Biomass-Derived Nitrogen-Doped Carbon Aerogel Counter Electrodes for Dye Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Photoanode
2.3. Synthesis of N Doped Carbon Aerogel
2.4. Preparation of Carbon Counter Electrode
2.5. Preparation of Platinum Electrode
2.6. Assembly of the Device
2.7. Characterization and Device Testing Techniques
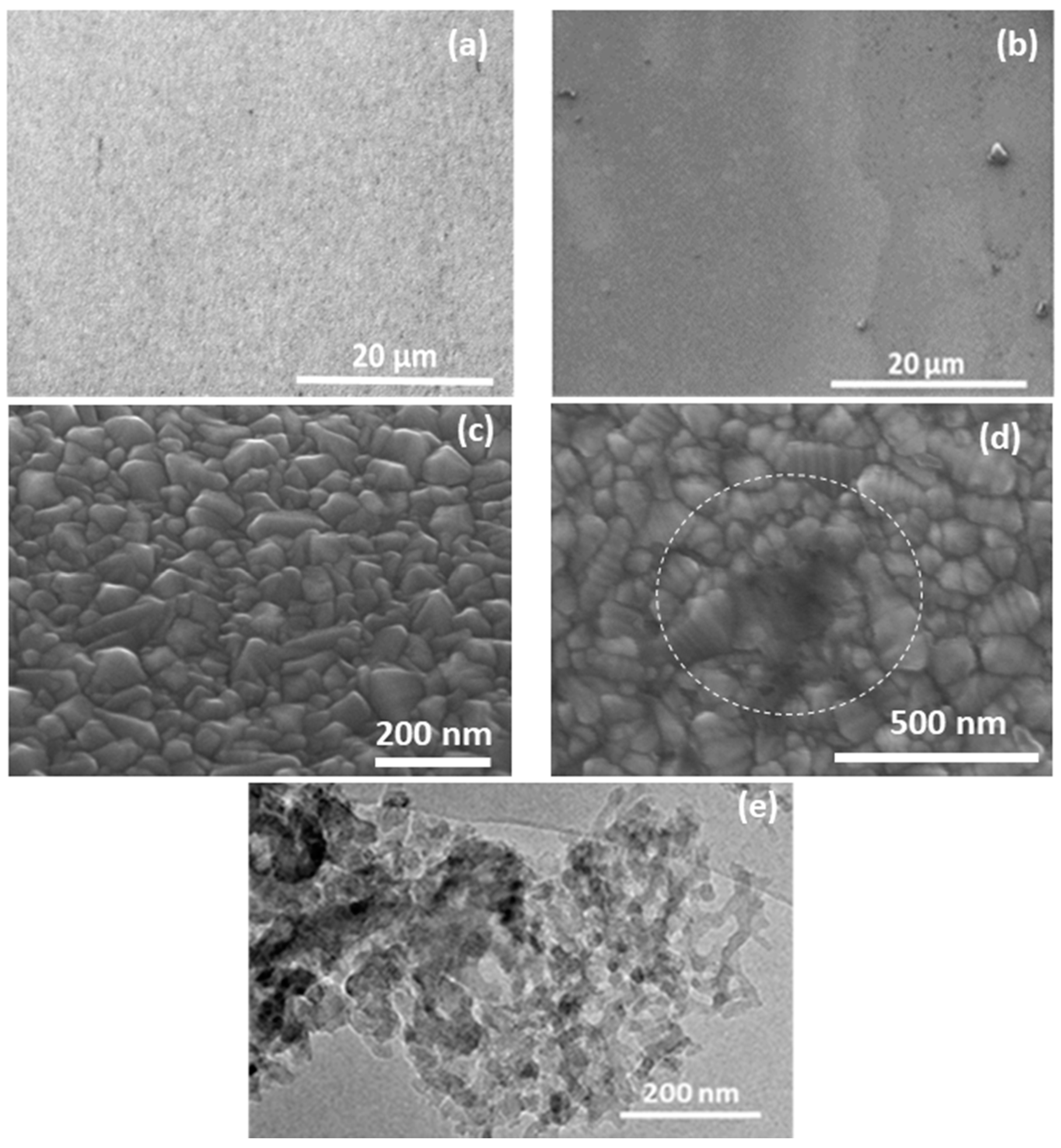
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Regan, B.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kay, A.; Grätzel, M. Low cost photovoltaic modules based on dye sensitized nanocrystalline titanium dioxide and carbon powder. Sol. Energy Mater. Sol. Cells 1996, 44, 99–117. [Google Scholar] [CrossRef]
- Freitag, M.; Daniel, Q.; Pazoki, M.; Sveinbjornsson, K.; Zhang, J.; Sun, L.; Hagfeldt, A.; Boschloo, G. High-efficiency dye-sensitized solar cells with molecular copper phenanthroline as solid hole conductor. Energy Environ. Sci. 2015, 8, 2634–2637. [Google Scholar] [CrossRef]
- Chava, R.K.; Lee, W.-M.; Oh, S.-Y.; Jeong, K.-U.; Yu, Y.-T. Improvement in light harvesting and device performance of dye sensitized solar cells using electrophoretic deposited hollow TiO2 nps scattering layer. Sol. Energy Mater. Sol. Cells 2017, 161, 255–262. [Google Scholar] [CrossRef]
- Grätzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- Halme, J.; Toivola, M.; Tolvanen, A.; Lund, P. Charge transfer resistance of spray deposited and compressed counter electrodes for dye-sensitized nanoparticle solar cells on plastic substrates. Sol. Energy Mater. Sol. Cells 2006, 90, 872–886. [Google Scholar] [CrossRef]
- Papageorgiou, N. Counter-electrode function in nanocrystalline photoelectrochemical cell configurations. Coord. Chem. Rev. 2004, 248, 1421–1446. [Google Scholar] [CrossRef]
- Kang, S.H.; Choi, S.H.; Kang, M.S.; Kim, J.Y.; Kim, H.S.; Hyeon, T.; Sung, Y.E. Nanorod-based dye-sensitized solar cells with improved charge collection efficiency. Adv. Mater. 2008, 20, 54–58. [Google Scholar] [CrossRef]
- Yasuo, C.; Ashraful, I.; Yuki, W.; Ryoichi, K.; Naoki, K.; Liyuan, H. Dye-sensitized solar cells with conversion efficiency of 11.1%. Jpn. J. Appl. Phys. 2006, 45, L638. [Google Scholar]
