Surface Modifications of Nanoparticles for Stability in Biological Fluids
Abstract
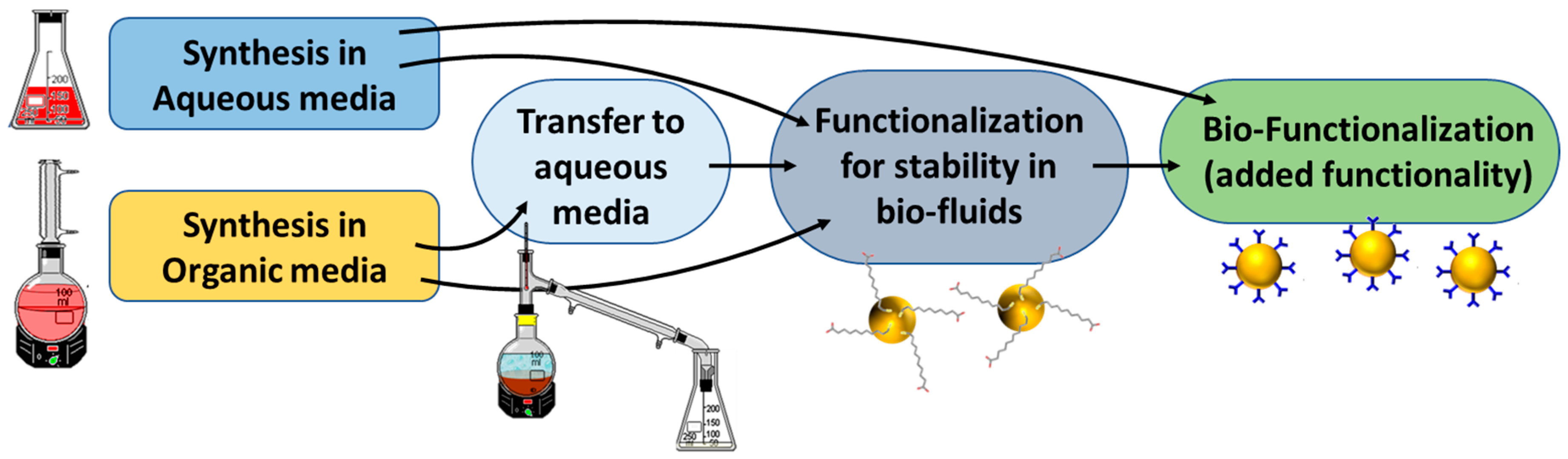
1. Introduction
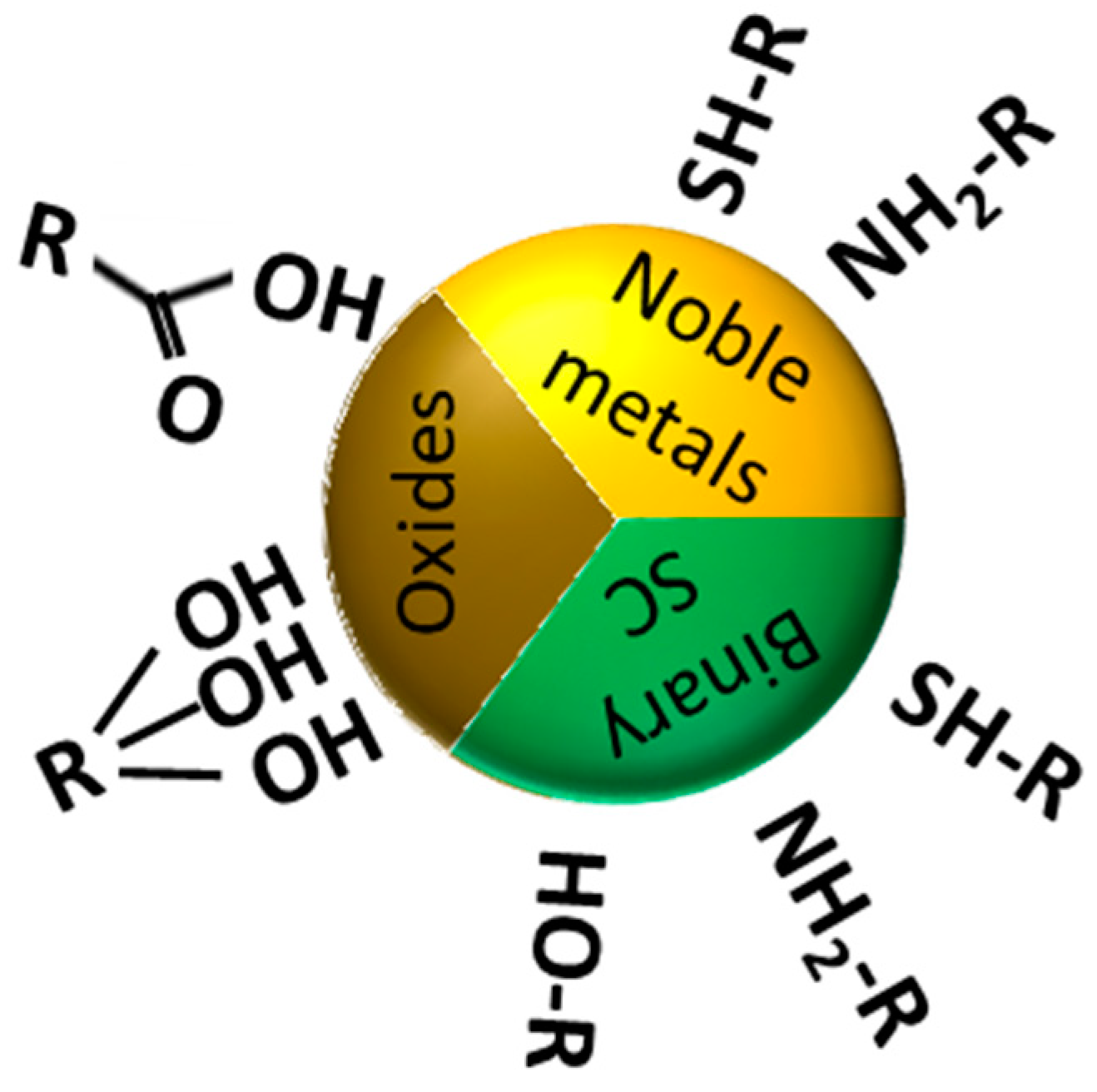
2. Surface Chemistry of Nanoparticles
3. PEG as Stabilizing Agent in Biological Systems
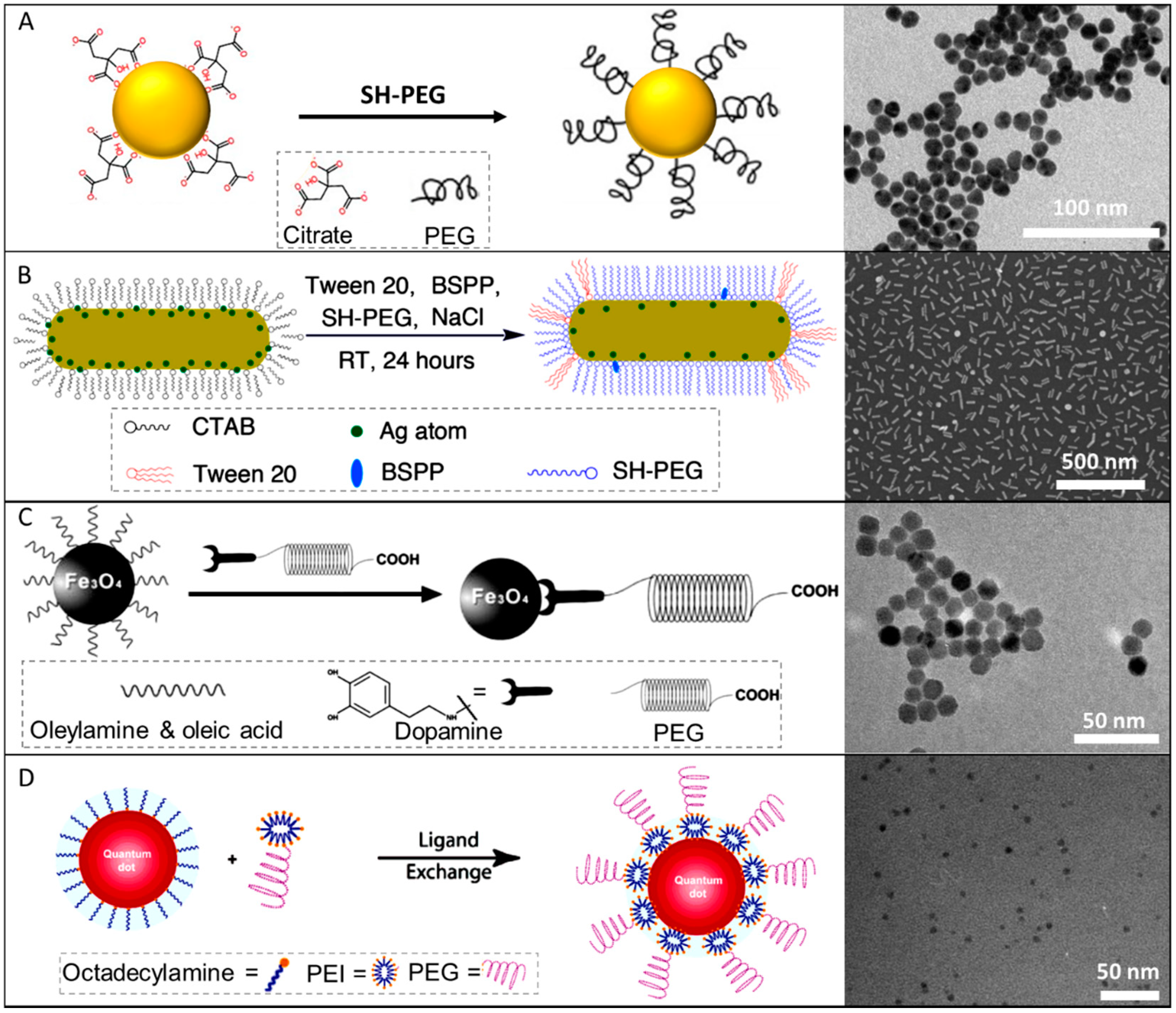
3.1. PEG Coating of Plasmonic NPs
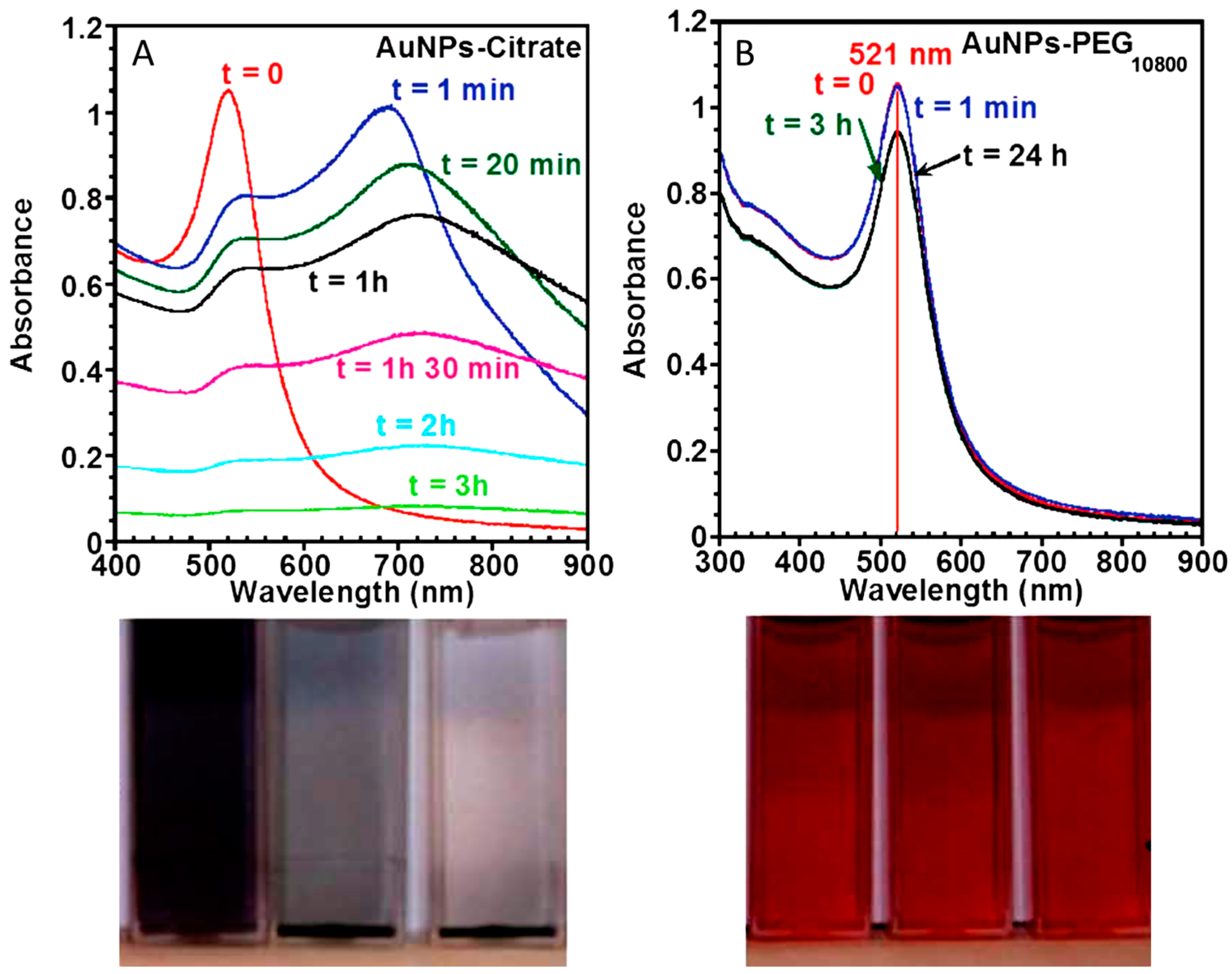
3.1.1. Citrate-Stabilized Particles
3.1.2. CTAB-Stabilized Au NPs
3.2. PEG Coating of Magnetic NPs
3.3. PEG Coating of Quantum Dots
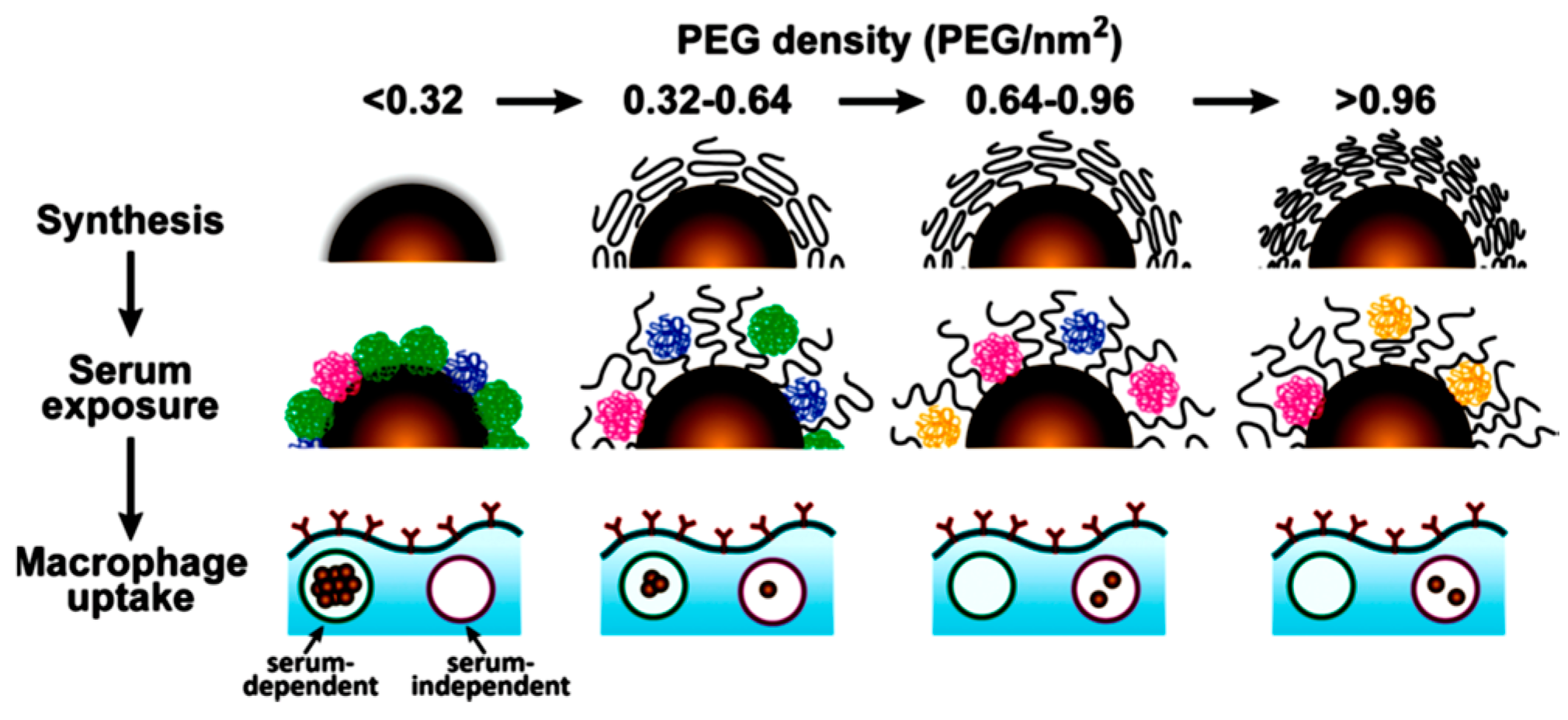
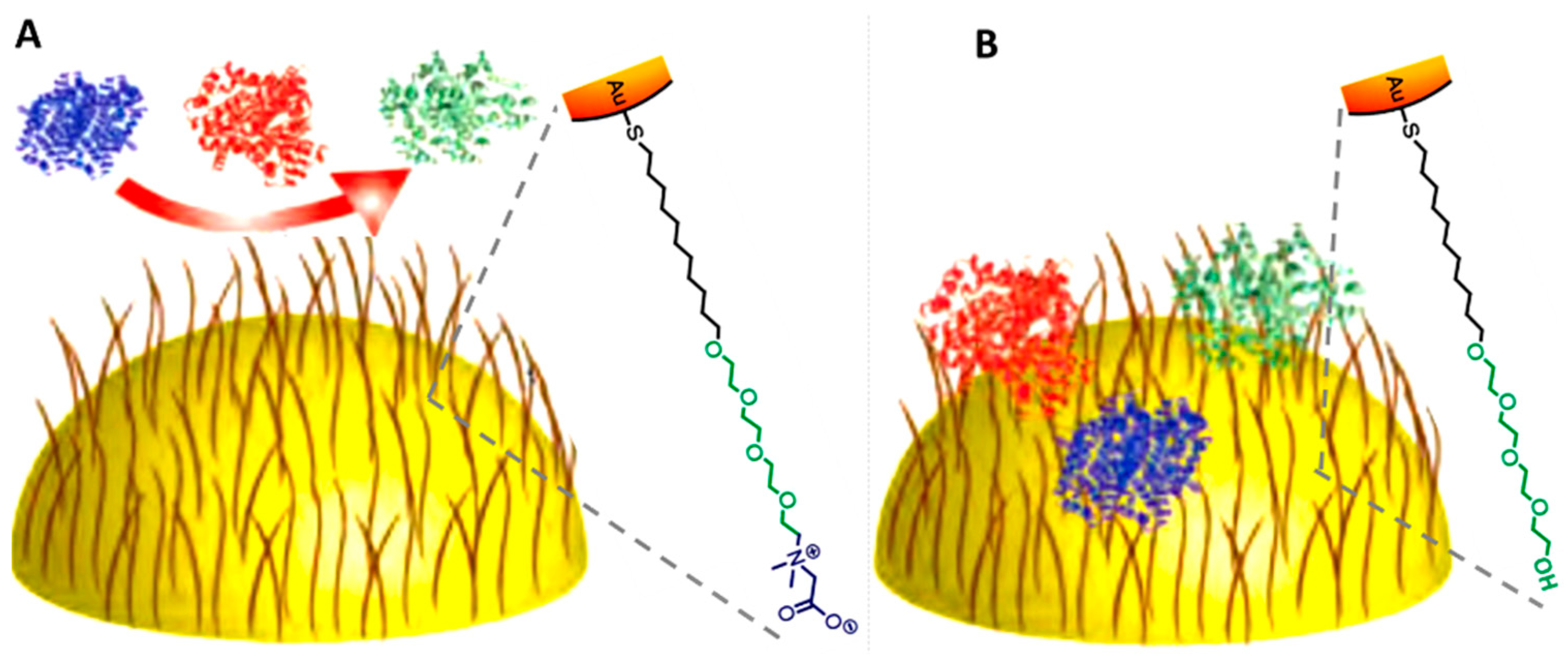
3.4. PEG Interaction with Proteins
3.5. PEG Drawbacks
4. Zwitterionic Ligands
4.1. Zwitterionic Coating of Plasmonic NPs
4.2. Zwitterionic Coating of QDs
4.3. Zwitterionic Coating of Magnetic NPs
4.4. Drawbacks of Zwitterionic Coatings
5. Lipid Bilayer
5.1. Lipid Bilayer Coating of Plasmonic NPs
5.2. Lipid Bilayer Coating of Fluorescent NPs
5.3. Lipid Bilayer Coating of Magnetic NPs
5.4. Drawbacks of Lipidic Coatings
6. Protein Coatings
6.1. Protein Coating of Plasmonic NPs
6.2. Protein Coating of QDs
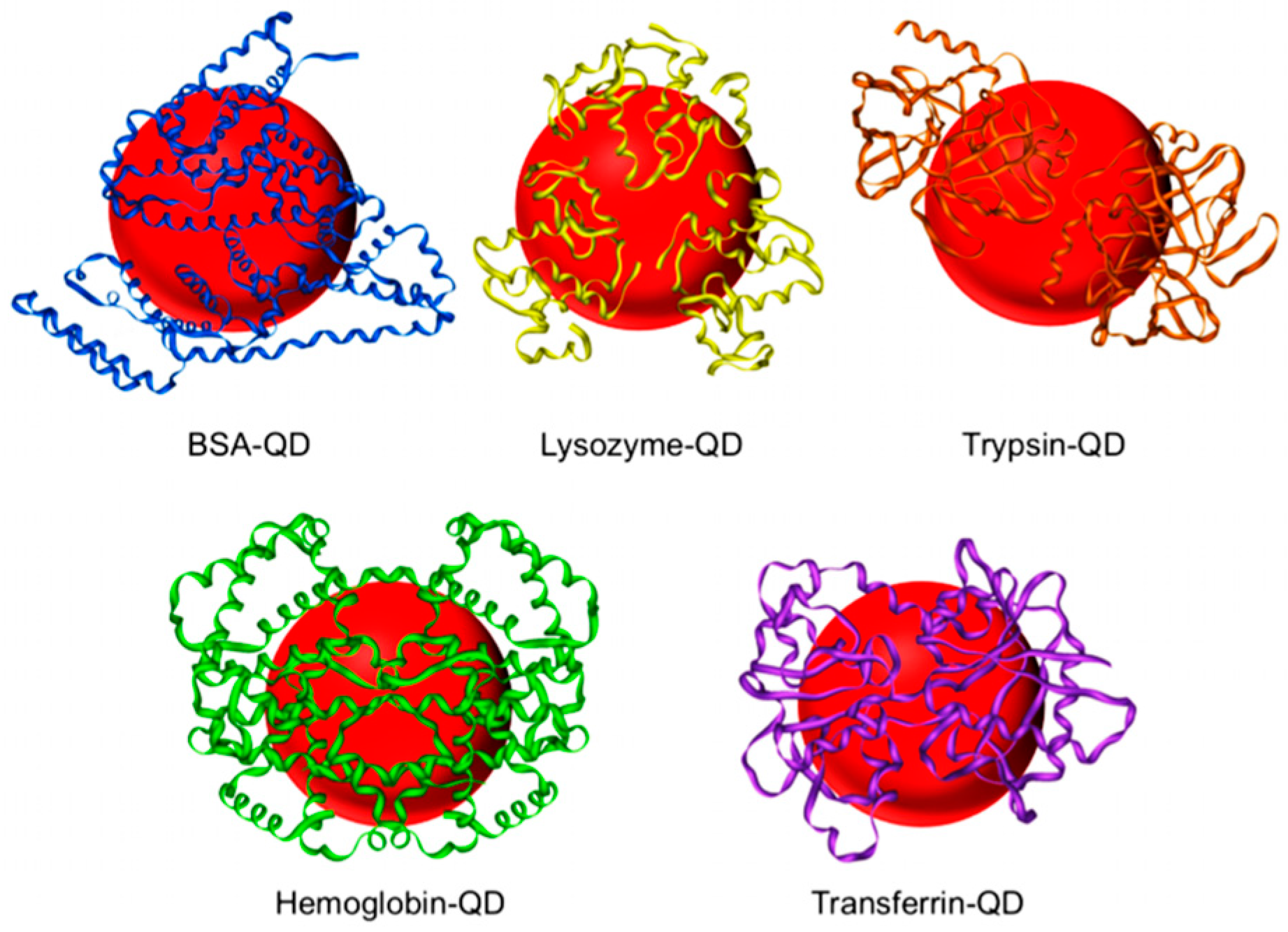
6.3. Protein Coating of Magnetic NPs
6.4. Drawbacks of Protein Coatings
7. Glycans
7.1. Glycans Coating of Noble NPs
7.2. Glycans Coating of QDs
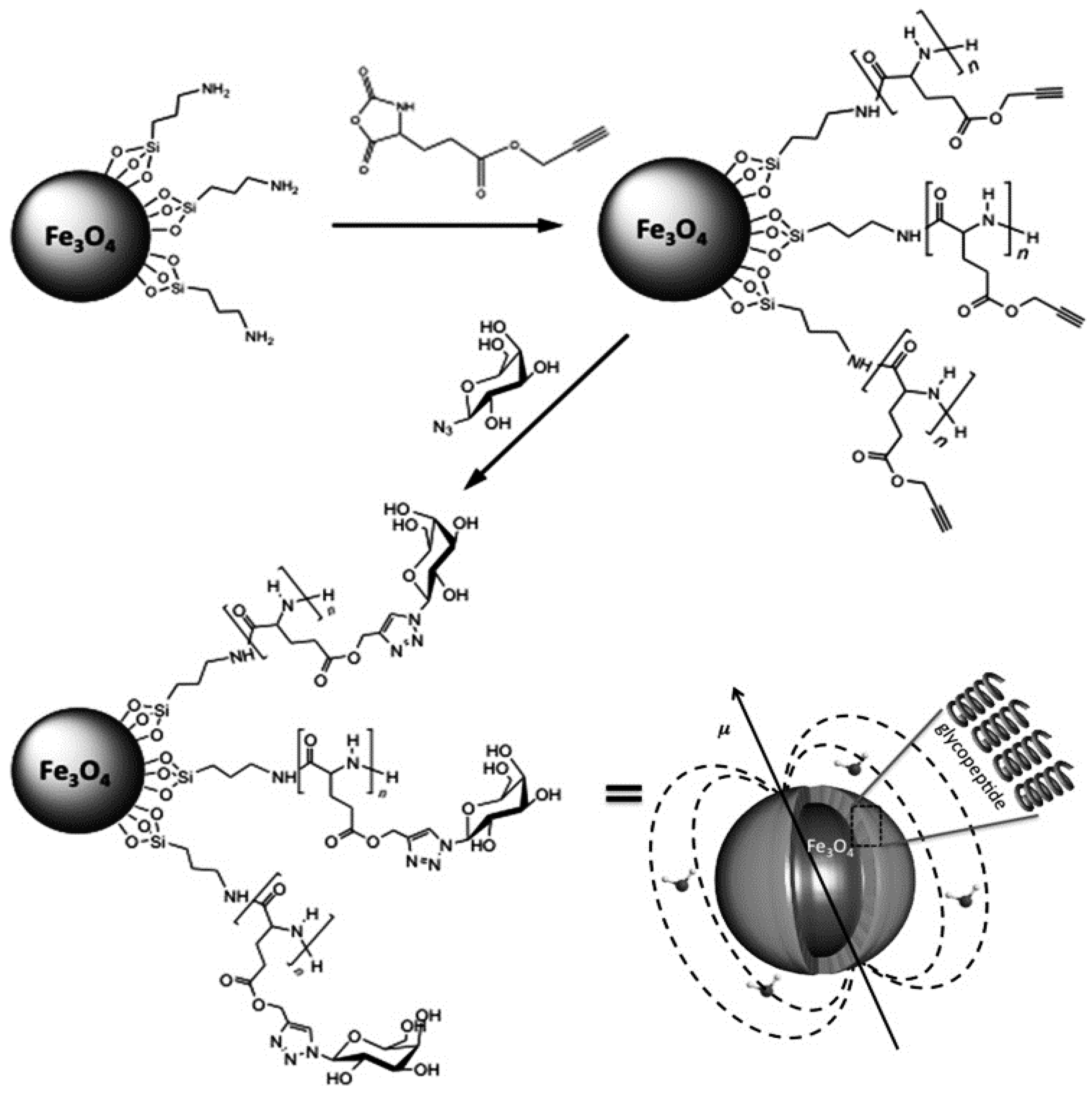
7.3. Glycans Coating of Magnetic NPs
7.4. Drawbacks of Glycans Coatings
8. Poly(Maleic Anhydride) Based Polymers
8.1. Poly(Maleic Anhydride) Based Polymers Coating of Plasmonic, Fluorescent and Magnetic NPs
8.2. Drawbacks of Glycans Coatings
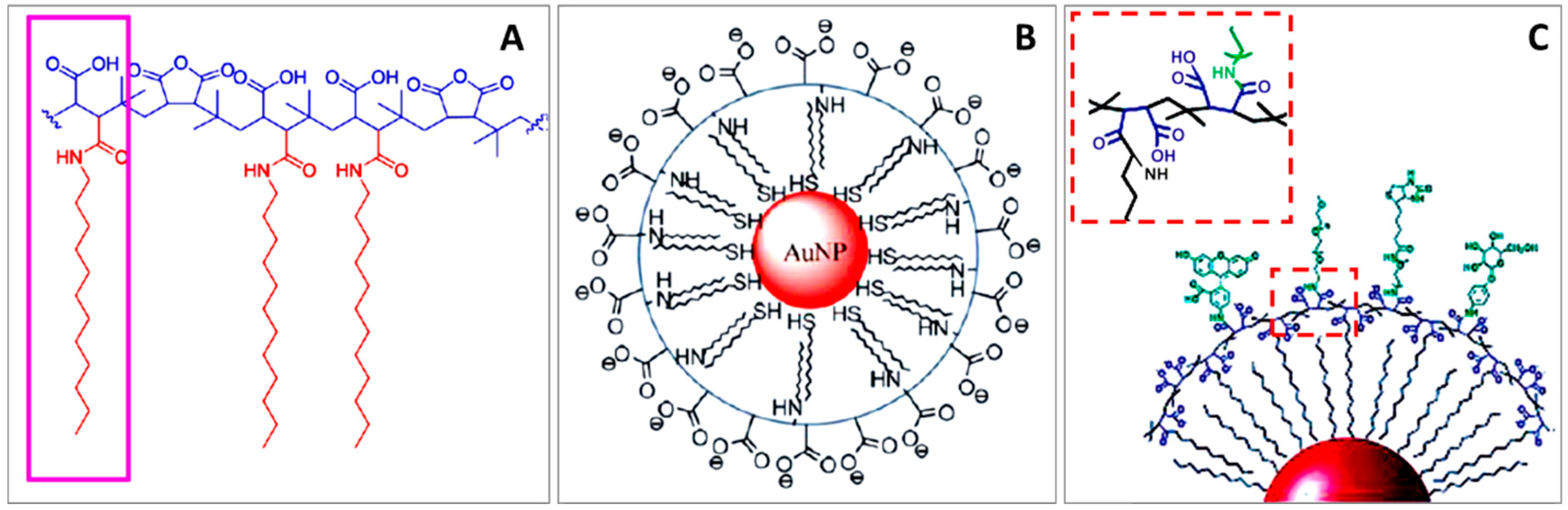
9. Mercaptoalkyl Acid Ligands
9.1. Mercaptoalkyl Acid Ligands on Plasmonic NPs
9.2. Mercaptoalkyl Acid Ligands on QDs
9.3. Mercaptoalkyl Acid Ligands on Magnetic NPs
9.4. Drawbacks of Mercaptoalkyl Acid Ligands
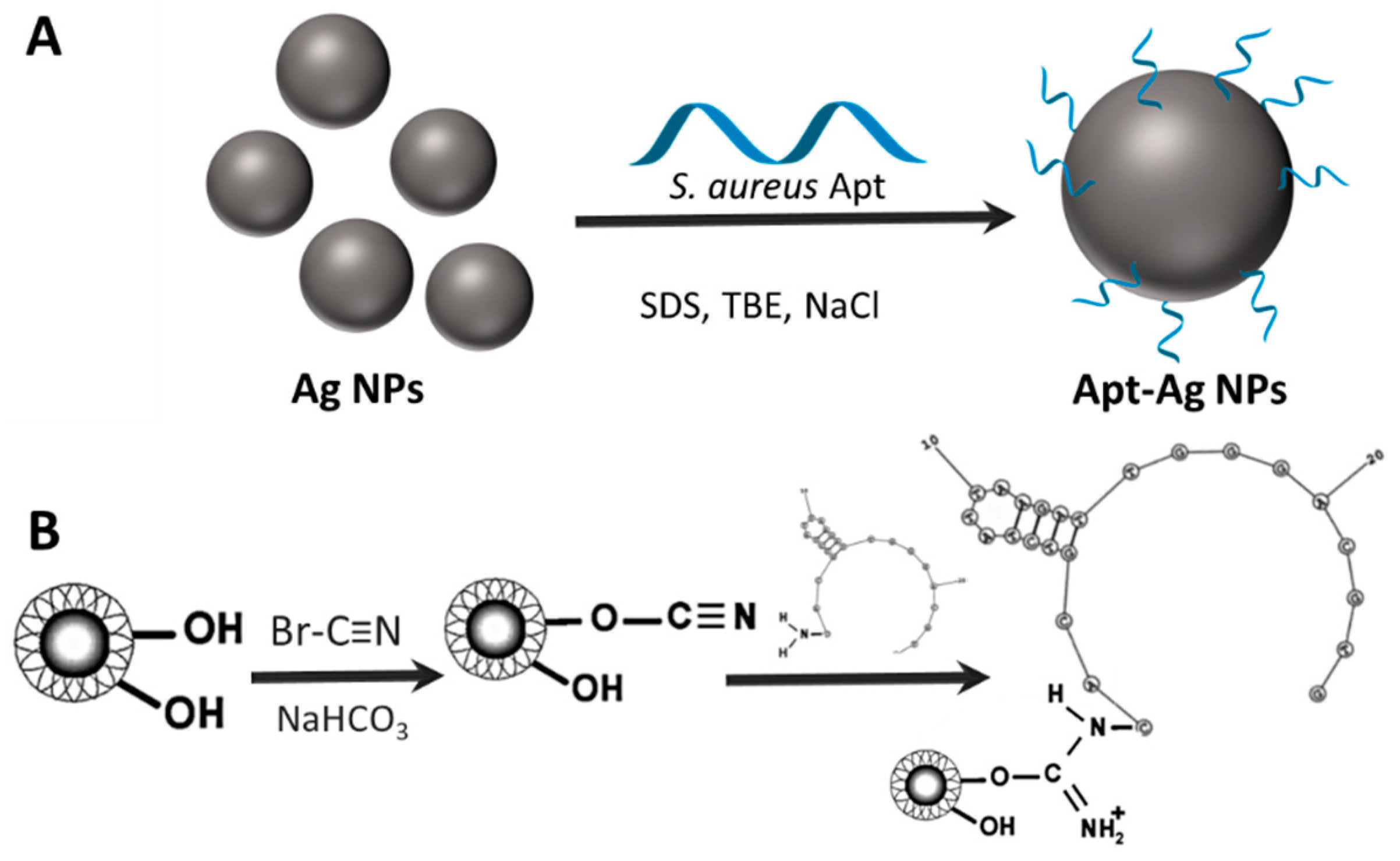
10. Aptamers
10.1. Aptamer Coating of Plasmonic NPs
10.2. Aptamer Coating of QDs
10.3. Aptamer Coating of Magnetic NPs
10.4. Drawbacks of Aptamer Coatings
11. NPs Immobilization on Colloidal Substrates
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wagner, V.; Dullaart, A.; Bock, A.K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006, 24, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomedicine 2013, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Santi, M.; Maccari, G.; Mereghetti, P.; Voliani, V.; Rocchiccioli, S.; Ucciferri, N.; Luin, S.; Signore, G. Rational design of a transferrin-binding peptide sequence tailored to targeted nanoparticle internalization. Bioconjugate Chem. 2017, 28, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Pradeep, T. Atomically precise clusters of noble metals: Emerging link between atoms and nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Li, S.; Thomas, A.; Kotov, N.A.; Haag, R. Functional graphene nanomaterials based architectures: Biointeractions, fabrications, and emerging biological applications. Chem. Rev. 2017, 117, 1826–1914. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, M.; Yoshimoto, K.; Sisido, M.; Ariga, K. Chemistry can make strict and fuzzy controls for bio-systems: DNA nanoarchitectonics and cell-macromolecular nanoarchitectonics. Bull. Chem. Soc. Jpn. 2017, 90, 967–1004. [Google Scholar] [CrossRef]
- Matsuura, K. Construction of functional biomaterials by biomolecular self-assembly. Bull. Chem. Soc. Jpn. 2017, 90, 873–884. [Google Scholar] [CrossRef]
- Yamamoto, E.; Kuroda, K. Colloidal mesoporous silica nanoparticles. Bull. Chem. Soc. Jpn. 2016, 89, 501–539. [Google Scholar] [CrossRef]
- Shirai, H.; Nguyen, M.T.; Čempel, D.; Tsukamoto, H.; Tokunaga, T.; Liao, Y.-C.; Yonezawa, T. Preparation of Au/Pd bimetallic nanoparticles by a microwave-induced plasma in liquid process. Bull. Chem. Soc. Jpn. 2017, 90, 279–285. [Google Scholar] [CrossRef]
- Koo Lee, Y.-E.; Kopelman, R. Chapter twenty-one—Nanoparticle pebble sensors in live cells. In Methods in Enzymology; Conn, P.M., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 504, pp. 419–470. [Google Scholar]
- Henriksen-Lacey, M.; Carregal-Romero, S.; Liz-Marzán, L.M. Current challenges toward in vitro cellular validation of inorganic nanoparticles. Bioconjugate Chem. 2017, 28, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Jessl, S.; Tebbe, M.; Guerrini, L.; Fery, A.; Alvarez-Puebla, R.A.; Pazos Perez, N. Silver-assisted synthesis of gold nanorods: The relation between silver additive and iodide impurities. Small 2018. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Minami, K.; Ebara, M.; Nakanishi, J. What are the emerging concepts and challenges in nano? Nanoarchitectonics, hand-operating nanotechnology and mechanobiology. Polym. J. 2016, 48, 371. [Google Scholar] [CrossRef]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. A review of clinical translation of inorganic nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Pazos-Perez, N.; Pazos, E.; Catala, C.; Mir-Simon, B.; Gómez-de Pedro, S.; Sagales, J.; Villanueva, C.; Vila, J.; Soriano, A.; García de Abajo, F.J.; et al. Ultrasensitive multiplex optical quantification of bacteria in large samples of biofluids. Sci. Rep. 2016, 6, 29014. Available online: http://www.nature.com/articles/srep29014#supplementary-information (accessed on 4 July 2018). [CrossRef] [PubMed]
