A Triton X-100-Based Microemulsion for the Removal of Hydrophobic Materials from Works of Art: SAXS Characterization and Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microemulsion Preparation
2.3. Solubility Tests
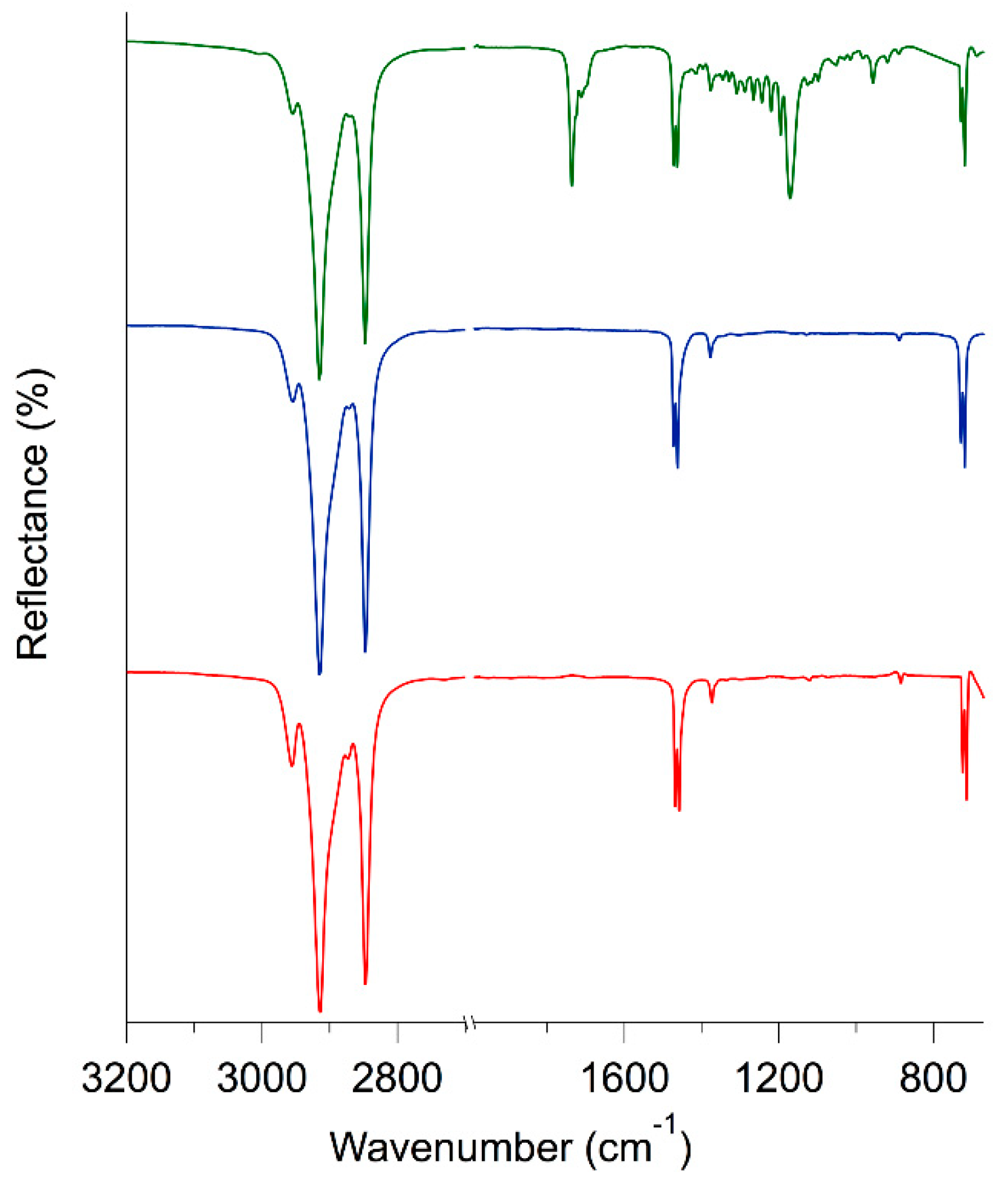
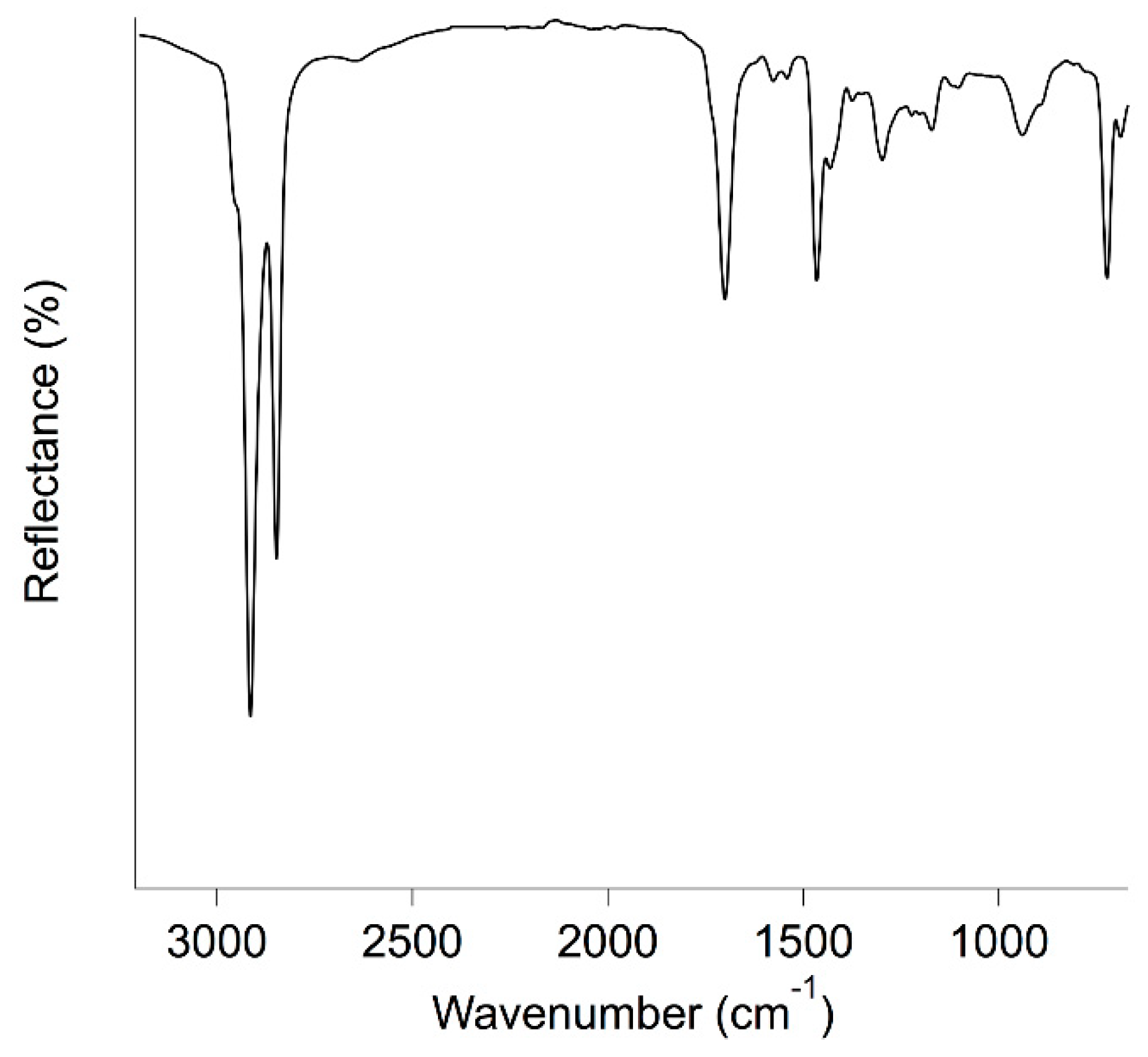
2.4. ATR FT-IR Spectroscopy
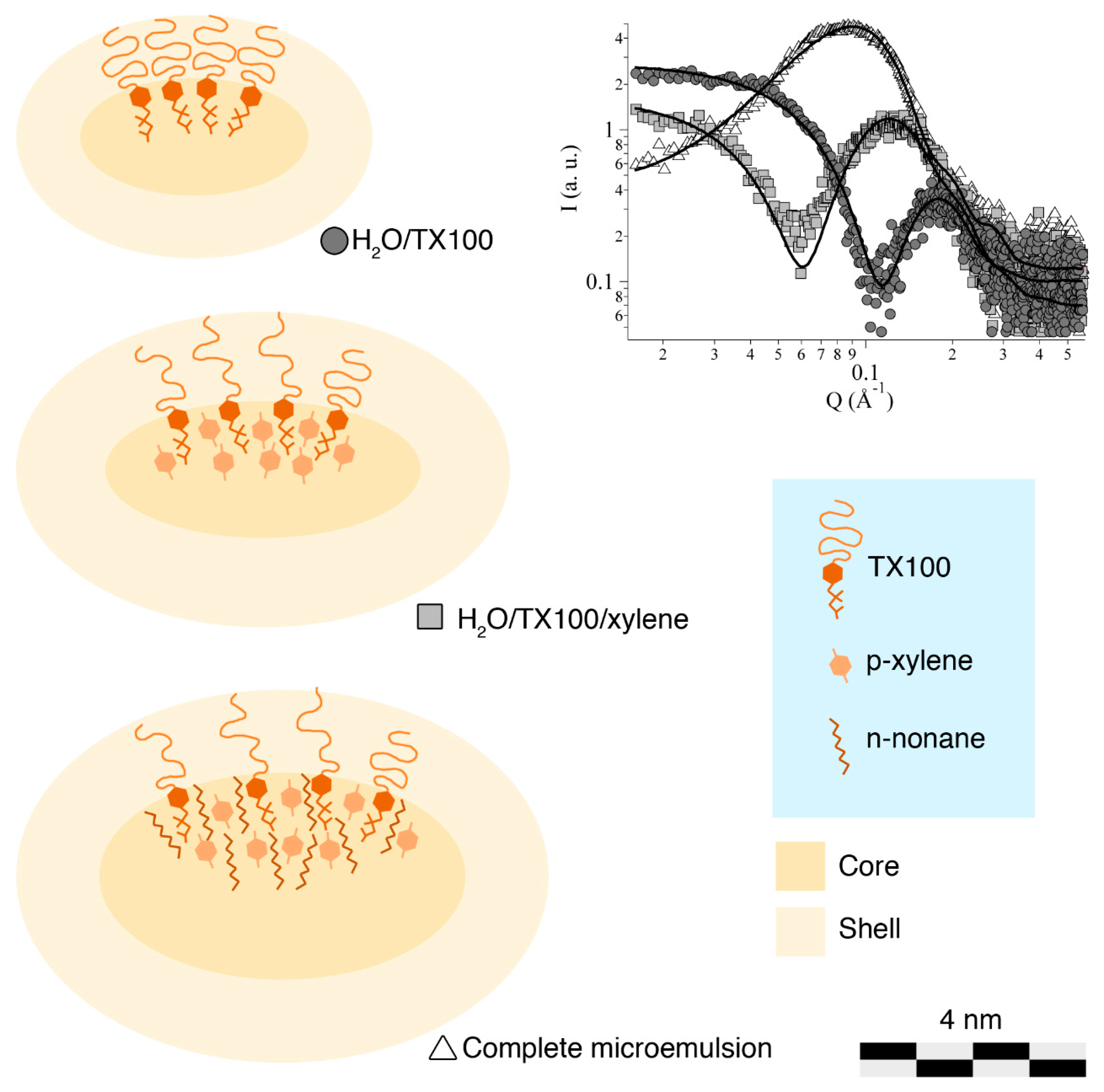
2.5. Small-Angle X-ray Scattering
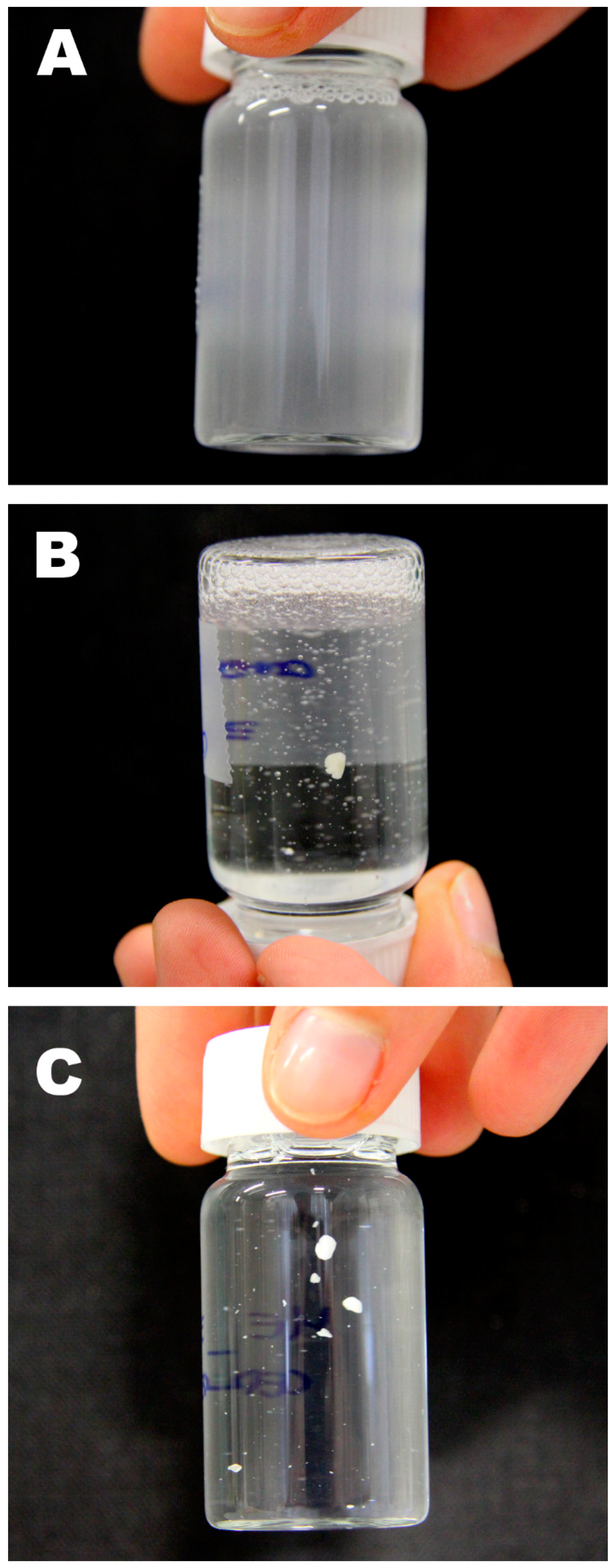
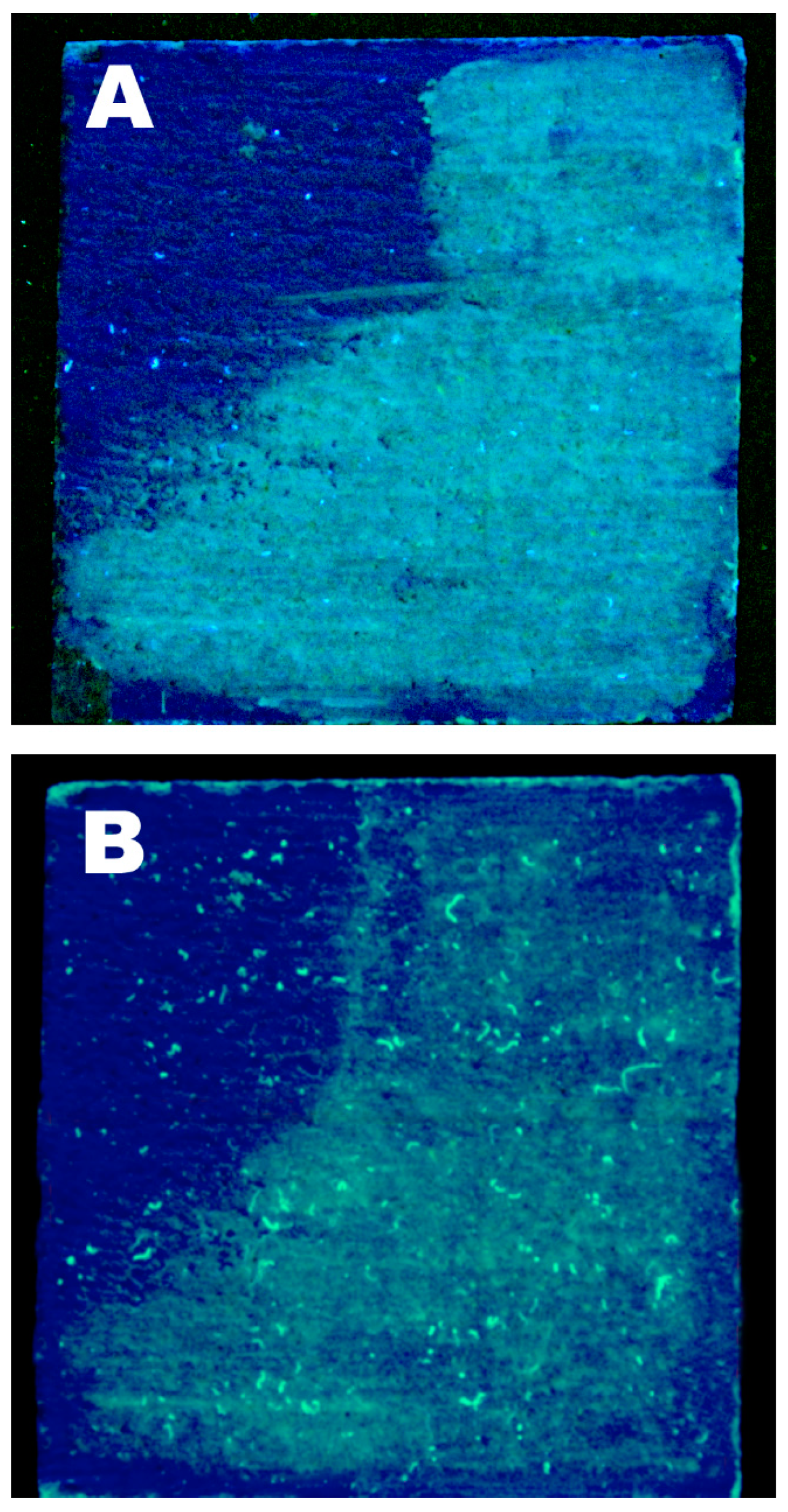
2.6. Laboratory Cleaning Test
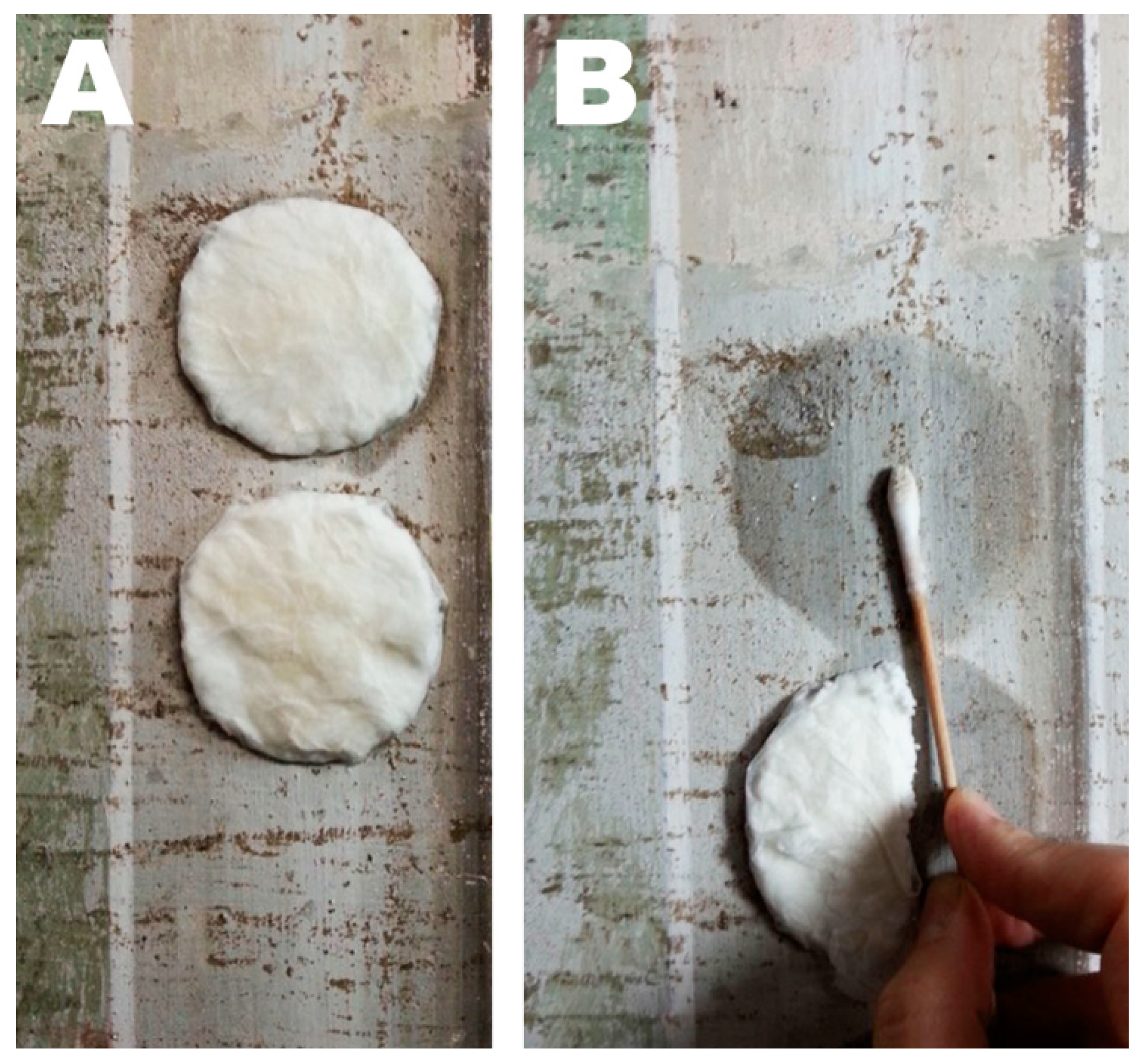
2.7. In Situ Cleaning Test
3. Results and Discussions
3.1. Characterization of Waxes
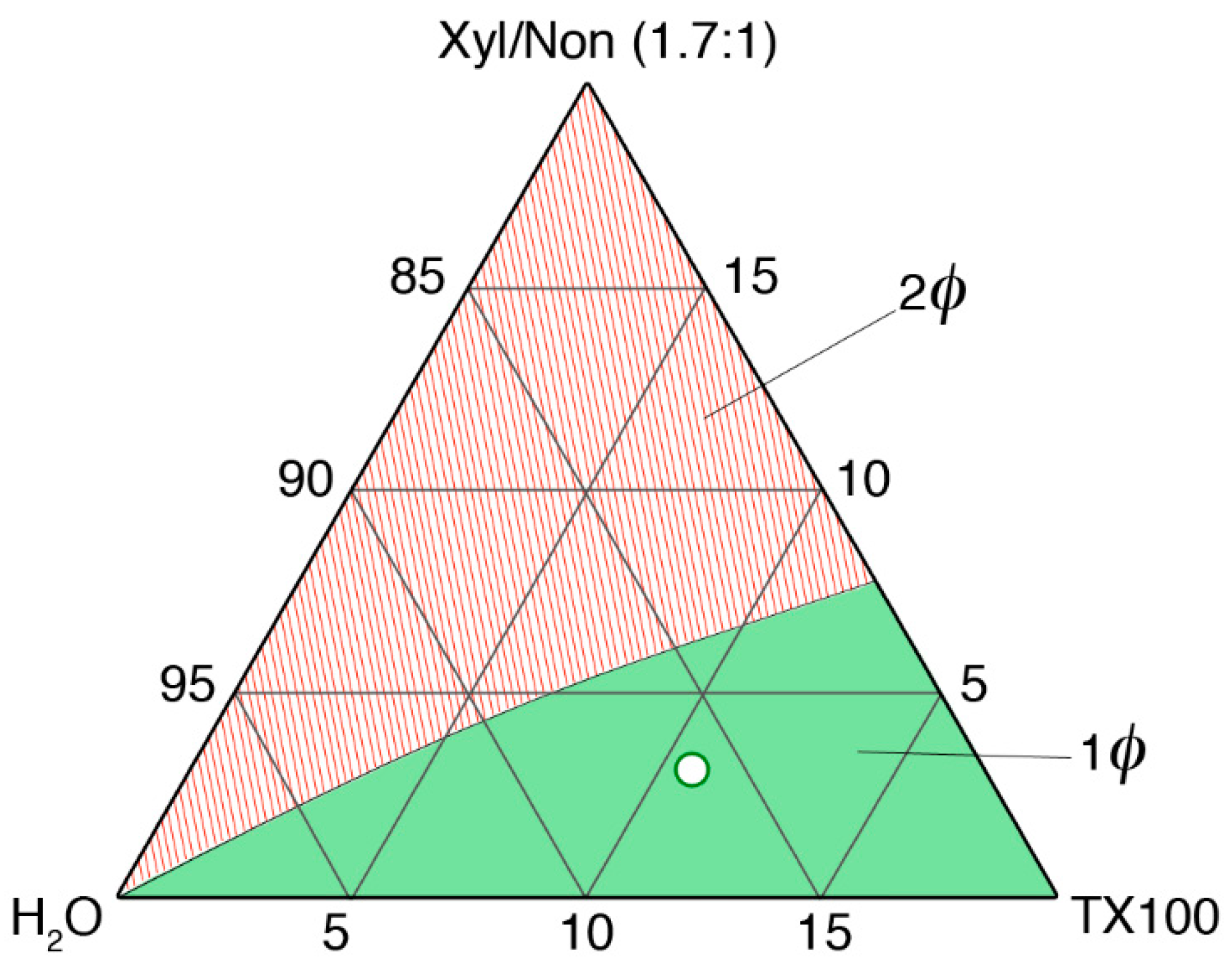
3.2. Formulation and Characterization of the Microemulsion
3.3. Wax Removal Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baglioni, P.; Chelazzi, D. Nanoscience for the Conservation of Works of Art; Royal Society of Chemistry: London, UK, 2013; ISBN 978-1-84973-566-7. [Google Scholar]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Nanotechnologies in the Conservation of Cultural Heritage: A Compendium of Materials and Techniques; Springer: Berlin, Germany, 2014; ISBN 978-94-017-9303-2. [Google Scholar]
