Single-Step Fabrication of Polymer Nanocomposite Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
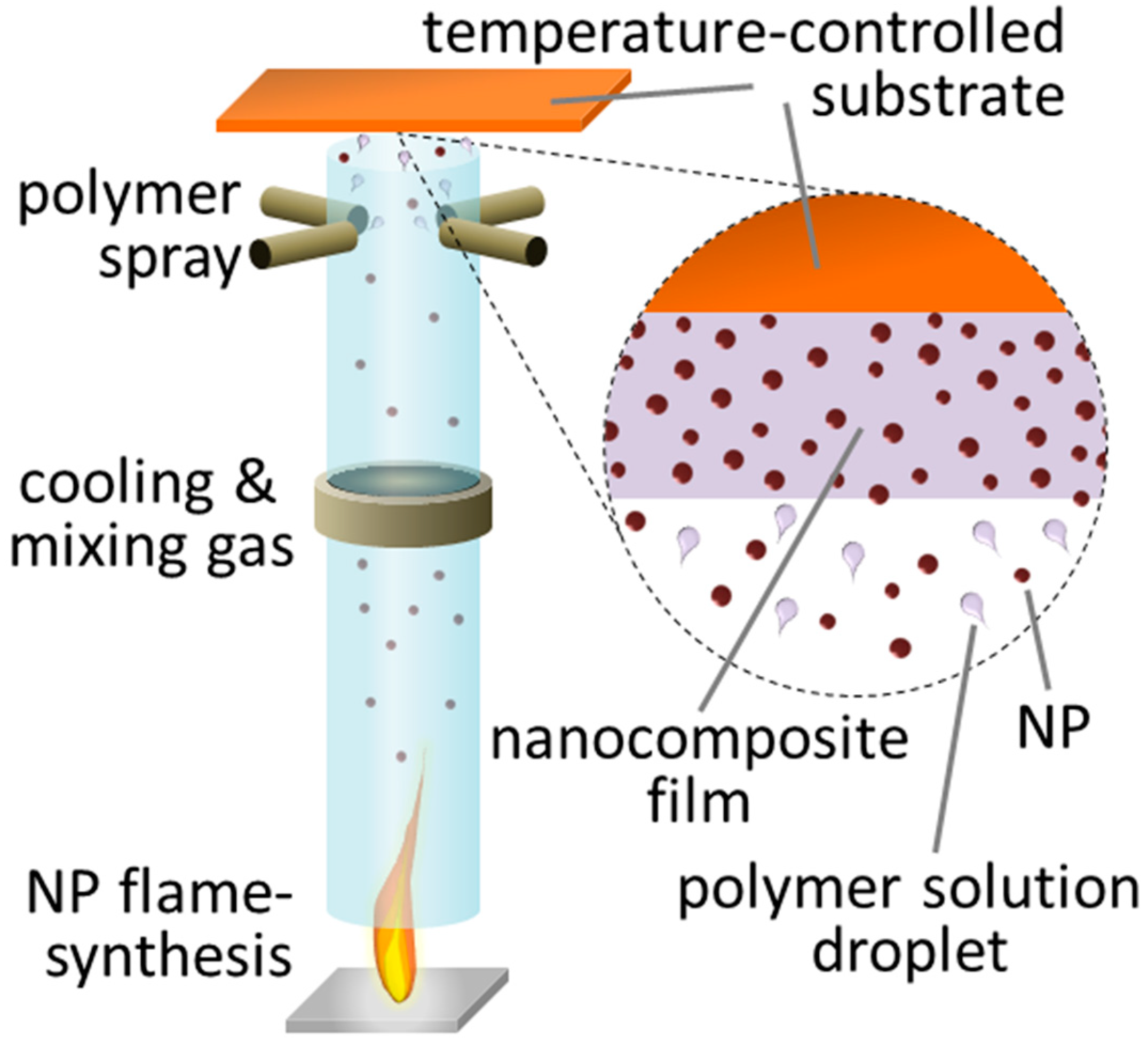
2.2. Fabrication
2.3. Characterization
3. Results and Discussion
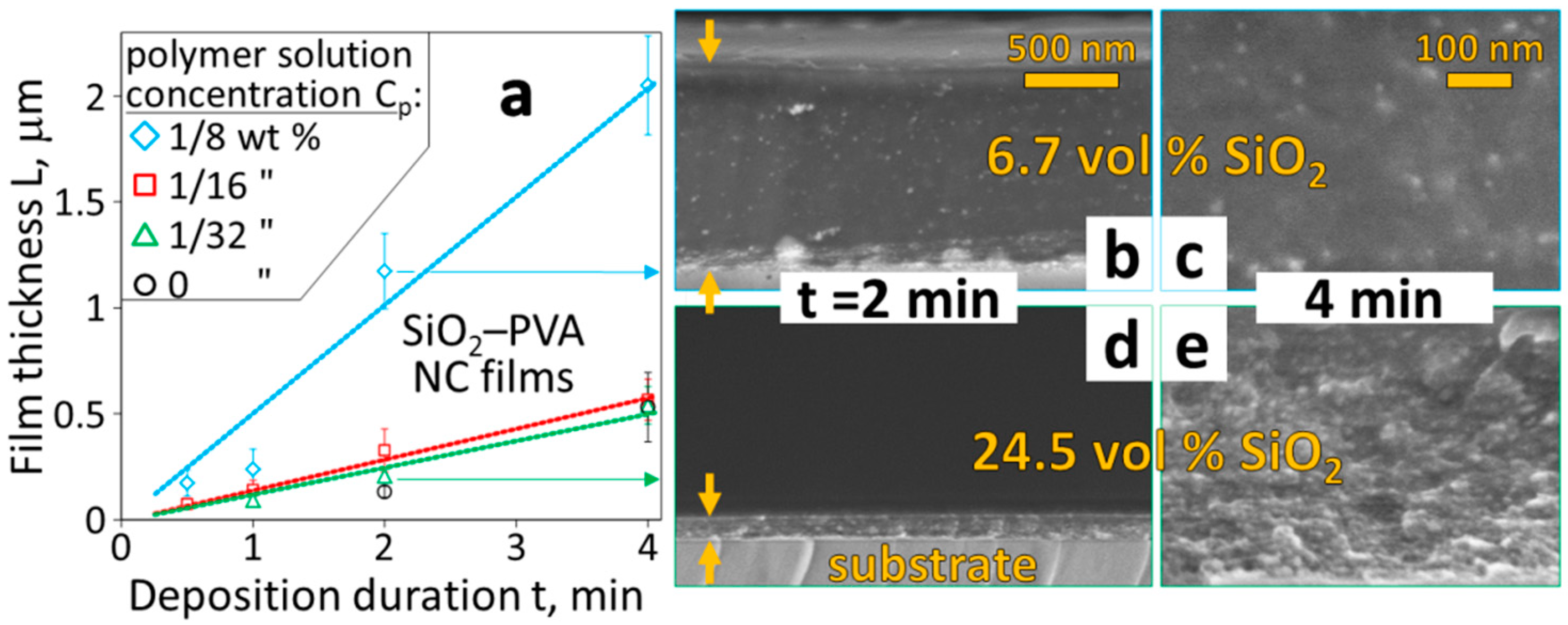
3.1. Films with Tunable High Loading of Filler Nanoparticles
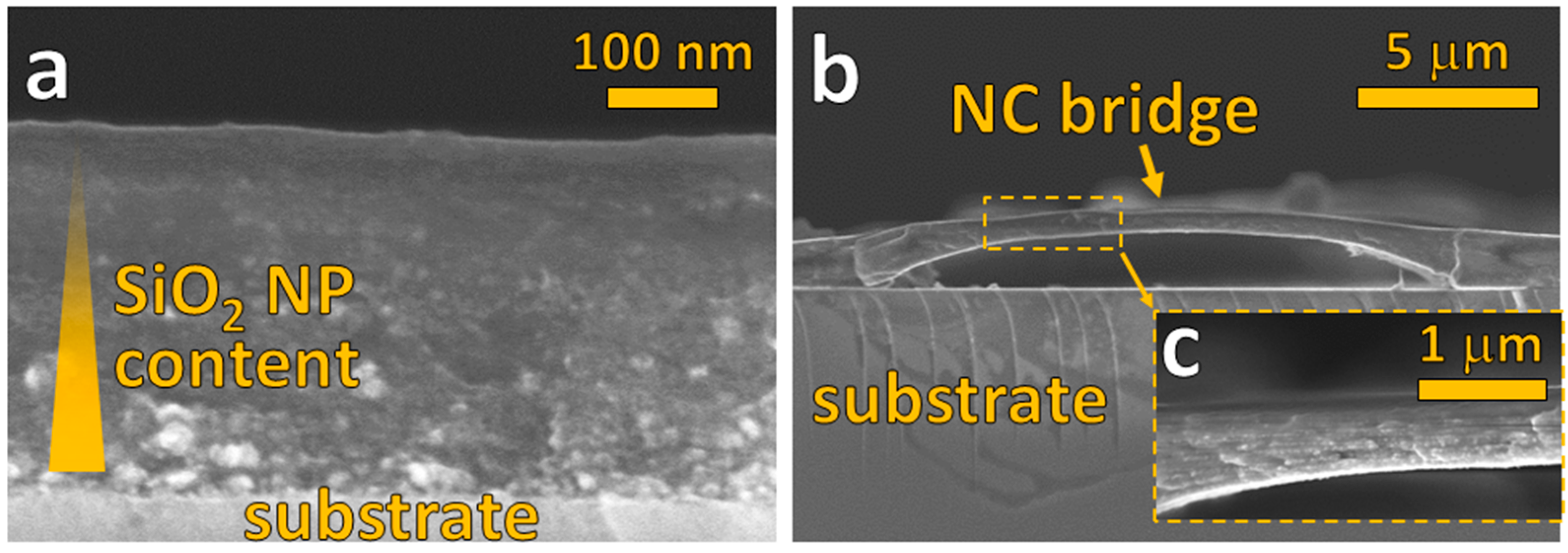
3.2. Multilayered Filler Content Gradients and Microstructures
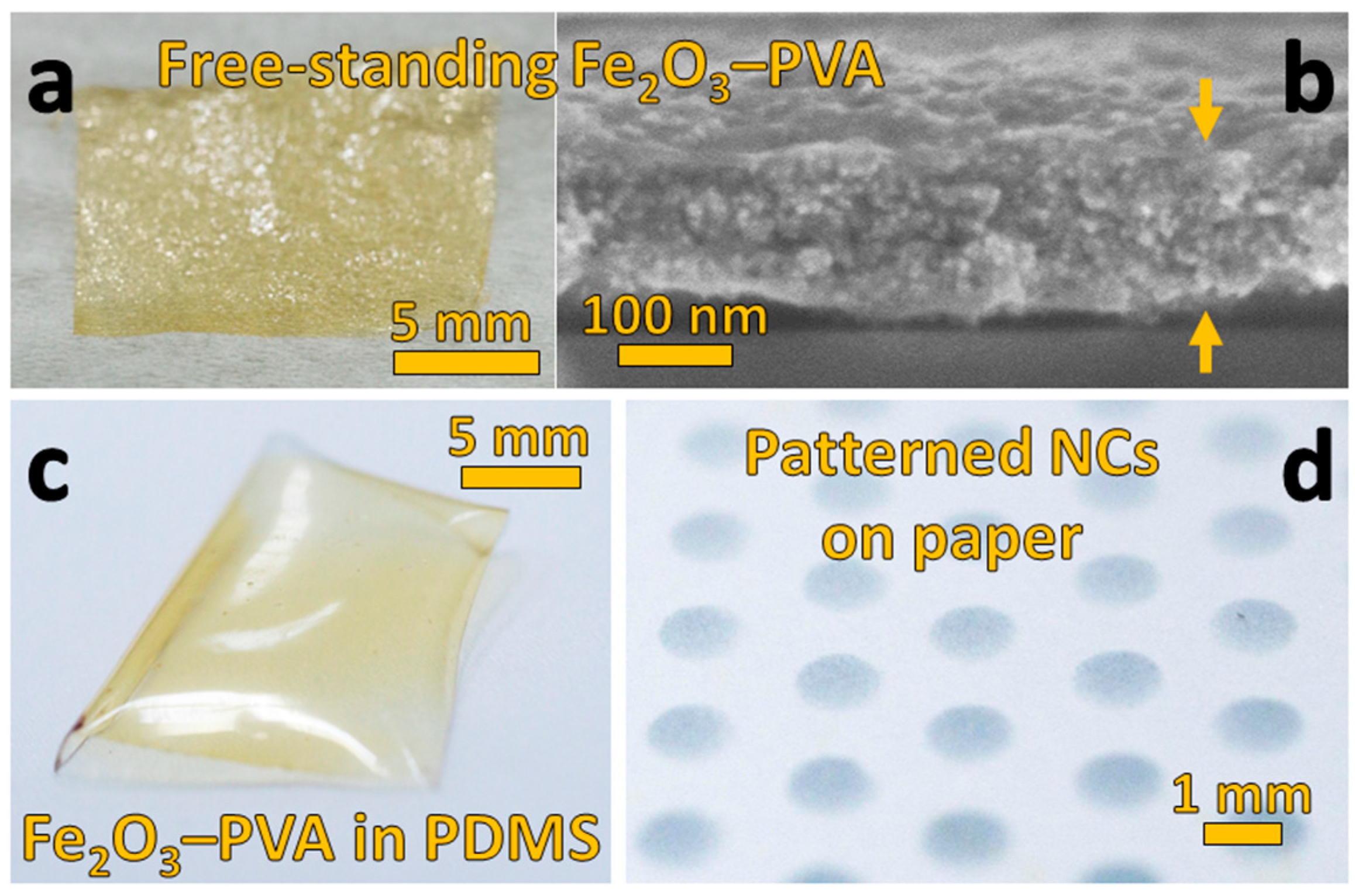
3.3. Free-Standing and PDMS-Embedded Nanocomposites
3.4. Patterned Nanocomposites on Paper
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hong, R.Y.; Chen, Q. Dispersion of inorganic nanoparticles in polymer matrices: Challenges and solutions. In Organic-Inorganic Hybrid Nanomaterials; Kalia, S., Haldorai, Y., Eds.; Springer: Cham, Switzerland, 2015; Volume 267, pp. 1–38. [Google Scholar]
- Sotiriou, G.A.; Blattmann, C.O.; Pratsinis, S.E. Flexible, multifunctional, magnetically actuated nanocomposite films. Adv. Funct. Mater. 2013, 23, 34–41. [Google Scholar] [CrossRef]
- Lashgari, S.; Karrabi, M.; Ghasemi, I.; Azizi, H.; Messori, M.; Paderni, K. Shape memory nanocomposite of poly(l-lactic acid)/graphene nanoplatelets triggered by infrared light and thermal heating. Express Polym. Lett. 2016, 10, 349–359. [Google Scholar] [CrossRef]
- Camenzind, A.; Schweizer, T.; Sztucki, M.; Pratsinis, S.E. Structure & strength of silica-PDMS nanocomposites. Polymer 2010, 51, 1796–1804. [Google Scholar]