- Gisbert-Quilis, P.; Masetti, M.; Morla-Folch, J.; Fitzgerald, J.M.; Pazos-Perez, N.; Garcia-Rico, E.; Giannini, V.; Alvarez-Puebla, R.A.; Guerrini, L. The structure of short and genomic DNA at the interparticle junctions of cationic nanoparticles. Adv. Mater. Interfaces 2017, 4, 1700724. [Google Scholar] [CrossRef]
- Morla-Folch, J.; Xie, H.N.; Gisbert-Quilis, P.; Gomez-de Pedro, S.; Pazos-Perez, N.; Alvarez-Puebla, R.A.; Guerrini, L. Ultrasensitive direct quantification of nucleobase modifications in DNA by surface-enhanced Raman scattering: The case of cytosine. Angew. Chem. Int. Ed. Engl. 2015, 54, 13650–13654. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Bobbio, A.; Miola, M.; Spriano, S. Micro- and nano-textured, hydrophilic and bioactive titanium dental implants. Surf. Coat. Technol. 2015, 276, 374–383. [Google Scholar] [CrossRef]
- Rausch, K.; Reuter, A.; Fischer, K.; Schmidt, M. Evaluation of nanoparticle aggregation in human blood serum. Biomacromolecules 2010, 11, 2836–2839. [Google Scholar] [CrossRef] [PubMed]
- Nichols, G.; Byard, S.; Bloxham, M.J.; Botterill, J.; Dawson, N.J.; Dennis, A.; Diart, V.; North, N.C.; Sherwood, J.D. A review of the terms agglomerate and aggregate with a recommendation for nomenclature used in powder and particle characterization. J. Pharm. Sci. 2002, 91, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.A.; Williams, D.R.M. Double layers and interparticle forces in colloid science and biology: Analytic results for the effect of ionic dispersion forces. Phys. Rev. Lett. 2004, 92, 248303. [Google Scholar] [CrossRef] [PubMed]
- Boström, M.; Williams, D.R.M.; Ninham, B.W. Specific ion effects: Why DLVO theory fails for biology and colloid systems. Phys. Rev. Lett. 2001, 87, 168103. [Google Scholar] [CrossRef] [PubMed]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R.H. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Morla-Folch, J.; Guerrini, L.; Pazos-Perez, N.; Arenal, R.; Alvarez-Puebla, R.A. Synthesis and optical properties of homogeneous nanoshurikens. ACS Photonics 2014, 1, 1237–1244. [Google Scholar] [CrossRef]
- Rahme, K.; Gauffre, F.; Marty, J.-D.; Payré, B.; Mingotaud, C. A systematic study of the stabilization in water of gold nanoparticles by poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymers. J. Phys. Chem. C 2007, 111, 7273–7279. [Google Scholar] [CrossRef]
- Rahme, K.; Vicendo, P.; Ayela, C.; Gaillard, C.; Payré, B.; Mingotaud, C.; Gauffre, F. A simple protocol to stabilize gold nanoparticles using amphiphilic block copolymers: Stability studies and viable cellular uptake. Chem. Eur. J. 2009, 15, 11151–11159. [Google Scholar] [CrossRef] [PubMed]
- Carril, M.; Padro, D.; Del Pino, P.; Carrillo-Carrion, C.; Gallego, M.; Parak, W.J. In situ detection of the protein corona in complex environments. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.D.; Kreyling, W.G.; Pfeiffer, C.; Schäffler, M.; Sarioglu, H.; Ristig, S.; Hirn, S.; Haberl, N.; Thalhammer, S.; Hauck, S.M.; et al. Colloidal stability and surface chemistry are key factors for the composition of the protein corona of inorganic gold nanoparticles. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Carrillo-Carrion, C.; Carril, M.; Parak, W.J. Techniques for the experimental investigation of the protein corona. Curr. Opin. Biotechnol. 2017, 46, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Pino, P.D.; Pelaz, B.; Zhang, Q.; Maffre, P.; Nienhaus, G.U.; Parak, W.J. Protein corona formation around nanoparticles—From the past to the future. Mater. Horiz. 2014, 1, 301–313. [Google Scholar] [CrossRef]
- Feliu, N.; Docter, D.; Heine, M.; del Pino, P.; Ashraf, S.; Kolosnjaj-Tabi, J.; Macchiarini, P.; Nielsen, P.; Alloyeau, D.; Gazeau, F.; et al. In vivo degeneration and the fate of inorganic nanoparticles. Chem. Soc. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Åberg, C.; Mahon, E.; Dawson, K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013, 8, 137. Available online: https://www.nature.com/articles/nnano.2012.237#supplementary-information (accessed on 4 July 2018). [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Hafner, J.H. Gold nanorod bioconjugates. Chem. Mater. 2005, 17, 4636–4641. [Google Scholar] [CrossRef]
- Rahme, K.; Nolan, M.T.; Doody, T.; McGlacken, G.P.; Morris, M.A.; O’Driscoll, C.; Holmes, J.D. Highly stable pegylated gold nanoparticles in water: Applications in biology and catalysis. RSC Adv. 2013, 3, 21016–21024. [Google Scholar] [CrossRef]
- Xie, J.; Xu, C.; Kohler, N.; Hou, Y.; Sun, S. Controlled pegylation of monodisperse fe3o4 nanoparticles for reduced non-specific uptake by macrophage cells. Adv. Mater. 2007, 19, 3163–3166. [Google Scholar] [CrossRef]
