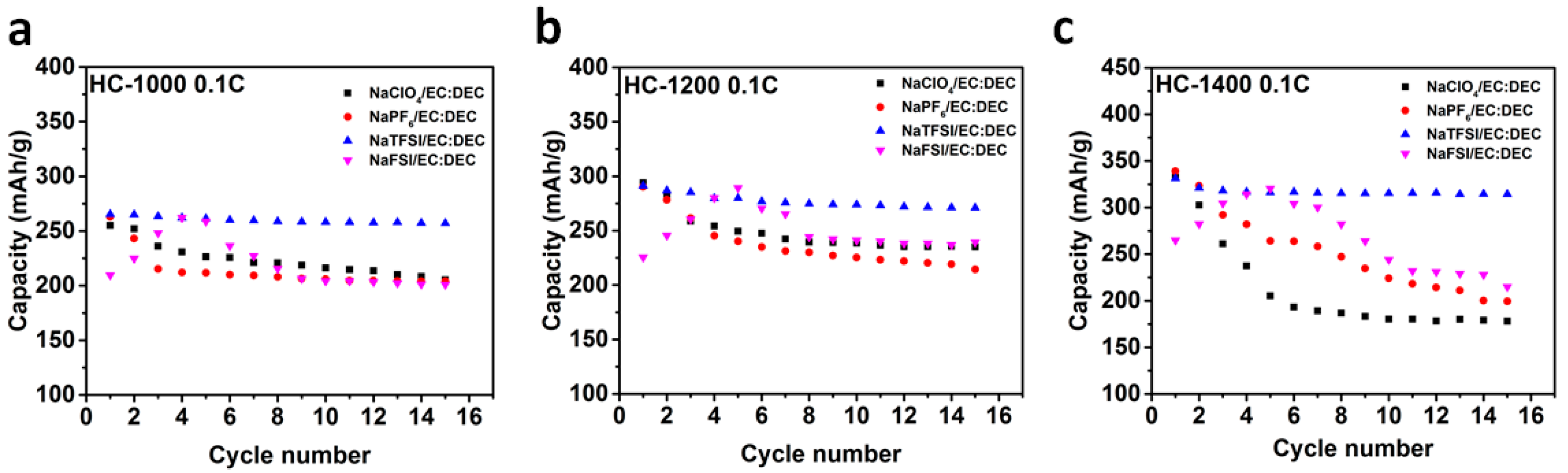
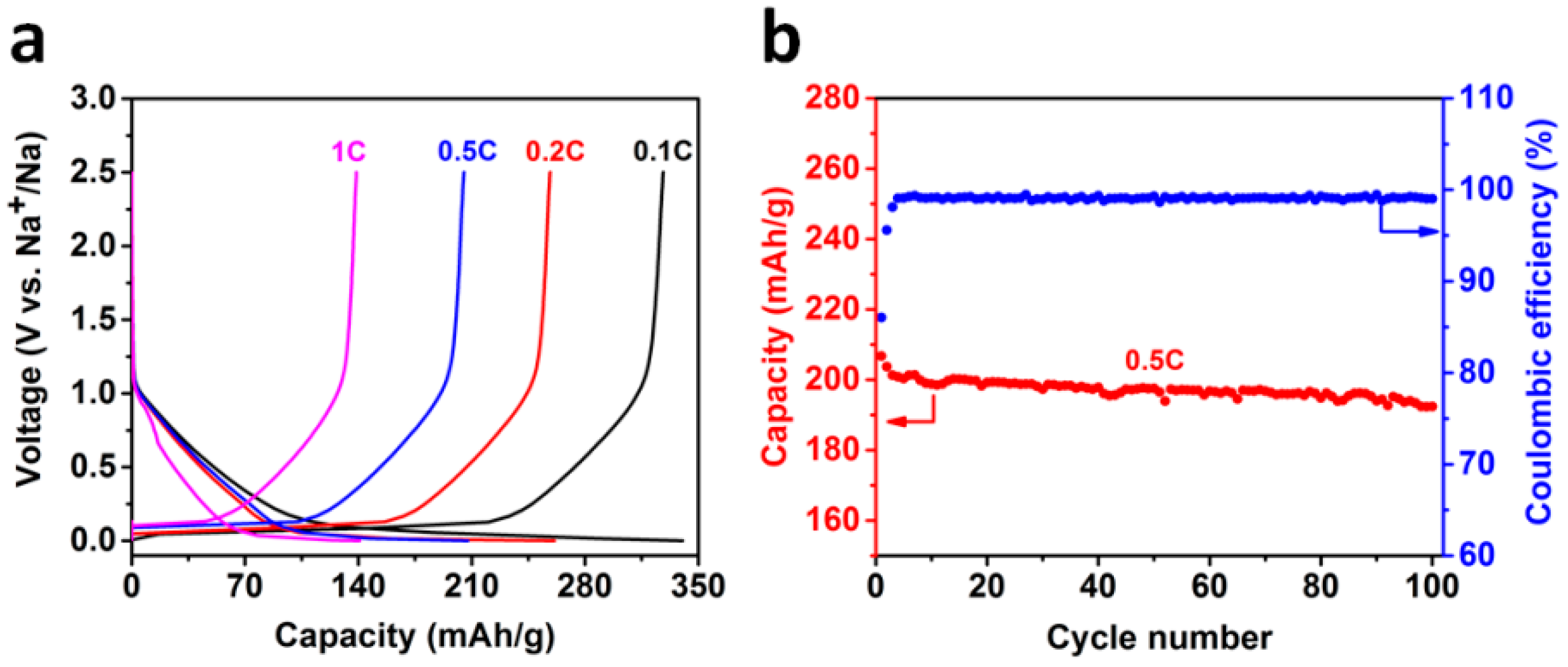
2.2. Sodium Storage Performance in Sodium Ion Batteries
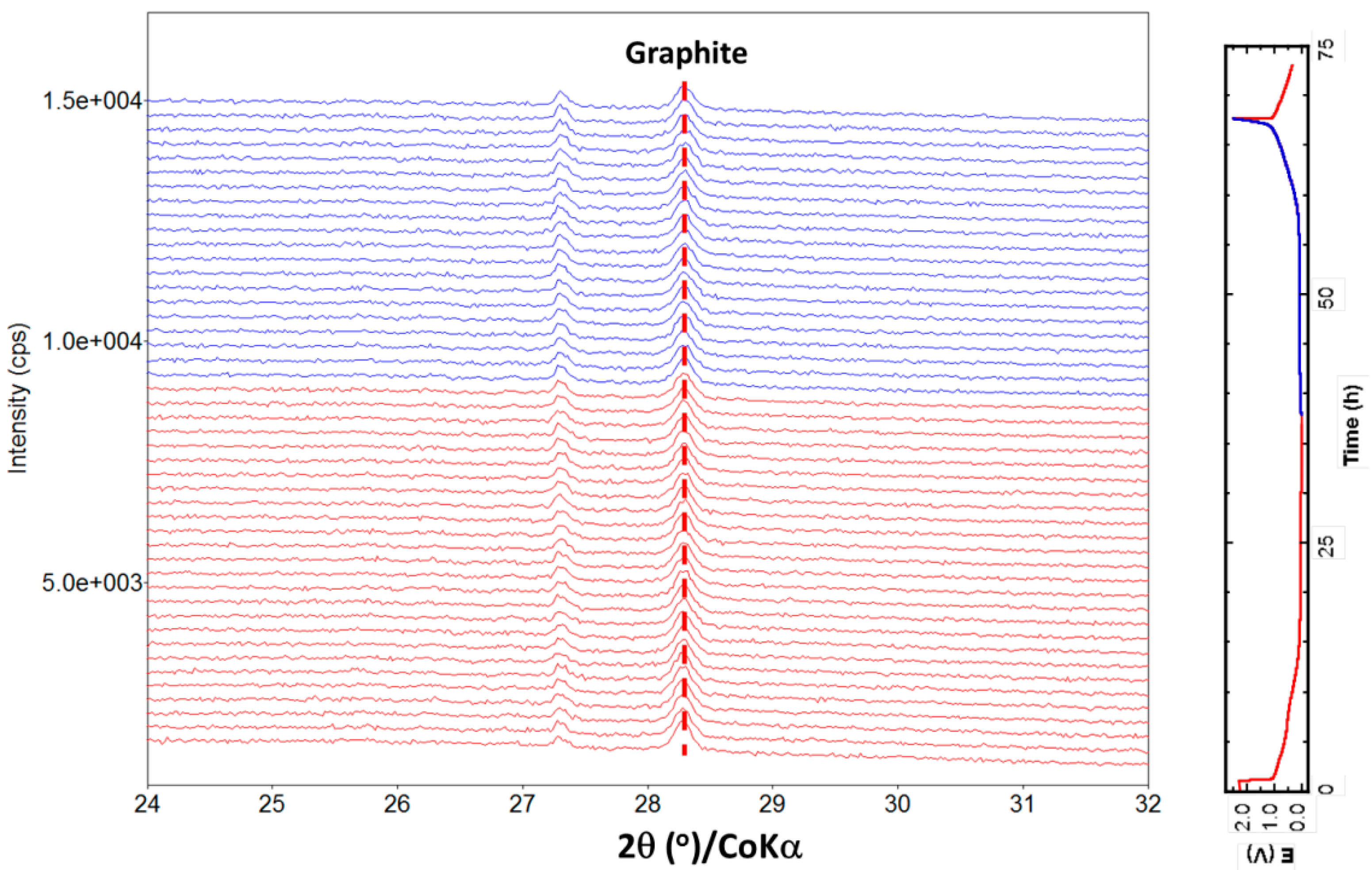
2.3. Structure Evolution
3. Methods
3.1. Materials Synthesis
3.2. Characterization
3.3. Electrochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Zu, C.X.; Li, H. Thermodynamic analysis on energy densities of batteries. Energy Environ. Sci. 2011, 4, 2614–2624. [Google Scholar] [CrossRef]
- Pan, H.L.; Lu, X.; Yu, X.Q.; Hu, Y.S.; Li, H.; Yang, X.Q.; Chen, L.Q. Sodium Storage and Transport Properties in Layered Na2Ti3O7 for Room-Temperature Sodium-Ion Batteries. Adv. Energy Mater. 2013, 3, 1186–1194. [Google Scholar] [CrossRef]
- Liu, S.; Tang, S.; Zhang, X.; Wang, A.; Yang, Q.-H.; Luo, J. Porous Al Current Collector for Dendrite-Free Na Metal Anodes. Nano Lett. 2017, 17, 5862–5868. [Google Scholar] [CrossRef] [PubMed]
- Delmas, C.; Braconnier, J.-J.; Fouassier, C.; Hagenmuller, P. Electrochemical intercalation of sodium in NaxCoO2 bronzes. Solid State Ionics 1981, 3–4, 165–169. [Google Scholar] [CrossRef]
- Berthelot, R.; Carlier, D.; Delmas, C. Electrochemical investigation of the P2-NaxCoO2 phase diagram. Nat. Mater. 2011, 10, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Komaba, S.; Takei, C.; Nakayama, T.; Ogata, A.; Yabuuchi, N. Electrochemical intercalation activity of layered NaCrO2 vs. LiCrO2. Electrochem. Commun. 2010, 12, 355–358. [Google Scholar] [CrossRef]
- Wang, X.; Tamaru, M.; Okubo, M.; Yamada, A. Electrode Properties of P2–Na2/3MnyCo1–yO2 as Cathode Materials for Sodium-Ion Batteries. J. Phys. Chem. C 2013, 117, 15545–15551. [Google Scholar] [CrossRef]
- Shinichi, K.; Tetsuri, N.; Atsushi, O.; Takaya, S.; Chikara, T.; Takada, S.; Hokura, A.; Nakai, I. Electrochemically Reversible Sodium Intercalation of Layered NaNi0.5Mn0.5O2 and NaCrO2. ECS Trans. 2009, 16, 43–55. [Google Scholar] [CrossRef]
- Vassilaras, P.; Toumar, A.J.; Ceder, G. Electrochemical properties of NaNi1/3Co1/3Fe1/3O2 as a cathode material for Na-ion batteries. Electrochem. Commun. 2014, 38, 79–81. [Google Scholar] [CrossRef]
