A Study on Microstructure and Properties of PHS Fiber Laser Welded Joints Obtained in Air Atmospheres
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
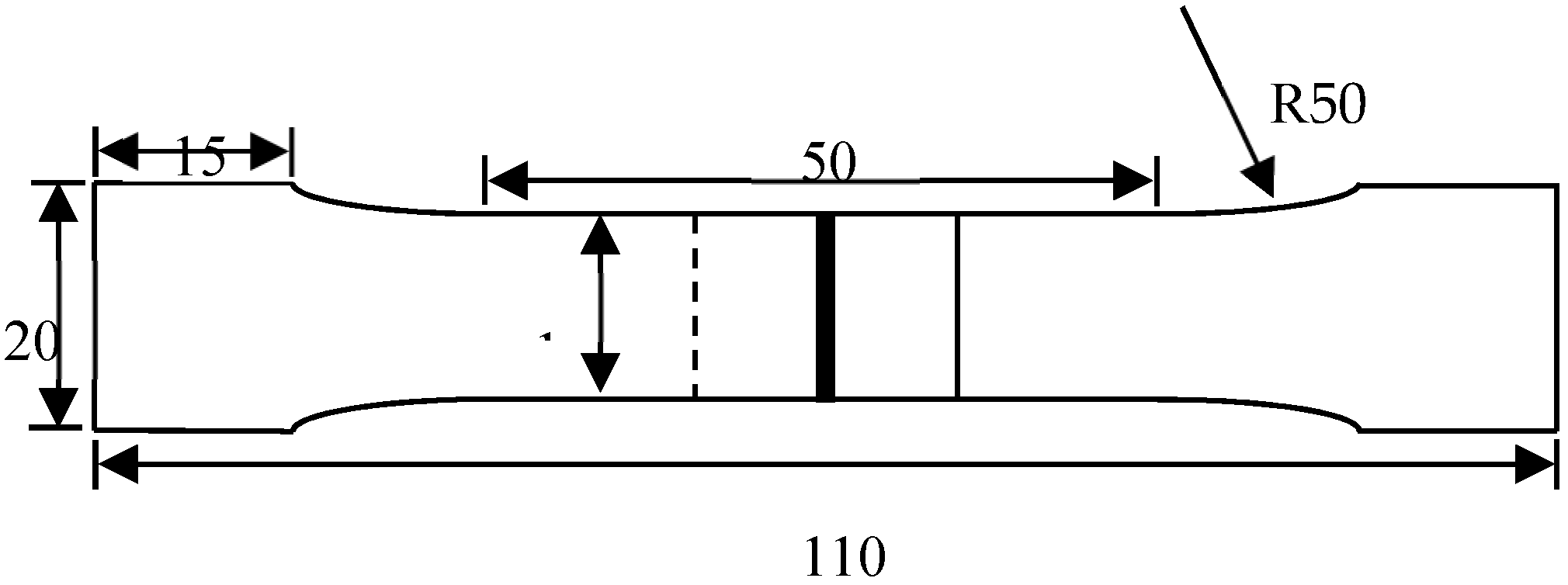
2.2. Experimental Methods
3. Results and Discussion
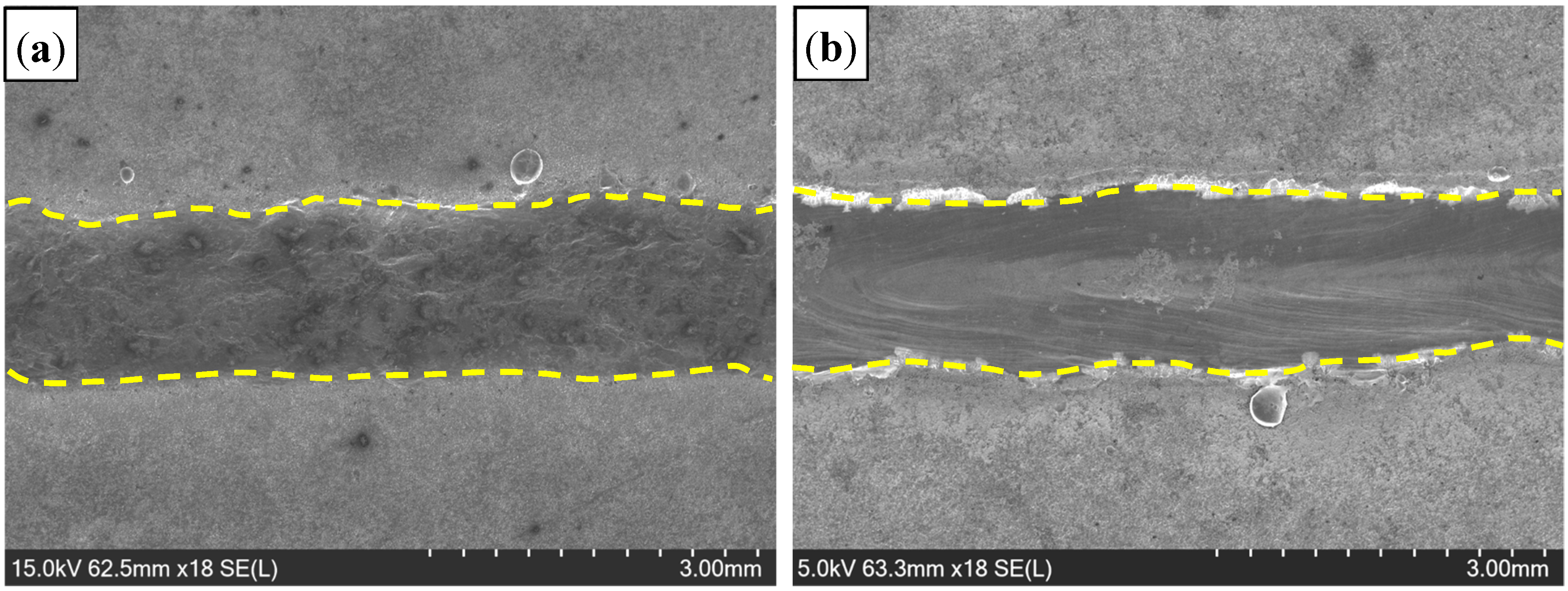
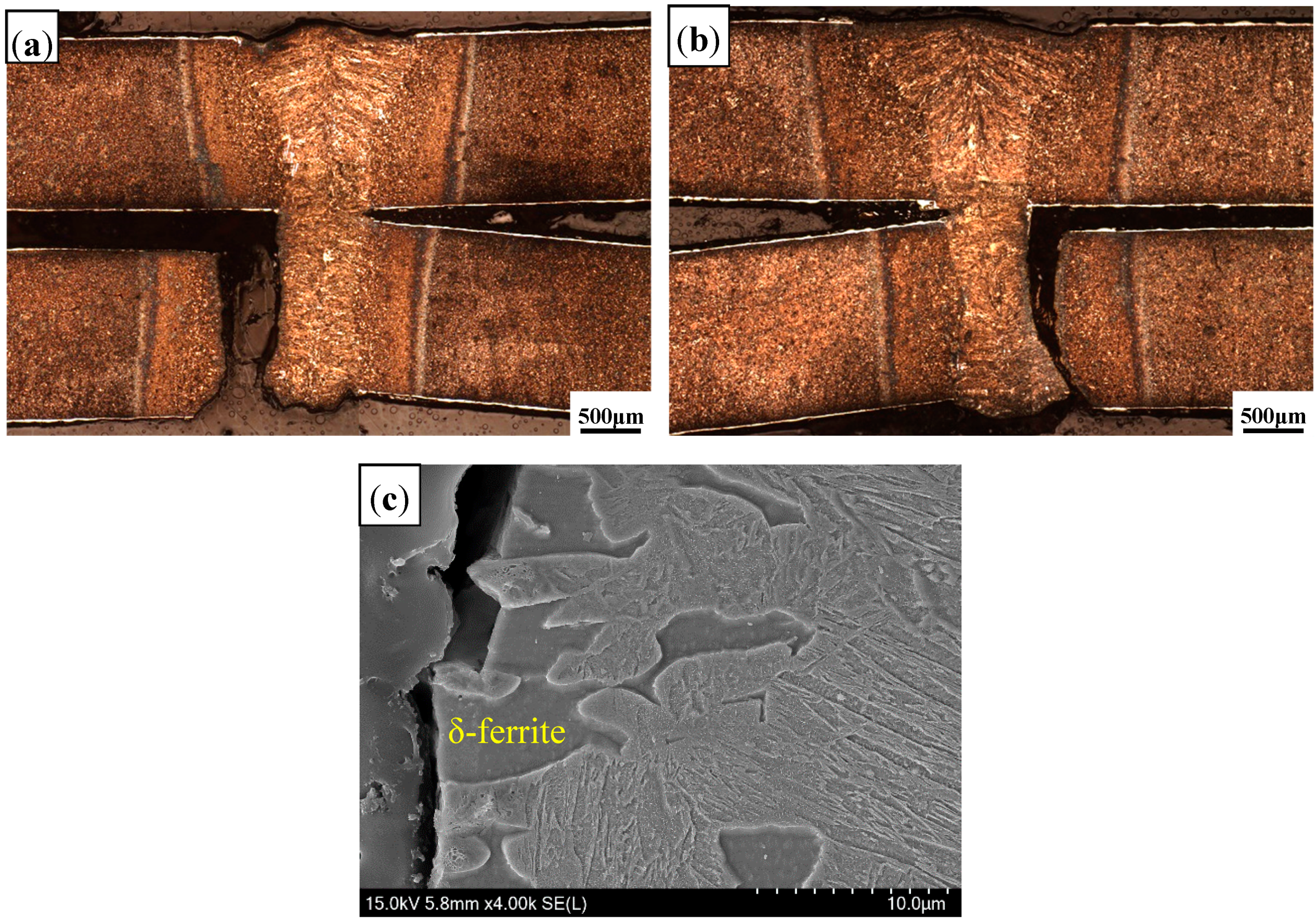
3.1. Appearance of Welded Seam
3.2. Microstructure
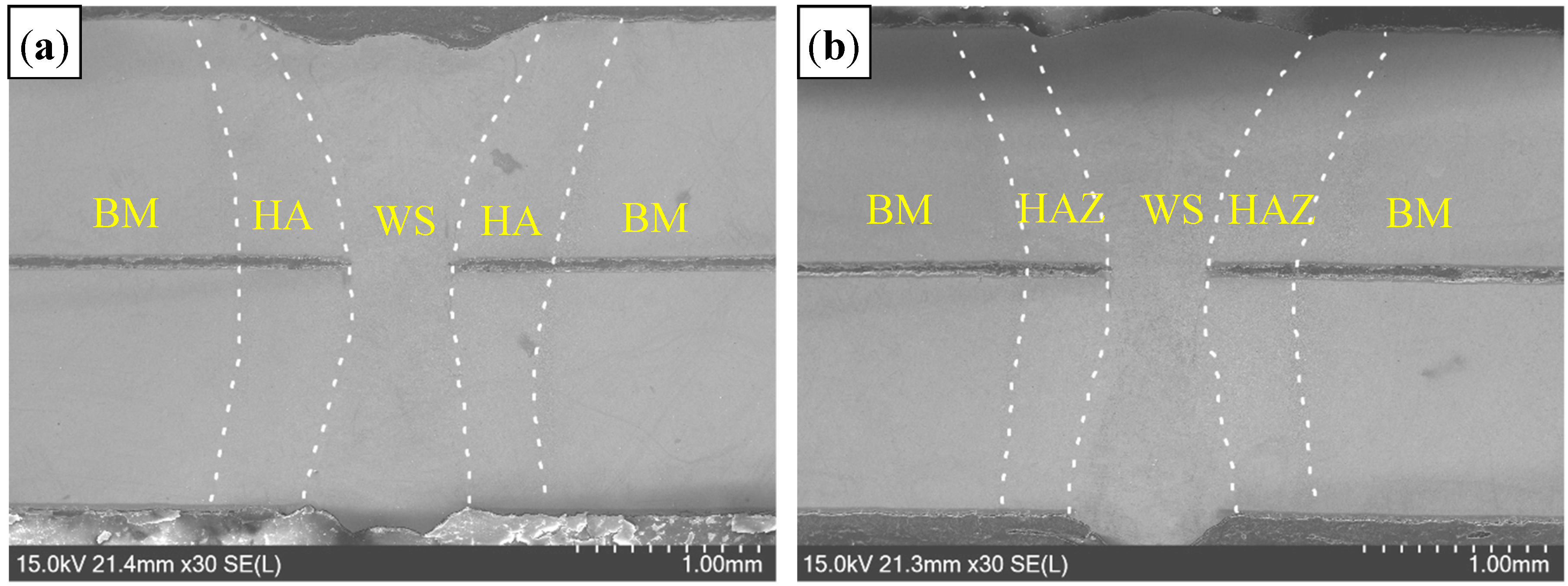
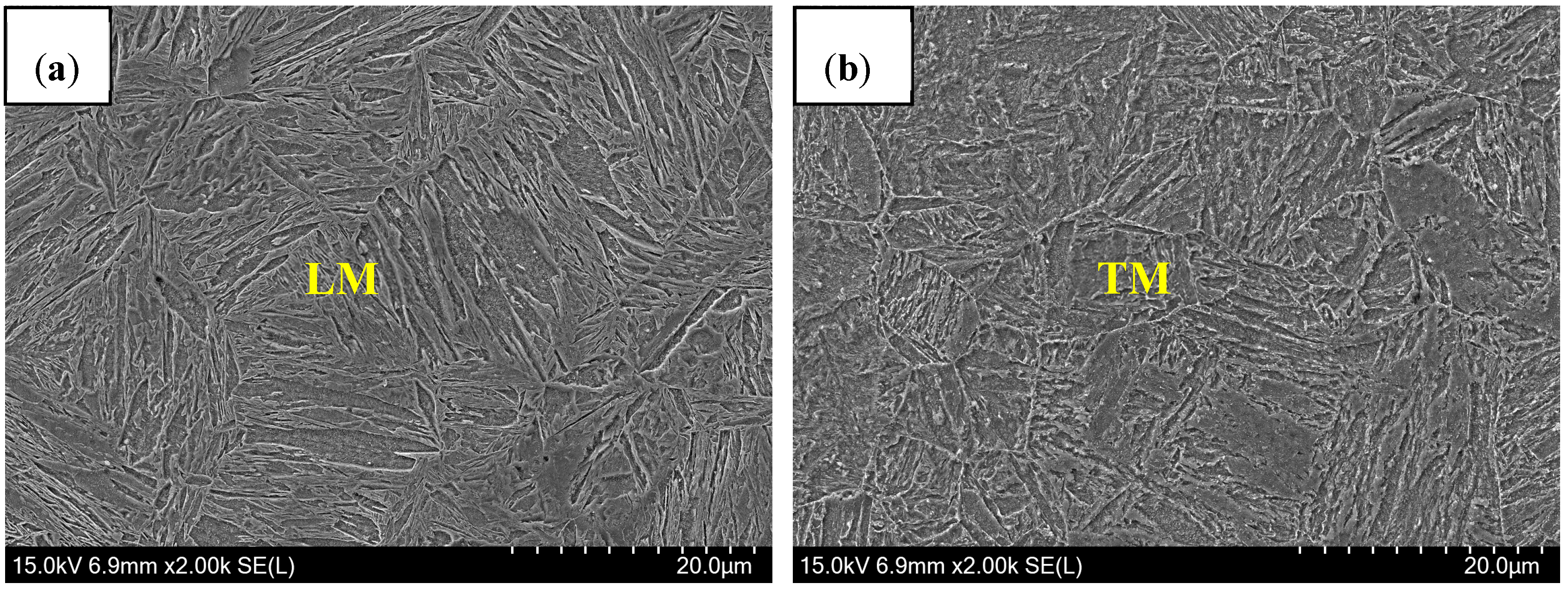
3.2.1. Microstructure of Welded Seam
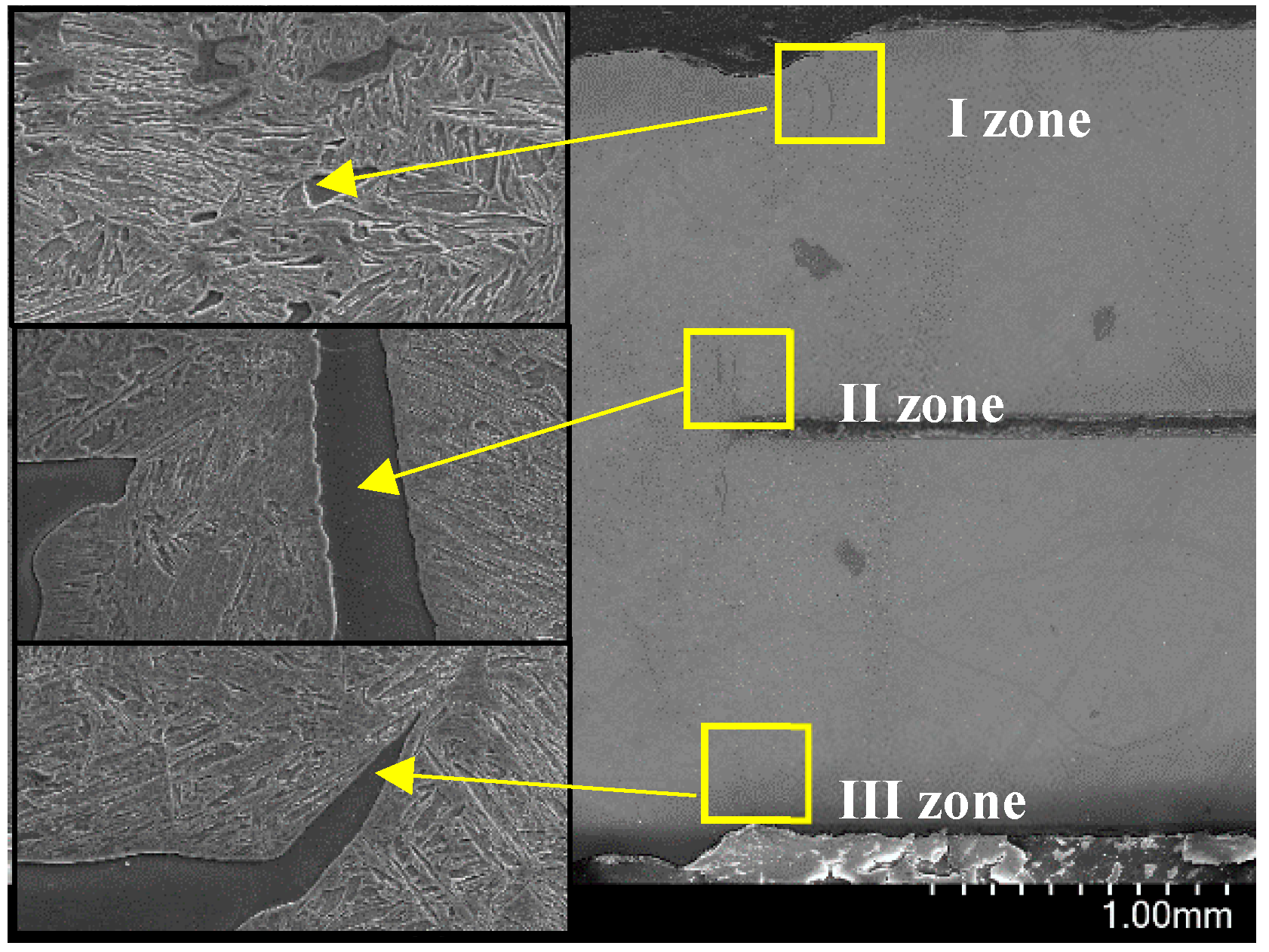
3.2.2. Inclusions of Welded Seam
K’ = a2Al·a3O/aAl2O3
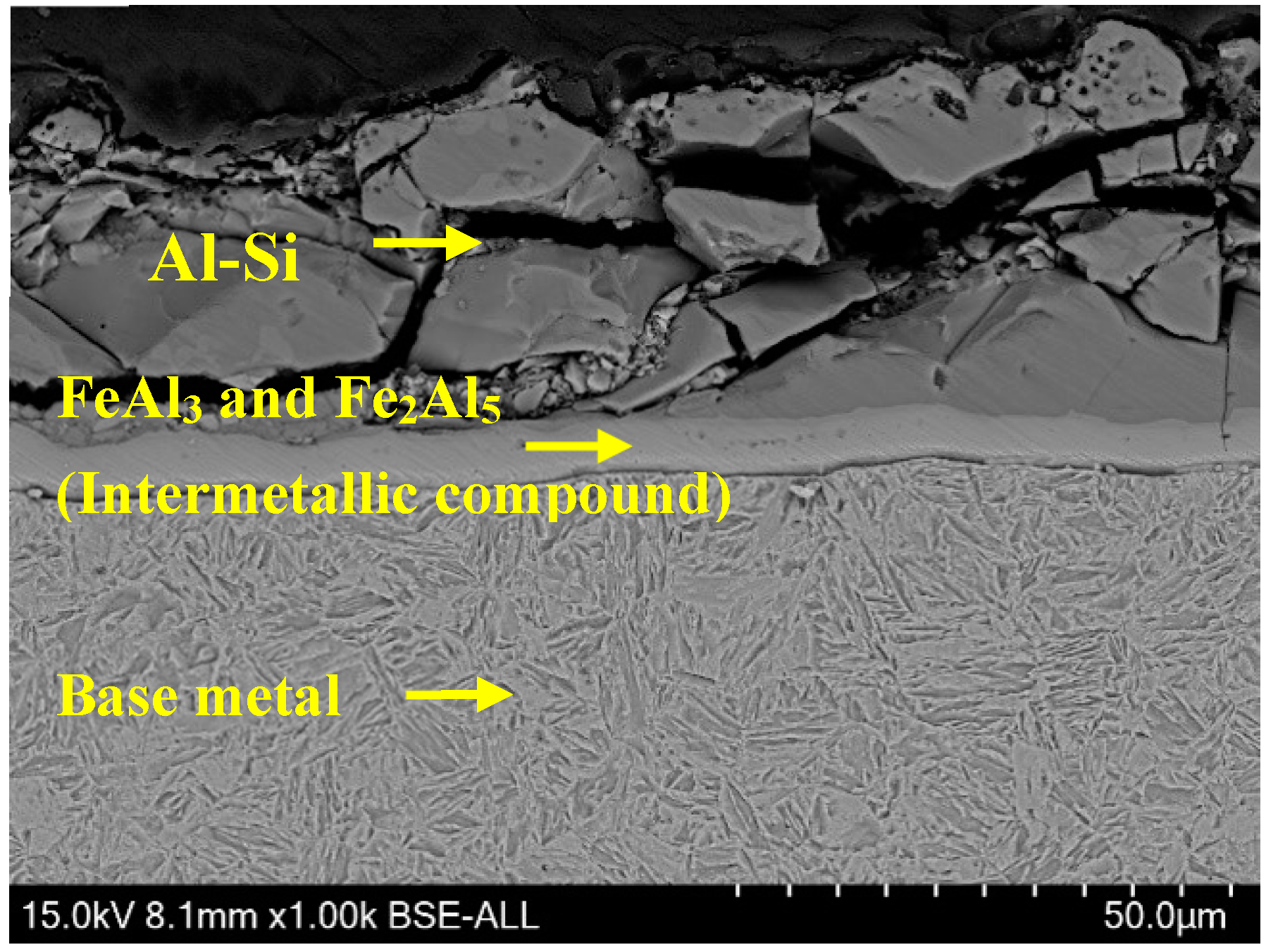
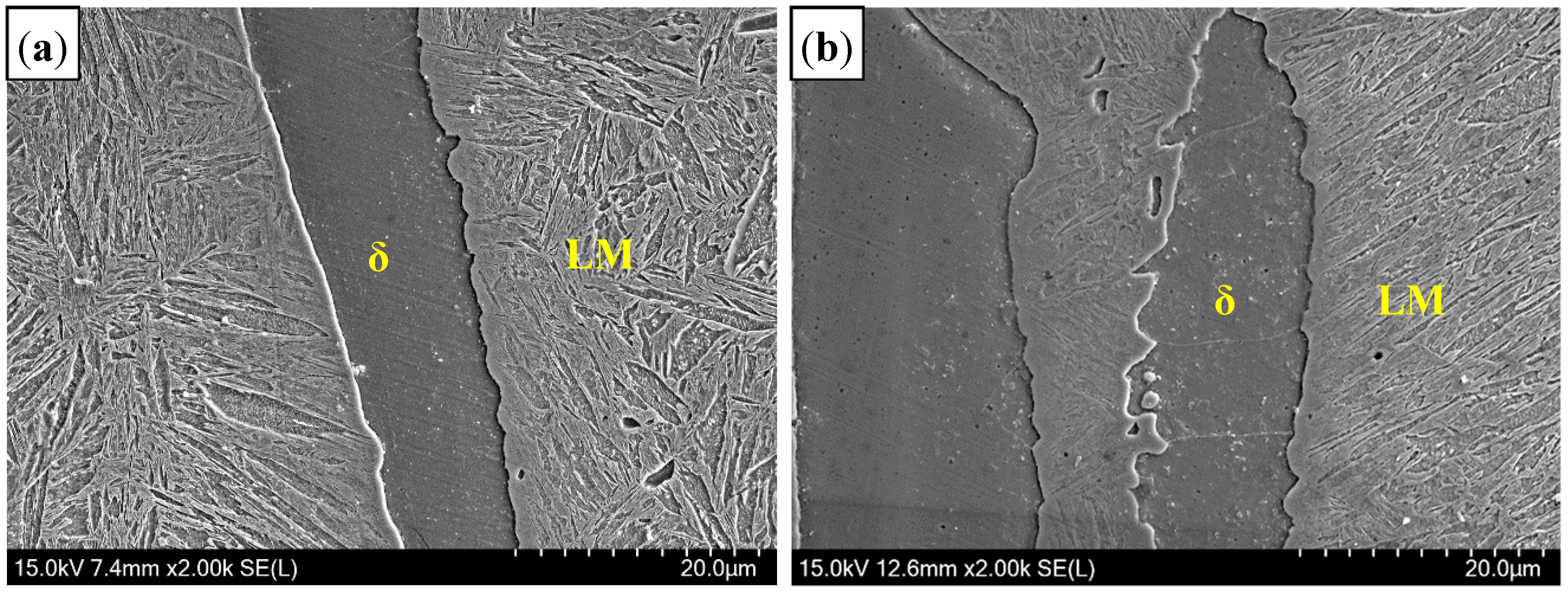
3.2.3. Microstructure of Fusion Line
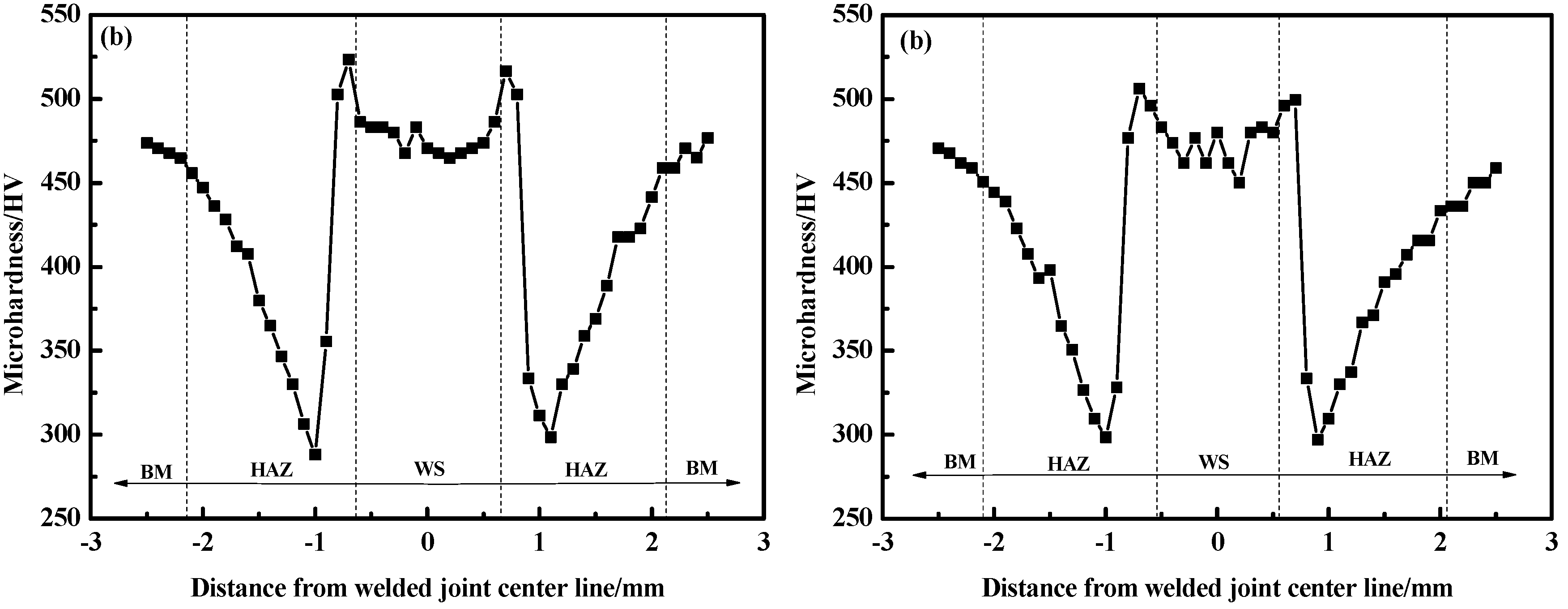
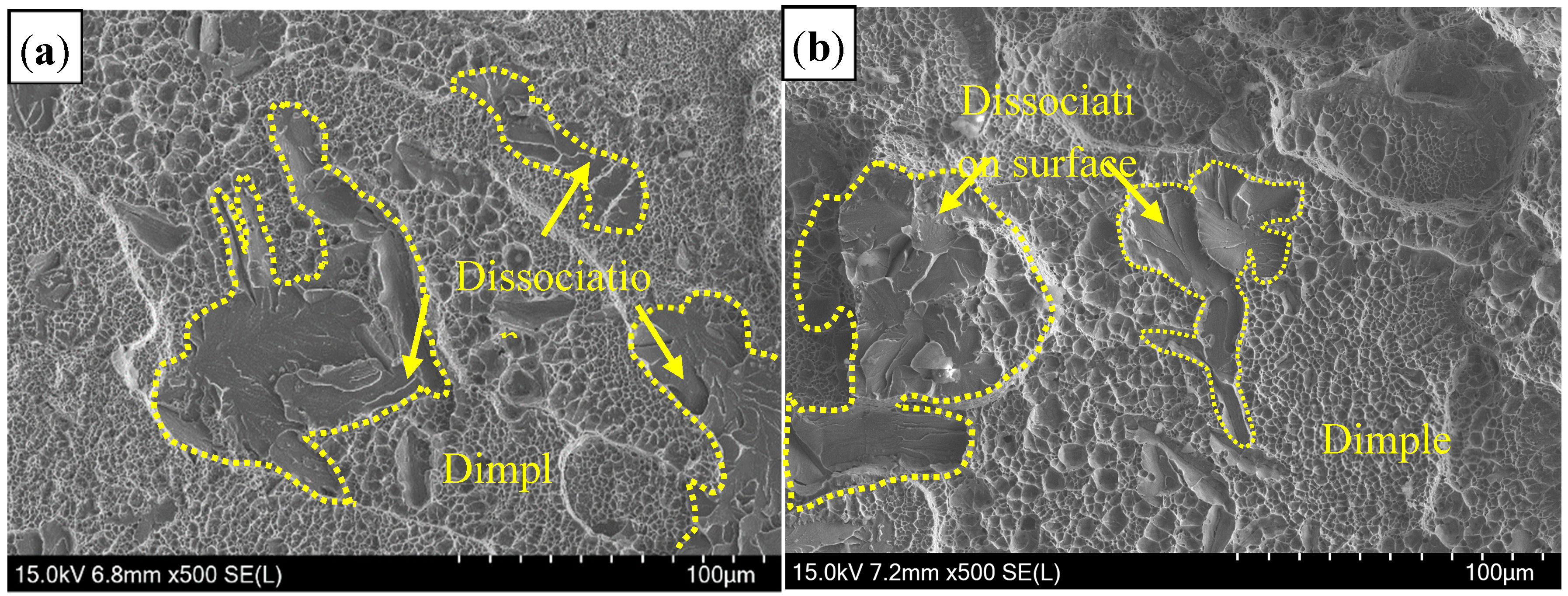