- Schulz, H.; Schimmöller, B.; Pratsinis, S.E.; Salz, U.; Bock, T. Radiopaque dental adhesives: Dispersion of flame-made Ta2O5/SiO2 nanoparticles in methacrylic matrices. J. Dent. 2008, 36, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.L.; Pan, T.R. Photopatternable conductive PDMS materials for microfabrication. Adv. Funct. Mater. 2008, 18, 1912–1921. [Google Scholar] [CrossRef]
- Yi, W.J.; Wang, Y.Y.; Wang, G.F.; Tao, X.M. Investigation of carbon black/silicone elastomer/dimethylsilicone oil composites for flexible strain sensors. Polym. Test. 2012, 31, 677–684. [Google Scholar] [CrossRef]
- Lei, Q.; Dancer, C.E.J.; Grovenor, C.R.M.; Grant, P.S. Preparation, microstructure and microwave dielectric properties of sprayed PFA/barium titanate composite films. Compos. Sci. Technol. 2016, 129, 198–204. [Google Scholar] [CrossRef]
- Southward, R.E.; Thompson, D.S.; Thompson, D.W.; Caplan, M.L.; St. Clair, A.K. Synthesis of reflective polyimide films via in situ silver(I) reduction. Chem. Mater. 1995, 7, 2171–2180. [Google Scholar] [CrossRef]
- Faupel, F.; Zaporojtchenko, V.; Strunskus, T.; Elbahri, M. Metal-polymer nanocomposites for functional applications. Adv. Eng. Mater. 2010, 12, 1177–1190. [Google Scholar] [CrossRef]
- Mädler, L.; Kammler, H.K.; Müller, R.; Pratsinis, S.E. Controlled synthesis of nanostructured particles by flame spray pyrolysis. J. Aerosol Sci. 2002, 33, 369–389. [Google Scholar] [CrossRef]
- Teleki, A.; Bjelobrk, N.; Pratsinis, S.E. Continuous surface functionalization of flame-made TiO2 nanoparticles. Langmuir 2010, 26, 5815–5822. [Google Scholar] [CrossRef] [PubMed]