- Barreca, S.; Bruno, M.; Oddo, L.; Orecchio, S. Preliminary study on analysis and removal of wax from a Carrara marble statue. Nat. Prod. Res. 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, T. Short Communication Removal of Paraffin Wax in the Re-treatment of Archaeological Iron. J. Am. Inst. Conserv. 2008, 47, 217–223. [Google Scholar] [CrossRef]
- Pan, A.; Chiussi, S.; Serra, J.; González, P.; León, B. Excimer laser removal of beeswax from galician granite monuments. J. Cult. Herit. 2009, 10, 48–52. [Google Scholar] [CrossRef]
- Pan, A.; Rebollar, E.; Conde, J.C.; Lusquiños, F.; Chiussi, S.; León, B. Experimental and theoretical study of the Nd:YAG laser removal of beeswax on Galician granite at 355 nm. Appl. Phys. A 2010, 100, 741–746. [Google Scholar] [CrossRef]
- Borgioli, L.; Caminati, G.; Gabrielli, G.; Ferroni, E. Removal of hydrophobic impurities from pictorial surfaces by means of heterogeneous systems. Sci. Technol. Cult. Herit. 1995, 4, 67–74. [Google Scholar]
- Baglioni, M.; Jàidar Benavides, Y.; Berti, D.; Giorgi, R.; Keiderling, U.; Baglioni, P. An amine-oxide surfactant-based microemulsion for the cleaning of works of art. J. Colloid Interface Sci. 2015, 440, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, M.; Jáidar Benavides, Y.; Desprat-Drapela, A.; Giorgi, R. Amphiphile-based nanofludis for the removal of styrene/acrylate coatings: Cleaning of stucco decoration in the Uaxactun archeological site (Guatemala). J. Cult. Herit. 