- Gao, F.; Wang, Y.; Zhang, J.; Shi, D.; Wang, M.; Humphry-Baker, R.; Wang, P.; Zakeeruddin, S.M.; Gratzel, M. A new heteroleptic ruthenium sensitizer enhances the absorptivity of mesoporous titania film for a high efficiency dye-sensitized solar cell. Chem. Commun. 2008, 2635–2637. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Ma, T. Platinum-free catalysts as counter electrodes in dye-sensitized solar cells. ChemSusChem 2012, 5, 1343–1357. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wei, J.; Wang, K.; Wu, D. Applications of carbon materials in photovoltaic solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 1461–1470. [Google Scholar] [CrossRef]
- Ramasamy, E.; Lee, W.J.; Lee, D.Y.; Song, J.S. Spray coated multi-wall carbon nanotube counter electrode for tri-iodide (i3-) reduction in dye-sensitized solar cells. Electrochem. Commun. 2008, 10, 1087–1089. [Google Scholar] [CrossRef]
- Hino, T.; Ogawa, Y.; Kuramoto, N. Preparation of functionalized and non-functionalized fullerene thin films on ito glasses and the application to a counter electrode in a dye-sensitized solar cell. Carbon 2006, 44, 880–887. [Google Scholar] [CrossRef]
- Wang, G.; Xing, W.; Zhuo, S. Application of mesoporous carbon to counter electrode for dye-sensitized solar cells. J. Power Sources 2009, 194, 568–573. [Google Scholar] [CrossRef]
- Ali, A.; Shehzad, K.; Ur-Rahman, F.; Shah, S.M.; Khurram, M.; Mumtaz, M.; Sagar, R.U.R. Flexible, low cost, and platinum-free counter electrode for efficient dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2016, 8, 25353–25360. [Google Scholar] [CrossRef] [PubMed]
- Battumur, T.; Mujawar, S.H.; Ambade, S.B.; Lee, D.S.; Lee, W.; Sukhbaatar, I.; Munkhbaatar, P.; Han, S.H.; Lee, S.H. Craphene based composites as a counter electrode for dye-sensitized solar cells. In Proceedings of the 2012 7th International Forum on Strategic Technology (IFOST), Tomsk, Russia, 18–21 September 2012; pp. 1–6. [Google Scholar]