3.3. Hardness and Tensile Shear Strength
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.N.; Sun, Q.; Zheng, Z.; Di, H.-S. Microstructure and fracture behavior of laser welded joints of DP steels with different heat inputs. Mater. Sci. Eng. A 2017, 699, 18–25. [Google Scholar] [CrossRef]
- Sun, Q.; Di, H.S.; Li, J.C.; Wang, X.-N. Effect of pulse frequency on microstructure and properties of welded joints for dual phase steel by pulsed laser welding. Mater. Des. 2016, 105, 201–211. [Google Scholar] [CrossRef]
- Iordachescu, D.; Blasco, M.; Lopez, R.; Cuesta, A.; Iordachescu, M.; Ocana, J. Recent achievements and trends in laser welding of thin plates. J. Optoelectron. Adv. Mater. 2011, 13, 981–985. [Google Scholar]
- Windmann, M.; Röttger, A.; Kügler, H.; Theisen, W. Laser beam welding of magnesium to coated high-strength steel 22MnB5. Int. J. Adv. Manuf. Technol. 2016, 87, 3149–3156. [Google Scholar] [CrossRef]
- Kim, C.; Kang, M.; Park, Y. Laser welding of Al-Si coated hot stamping steel. Procedia Eng. 2011, 10, 2226–2231. [Google Scholar] [CrossRef]
- Kang, M.; Kim, C.; Lee, J. Weld strength of laser-welded hot-press-forming steel. J. Laser Appl. 2012, 24, 367–377. [Google Scholar] [CrossRef]
- Nakkalil, R.; Richards, N.L.; Chaturvedi, M.C. Fusion zone microstructure of electron beam welded incoloy 903. Scr. Metall. Mater. 1992, 26, 545–550. [Google Scholar] [CrossRef]
- Cam, G. Welding in the World; Springer: Berlin, Germany, 1999. [Google Scholar]
- Mazumder, J. Laser beam welding. In ASM Handbook; ASM International: Almere, The Netherlands, 1993; Volume 12, pp. 262–269. [Google Scholar]