- Starsich, F.H.L.; Hirt, A.M.; Stark, W.J.; Grass, R.N. Gas-phase synthesis of magnetic metal/polymer nanocomposites. Nanotechnology 2014, 25, 505602. [Google Scholar] [CrossRef] [PubMed]
- Teleki, A.; Heine, M.C.; Krumeich, F.; Akhtar, M.K.; Pratsinis, S.E. In situ coating of flame-made TiO2 particles with nanothin SiO2 films. Langmuir 2008, 24, 12553–12558. [Google Scholar] [CrossRef] [PubMed]
- Tricoli, A.; Graf, M.; Mayer, F.; Kuhne, S.; Hierlemann, A.; Pratsinis, S.E. Micropatterning layers by flame aerosol deposition-annealing. Adv. Mater. 2008, 20, 3005–3010. [Google Scholar] [CrossRef]
- Zhao, X.; Hinchliffe, C.; Johnston, C.; Dobson, P.J.; Grant, P.S. Spray deposition of polymer nanocomposite films for dielectric applications. Mater. Sci. Eng. B Adv. 2008, 151, 140–145. [Google Scholar] [CrossRef]
- Mädler, L.; Stark, W.J.; Pratsinis, S.E. Simultaneous deposition of au nanoparticles during flame synthesis of TiO2 and SiO2. J. Mater. Res. 2003, 18, 115–120. [Google Scholar] [CrossRef]
- Waser, O.; Groehn, A.J.; Eggersdorfer, M.L.; Pratsinis, S.E. Air entrainment during flame aerosol synthesis of nanoparticles. Aerosol Sci. Technol. 2014, 48, 1195–1206. [Google Scholar] [CrossRef]
- Suter, M.; Ergeneman, O.; Zurcher, J.; Moitzi, C.; Pane, S.; Rudin, T.; Pratsinis, S.E.; Nelson, B.J.; Hierold, C. A photopatternable superparamagnetic nanocomposite: Material characterization and fabrication of microstructures. Sens. Actuator B Chem. 2011, 156, 433–443. [Google Scholar] [CrossRef]