- Xu, J.; Lee, D.H.; Raphaële, J.C.; Yu, X.Q.; Michal, L.; Andrew, J.P.; Pintacuda, G.; Yang, X.-Q.; Grey, C.P.; Meng, Y.S. Identifying the Critical Role of Li Substitution in P2–Nax[LiyNizMn1–y–z]O2 (0 < x, y, z < 1) Intercalation Cathode Materials for High-Energy Na-Ion Batteries. Chem. Mater. 2014, 26, 1260–1269. [Google Scholar] [CrossRef]
- Gupta, A.; Buddie, M.C.; Goodenough, J.B. Na2Ni2TeO6: Evaluation as a cathode for sodium battery. J. Power Sources 2013, 243, 817–821. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, R.; Hu, Y.-S.; Avdeev, M.; Chen, L. P2-Na0.6Cr0.6Ti0.4O2 cation-disordered electrode for high-rate symmetric rechargeable sodium-ion batteries. Nat. Commun. 2015, 6, 6954. [Google Scholar] [CrossRef] [PubMed]
- Carlier, D.; Cheng, J.H.; Berthelot, R.; Guignard, M.; Yoncheva, M.; Stoyanova, R.; Hwang, B.J.; Delmas, C. The P2-Na2/3Co2/3Mn1/3O2 phase: structure, physical properties and electrochemical behavior as positive electrode in sodium battery. Dalton Trans. 2011, 40, 9306–9312. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, N.; Kajiyama, M.; Iwatate, J.; Nishikawa, H.; Hitomi, S.; Okuyama, R.; Usui, R.; Yamada, Y.; Komaba, S. P2-type NaxFe1/2Mn1/2 O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 2012, 11, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.; Hernan, L.; Morales, J.; Sanchez, L.; Pena, J.S.; Aranda, M. Synthesis and characterization of high-temperature hexagonal P2-Na0.6MnO2 and its electrochemical behaviour as cathode in sodium cells. J. Mater. Chem. 2002, 12, 1142–1147. [Google Scholar] [CrossRef]
- Mu, L.Q.; Xu, S.Y.; Li, Y.M.; Hu, Y.-S.; Li, H.; Chen, L.Q.; Huang, X.J. Prototype Sodium-Ion Batteries Using an Air-Stable and Co/Ni-Free O3-Layered Metal Oxide Cathode. Adv. Mater. 2015, 27, 6928–6933. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, N.; Yoshida, H.; Komaba, S. Crystal Structures and Electrode Performance of Alpha-NaFeO2 for Rechargeable Sodium Batteries. Electrochemical 2012, 80, 716–719. [Google Scholar] [CrossRef]