- Li, G.R.; Wang, F.; Song, J.; Xiong, F.Y.; Gao, X.P. Tin-conductive carbon black composite as counter electrode for dye-sensitized solar cells. Electrochim. Acta 2012, 65, 216–220. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Wang, Y.; Huang, Q.; Dai, C.; Gamboa, S.; Sebastian, P.J. Structure and electrochemical properties of carbon aerogels synthesized at ambient temperatures as supercapacitors. J. Non-Cryst. Solids 2008, 354, 19–24. [Google Scholar] [CrossRef]
- Zhao, B.; Huang, H.; Jiang, P.; Zhao, H.; Huang, X.; Shen, P.; Wu, D.; Fu, R.; Tan, S. Flexible counter electrodes based on mesoporous carbon aerogel for high-performance dye-sensitized solar cells. J. Phys. Chem. C 2011, 115, 22615–22621. [Google Scholar] [CrossRef]
- Briscoe, J.; Marinovic, A.; Sevilla, M.; Dunn, S.; Titirici, M. Biomass-derived carbon quantum dot sensitizers for solid-state nanostructured solar cells. Angew. Chem. Int. Ed. 2015, 54, 4463–4468. [Google Scholar] [CrossRef] [PubMed]
- Marinovic, A.; Kiat, L.S.; Dunn, S.; Titirici, M.-M.; Briscoe, J. Carbon-nanodot solar cells from renewable precursors. ChemSusChem 2017, 10, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, Z.; Chang, J.; Liu, X.; Wu, D.; Xu, F.; Guo, Y.; Jiang, K. Nitrogen-doped porous carbons as electrode materials for high-performance supercapacitor and dye-sensitized solar cell. ACS Appl. Mater. Interfaces 2015, 7, 20234–20244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, H.; Xu, H.; Zhang, Q.; Sun, H.; Zhang, L. Nitrogen-doped carbon microspheres counter electrodes for dye-sensitized solar cells by microwave assisted method. RSC Adv. 2016, 6, 58064–58068. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Antonietti, M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Preuss, K.; Tanase, L.C.; Teodorescu, C.M.; Abrahams, I.; Titirici, M.M. Sustainable metal-free carbogels as oxygen reduction electrocatalysts. J. Mater. Chem. A 2017, 5, 16336–16343. [Google Scholar] [CrossRef]
- Soosaimanickam, A.; Xuan, L.; Ann-Louise, A.; Pelin, Y.; Steve, D.; Moorthy, B.S.; Joe, B. Photo-enhanced catalytic activity of spray-coated Cu2SnSe3 nanoparticle counter electrode for dye-sensitised solar cells. Phys. Status Solidi (RRL) Rapid Res. Lett. 2016, 10, 739–744. [Google Scholar]
