A Simple, Quick and Eco-Friendly Strategy of Synthesis Nanosized α-LiFeO2 Cathode with Excellent Electrochemical Performance for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
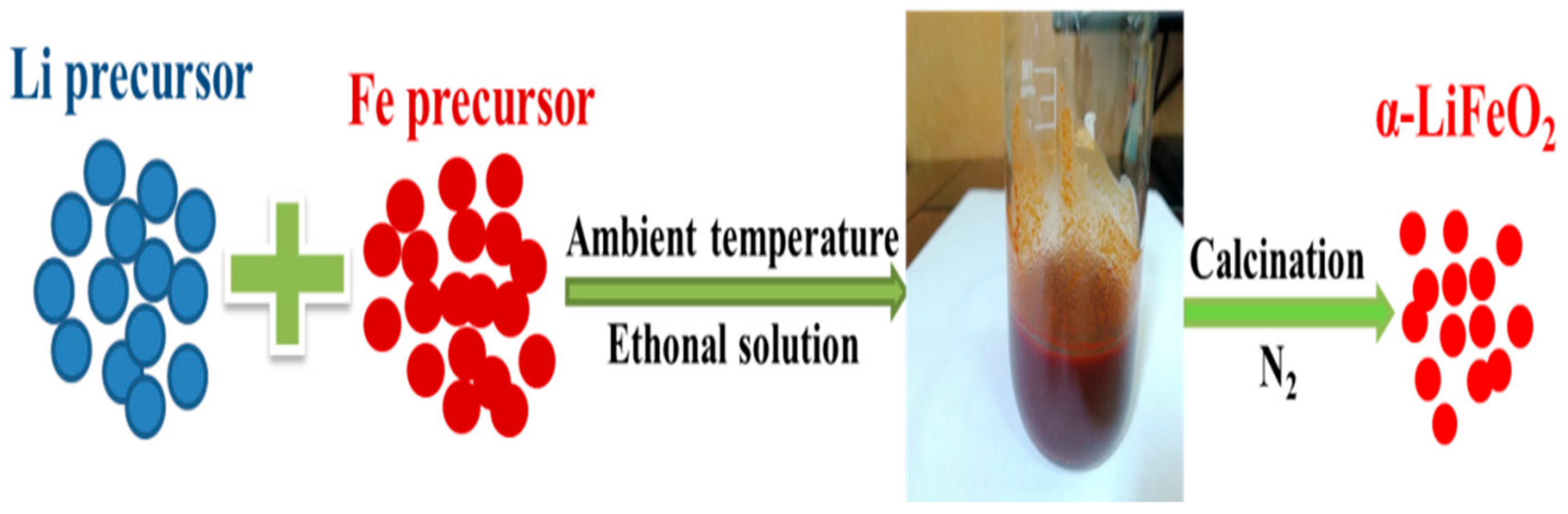
2.1. Synthesis of α-LiFeO2 Cathode Materials
2.2. Characterizations of α-LiFeO2 Cathode Materials
2.3. Electrochemical Tests of α-LiFeO2 Cathode Materials
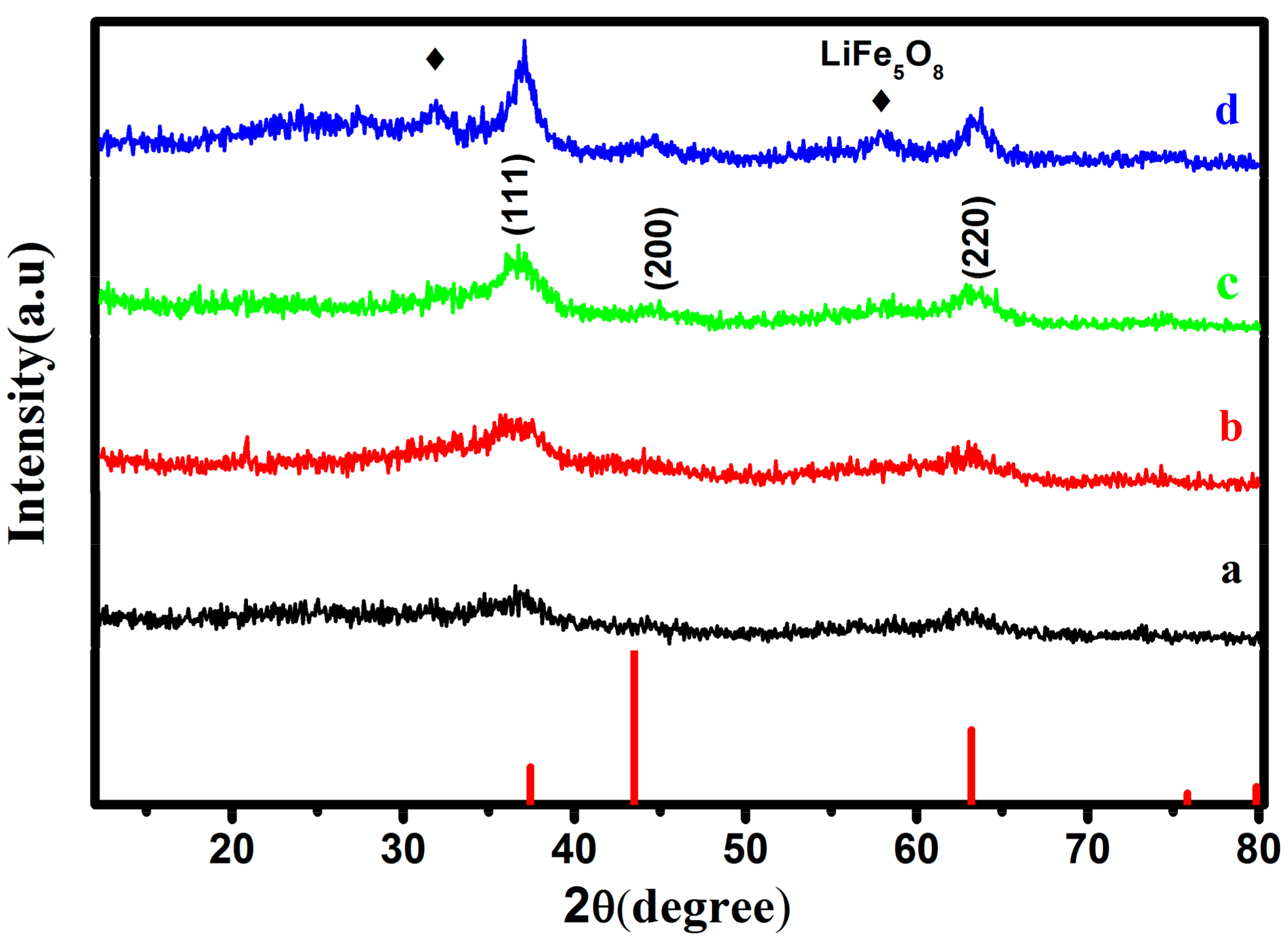
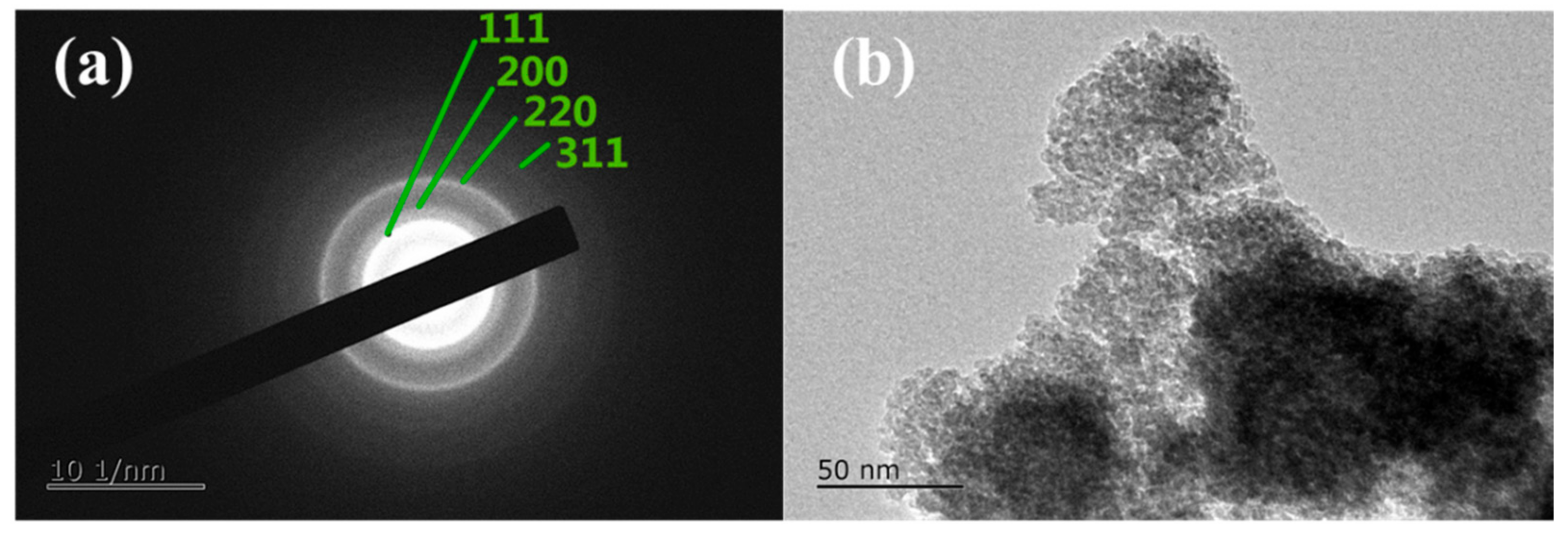
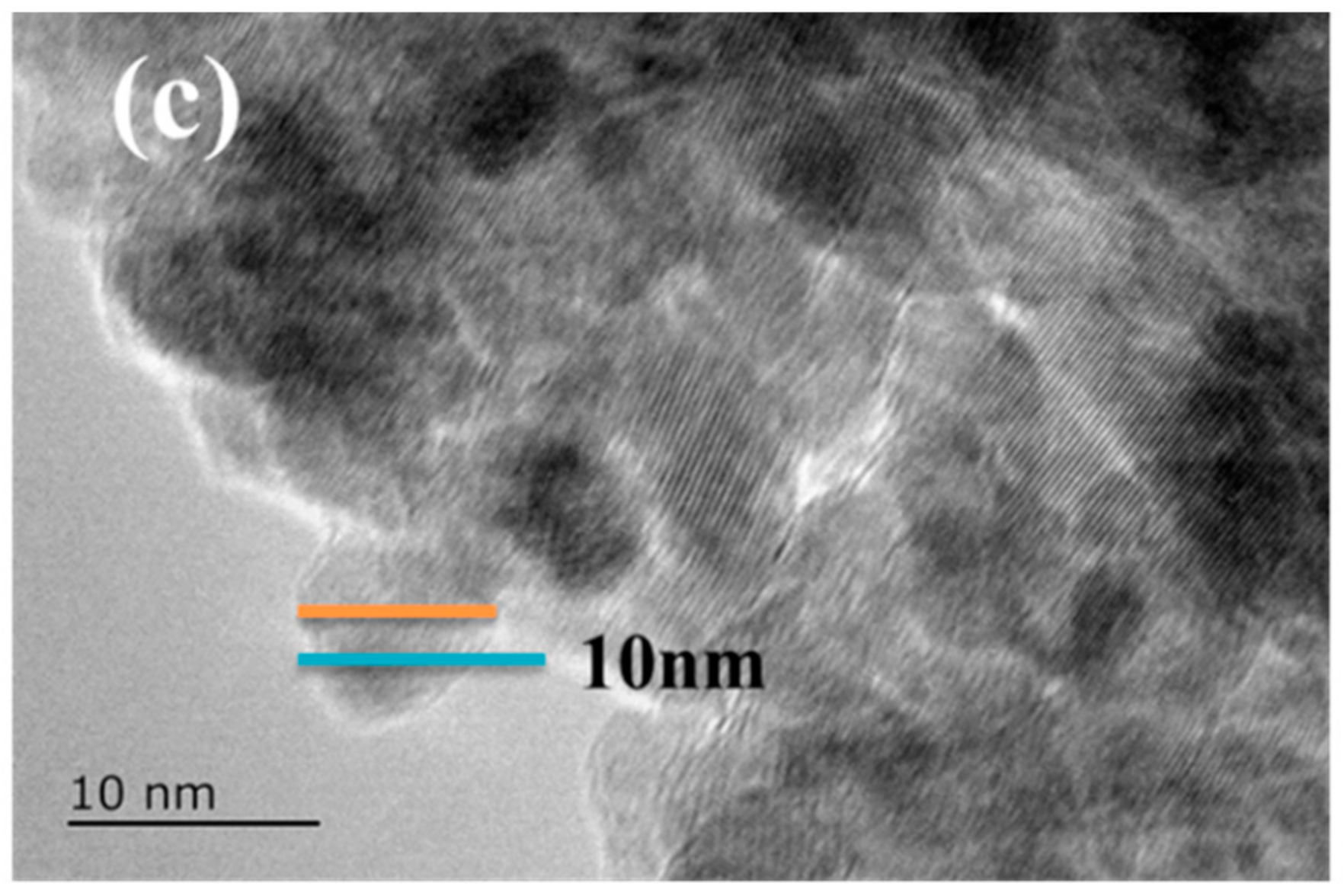
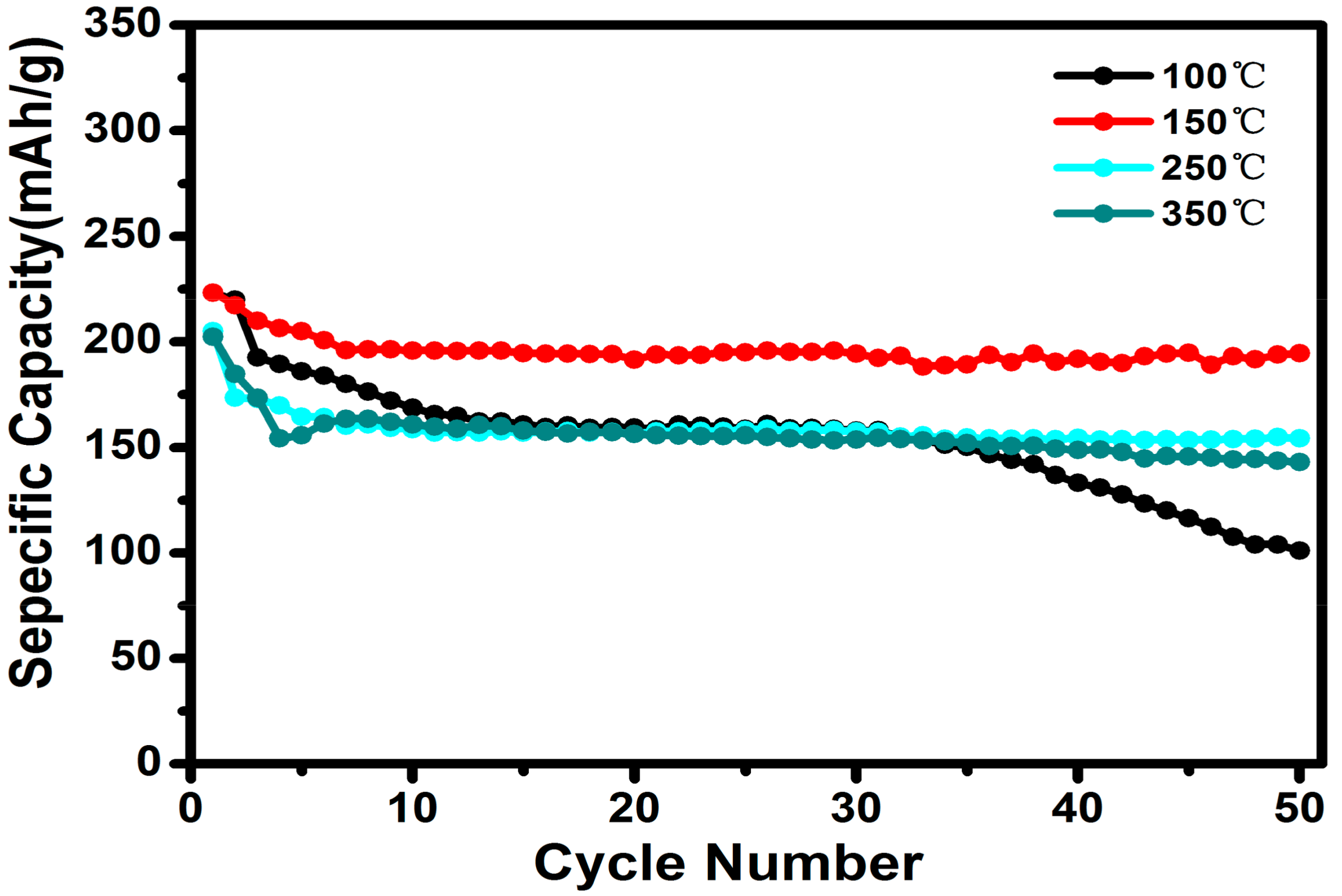
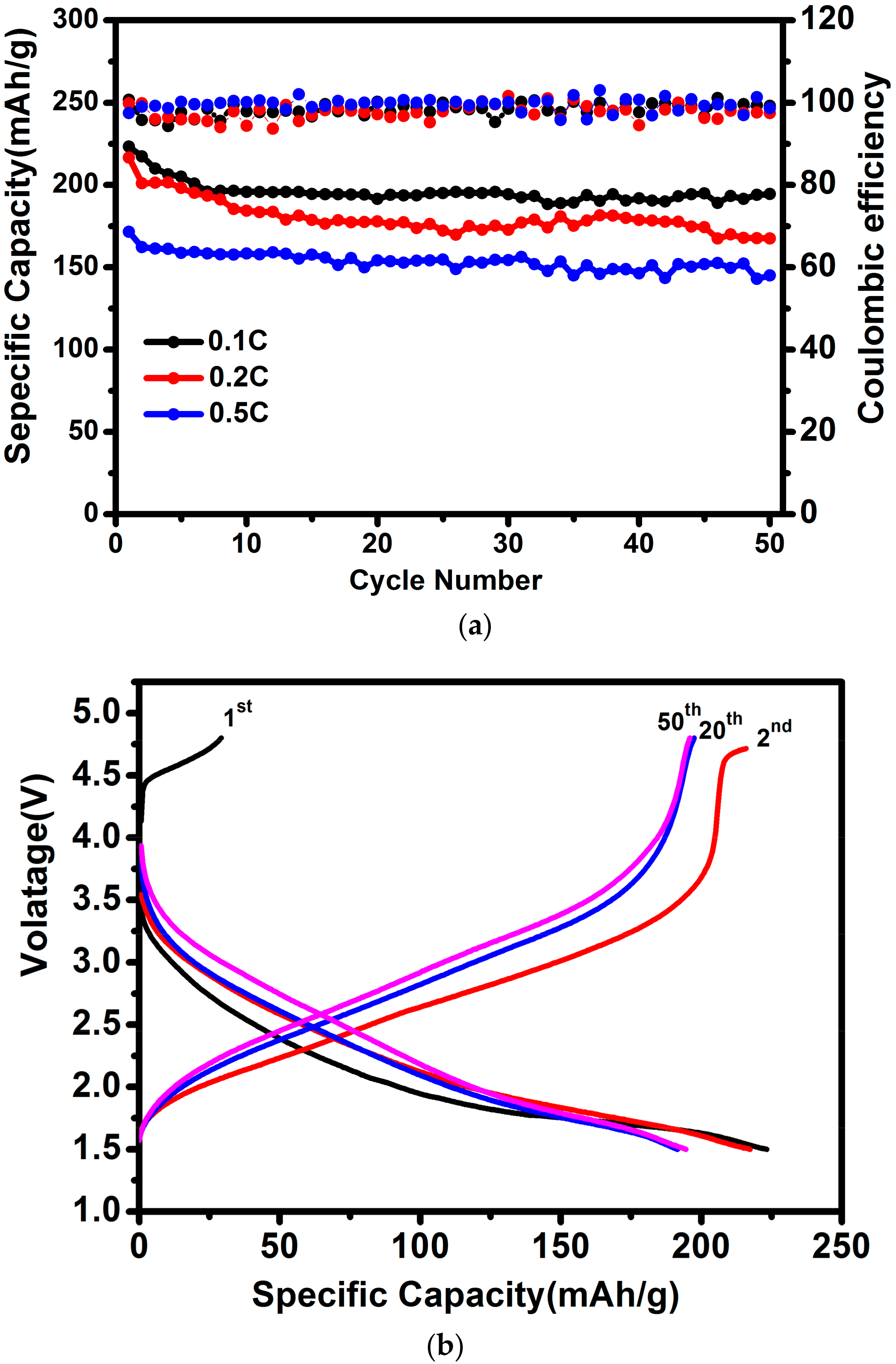
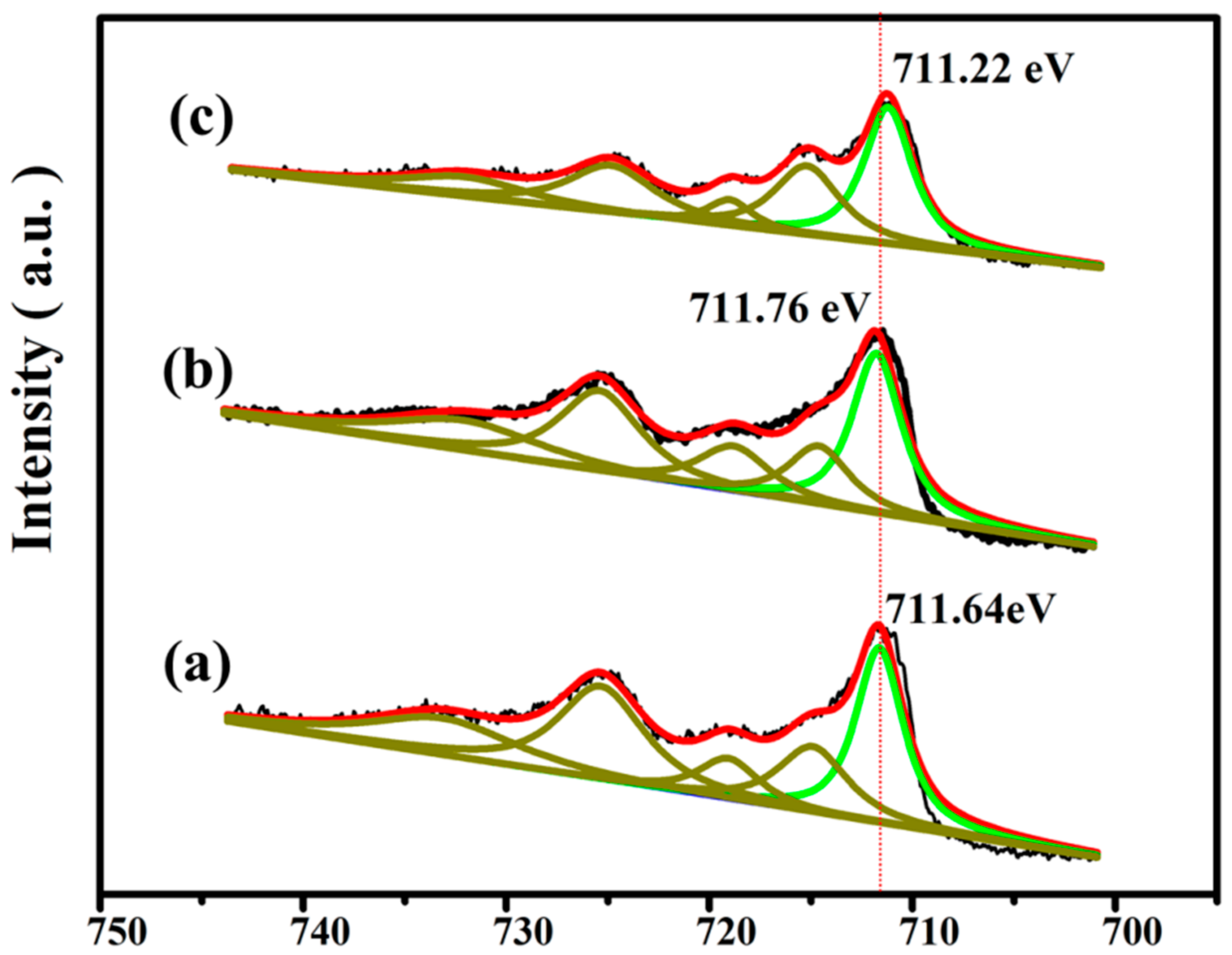
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dysart, A.D.; Phuah, X.L.; Shrestha, K.L.; Ariga, K.; Pol, V.G. Room and elevated temperature lithium-ion storage in structurally submicron carbon spheres with mechanistic. Carbon 2018, 134, 334–344. [Google Scholar] [CrossRef]
- Zheng, J.; Lochala, J.A.; Kwok, A.; Deng, Z.D.; Xiao, J. Research Progress towards Understanding the Unique Interfaces between Concentrated Electrolytes and Electrodes for Energy Storage Applications. Adv. Sci. 2017, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Aifantis, K.E.; Hackney, S.A.; Kumar, R.V. High Energy Density Lithium Batteries: Materials, Engineering, Application; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Kanno, R.T.; Shirane, Y.; Kawamoto, Y.; Takana, M.; Ohashi, M.; Yamaguchi, Y. Synthesis, structure, and