- Duan, H.; Nie, S. Cell-penetrating quantum dots based on multivalent and endosome-disrupting surface coatings. J. Am. Chem. Soc. 2007, 129, 3333–3338. [Google Scholar] [CrossRef] [PubMed]
- Voliani, V.; Luin, S.; Ricci, F.; Beltram, F. Single-step bifunctional coating for selectively conjugable nanoparticles. Nanoscale 2010, 2, 2783–2789. [Google Scholar] [CrossRef] [PubMed]
- Holmlin, R.E.; Chen, X.; Chapman, R.G.; Takayama, S.; Whitesides, G.M. Zwitterionic SAMS that resist nonspecific adsorption of protein from aqueous buffer. Langmuir 2001, 17, 2841–2850. [Google Scholar] [CrossRef]
- Pillai, P.P.; Huda, S.; Kowalczyk, B.; Grzybowski, B.A. Controlled pH stability and adjustable cellular uptake of mixed-charge nanoparticles. J. Am. Chem. Soc. 2013, 135, 6392–6395. [Google Scholar] [CrossRef] [PubMed]
- Chairam, S.; Somsook, E. Starch vermicelli template for synthesis of magnetic iron oxide nanoclusters. J. Magn. Magn. Mater. 2008, 320, 2039–2043. [Google Scholar] [CrossRef]
- Berry, C.C.; Wells, S.; Charles, S.; Curtis, A.S.G. Dextran and albumin derivatised iron oxide nanoparticles: Influence on fibroblasts in vitro. Biomaterials 2003, 24, 4551–4557. [Google Scholar] [CrossRef]
- Park, J.-H.; Im, K.-H.; Lee, S.-H.; Kim, D.-H.; Lee, D.-Y.; Lee, Y.-K.; Kim, K.-M.; Kim, K.-N. Preparation and characterization of magnetic chitosan particles for hyperthermia application. J. Magn. Magn. Mater. 2005, 293, 328–333. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Z.; Lu, W.; Zhang, R.; Huang, Q.; Tian, M.; Li, L.; Liang, D.; Li, C. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials 2009, 30, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shipton, M.K.; Ryan, J.; Kaufman, E.D.; Franzen, S.; Feldheim, D.L. Synthesis, stability, and cellular internalization of gold nanoparticles containing mixed peptide–poly(ethylene glycol) monolayers. Anal. Chem. 2007, 79, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Niidome, T.; Yamagata, M.; Okamoto, Y.; Akiyama, Y.; Takahashi, H.; Kawano, T.; Katayama, Y.; Niidome, Y. PEG-modified gold nanorods with a stealth character for in vivo applications. J. Control. Release 2006, 114, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Boca, S.C.; Astilean, S. Detoxification of gold nanorods by conjugation with thiolated poly(ethylene glycol) and their assessment as sers-active carriers of Raman tags. Nanotechnology 2010, 21, 235601. [Google Scholar] [CrossRef] [PubMed]
- Kinnear, C.; Dietsch, H.; Clift, M.J.D.; Endes, C.; Rothen-Rutishauser, B.; Petri-Fink, A. Gold nanorods: Controlling their surface chemistry and complete detoxification by a two-step place exchange. Angew. Chem. Int. Ed. 2013, 52, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, M. Fast loading of PEG-SH on CTAB-protected gold nanorods. RSC Adv. 2014, 4, 17760–17767. [Google Scholar] [CrossRef]
- Thierry, B.; Ng, J.; Krieg, T.; Griesser, H.J. A robust procedure for the functionalization of gold nanorods and noble metal nanoparticles. Chem. Commun. 2009, 1724–1726. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.S.; Lee, N.; Kim, T.; Kim, H.; Yu, T.; Song, I.C.; Moon, W.K.; Hyeon, T. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew. Chem. Int. Ed. 2008, 47, 8438–8441. [Google Scholar] [CrossRef] [PubMed]
- Sakura, T.; Takahashi, T.; Kataoka, K.; Nagasaki, Y. One-pot preparation of mono-dispersed and physiologically stabilized gold colloid. Colloid Polym. Sci. 2005, 284, 97–101. [Google Scholar] [CrossRef]
- Dubertret, B.; Skourides, P.; Norris, D.J.; Noireaux, V.; Brivanlou, A.H.; Libchaber, A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 2002, 298, 1759–1762. [Google Scholar] [CrossRef] [PubMed]
- García, K.P.; Zarschler, K.; Barbaro, L.; Barreto, J.A.; O’Malley, W.; Spiccia, L.; Stephan, H.; Graham, B. Zwitterionic-coated “stealth” nanoparticles for biomedical applications: Recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small 2014, 10, 2516–2529. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; del Pino, P.; Maffre, P.; Hartmann, R.; Gallego, M.; Rivera-Fernández, S.; de la Fuente, J.M.; Nienhaus, G.U.; Parak, W.J. Surface functionalization of nanoparticles with polyethylene glycol: Effects on protein adsorption and cellular uptake. ACS Nano 2015, 9, 6996–7008. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Ipe, B.I.; Misra, P.; Lee, J.H.; Bawendi, M.G.; Frangioni, J.V. Tissue- and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett. 2009, 9, 2354–2359. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Hühn, J.; Carrillo-Carrion, C.; Soliman, M.G.; Pfeiffer, C.; Valdeperez, D.; Masood, A.; Chakraborty, I.; Zhu, L.; Gallego, M.; Yue, Z.; et al. Selected standard protocols for the synthesis, phase transfer, and characterization of inorganic colloidal nanoparticles. Chem. Mater. 2017, 29, 399–461. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Green, L.A.W. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Rahme, K.; Chen, L.; Hobbs, R.G.; Morris, M.A.; O’Driscoll, C.; Holmes, J.D. Pegylated gold nanoparticles: Polymer quantification as a function of PEG lengths and nanoparticle dimensions. RSC Adv. 2013, 3, 6085–6094. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, Y.; Lu, X.; Thai, T.; Lee, N.A.; Bach, U.; Gooding, J.J. Biocompatible gold nanorods: One-step surface functionalization, highly colloidal stability, and low cytotoxicity. Langmuir 2015, 31, 4973–4980. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Nakshatri, H.; Irudayaraj, J. Identity profiling of cell surface markers by multiplex gold nanorod probes. Nano Lett. 2007, 7, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. Pegylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 2003, 55, 403–419. [Google Scholar] [CrossRef]