- Yuhua, X.; Jun, L.; Hao, C.; Ruigang, W.; Dingqiang, L.; Jia, Q.; Liming, D. Nitrogen-doped graphene foams as metal-free counter electrodes in high-performance dye-sensitized solar cells. Angew. Chem. Int. Ed. 2012, 51, 12124–12127. [Google Scholar]
- Song, L.; Luo, Q.; Zhao, F.; Li, Y.; Lin, H.; Qu, L.; Zhang, Z. Dually functional, n-doped porous graphene foams as counter electrodes for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2014, 16, 21820–21826. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Cai, X.; Wu, H.; Yu, X.; Peng, M.; Yan, K.; Zou, D. Nitrogen-doped graphene for dye-sensitized solar cells and the role of nitrogen states in triiodide reduction. Energy Environ. Sci. 2013, 6, 3356–3362. [Google Scholar] [CrossRef]
- Potscavage, W.J.; Sharma, A.; Kippelen, B. Critical interfaces in organic solar cells and their influence on the open-circuit voltage. Acc. Chem. Res. 2009, 42, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Xie, Y.; Ropp, M.; Galipeau, D.; Bailey, S.; Qiao, Q. Dye-sensitized solar cells based on low cost nanoscale carbon/TiO2 composite counter electrode. Energy Environ. Sci. 2009, 2, 426–429. [Google Scholar] [CrossRef]
- Balamurugan, J.; Pandurangan, A.; Kim, N.H.; Lee, J.H. Facile synthesis of high quality multi-walled carbon nanotubes on novel 3d kit-6: Application in high performance dye-sensitized solar cells. Nanoscale 2015, 7, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Mulder, W.H.; Sluyters, J.H.; Pajkossy, T.; Nyikos, L. Tafel current at fractal electrodes: Connection with admittance spectra. J. Electroanal. Chem. Interfacial Electrochem. 1990, 285, 103–115. [Google Scholar] [CrossRef]
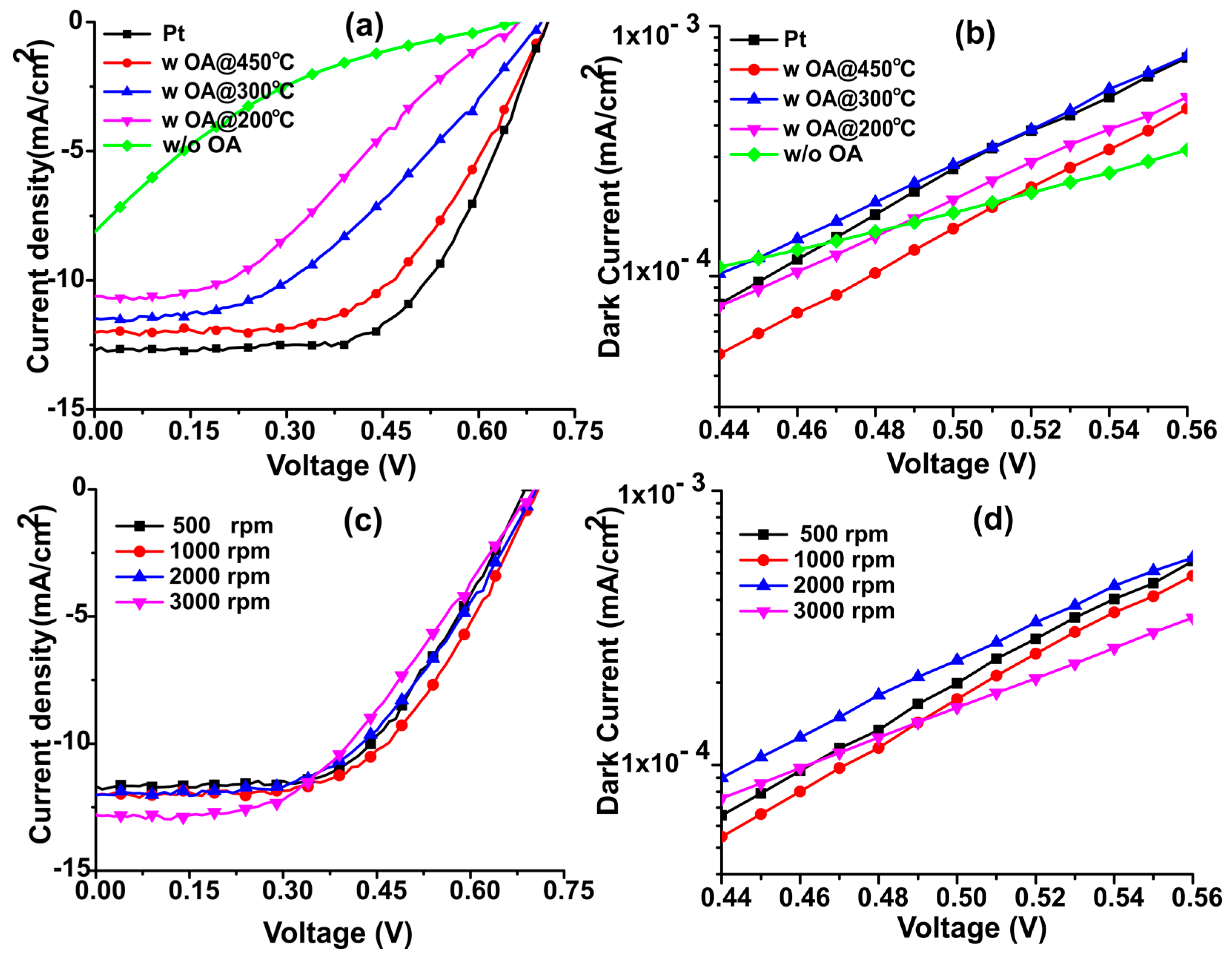


| Samples | Annealing Temperature (°C) | Current Density (mA/cm2) | Open Circuit Voltage (V) | Fill Factor | Efficiency (%) | Rs (Ω) |
|---|---|---|---|---|---|---|
| Platinum | 450 | 12.04 ± 0.58 | 0.71 ± 0.02 | 0.58 ± 0.02 | 5.08 ± 0.24 | 78.38 |