- Gaume, J.; Taviot-Gueho, C.; Cros, S.; Rivaton, A.; Thérias, S.; Gardette, J.-L. Optimization of PVA clay nanocomposite for ultra-barrier multilayer encapsulation of organic solar cells. Sol. Energy Mater. Sol. C 2012, 99, 240–249. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, L.Q.; Liu, H.L.; Dong, Y.C.; Yu, Y.C.; Tian, M. Lead magnesium niobate-filled silicone dielectric elastomer with large actuated strain. J. Appl. Polym. Sci. 2012, 125, 2196–2201. [Google Scholar] [CrossRef]
- Schneider, R.; Lüthi, S.R.; Albrecht, K.; Brülisauer, M.; Bernard, A.; Geiger, T. Transparent silicone calcium fluoride nanocomposite with improved thermal conductivity. Macromol. Mater. Eng. 2015, 300, 80–85. [Google Scholar] [CrossRef]
- Im, H.; Kim, J. Thermal conductivity of a graphene oxide–carbon nanotube hybrid/epoxy composite. Carbon 2012, 50, 5429–5440. [Google Scholar] [CrossRef]
- Libanori, R.; Erb, R.M.; Reiser, A.; Le Ferrand, H.; Suess, M.J.; Spolenak, R.; Studart, A.R. Stretchable heterogeneous composites with extreme mechanical gradients. Nat. Commun. 2012, 3, 1265. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Yuan, Q.; Niu, Y.; Chen, J.; Wang, Q.; Wang, H. Ultrahigh electric displacement and energy density in gradient layer-structured BaTiO3/PVDF nanocomposites with an interfacial barrier effect. J. Mater. Chem. A 2017, 5, 10849–10855. [Google Scholar] [CrossRef]
- Suter, M.; Ergeneman, O.; Zürcher, J.; Schmid, S.; Camenzind, A.; Nelson, B.J.; Hierold, C. Superparamagnetic photocurable nanocomposite for the fabrication of microcantilevers. J. Micromech. Microeng. 2011, 21, 25023. [Google Scholar] [CrossRef]