- Rubio-Garcia, J.; Coppel, Y.; Lecante, P.; Mingotaud, C.; Chaudret, B.; Gauffre, F.; Kahn, M.L. One-step synthesis of metallic and metal oxide nanoparticles using amino-PEG oligomers as multi-purpose ligands: Size and shape control, and quasi-universal solvent dispersibility. Chem. Commun. 2011, 47, 988–990. [Google Scholar] [CrossRef] [PubMed]
- Kohler, N.; Fryxell, G.E.; Zhang, M. A bifunctional poly(ethylene glycol) silane immobilized on metallic oxide-based nanoparticles for conjugation with cell targeting agents. J. Am. Chem. Soc. 2004, 126, 7206–7211. [Google Scholar] [CrossRef] [PubMed]
- Thiry, M.; Boldt, K.; Nikolic, M.S.; Schulz, F.; Ijeh, M.; Panicker, A.; Vossmeyer, T.; Weller, H. Fluorescence properties of hydrophilic semiconductor nanoparticles with tridentate polyethylene oxide ligands. ACS Nano 2011, 5, 4965–4973. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Ying, L.; Hong, X.; Hall, E.A.; Abell, C.; Klenerman, D. A compact functional quantum dot–DNA conjugate: Preparation, hybridization, and specific label-free DNA detection. Langmuir 2008, 24, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Tyrakowski, C.M.; Snee, P.T. A primer on the synthesis, water-solubilization, and functionalization of quantum dots, their use as biological sensing agents, and present status. Phys. Chem. Chem. Phys. 2014, 16, 837–855. [Google Scholar] [CrossRef] [PubMed]
- Susumu, K.; Uyeda, H.T.; Medintz, I.L.; Pons, T.; Delehanty, J.B.; Mattoussi, H. Enhancing the stability and biological functionalities of quantum dots via compact multifunctional ligands. J. Am. Chem. Soc. 2007, 129, 13987–13996. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, M.; Jonczyk, R.; Walter, J.-G.; Springer, S.; Lavrentieva, A.; Stahl, F.; Green, M.; Scheper, T. Aqueous synthesis of pegylated quantum dots with increased colloidal stability and reduced cytotoxicity. Bioconjugate Chem. 2016, 27, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Patri, A.K.; Zheng, J.; Clogston, J.D.; Ayub, N.; Aggarwal, P.; Neun, B.W.; Hall, J.B.; McNeil, S.E. Interaction of colloidal gold nanoparticles with human blood: Effects on particle size and analysis of plasma protein binding profiles. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.D.; Olsen, J.B.; Guo, H.; Emili, A.; Chan, W.C.W. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Unsworth, L.D.; Sheardown, H.; Brash, J.L. Protein-resistant poly(ethylene oxide)-grafted surfaces: Chain density-dependent multiple mechanisms of action. Langmuir 2008, 24, 1924–1929. [Google Scholar] [CrossRef] [PubMed]
- Szleifer, I. Protein adsorption on surfaces with grafted polymers: A theoretical approach. Biophys. J. 1997, 72, 595–612. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle pegylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Uehara, Y.; Moriyoshi, N.; Ishida, T.; Kiwada, H. Anti-PEG IGM production by siRNA encapsulated in a pegylated lipid nanocarrier is dependent on the sequence of the siRNA. J. Control. Release 2011, 151, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Van Ghelue, M.; Ribeiro, A.; Solheim, B.; Akkermans, A.D.; Bisseling, T.; Pawlowski, K. Sucrose synthase and enolase expression in actinorhizal nodules of alnus glutinosa: Comparison with legume nodules. Mol. Gen. Genet. 1996, 250, 437–446. [Google Scholar] [PubMed]
- Andersen, A.J.; Robinson, J.T.; Dai, H.; Hunter, A.C.; Andresen, T.L.; Moghimi, S.M. Single-walled carbon nanotube surface control of complement recognition and activation. ACS Nano 2013, 7, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Hamad, I.; Al-Hanbali, O.; Hunter, A.C.; Rutt, K.J.; Andresen, T.L.; Moghimi, S.M. Distinct polymer architecture mediates switching of complement activation pathways at the nanosphere-serum interface: Implications for stealth nanoparticle engineering. ACS Nano 2010, 4, 6629–6638. [Google Scholar] [CrossRef] [PubMed]
- Tonga, G.Y.; Saha, K.; Rotello, V.M. 25th anniversary article: Interfacing nanoparticles and biology: New strategies for biomedicine. Adv. Mater. 2014, 26, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Y.; Li, H.; Huang, N.; Jin, Q.; Ren, K.; Ji, J. Enhanced retention and cellular uptake of nanoparticles in tumors by controlling their aggregation behavior. ACS Nano 2013, 7, 6244–6257. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.C.; Zhang, Q.; Xia, Y. The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nat. Nanotechnol. 2011, 6, 385. Available online: https://www.nature.com/articles/nnano.2011.58#supplementary-information (accessed on 4 July 2018). [CrossRef] [PubMed]