- Wang, Y.S.; Liu, J.; Lee, B.; Qiao, R.M.; Yang, Z.Z.; Xu, S.Y.; Yu, X.Q.; Gu, L.; Hu, Y.-S.; Yang, W.L.; et al. Ti-substituted tunnel-type Na0.44MnO2 oxide as a negative electrode for aqueous sodium-ion batteries. Nat. Commun. 2015, 6, 6401. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Ponraj, R.; Kannan, A.G.; Lee, H.-W.; Fathi, R.; Ruffo, R.; Mari, C.M.; Kim, D.K. Diffusion behavior of sodium ions in Na0.44MnO2 in aqueous and non-aqueous electrolytes. J. Power Sources 2013, 244, 758–763. [Google Scholar] [CrossRef]
- Wang, Y.S.; Mu, L.Q.; Liu, J.; Yang, Z.Z.; Yu, X.Q.; Gu, L.; Hu, Y.-S.; Li, H.; Yang, X.-Q.; Chen, L.Q.; et al. A Novel High Capacity Positive Electrode Material with Tunnel-Type Structure for Aqueous Sodium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1501005. [Google Scholar] [CrossRef]
- Jian, Z.L.; Han, W.Z.; Lu, X.; Yang, H.X.; Hu, Y.-S.; Zhou, J.; Zhou, Z.B.; Li, J.Q.; Chen, W.; Chen, D.F.; et al. Superior Electrochemical Performance and Storage Mechanism of Na3V2(PO4)3 Cathode for Room-Temperature Sodium-Ion Batteries. Adv. Energy Mater. 2013, 3, 156–160. [Google Scholar] [CrossRef]
- Wessells, C.D.; Huggins, R.A.; Cui, Y. Copper hexacyanoferrate battery electrodes with long cycle life and high power. Nat. Commun. 2011, 2, 1563. [Google Scholar] [CrossRef] [PubMed]
- Wessells, C.D.; Peddada, S.V.; Huggins, R.A.; Cui, Y. Nickel Hexacyanoferrate Nanoparticle Electrodes For Aqueous Sodium and Potassium Ion Batteries. Nano Lett. 2011, 11, 5421–5425. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Han, X.G.; Xu, Y.H.; Liu, Y.H.; Zheng, S.Y.; Xu, K.; Hu, L.B.; Wang, C.S. Electrospun Sb/C Fibers for a Stable and Fast Sodium-Ion Battery Anode. Acs Nano 2013, 7, 6378–6386. [Google Scholar] [CrossRef]
- Darwiche, A.; Marino, C.; Sougrati, M.T.; Fraisse, B.; Stievano, L.; Monconduit, L. Better Cycling Performances of Bulk Sb in Na-Ion Batteries Compared to Li-Ion Systems: An Unexpected Electrochemical Mechanism. J. Am. Chem. Soc. 2012, 134, 20805–20811. [Google Scholar] [CrossRef] [PubMed]