- Tadamalle, A. Estimation of weld pool geometry and cooling rate in laser welding. In Proceedings of the 5th International and 26th All India Manufacturing Technology, Design and Research Conference (AIMTDR), Guwahati, India, 12–14 December 2014. [Google Scholar]
- Bárta, J.; Vrtochová, T.; Krampoťák, P. The shielding gas influence on the laser beam welding of 2205 duplex stainless steel. Adv. Mater. Res. 2013, 772, 89–93. [Google Scholar] [CrossRef]
- Keskitalo, M.; Mäntyjärvi, K.; Sundqvist, J.; Powell, J.; Kaplan, A.F.H. Laser welding of duplex stainless steel with nitrogen as shielding gas. J. Mater. Process. Technol. 2015, 216, 381–384. [Google Scholar] [CrossRef]
- Yadaiah, N.; Bag, S.; Paul, C.P.; Kukreja, L.M. Influence of self-protective atmosphere in fiber laser welding of austenitic stainless steel. Int. J. Adv. Manuf. Technol. 2015, 86, 853–870. [Google Scholar] [CrossRef]
- Arata, Y.; Abe, N.; Oda, T. Fundamental phenomena in high power CO2 laser welding (report I): Atmospheric laser welding (welding physics, process & instrument). Trans. JWRI 1985, 14, 5–11. [Google Scholar]
- Yu, H.Y.; Bao, L.; Deng, Y.Z.; Cao, W. Forming response of ultra-high strength steel sheet to stamping speed during hot forming. Adv. Mater. Res. 2010, 160–162, 123–129. [Google Scholar] [CrossRef]
- Moon, J.H.; Seo, P.K.; Kang, C.G. A study on mechanical properties of laser-welded blank of a boron sheet steel by laser ablation variable of Al-Si coating layer. Int. J. Precis. Eng. Manuf. 2013, 14, 283–288. [Google Scholar] [CrossRef]