- Rascol, E.; Daurat, M.; Da Silva, A.; Maynadier, M.; Dorandeu, C.; Charnay, C.; Garcia, M.; Lai-Kee-Him, J.; Bron, P.; Auffan, M.; et al. Biological fate of Fe3O4 core-shell mesoporous silica nanoparticles depending on particle surface chemistry. Nanomaterials 2017, 7, 162. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse applications of nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zhang, Z.; Chen, S.; Bryers, J.D.; Jiang, S. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 2007, 28, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, H.; Cao, Z.; Keefe, A.; Wang, J.; Jiang, S. Multifunctional and degradable zwitterionic nanogels for targeted delivery, enhanced MR imaging, reduction-sensitive drug release, and renal clearance. Biomaterials 2011, 32, 4604–4608. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Miranda, O.R.; Moyano, D.F.; Walden, C.A.; Giri, K.; Bhattacharya, R.; Robertson, J.D.; Rotello, V.M.; Reid, J.M.; Mukherjee, P. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PLoS ONE 2011, 6, e24374. [Google Scholar] [CrossRef] [PubMed]
- Moyano, D.F.; Saha, K.; Prakash, G.; Yan, B.; Kong, H.; Yazdani, M.; Rotello, V.M. Fabrication of corona-free nanoparticles with tunable hydrophobicity. ACS Nano 2014, 8, 6748–6755. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Moyano, D.F.; Parnsubsakul, A.; Papadopoulos, A.; Wang, L.-S.; Landis, R.F.; Das, R.; Rotello, V.M. Ultrastable and biofunctionalizable gold nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 14096–14101. [Google Scholar] [CrossRef] [PubMed]
- Breus, V.V.; Heyes, C.D.; Tron, K.; Nienhaus, G.U. Zwitterionic biocompatible quantum dots for wide pH stability and weak nonspecific binding to cells. ACS Nano 2009, 3, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Muro, E.; Pons, T.; Lequeux, N.; Fragola, A.; Sanson, N.; Lenkei, Z.; Dubertret, B. Small and stable sulfobetaine zwitterionic quantum dots for functional live-cell imaging. J. Am. Chem. Soc. 2010, 132, 4556–4557. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, L.; Wang, S.; White, A.D.; Jiang, S. Functionalizable and ultra stable nanoparticles coated with zwitterionic poly(carboxybetaine) in undiluted blood serum. Biomaterials 2009, 30, 5617–5621. [Google Scholar] [CrossRef] [PubMed]
- Zhan, N.; Palui, G.; Grise, H.; Tang, H.; Alabugin, I.; Mattoussi, H. Combining ligand design with photoligation to provide compact, colloidally stable, and easy to conjugate quantum dots. ACS Appl. Mater. Interfaces 2013, 5, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Agasti, S.S.; Chompoosor, A.; You, C.C.; Ghosh, P.; Kim, C.K.; Rotello, V.M. Photoregulated release of caged anticancer drugs from gold nanoparticles. J. Am. Chem. Soc. 2009, 131, 5728–5729. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Martin, J.D.; Lee, J.; Harris, D.K.; Fukumura, D.; Jain, R.K.; Bawendi, M. Spatial charge configuration regulates nanoparticle transport and binding behavior in vivo. Angew. Chem. Int. Ed. 2013, 52, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Ghosh, P.; Pagliuca, C.; Zhu, Z.-J.; Menichetti, S.; Rotello, V.M. Entrapment of hydrophobic drugs in nanoparticle monolayers with efficient release into cancer cells. J. Am. Chem. Soc. 2009, 131, 1360–1361. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Choi, H.S.; Zimmer, J.P.; Tanaka, E.; Frangioni, J.V.; Bawendi, M. Compact cysteine-coated CdSe(ZnCdS) quantum dots for in vivo applications. J. Am. Chem. Soc. 2007, 129, 14530–14531. [Google Scholar] [CrossRef] [PubMed]
- Gaponik, N.; Talapin, D.V.; Rogach, A.L.; Hoppe, K.; Shevchenko, E.V.; Kornowski, A.; Eychmüller, A.; Weller, H. Thiol-capping of CdTe nanocrystals: An alternative to organometallic synthetic routes. J. Phys. Chem. B 2002, 106, 7177–7185. [Google Scholar] [CrossRef]
- Vinayaka, A.C.; Thakur, M.S. Photoabsorption and resonance energy transfer phenomenon in CdTe–protein bioconjugates: An insight into QD–biomolecular interactions. Bioconjugate Chem. 2011, 22, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Haifeng, B.; Erkang, W.; Shaojun, D. One-pot synthesis of CdTe nanocrystals and shape control of luminescent CdTe–cystine nanocomposites. Small 2006, 2, 476–480. [Google Scholar] [CrossRef]
- Talapin, D.V.; Rogach, A.L.; Shevchenko, E.V.; Kornowski, A.; Haase, M.; Weller, H. Dynamic distribution of growth rates within the ensembles of colloidal II–VI and III–V semiconductor nanocrystals as a factor governing their photoluminescence efficiency. J. Am. Chem. Soc. 2002, 124, 5782–5790. [Google Scholar] [CrossRef] [PubMed]
- Mamedova, N.N.; Kotov, N.A.; Rogach, A.L.; Studer, J. Albumin–CdTe nanoparticle bioconjugates: Preparation, structure, and interunit energy transfer with antenna effect. Nano Lett. 2001, 1, 281–286. [Google Scholar] [CrossRef]