2015, 16, 862–868. [Google Scholar] [CrossRef]
- Baglioni, M.; Raudino, M.; Berti, D.; Keiderling, U.; Bordes, R.; Holmberg, K.; Baglioni, P. Nanostructured fluids from degradable nonionic surfactants for the cleaning of works of art from polymer contaminants. Soft Matter 2014, 10, 6798–6809. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, M.; Rengstl, D.; Berti, D.; Bonini, M.; Giorgi, R.; Baglioni, P. Removal of acrylic coatings from works of art by means of nanofluids: Understanding the mechanism at the nanoscale. Nanoscale 2010, 2, 1723. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, M.; Giorgi, R.; Berti, D.; Baglioni, P. Smart cleaning of cultural heritage: A new challenge for soft nanoscience. Nanoscale 2012, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Raudino, M.; Selvolini, G.; Montis, C.; Baglioni, M.; Bonini, M.; Berti, D.; Baglioni, P. Polymer Films Removed from Solid Surfaces by Nanostructured Fluids: Microscopic Mechanism and Implications for the Conservation of Cultural Heritage. ACS Appl. Mater. Interfaces 2015, 7, 6244–6253. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, M.; Montis, C.; Brandi, F.; Guaragnone, T.; Meazzini, I.; Baglioni, P.; Berti, D. Dewetting acrylic polymer films with water/propylene carbonate/surfactant mixtures—Implications for cultural heritage conservation. Phys. Chem. Phys. 2017. [Google Scholar] [CrossRef] [PubMed]
- Raudino, M.; Giamblanco, N.; Montis, C.; Berti, D.; Marletta, G.; Baglioni, P. Probing the Cleaning of Polymeric Coatings by Nanostructured Fluids: A QCM-D Study. Langmuir 2017, 33, 5675–5684. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, M.; Montis, C.; Chelazzi, D.; Giorgi, R.; Berti, D.; Baglioni, P. Polymer Film Dewetting by Water/Surfactant/Good-Solvent Mixtures: A Mechanistic Insight and Its Implications for the Conservation of Cultural Heritage. Angew. Chem. Int. Ed. 2018, 57, 7355–7359. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, T. Colloid Science: Principles, Methods and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 978-1-4443-2018-3. [Google Scholar]
- Evans, D.F.; Wennerström, H. The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet; Wiley: Hoboken, NJ, USA, 1999; ISBN 978-0-471-24247-5. [Google Scholar]
- Holmberg, K. Handbook of Applied Surface and Colloid Chemistry; Wiley: Hoboken, NJ, USA, 2002; ISBN 978-0-471-49083-8. [Google Scholar]