electrochemical properties of a new lithium iron oxide, LiFeO2, with a corrugated layer structure. J. Electrochem. Soc. 1996, 143, 2435–2442. [Google Scholar] [CrossRef]
- Kanno, R.; Shirane, T.; Inaba, Y.; Kawamoto, Y. Synthesis and electrochemical properties of lithium iron oxides with layer-related structures. J. Power Sources 1997, 68, 145–152. [Google Scholar] [CrossRef]
- Li, J.G.; Luo, J.; Wang, L.; He, X.M. Recent advances in the LiFeO2-based materials for Li-ion batteries. Int. J. Electrochem. Sci. 2011, 6, 1550–1561. [Google Scholar]
- Sakurai, Y.; Arai, H.; Okada, S.; Yamaki, J. Low temperature synthesis and electrochemical characteristics of LiFeO2 cathodes. J. Power Sources 1997, 68, 711–715. [Google Scholar] [CrossRef]
- Sakurai, Y.; Arai, H.; Yamaki, J. Preparation of electrochemically active a-LiFeO2 at low temperature. Solid State Ion. 1998, 113–115, 29–34. [Google Scholar] [CrossRef]
- Li, K.Y.; Hao, C.; Shua, F.F.; Chen, K.F. Low temperature synthesis of Fe2O3 and LiFeO2 as cathode materials for lithium-ion batteries. Electrochim. Acta 2014, 136, 10–18. [Google Scholar] [CrossRef]