- Palacio, M.; Bhushan, B.; Ferrell, N.; Hansford, D. Nanomechanical characterization of polymer beam structures for biomems applications. Sens. Actuator A Phys. 2007, 135, 637–650. [Google Scholar] [CrossRef]
- Strobel, R.; Pratsinis, S.E. Flame aerosol synthesis of smart nanostructured materials. J. Mater. Chem. 2007, 17, 4743–4756. [Google Scholar] [CrossRef]
- Li, D.; Teoh, W.Y.; Selomulya, C.; Woodward, R.C.; Munroe, P.; Amal, R. Insight into microstructural and magnetic properties of flame-made γ-Fe2O3 nanoparticles. J. Mater. Chem. 2007, 17, 4876–4884. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Q.; Guo, F.; Gu, J.; Zhang, J. Preparation and characterization of magnetic polyimide hybrid thin films. J. Appl. Polym. Sci. 2012, 125, 725–730. [Google Scholar] [CrossRef]
- Lipomi, D.J.; Lee, J.A.; Vosgueritchian, M.; Tee, B.C.K.; Bolander, J.A.; Bao, Z. Electronic properties of transparent conductive films of PEDOT:PSS on stretchable substrates. Chem. Mater. 2012, 24, 373–382. [Google Scholar] [CrossRef]
- Cruz, S.; Rocha, L.A.; Viana, J.C. Enhanced printability of thermoplastic polyurethane substrates by silica particles surface interactions. Appl. Surf. Sci. 2016, 360, 198–206. [Google Scholar] [CrossRef]