- Mitra, U.; Eagar, T.W. Slag-metal reactions during welding: Part I evaluation and reassessment of existing theories. Metall. Mater. Trans. B 1991, 22, 65–71. [Google Scholar] [CrossRef]
- Lau, T.; Weatherly, G.C.; Mclean, A. The sources of oxygen and nitrogen contamination in submerged arc welding using CaO-A12O3 based fluxes. Weld. J. 1985, 64, 343–344. [Google Scholar]
- Saha, D.C.; Biro, E.; Gerlich, A.P.; Zhou, N.Y. Fusion zone microstructure evolution of fiber laser welded press-hardened steels. Scr. Mater. 2016, 121, 18–22. [Google Scholar] [CrossRef]
- Straumal, B.B.; Mazilkin, A.A.; Baretzky, B. Grain boundary complexions and pseudopartial wetting. Curr. Opin. Solid State Mater. Sci. 2016, 20, 247–256. [Google Scholar] [CrossRef]
- Tseng, C.C.; Shen, Y.; Thompson, S.W.; Krauss, M.G. Fracture and the formation of sigma phase, M23C6, and austenite from delta-ferrite in an AlSi304L stainless steel. Metall. Mater. Trans. A 1994, 25, 1147–1158. [Google Scholar] [CrossRef]
- Yi, H.L.; Lee, K.Y.; Bhadeshia, H.K.D.H. Stabilisation of ferrite in hot rolled δ-TRIP steel. Mater. Sci. Technol. 2011, 27, 525–529. [Google Scholar] [CrossRef]
- Liang, G.F.; Wan, C.Q.; Wu, J.C.; Zhu, G.M.; Yu, Y.; Fang, Y. In situ, observation of growth behavior and morphology of delta-ferrite as function of solidification rate in an AISI304 stainless steel. Acta Metall. Sin.-Engl. 2006, 19, 441–448. [Google Scholar] [CrossRef]
- Zhang, J.M.; Zhang, J.F.; Yang, Z.G.; Li, G.Y.; Yao, G.; Li, S.X.; Hui, W.J.; Weng, Y.Q. Estimation of maximum inclusion size and fatigue strength in high-strength ADF1 steel. Mater. Sci. Eng. A 2005, 394, 126–131. [Google Scholar] [CrossRef]