- Komaba, S.; Matsuura, Y.; Ishikawa, T.; Yabuuchi, N.; Murata, W.; Kuze, S. Redox reaction of Sn-polyacrylate electrodes in aprotic Na cell. Electrochem. Commun. 2012, 21, 65–68. [Google Scholar] [CrossRef]
- Xiao, L.F.; Cao, Y.L.; Xiao, J.; Wang, W.; Libor, K.; Nie, Z.M.; Liu, J. High capacity, reversible alloying reactions in SnSb/C nanocomposites for Na-ion battery applications. Chem. Commun. 2012, 48, 3321–3323. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wu, X.; Cao, Y.; Ai, X.; Yang, H. High Capacity and Rate Capability of Amorphous Phosphorus for Sodium Ion Batteries. Angew. Chem. Int. Ed. 2013, 125, 4731–4734. [Google Scholar] [CrossRef]
- Kim, Y.; Park, Y.; Choi, A.; Choi, N.-S.; Kim, J.; Lee, J.; Ryu, J.H.; Seung, M.O.; Lee, K.T. An Amorphous Red Phosphorus/Carbon Composite as a Promising Anode Material for Sodium Ion Batteries. Adv. Mater. 2013, 25, 3045–3049. [Google Scholar] [CrossRef] [PubMed]
- Senguttuvan, P.; Rousse, G.; Seznec, V.; Tarascon, J.-M.; Palacín, M.R. Na2Ti3O7: Lowest Voltage Ever Reported Oxide Insertion Electrode for Sodium Ion Batteries. Chem. Mater. 2011, 23, 4109–4111. [Google Scholar] [CrossRef]
- Wu, D.; Li, X.; Xu, B.; Wu, N.; Liu, L.; Ceder, G. NaTiO2: A layered anode material for sodium-ion batteries. Energy Environ. Sci. 2015, 8, 195–202. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, L.; Pan, H.L.; Lu, X.; Gu, L.; Hu, Y.-S.; Li, H.; Armand, M.; Ikuhara, Y.; Chen, L.Q.; et al. Direct atomic-scale confirmation of three-phase storage mechanism in Li4Ti5O12 anodes for room-temperature sodium-ion batteries. Nat. Commun. 2013, 4, 1870. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Yu, X.Q.; Xu, S.Y.; Bai, J.M.; Xiao, R.J.; Hu, Y.-S.; Li, H.; Yang, X.-Q.; Chen, L.Q.; Huang, X.J. A zero-strain layered metal oxide as the negative electrode for long-life sodium-ion batteries. Nat. Commun. 2013, 4, 2365. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, L.; Zhu, Z.; Hu, Z.; Zhao, Q.; Chen, J. All Organic Sodium-Ion Batteries with Na4C8H2O6. Angew. Chem. Int. Ed. 2014, 53, 5892–5896. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhao, J.M.; Hu, Y.-S.; Li, H.; Zhou, Z.B.; Armand, M.; Chen, L.Q. Disodium Terephthalate (Na2C8H4O4) as High Performance Anode Material for Low-Cost Room-Temperature Sodium-Ion Battery. Adv. Energy Mater 2012, 2, 962–965. [Google Scholar] [CrossRef]