- Rao, K.S.; Goyal, P.S.; Dasannacharya, B.A.; Kelkar, V.K.; Manohar, C.; Menon, S.V.G. Small angle neutron scattering from micellar solutions of triton X-100. Pramana 1991, 37, 311–319. [Google Scholar] [CrossRef]
- Stubičar, N.; Matejaš, J.; Zipper, P.; Wilfing, R. Size, Shape and Internal Structure of Triton X-100 Micelles Determined by Light and Small-Angle X-Ray Scattering Techniques. In Surfactants in Solution; Springer: Boston, MA, USA, 1989; pp. 181–195. ISBN 978-1-4615-7986-1. [Google Scholar]
- Paradies, H.H. Shape and size of a nonionic surfactant micelle. Triton X-100 in aqueous solution. J. Phys. Chem. 1980, 84, 599–607. [Google Scholar] [CrossRef]
- Robson, R.J.; Dennis, E.A. The size, shape, and hydration of nonionic surfactant micelles. Triton X-100. J. Phys. Chem. 1977, 81, 1075–1078. [Google Scholar] [CrossRef]
- Charlton, I.D.; Doherty, A.P. Electrolyte-Induced Structural Evolution of Triton X-100 Micelles. J. Phys. Chem. B 2000, 104, 8327–8332. [Google Scholar] [CrossRef]
- Warth, A.H. The Chemistry and Technology of Waxes, 2nd ed.; Reinhold Publishing Co.: New York, NY, USA, 1956. [Google Scholar]