- Rahman, M.M.; Glushenkov, A.M.; Chen, Z.; Dai, X.J.; Ramireddy, T.; Chen, Y. Clusters of a-LiFeO2 nanoparticles incorporated into multi-walled carbon nanotubes: A lithium-ion battery cathode with enhanced lithium storage properties. Phys. Chem. Chem. Phys. 2013, 15, 20371–20378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, M.M.; Wang, Z.H.; Xian, X.C. Enhanced high-rate performance of Li4Ti5O12 microspheres/multiwalled carbon nanotubes composites prepared by electrostatic self-assembly. Electrochim. Acta 2018, 276, 73–80. [Google Scholar] [CrossRef]
- Morales, J.; Santos-Pena, J. Highly electroactive nanosized a-LiFeO2. Electrochem. Commun. 2007, 9, 2116–2120. [Google Scholar] [CrossRef]
- Hirayama, M.; Tomita, H.; Kubota, K.; Kanno, R. Synthesis and electrochemical properties of nanosized LiFeO2 particles with a layered rock salt structure for lithium batteries. Mater. Res. Bull. 2012, 47, 79–84. [Google Scholar] [CrossRef]
- Wang, Y.R.; Wang, J.; Liao, H.T.; Qian, X.F.; Wang, M. Facile synthesis of electrochemically active α-LiFeO2 nanoparticles in absolute ethanol at ambient temperature. RSC Adv. 2014, 4, 3753–3757. [Google Scholar]
- Liu, H.; Ji, P.; Han, X. Rheological phase synthesis of nanosized a-LiFeO2 with higher crystallinity degree for cathode material of lithium-ion batteries. Mater. Chem. Phys. 2016, 183, 152–157. [Google Scholar] [CrossRef]
- Ma, Y.M.; Zhu, Y.C.; Yu, Y.; Mei, T.; Xing, Z.; Zhang, X.; Qian, Y.T. Low Temperature Synthesis of α-LiFeO2 Nanoparticles and its behavior as cathode materials for Li-ion batteries. Int. J. Electrochem. Sci. 2012, 7, 4657–4662. [Google Scholar]