- Wei, H.; Insin, N.; Lee, J.; Han, H.-S.; Cordero, J.M.; Liu, W.; Bawendi, M.G. Compact zwitterion-coated iron oxide nanoparticles for biological applications. Nano Lett. 2012, 12, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.Y.; Mao, C.Q.; Du, X.J.; Du, J.Z.; Wang, F.; Wang, J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv. Mater. 2012, 24, 5476–5480. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, V.; Wan, C.; Tamm, L.K. Domain coupling in asymmetric lipid bilayers. Biochim. Biophys. Acta 2009, 1788, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Mashaghi, S.; Jadidi, T.; Koenderink, G.; Mashaghi, A. Lipid nanotechnology. Int. J. Mol. Sci. 2013, 14, 4242–4282. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, D.; Caracciolo, G.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Anchordoquy, T.J.; Lagana, A. Surface chemistry and serum type both determine the nanoparticle-protein corona. J. Proteom. 2015, 119, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, D.; Mote, K.R.; MacLaughlin, C.M.; Biswas, N.; Chandra, B.; Basu, J.K.; Walker, G.C.; Madhu, P.K.; Maiti, S. Cell-membrane-mimicking lipid-coated nanoparticles confer Raman enhancement to membrane proteins and reveal membrane-attached amyloid-β conformation. ACS Nano 2015, 9, 9070–9077. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.; MacLaughlin, C.M.; Gunari, N.; Walker, G.C. Phospholipid membrane encapsulation of nanoparticles for surface-enhanced Raman scattering. Langmuir 2011, 27, 7024–7033. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Liu, Y.; West, A.; Schuler, E.E.; Yehl, K.; Dyer, R.B.; Kindt, J.T.; Salaita, K. Quantum dots encapsulated within phospholipid membranes: Phase-dependent structure, photostability, and site-selective functionalization. J. Am. Chem. Soc. 2014, 136, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Nyalosaso, J.L.; Rascol, E.; Pisani, C.; Dorandeu, C.; Dumail, X.; Maynadier, M.; Gary-Bobo, M.; Kee Him, J.L.; Bron, P.; Garcia, M.; et al. Synthesis, decoration, and cellular effects of magnetic mesoporous silica nanoparticles. RSC Adv. 2016, 6, 57275–57283. [Google Scholar] [CrossRef]
- Wang, M.; Petersen, N.O. Characterization of phospholipid-encapsulated gold nanoparticles: A versatile platform to study drug delivery and cellular uptake mechanisms. Can. J. Chem. 2014, 93, 265–271. [Google Scholar] [CrossRef]
- Albanese, A.; Chan, W.C.W. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano 2011, 5, 5478–5489. [Google Scholar] [CrossRef] [PubMed]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time evolution of the nanoparticle protein corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Walczyk, D.; Bombelli, F.B.; Monopoli, M.P.; Lynch, I.; Dawson, K.A. What the cell “sees” in bionanoscience. J. Am. Chem. Soc. 2010, 132, 5761–5768. [Google Scholar] [CrossRef] [PubMed]
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.E.; Salvatore, F.; Tasciotti, E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine 2016, 11, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Karolin, J.; Johansson, L.B.A.; Strandberg, L.; Ny, T. Fluorescence and absorption spectroscopic properties of dipyrrometheneboron difluoride (bodipy) derivatives in liquids, lipid membranes, and proteins. J. Am. Chem. Soc. 1994, 116, 7801–7806. [Google Scholar] [CrossRef]
- Rosen, B.P. Bacterial resistance to heavy metals and metalloids. JBIC J. Biol. Inorg. Chem. 1996, 1, 273–277. [Google Scholar] [CrossRef]
- Brewer, S.H.; Glomm, W.R.; Johnson, M.C.; Knag, M.K.; Franzen, S. Probing BSA binding to citrate-coated gold nanoparticles and surfaces. Langmuir 2005, 21, 9303–9307. [Google Scholar] [CrossRef] [PubMed]
- Tebbe, M.; Kuttner, C.; Männel, M.; Fery, A.; Chanana, M. Colloidally stable and surfactant-free protein-coated gold nanorods in biological media. ACS Appl. Mater. Interfaces 2015, 7, 5984–5991. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, M.; Sousa, F.; Wenk, A.; Sitia, L.; Hirn, S.; Schleh, C.; Haberl, N.; Violatto, M.; Canovi, M.; Andreozzi, P.; et al. Blood protein coating of gold nanoparticles as potential tool for organ targeting. Biomaterials 2014, 35, 3455–3466. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Baldelli Bombelli, F.; Dawson, K.A. Physical–chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Chanana, M.; Rivera_Gil, P.; Correa-Duarte, M.A.; Liz-Marzán, L.M.; Parak, W.J. Physicochemical properties of protein-coated gold nanoparticles in biological fluids and cells before and after proteolytic digestion. Angew. Chem. Int. Ed. 2013, 52, 4179–4183. [Google Scholar] [CrossRef] [PubMed]
- Chanana, M.; Correa-Duarte, M.A.; Liz-Marzán, L.M. Insulin-coated gold nanoparticles: A plasmonic device for studying metal–protein interactions. Small 2011, 7, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Strozyk, M.S.; Chanana, M.; Pastoriza-Santos, I.; Pérez-Juste, J.; Liz-Marzán, L.M. Protein/polymer-based dual-responsive gold nanoparticles with pH-dependent thermal sensitivity. Adv. Funct. Mater. 2012, 22, 1436–1444. [Google Scholar] [CrossRef]