- Alcántara, R.; Jiménez-Mateos, J.M.; Lavela, P.; Tirado, J.L. Carbon black: a promising electrode material for sodium-ion batteries. Electrochem. Commun. 2001, 3, 639–642. [Google Scholar] [CrossRef]
- Li, Y.M.; Xu, S.Y.; Wu, X.Y.; Yu, J.Z.; Wang, Y.S.; Hu, Y.-S.; Li, H.; Chen, L.Q.; Huang, X.J. Amorphous monodispersed hard carbon micro-spherules derived from biomass as a high performance negative electrode material for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 71–77. [Google Scholar] [CrossRef]
- Wu, L.; Buchholz, D.; Vaalma, C.; Giffin, G.A.; Passerini, S. Apple-Biowaste-Derived Hard Carbon as a Powerful Anode Material for Na-Ion Batteries. Chem. Electr. Chem. 2016, 3, 292–298. [Google Scholar] [CrossRef]
- Elmira, M.L.; Jia, D.; Cui, K.; Alireza, K.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-Density Sodium and Lithium Ion Battery Anodes from Banana Peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.S.; Titirici, M.M.; Chen, L.; Huang, X. Hard Carbon Microtubes Made from Renewable Cotton as High-Performance Anode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600659. [Google Scholar] [CrossRef]
- Lv, W.M.; Wen, F.S.; Xiang, J.Y.; Zhao, J.; Li, L.; Wang, L.M.; Liu, Z.Y.; Tian, Y.J. Peanut shell derived hard carbon as ultralong cycling anodes for lithium and sodium batteries. Electrochim. Acta 2015, 176, 533–541. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. High capacity anode materials for rechargeable sodium-ion batteries. J. Electrochem. Soc. 2000, 147, 1271–1273. [Google Scholar] [CrossRef]
- Shinichi, K.; Wataru, M.; Toru, I.; Naoaki, Y.; Tomoaki, O.; Tetsuri, N.; Atsushi, O.; Kazuma, G.; Kazuya, F. Electrochemical Na Insertion and Solid Electrolyte Interphase for HardCarbon Electrodes and Application to Na-Ion Batteries. Adv. Funct. Mater. 2011, 21, 3859–3867. [Google Scholar] [CrossRef]