- Masae, M.; Pitsuwan, P.; Sikong, L.; Kongsong, P.; Kooptarnond, K.; Phoempoon, P. Thermo-physical characterization of paraffin and beeswax on cotton fabric. Thammasat Int. J. Sci. Technol. 2014, 19, 69–77. [Google Scholar]
- Svečnjak, L.; Baranović, G.; Vinceković, M.; Prđun, S.; Bubalo, D.; Gajger, I.T. An approach for routine analytical detection of beeswax adulteration using ftir-atr spectroscopy. J. Apic. Sci. 2015, 59, 37–49. [Google Scholar] [CrossRef]
- Hepburn, H.R. Composition and Synthesis of Beeswax. In Honeybees and Wax; Springer: Berlin/Heidelberg, Germany, 1986; pp. 44–56. [Google Scholar]
- Goyal, P.S.; Menon, S.V.G.; Dasannacharya, B.A.; Thiyagarajan, P. Small-angle neutron-scattering study of micellar structure and interparticle interactions in Triton X-100 solutions. Phys. Rev. E 1995, 51, 2308–2315. [Google Scholar] [CrossRef]
- Brown, W.; Rymden, R.; Van Stam, J.; Almgren, M.; Svensk, G. Static and dynamic properties of nonionic amphiphile micelles: Triton X-100 in aqueous solution. J. Phys. Chem. 1989, 93, 2512–2519. [Google Scholar] [CrossRef]
- Kushner, L.M.; Hubbard, W.D. Viscometric and Turbidimetric Measurements of Dilute Aqueous Solutions of a Non-ionic Detergent. J. Phys. Chem. 1954, 58, 1163–1167. [Google Scholar] [CrossRef]