- Kim, J.; Manthiram, A. Synthesis and Lithium Intercalation Properties of Nanocrystalline Lithium Iron Oxides. J. Electrochem. Soc. 1999, 146, 4371–4374. [Google Scholar] [CrossRef]
- Lee, Y.S.; Yoon, C.S.; Sun, Y.K.; Kobayakawa, K. Synthesis of nano-crystalline LiFeO2 material with advanced battery performance. Electrochem. Commun. 2002, 4, 727–731. [Google Scholar] [CrossRef]
- Shirane, T.; Kanno, R.; Kawamoto, Y.; Takeda, Y.; Takano, M. Structure and physical properties of lithium iron oxide, LiFeO2, synthesized by ionic exchange reaction. Solid State Ion. 1995, 79, 227–233. [Google Scholar] [CrossRef]
- Cook, W.; Manley, M. Raman characterization of α- and β-LiFe5O8 prepared through a solid-state reaction pathway. J. Solid State Chem. 2010, 183, 322–326. [Google Scholar] [CrossRef]
- El-Shaarawy, M.G.; Maklad, M.H.; Rashad, M.M. Structural, AC conductivity, dielectric behavior and magnetic properties of Mg-substituted LiFe5O8 powders synthesized by sol–gel auto-combustion method. J. Mater. Sci. Mater. Electron. 2015, 26, 6040–6050. [Google Scholar] [CrossRef]