- Wang, J.; Yue, Y.; Chen, G.; Xia, J. Protease-promoted drug delivery using peptide-functionalized gold nanoparticles. Soft Matter 2011, 7, 7217–7222. [Google Scholar] [CrossRef]
- He, X.; Gao, L.; Ma, N. One-step instant synthesis of protein-conjugated quantum dots at room temperature. Sci. Rep. 2013, 3, 2825. Available online: https://www.nature.com/articles/srep02825#supplementary-information (accessed on 4 July 2018). [CrossRef] [PubMed]
- Bychkova, A.V.; Sorokina, O.N.; Pronkin, P.G.; Tatikolov, A.S.; Kovarski, A.L.; Rosenfeld, M.A. Protein-Coated Magnetic Nanoparticles: Creation and Investigation. Ph.D. Thesis, Sumy State University, Sumy, Ukraine, 2013. [Google Scholar]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Freeze, H.H.; Stanley, P.; Bertozzi, C.R.; Hart, G.W.; Etzler, M.E. (Eds.) Essentials of Glycobiology; Cold Spring Harbor: Laurel Hollow, NY, USA, 2009. [Google Scholar]
- Marradi, M.; Chiodo, F.; Garcia, I.; Penades, S. Glyconanoparticles as multifunctional and multimodal carbohydrate systems. Chem. Soc. Rev. 2013, 42, 4728–4745. [Google Scholar] [CrossRef] [PubMed]
- Fuente, J.M.D.L.; Barrientos, A.G.; Rojas, T.C.; Rojo, J.; Cañada, J.; Fernández, A.; Penadés, S. Gold glyconanoparticles as water-soluble polyvalent models to study carbohydrate interactions. Angew. Chem. Int. Ed. 2001, 40, 2257–2261. [Google Scholar] [CrossRef]
- García, I.; Sánchez-Iglesias, A.; Henriksen-Lacey, M.; Grzelczak, M.; Penadés, S.; Liz-Marzán, L.M. Glycans as biofunctional ligands for gold nanorods: Stability and targeting in protein-rich media. J. Am. Chem. Soc. 2015, 137, 3686–3692. [Google Scholar] [CrossRef] [PubMed]
- Kikkeri, R.; Laurino, P.; Odedra, A.; Seeberger, P.H. Synthesis of carbohydrate-functionalized quantum dots in microreactors. Angew. Chem. Int. Ed. 2010, 49, 2054–2057. [Google Scholar] [CrossRef] [PubMed]
- Ninjbadgar, T.; Brougham, D.F. Epoxy ring opening phase transfer as a general route to water dispersible superparamagnetic Fe3O4 nanoparticles and their application as positive MRI contrast agents. Adv. Funct. Mater. 2011, 21, 4769–4775. [Google Scholar] [CrossRef]
- Borase, T.; Ninjbadgar, T.; Kapetanakis, A.; Roche, S.; O’Connor, R.; Kerskens, C.; Heise, A.; Brougham, D.F. Stable aqueous dispersions of glycopeptide-grafted selectably functionalized magnetic nanoparticles. Angew. Chem. Int. Ed. 2013, 52, 3164–3167. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.A.; Sperling, R.A.; Li, J.K.; Yang, T.Y.; Li, P.Y.; Zanella, M.; Chang, W.H.; Parak, W.J. Design of an amphiphilic polymer for nanoparticle coating and functionalization. Small 2008, 4, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Hühn, D.; Kantner, K.; Geidel, C.; Brandholt, S.; De Cock, I.; Soenen, S.J.H.; Rivera_Gil, P.; Montenegro, J.-M.; Braeckmans, K.; Müllen, K.; et al. Polymer-coated nanoparticles interacting with proteins and cells: Focusing on the sign of the net charge. ACS Nano 2013, 7, 3253–3263. [Google Scholar] [CrossRef] [PubMed]
- Jimenez de Aberasturi, D.; Serrano-Montes, A.B.; Langer, J.; Henriksen-Lacey, M.; Parak, W.J.; Liz-Marzán, L.M. Surface enhanced Raman scattering encoded gold nanostars for multiplexed cell discrimination. Chem. Mater. 2016, 28, 6779–6790. [Google Scholar] [CrossRef]
- Zhang, F.; Lees, E.; Amin, F.; Rivera_Gil, P.; Yang, F.; Mulvaney, P.; Parak, W.J. Polymer-coated nanoparticles: A universal tool for biolabelling experiments. Small 2011, 7, 3113–3127. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Jimenez de Aberasturi, D.; Pazos-Perez, N.; Guerrini, L.; Masood, A.; Alvarez-Puebla Ramon, A.; Feliu, N.; Parak Wolfgang, J. Ion-selective ligands: How colloidal nano- and micro-particles can introduce new functionalities. Z. Phys. Chem. 2018. [Google Scholar] [CrossRef]
- Mir-Simon, B.; Reche-Perez, I.; Guerrini, L.; Pazos-Perez, N.; Alvarez-Puebla, R.A. Universal one-pot and scalable synthesis of SERS encoded nanoparticles. Chem. Mater. 2015, 27, 950–958. [Google Scholar] [CrossRef]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [PubMed]
- Mattoussi, H.; Mauro, J.M.; Goldman, E.R.; Anderson, G.P.; Sundar, V.C.; Mikulec, F.V.; Bawendi, M.G. Self-assembly of CdSe–ZnS quantum dot bioconjugates using an engineered recombinant protein. J. Am. Chem. Soc. 2000, 122, 12142–12150. [Google Scholar] [CrossRef]
- Aldana, J.; Wang, Y.A.; Peng, X. Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. J. Am. Chem. Soc. 2001, 123, 8844–8850. [Google Scholar] [CrossRef] [PubMed]
- Breus, V.V.; Heyes, C.D.; Nienhaus, G.U. Quenching of CdSe–ZnS core–shell quantum dot luminescence by water-soluble thiolated ligands. J. Phys. Chem. C 2007, 111, 18589–18594. [Google Scholar] [CrossRef]