- Tanford, C.; Nozaki, Y.; Rohde, M.F. Size and shape of globular micelles formed in aqueous solution by n-alkyl polyoxyethylene ethers. J. Phys. Chem. 1977, 81, 1555–1560. [Google Scholar] [CrossRef]
- Kline, S.R. Reduction and analysis of SANS and USANS data using IGOR Pro. J. Appl. Crystallogr. 2006, 39, 895–900. [Google Scholar] [CrossRef]
- Regert, M.; Colinart, S.; Degrand, L.; Decavallas, O. Chemical Alteration and Use of Beeswax Through Time: Accelerated Ageing Tests and Analysis of Archaeological Samples from Various Environmental Contexts. Archaeometry 2001, 43, 549–569. [Google Scholar] [CrossRef]
| Wax | Solvents | |||||
|---|---|---|---|---|---|---|
| Ligroin | n-Nonane | p-Xylene | Limonene | Turpentine | Chloroform | |
| Beeswax | Emulsified | Emulsified | Solubilized | Swollen | Swollen | Solubilized |
| Paraffin | Solubilized | Solubilized | Solubilized | Solubilized | Solubilized | Solubilized |
| Ceresin | Swollen | Swollen | Swollen | Swollen | Swollen | Swollen |
| Systems | |||
|---|---|---|---|
| Fitting Parameters | H2O/TX100 | H2O/TX100/xylene | Microemulsion |
| a (Å) | 20.2 ± 0.2 | 27.9 ± 1.3 | 32.4 ± 0.1 |
| b (Å) | 10.4 ± 0.2 | 12.1 ± 2.4 | 18.3 ± 0.1 |
| t (Å) | 11.1 ± 0.1 | 15.8 ± 3.3 | 14.7 ± 0.2 |
| SLDcore (10−6 Å−2) | 7.8 | 7.8 | 7.8 |
| SLDshell (10−6 Å−2) | 10.8 | 10.0 | 9.9 |