- Rahman, M.M.; Wang, J.Z.; Hassan, M.F.; Chou, S.; Chen, Z.; Liu, H.K. Nanocrystalline porous a-LiFeO2–C composite—An environmentally friendly cathode for the lithium-ion battery. Energy Environ. Sci. 2011, 4, 952–957. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.Q.; Wang, L.P.; Wu, Y.; Zhao, H.Y. Synthesis of Li2FeSiO4/C nanocomposite via a hydrothermal-assisted sol–gel process. Solid State Ion. 2015, 276, 33–39. [Google Scholar] [CrossRef]
- Wang, X.; Gao, L.S.; Zhou, F.; Zhang, Z.D.; Jia, M.R.; Tang, C.M.; Shen, T.; Zheng, H.G. Large-scale synthesis of a-LiFeO2 nanorods by low-temperature molten salt synthesis (MSS) method. J. Cryst. Growth 2004, 265, 220–223. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Liu, S.S.; Wang, Z.W.; Cai, Y.; Tan, M.; Liu, X.Q. LiSixMn2-xO4 (x ≤ 0.10) cathode materials with improved electrochemical properties prepared via a simple solid-state method for high-performance lithium-ion batteries. Ceram. Int. 2016, 42, 13442–13448. [Google Scholar] [CrossRef]
- Loukya, B.; Negi, D.S.; Sahu, R.; Pachauri, N.; Datta, A.; Gupta, R. Structural characterization of epitaxial LiFe5O8 thin films grown by chemical vapor deposition. J. Alloys Compd. 2016, 668, 187–193. [Google Scholar] [CrossRef]
- Wu, S.H.; Liu, H.Y. Preparation of α-LiFeO2-based cathode materials by an ionic exchange method. J. Power Sources 2007, 174, 789–794. [Google Scholar] [CrossRef]