| N-dC with OA | 450 | 11.62 ± 0.40 | 0.70 ± 0.01 | 0.56 ± 0.01 | 4.47 ± 0.21 | 84.81 |
| N-dC with OA | 300 | 11.45 ± 0.44 | 0.69 ± 0.01 | 0.44 ± 0.02 | 3.47 ± 0.16 | 89.19 |
| N-dC with OA | 200 | 10.85 ± 0.56 | 0.67 ± 0.02 | 0.33 ± 0.03 | 2.39 ± 0.20 | 96.23 |
| N-dC w/o OA | 200 | 8.06 ± 0.20 | 0.66 ± 0.03 | 0.15 ± 0.01 | 0.78 ± 0.03 | 145.01 |
| Spin Speeds (rpm) | Current Density (mA/cm2) | Open Circuit Voltage (V) | Fill Factor | Efficiency (%) | Rs (Ω) |
|---|---|---|---|---|---|
| 500 | 11.42 ± 0.36 | 0.68 ± 0.01 | 0.56 ± 0.01 | 4.40 ± 0.13 | 86.09 |
| 1000 | 11.62 ± 0.4 | 0.70 ± 0.01 | 0.56 ± 0.01 | 4.47 ± .21 | 84.81 |
| 2000 | 11.59 ± 0.28 | 0.70 ± 0.01 | 0.54 ± 0.02 | 4.31 ± 0.19 | 84.06 |
| 3000 | 11.71 ± 0.41 | 0.70 ± 0.01 | 0.50 ± 0.02 | 4.01 ± 0.26 | 80.01 |
| Sample | 2Rct (Ω) | 2Rs (Ω) | CPE-T (µF) | CPE-P | C/2 (µF) | Rs* (Ω) |
|---|---|---|---|---|---|---|
| Pt | 0.70 | 17.73 | 0.92 | 0.86 | 1.60 | 78.38 |
| N-dC (450 °C) | 0.83 | 17.63 | 0.84 | 0.80 | 1.38 | 84.81 |
| N-dC (200 °C) | 0.65 | 21.73 | 0.91 | 0.85 | 1.56 | 96.23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butt, M.T.Z.; Preuss, K.; Titirici, M.-M.; Rehman, H.U.; Briscoe, J. Biomass-Derived Nitrogen-Doped Carbon Aerogel Counter Electrodes for Dye Sensitized Solar Cells. Materials 2018, 11, 1171. https://doi.org/10.3390/ma11071171
Butt MTZ, Preuss K, Titirici M-M, Rehman HU, Briscoe J. Biomass-Derived Nitrogen-Doped Carbon Aerogel Counter Electrodes for Dye Sensitized Solar Cells. Materials. 2018; 11(7):1171. https://doi.org/10.3390/ma11071171
Chicago/Turabian StyleButt, Mira Tul Zubaida, Kathrin Preuss, Maria-Magdalena Titirici, Habib Ur Rehman, and Joe Briscoe. 2018. "Biomass-Derived Nitrogen-Doped Carbon Aerogel Counter Electrodes for Dye Sensitized Solar Cells" Materials 11, no. 7: 1171. https://doi.org/10.3390/ma11071171
APA StyleButt, M. T. Z., Preuss, K., Titirici, M.-M., Rehman, H. U., & Briscoe, J. (2018). Biomass-Derived Nitrogen-Doped Carbon Aerogel Counter Electrodes for Dye Sensitized Solar Cells. Materials, 11(7), 1171. https://doi.org/10.3390/ma11071171