- De Villiers, C.A.; Lapsley, M.C.; Hall, E.A.H. A step towards mobile arsenic measurement for surface waters. Analyst 2015, 140, 2644–2655. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Thode, C.J.; Mabry, J.K.; Harrell, J.W.; Nikles, D.E.; Sun, K.; Wang, L.M. Self-assembly of magnetic biofunctional nanoparticles. J. Appl. Phys. 2005, 97, 10Q901. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Liu, L.; Tian, L.; He, N. An integrated and sensitive detection platform for biosensing application based on Fe@Au magnetic nanoparticles as bead array carries. Biosens. Bioelectron. 2010, 26, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat. Rev. Drug Discov. 2014, 13, 813. [Google Scholar] [CrossRef] [PubMed]
- Koren, E.; Apte, A.; Jani, A.; Torchilin, V.P. Multifunctional pegylated 2C5-immunoliposomes containing pH-sensitive bonds and tat peptide for enhanced tumor cell internalization and cytotoxicity. J. Control. Release 2012, 160, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537. [Google Scholar] [CrossRef] [PubMed]
- Catala, C.; Mir-Simon, B.; Feng, X.; Cardozo, C.; Pazos-Perez, N.; Pazos, E.; Pedro, S.G.D.; Guerrini, L.; Soriano, A.; Vila, J.; et al. Online SERS quantification of staphylococcus aureus and the application to diagnostics in human fluids. Adv. Mater. Technol. 2016, 1, 1600163. [Google Scholar] [CrossRef]
- Zhou, D.; Piper, J.D.; Abell, C.; Klenerman, D.; Kang, D.J.; Ying, L. Fluorescence resonance energy transfer between a quantum dot donor and a dye acceptor attached to DNA. Chem. Commun. 2005, 4807–4809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Y.; Johnson, L.W. Single quantum-dot-based aptameric nanosensor for cocaine. Anal. Chem. 2009, 81, 3051–3055. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.G.; Nagaoka, Y.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Aptamer conjugated magnetic nanoparticles as nanosurgeons. Nanotechnology 2010, 21, 455102. [Google Scholar] [CrossRef] [PubMed]
- Delaviz, N.; Gill, P.; Ajami, A.; Aarabi, M. Aptamer-conjugated magnetic nanoparticles for the efficient removal of HCV particles from human plasma samples. RSC Adv. 2015, 5, 79433–79439. [Google Scholar] [CrossRef]
- Pazos, E.; Garcia-Algar, M.; Penas, C.; Nazarenus, M.; Torruella, A.; Pazos-Perez, N.; Guerrini, L.; Vázquez, M.E.; Garcia-Rico, E.; Mascareñas, J.L.; et al. Surface-enhanced Raman scattering surface selection rules for the proteomic liquid biopsy in real samples: Efficient detection of the oncoprotein C-MYC. J. Am. Chem. Soc. 2016, 138, 14206–14209. [Google Scholar] [CrossRef] [PubMed]
- Pazos-Perez, N.; Borke, T.; Andreeva, D.V.; Alvarez-Puebla, R.A. Silver coated aluminium microrods as highly colloidal stable SERS platforms. Nanoscale 2011, 3, 3265–3268. [Google Scholar] [CrossRef] [PubMed]


| PEG-SH (Mw) | DLS (v)/PEG Layer (nm) | Weight Loss (%) T > 320 °C | NPEG per 15 nm AuNP | Footprint (nm2) | Grafting Density (nm2) |
|---|---|---|---|---|---|
| 2100 | 2.83 ± 0.66 | 6.7 | 695 ± 87 | 0.25 | 3.93 |
| 5400 | 7.79 ± 1.0 | 9.9 | 424 ± 53 | 0.42 | 2.4 |
| 10,800 | 12.77 ± 1.5 | 12 | 278 ± 42 | 0.63 | 1.57 |
| 19,500 | 21.61 ± 2.5 | 10.82 | 123 ± 16.5 | 1.33 | 0.75 |
| 29,500 | 25.6 ± 3.0 | 10 | 81 ± 10 | 2.18 | 0.46 |
| 51,400 | 37.15 ± 4.0 | 10.85 | 50 ± 6 | 3.15 | 0.32 |
| Diameter (nm)/EM | Diameter (nm)/DLS | Weight Loss (%) T > 320 °C | NPEG per AuNP | Footprint (nm2) | Grafting Density (nm2) |
|---|---|---|---|---|---|
| 15 ± 1.8 | 59 ± 3.5 | 14.25 | 278 ± 42 | 0.63 | 1.57 |
| 30 ± 3.5 | 72 ± 5 | 5.7 | 916 ± 106 | 0.78 | 1.29 |
| 62.5 ± 6 | 102 ± 9 | 1.64 | 2572 ± 402 | 1.25 | 0.8 |
| 93 ± 12 | 138 ± 10 | 1.41 | 6778 ± 814 | 1.05 | 0.96 |
| 115 ± 10 | 165 ± 14 | 1.45 | 12,960 ± 1227 | 0.8 | 1.25 |
| Material | |||
|---|---|---|---|
| Stabilizing Molecule | Plasmonic Particles | Magnetic Particles | Quantum Dots |
| PEG | SH-PEG | Hydroxyl-PEG (dopamine-PEG) | PEI-PEG |
| SH-PEG | |||
| Zwitterionic ligands | SH-zwitterionic | Dopamine-zwitterionic | SH-zwitterionic |
| Lipid bilayers | DOPC/ESM/Chol | DMPC | POPC/POPG |
| Protein coatings | Serum Albumin Insulin Lactoglobulin Ovalbumin | Serum Albumin Thrombin | Serum Albumin Lysozyme Trypsin Hemoglobin Transferrin |
| Glycans | SH-glycoconjugates | Azide-Galactose | Thioethoxy-galactopyranoside |
| Poly(maleic anhydride) based polymers | polymer(isobutylene-altmaleic anhydride)/dodecylamine/bis(6-aminohexyl)amine | ||
| Mercaptoalkyl acid ligands | MUA | ||
| Aptamers | SH-Apt/SDS/TBE/NaCl | NH2-Apt | SH-Apt |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials 2018, 11, 1154. https://doi.org/10.3390/ma11071154
Guerrini L, Alvarez-Puebla RA, Pazos-Perez N. Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials. 2018; 11(7):1154. https://doi.org/10.3390/ma11071154
Chicago/Turabian StyleGuerrini, Luca, Ramon A. Alvarez-Puebla, and Nicolas Pazos-Perez. 2018. "Surface Modifications of Nanoparticles for Stability in Biological Fluids" Materials 11, no. 7: 1154. https://doi.org/10.3390/ma11071154
APA StyleGuerrini, L., Alvarez-Puebla, R. A., & Pazos-Perez, N. (2018). Surface Modifications of Nanoparticles for Stability in Biological Fluids. Materials, 11(7), 1154. https://doi.org/10.3390/ma11071154