- Lee, Y.S.; Satob, S.; Sun, Y.K.; Kobayakawab, K.; Satob, Y. A new type of orthorhombic LiFeO2 with advanced battery performance and its structural change during cycling. J. Power Sources 2003, 119–121, 285–289. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Tee, D.W.; Mantia, F.L. Synthesis of tetrahedral LiFeO2 and its behavior as a cathode in rechargeable lithium batteries. J. Am. Chem. Soc. 2008, 130, 3554–3559. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, M.; Tomita, H.; Kubota, K.; Kanno, R. Structure and electrode reactions of layered rock salt LiFeO2 nanoparticles for lithium battery cathode. J. Power Sources 2011, 196, 6809–6814. [Google Scholar] [CrossRef]
- Catti, M.; Montero, C.M. First-principles modelling of lithium iron oxides as battery cathode materials. J. Power Sources 2011, 196, 3955–3961. [Google Scholar] [CrossRef]
- Moralesa, J.; Santos-Pe, J.; Trocolia, R.; Frangerb, S. Insights into the electrochemical activity of nanosized α-LiFeO2. Electrochim. Acta 2008, 53, 6366–6371. [Google Scholar] [CrossRef]
- Rahmana, M.M.; Wang, J.Z.; Chen, Z.X.; Liu, H.K. Synthesis of carbon coated nanocrystalline porous α-LiFeO2 composite and its application as anode for the lithium ion battery. J. Alloys Compd. 2011, 509, 5408–5413. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.Z.; Chou, S.L.; Liu, H.K.; Ozawa, K.; Li, H. Polypyrrole-coated α-LiFeO2 nanocomposite with enhanced electrochemical properties for lithium-ion batteries. Electrochim. Acta 2013, 108, 820–826. [Google Scholar] [CrossRef]