- Yao, B.; Zhang, J.; Kou, T.Y.; Song, Y.; Liu, T.Y.; Li, Y. Paper-based electrodes for flexible energy storage devices. Adv. Sci. 2017, 4, 1700107. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, J.M.; Aromaa, M.; Teisala, H.; Tuominen, M.; Stepien, M.; Saarinen, J.J.; Toivakka, M.; Kuusipalo, J. Nanoparticle deposition from liquid flame spray onto moving roll-to-roll paperboard material. Aerosol Sci. Technol. 2011, 45, 827–837. [Google Scholar] [CrossRef]
- Xu, T.; Qiao, Q. Conjugated polymer-inorganic semiconductor hybrid solar cells. Energy Environ. Sci. 2011, 4, 2700–2720. [Google Scholar] [CrossRef]
- Blattmann, C.O.; Pratsinis, S.E. In situ measurement of conductivity during nanocomposite film deposition. Appl. Surf. Sci. 2016, 371, 329–336. [Google Scholar] [CrossRef]
- Lessing, J.; Glavan, A.C.; Walker, S.B.; Keplinger, C.; Lewis, J.A.; Whitesides, G.M. Inkjet printing of conductive inks with high lateral resolution on omniphobic “RF paper” for paper-based electronics and MEMS. Adv. Mater. 2014, 26, 4677–4682. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blattmann, C.O.; Pratsinis, S.E. Single-Step Fabrication of Polymer Nanocomposite Films. Materials 2018, 11, 1177. https://doi.org/10.3390/ma11071177
Blattmann CO, Pratsinis SE. Single-Step Fabrication of Polymer Nanocomposite Films. Materials. 2018; 11(7):1177. https://doi.org/10.3390/ma11071177
Chicago/Turabian StyleBlattmann, Christoph O., and Sotiris E. Pratsinis. 2018. "Single-Step Fabrication of Polymer Nanocomposite Films" Materials 11, no. 7: 1177. https://doi.org/10.3390/ma11071177
APA StyleBlattmann, C. O., & Pratsinis, S. E. (2018). Single-Step Fabrication of Polymer Nanocomposite Films. Materials, 11(7), 1177. https://doi.org/10.3390/ma11071177