| SLDsolvent (10−6 Å−2) | 9.4 | 9.4 | 9.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baglioni, M.; Poggi, G.; Ciolli, G.; Fratini, E.; Giorgi, R.; Baglioni, P. A Triton X-100-Based Microemulsion for the Removal of Hydrophobic Materials from Works of Art: SAXS Characterization and Application. Materials 2018, 11, 1144. https://doi.org/10.3390/ma11071144
Baglioni M, Poggi G, Ciolli G, Fratini E, Giorgi R, Baglioni P. A Triton X-100-Based Microemulsion for the Removal of Hydrophobic Materials from Works of Art: SAXS Characterization and Application. Materials. 2018; 11(7):1144. https://doi.org/10.3390/ma11071144
Chicago/Turabian StyleBaglioni, Michele, Giovanna Poggi, Giulia Ciolli, Emiliano Fratini, Rodorico Giorgi, and Piero Baglioni. 2018. "A Triton X-100-Based Microemulsion for the Removal of Hydrophobic Materials from Works of Art: SAXS Characterization and Application" Materials 11, no. 7: 1144. https://doi.org/10.3390/ma11071144
APA StyleBaglioni, M., Poggi, G., Ciolli, G., Fratini, E., Giorgi, R., & Baglioni, P. (2018). A Triton X-100-Based Microemulsion for the Removal of Hydrophobic Materials from Works of Art: SAXS Characterization and Application. Materials, 11(7), 1144. https://doi.org/10.3390/ma11071144