- Zhang, B.; Ghimbeu, C.M.; Laberty, C.; Vix, Guterl, C.; Tarascon, J.M. Correlation Between Microstructure and Na Storage Behavior in Hard Carbon. Adv. Energy Mater. 2016, 6, 1501588. [Google Scholar] [CrossRef]
| Electrode Electrolyte | HC-1000 1st Charge/Discharge (ICE) | HC-1200 1st Charge/Discharge (ICE) | HC-1400 1st Charge/Discharge (ICE) |
|---|---|---|---|
| NaPF6/EC:DEC | 262/349 (75.1%) | 290/362 (80.1%) | 337/391 (86.1%) |
| NaClO4/EC:DEC | 260/347(74.8%) | 296/376(78.5%) | 332/401(82.7%) |
| NaTFSI/EC:DEC | 264/354(74.5%) | 291/368(79.1%) | 332/378(88.03%) |
| NaFSI/EC:DEC | 209/287(72.8%) | 225/286(78.6%) | 264/317(83.1%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Feng, Z.; Zhu, W.; Gariépy, V.; Gagnon, C.; Provencher, M.; Laul, D.; Veillette, R.; Trudeau, M.L.; Guerfi, A.; et al. High Capacity and High Efficiency Maple Tree-Biomass-Derived Hard Carbon as an Anode Material for Sodium-Ion Batteries. Materials 2018, 11, 1294. https://doi.org/10.3390/ma11081294
Wang Y, Feng Z, Zhu W, Gariépy V, Gagnon C, Provencher M, Laul D, Veillette R, Trudeau ML, Guerfi A, et al. High Capacity and High Efficiency Maple Tree-Biomass-Derived Hard Carbon as an Anode Material for Sodium-Ion Batteries. Materials. 2018; 11(8):1294. https://doi.org/10.3390/ma11081294
Chicago/Turabian StyleWang, Yuesheng, Zimin Feng, Wen Zhu, Vincent Gariépy, Catherine Gagnon, Manon Provencher, Dharminder Laul, René Veillette, Michel L. Trudeau, Abdelbast Guerfi, and et al. 2018. "High Capacity and High Efficiency Maple Tree-Biomass-Derived Hard Carbon as an Anode Material for Sodium-Ion Batteries" Materials 11, no. 8: 1294. https://doi.org/10.3390/ma11081294
APA StyleWang, Y., Feng, Z., Zhu, W., Gariépy, V., Gagnon, C., Provencher, M., Laul, D., Veillette, R., Trudeau, M. L., Guerfi, A., & Zaghib, K. (2018). High Capacity and High Efficiency Maple Tree-Biomass-Derived Hard Carbon as an Anode Material for Sodium-Ion Batteries. Materials, 11(8), 1294. https://doi.org/10.3390/ma11081294