- Abdel-Ghany, A.E.; Mauger, A.; Groult, H.; Zaghib, K.; Julien, C.M. Structural properties and electrochemistry of a-LiFeO2. J. Power Sources 2012, 197, 285–291. [Google Scholar] [CrossRef]
- Ju, B.W.; Wang, X.Y.; Wu, C.; Yang, X.K.; Shu, H.B.; Bai, Y.S.; Wen, W.C.; Yi, X. Electrochemical performance of the graphene/Y2O3/LiMn2O4 hybrid as cathode for lithium-ion battery. J. Alloys Compd. 2014, 584, 454–460. [Google Scholar] [CrossRef]
- Wu, H.; Li, H.; Sun, G.; Ma, S.L.; Yang, X.J. Synthesis, characterization and electromagnetic performance of nanocomposites of grapheme with a-LiFeO2 and b-LiFe5O8. J. Mater. Chem. C 2015, 3, 5457–5466. [Google Scholar] [CrossRef]
- Buyukyazi, M.; Mathurn, S. 3D nanoarchitectures of α-LiFeO2 and α-LiFeO2/C nanofibers for high power lithium-ion batteries. Nano Energy 2015, 13, 28–35. [Google Scholar] [CrossRef]
- Tu, J.; Wu, K.; Tang, H.; Zhou, H.H.; Jiao, S.Q. Mg–Ti co-doping behavior of porous LiFePO4 microspheres for high-rate lithium-ion batteries. J. Mater. Chem. A 2017, 5, 17021–17028. [Google Scholar] [CrossRef]

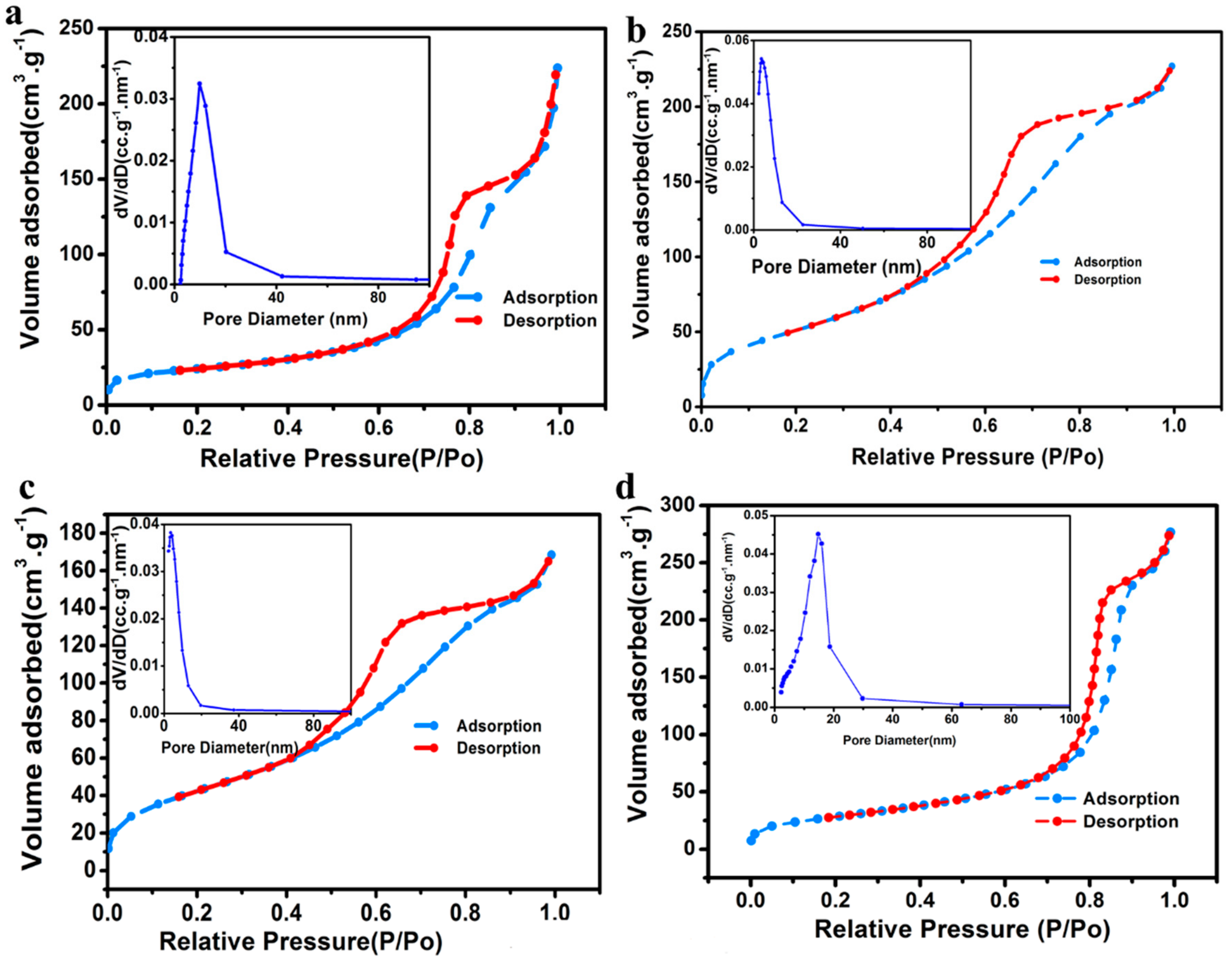
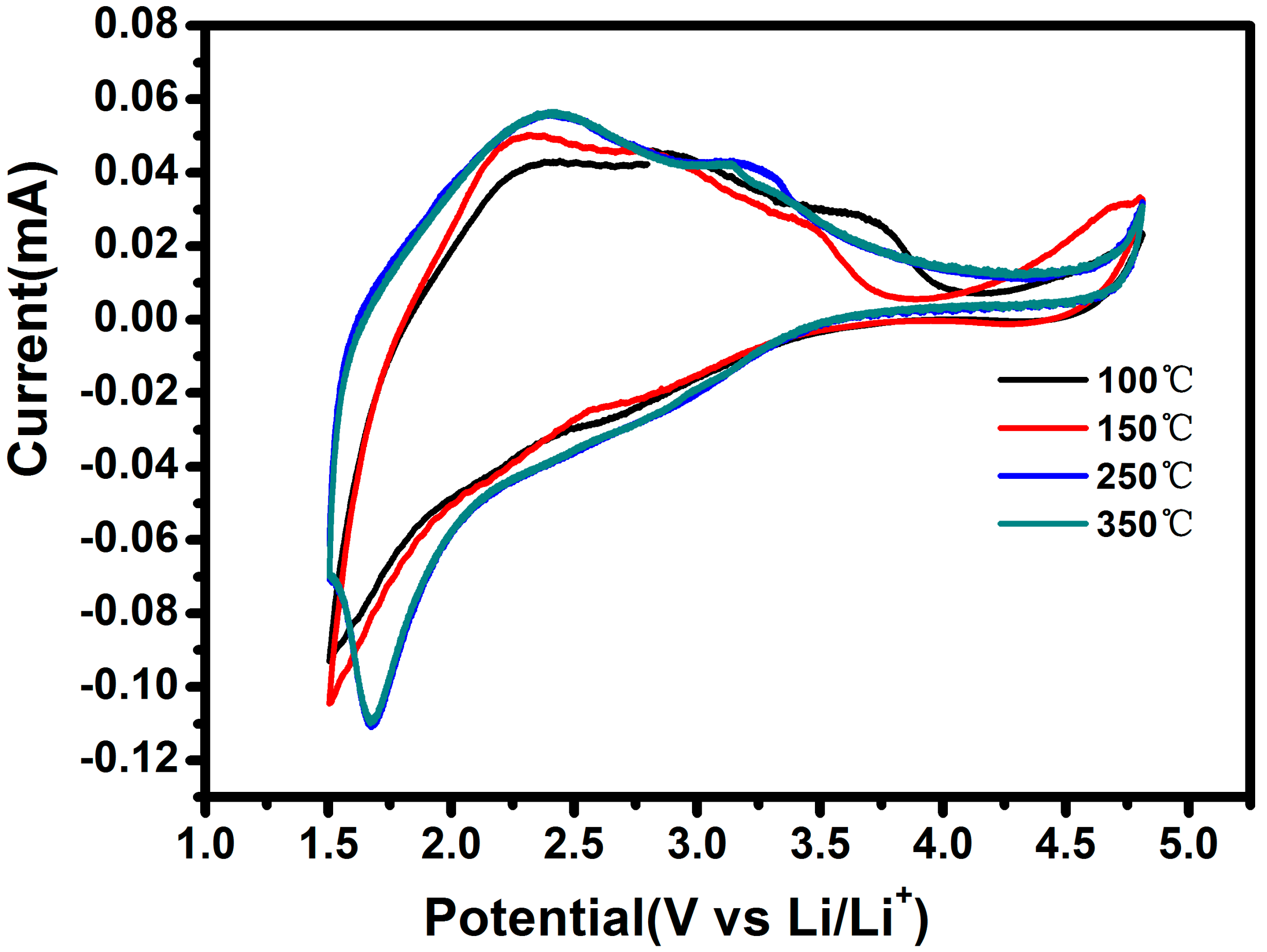
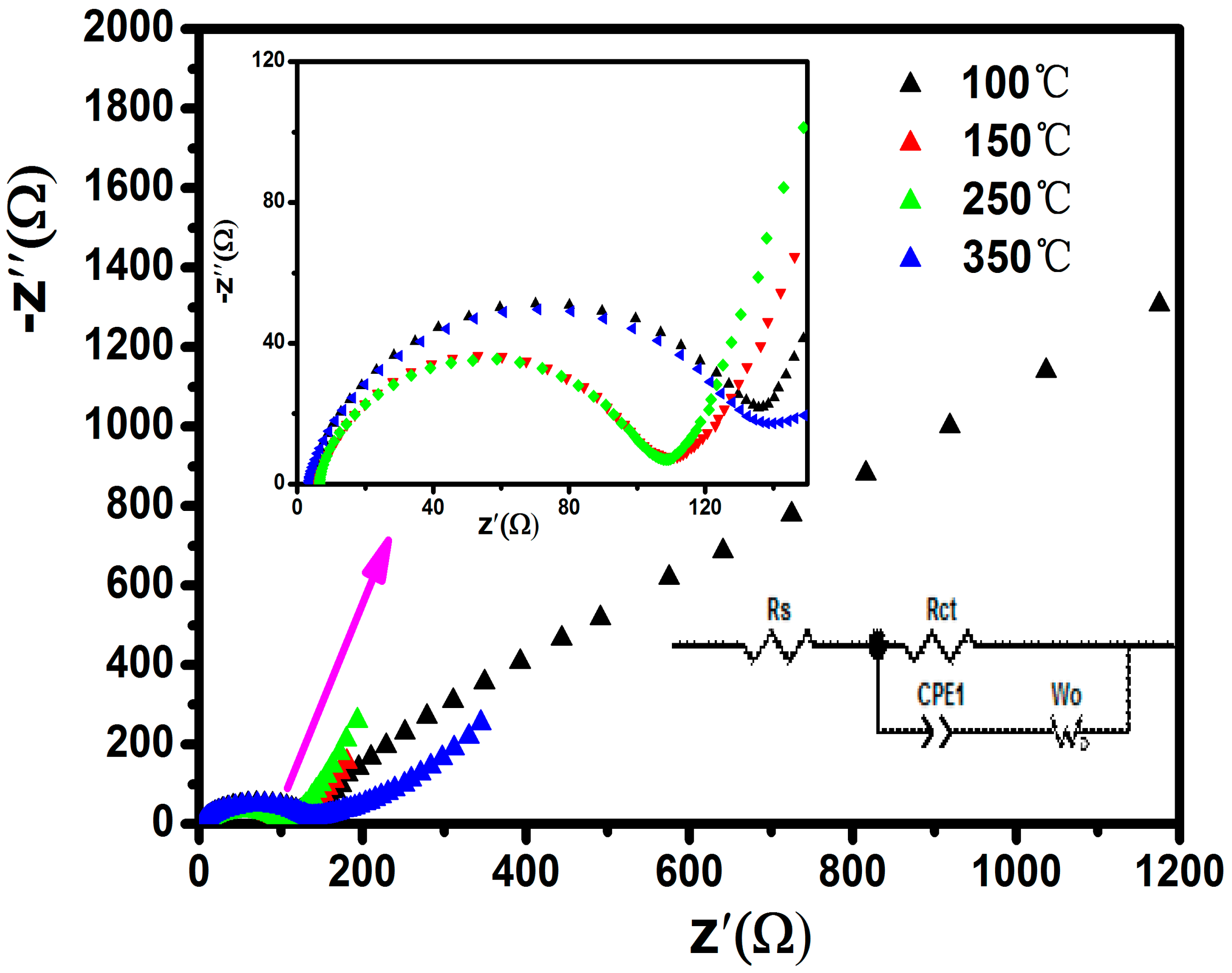
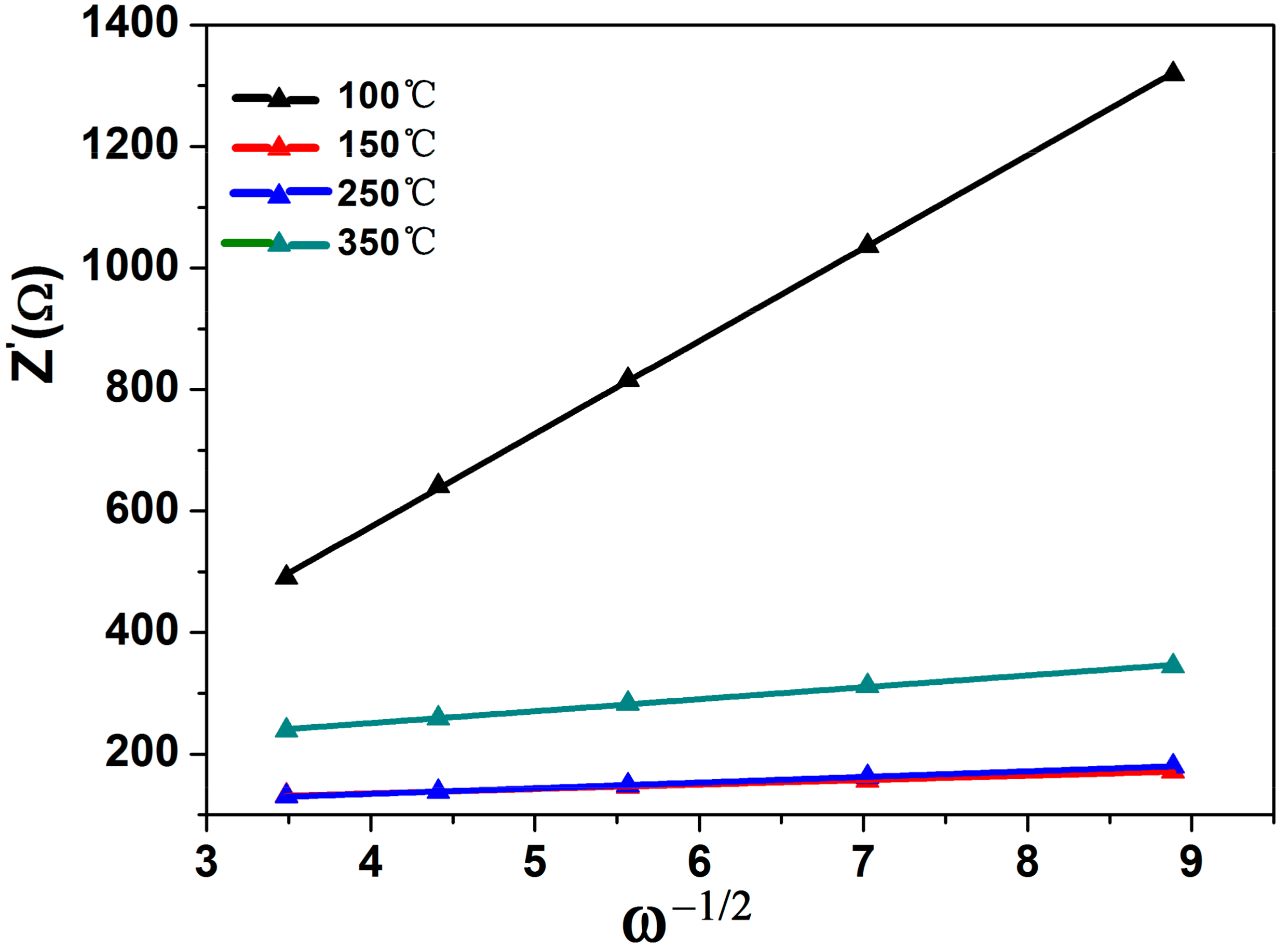
| Sample | SBET/m2·g−1 | Pore Diameter/nm |
|---|---|---|
| 100 °C | 82.11 | 8.46 |
| 150 °C | 194.48 | 3.27 |
| 250 °C | 159.30 | 3.61 |
| 350 °C | 103.42 | 8.28 |
| Sample | Rct/Ω | σ/S | DLi+/cm2·S−1 |
|---|---|---|---|
| 100 °C | 152.8 | 152.83 | 2.98 × 10−13 |
| 150 °C | 94.4 | 7.29 | 1.3 × 10−10 |
| 250 °C | 94.59 | 9.28 | 8.08 × 10−11 |
| 350 °C | 121.7 | 19.55 | 9.11 × 10−12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Zhao, H.; Liu, X. A Simple, Quick and Eco-Friendly Strategy of Synthesis Nanosized α-LiFeO2 Cathode with Excellent Electrochemical Performance for Lithium-Ion Batteries. Materials 2018, 11, 1176. https://doi.org/10.3390/ma11071176
Hu Y, Zhao H, Liu X. A Simple, Quick and Eco-Friendly Strategy of Synthesis Nanosized α-LiFeO2 Cathode with Excellent Electrochemical Performance for Lithium-Ion Batteries. Materials. 2018; 11(7):1176. https://doi.org/10.3390/ma11071176
Chicago/Turabian StyleHu, Youzuo, Hongyuan Zhao, and Xingquan Liu. 2018. "A Simple, Quick and Eco-Friendly Strategy of Synthesis Nanosized α-LiFeO2 Cathode with Excellent Electrochemical Performance for Lithium-Ion Batteries" Materials 11, no. 7: 1176. https://doi.org/10.3390/ma11071176
APA StyleHu, Y., Zhao, H., & Liu, X. (2018). A Simple, Quick and Eco-Friendly Strategy of Synthesis Nanosized α-LiFeO2 Cathode with Excellent Electrochemical Performance for Lithium-Ion Batteries. Materials, 11(7), 1176. https://doi.org/10.3390/ma11071176