- Ohta, H.; Suito, H. Activities in CaO-SiO2-Al2O3, slags and deoxidation equilibria of Si and Al. Metall. Mater. Trans. B 1996, 27, 943–953. [Google Scholar] [CrossRef]
- Krawiec, H.; Vignal, V.; Heintz, O.; Krawietz, H.; Chauveau, E. Dissolution of chromium-enriched inclusions and pitting corrosion of resulfurized stainless steels. Metall. Mater. Trans. A 2006, 37, 1541–1549. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.N.; Zhu, G.J.; Chen, C.J.; Hou, J.X.; Zhang, S.H.; Jing, H.M. Effect of laser welding process parameters on microstructure and mechanical properties on butt joint of new hot-rolled nano-scale precipitate strengthen steel. Acta Metall. Sin.-Engl. 2014, 27, 521–529. [Google Scholar] [CrossRef]

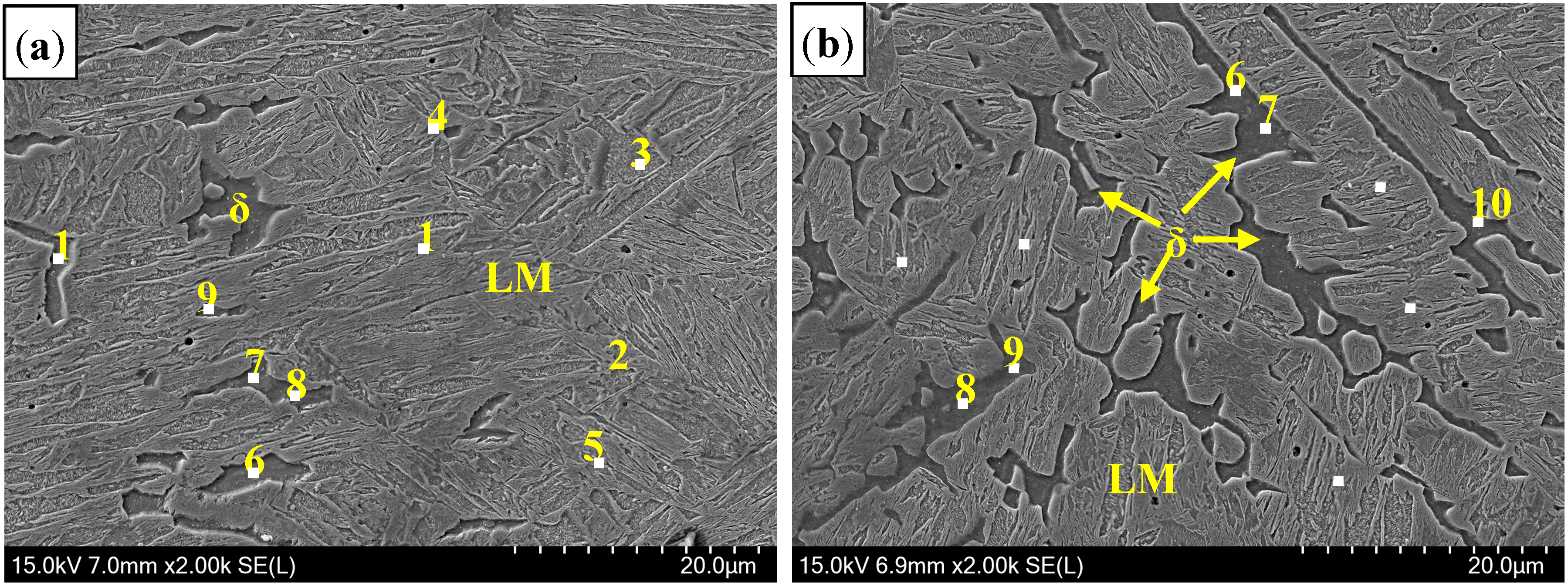
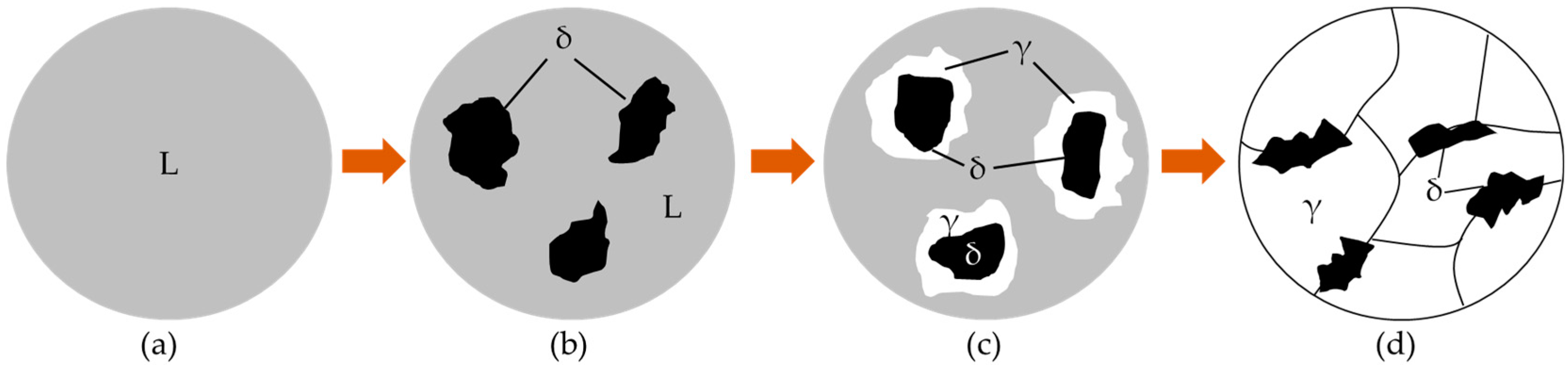
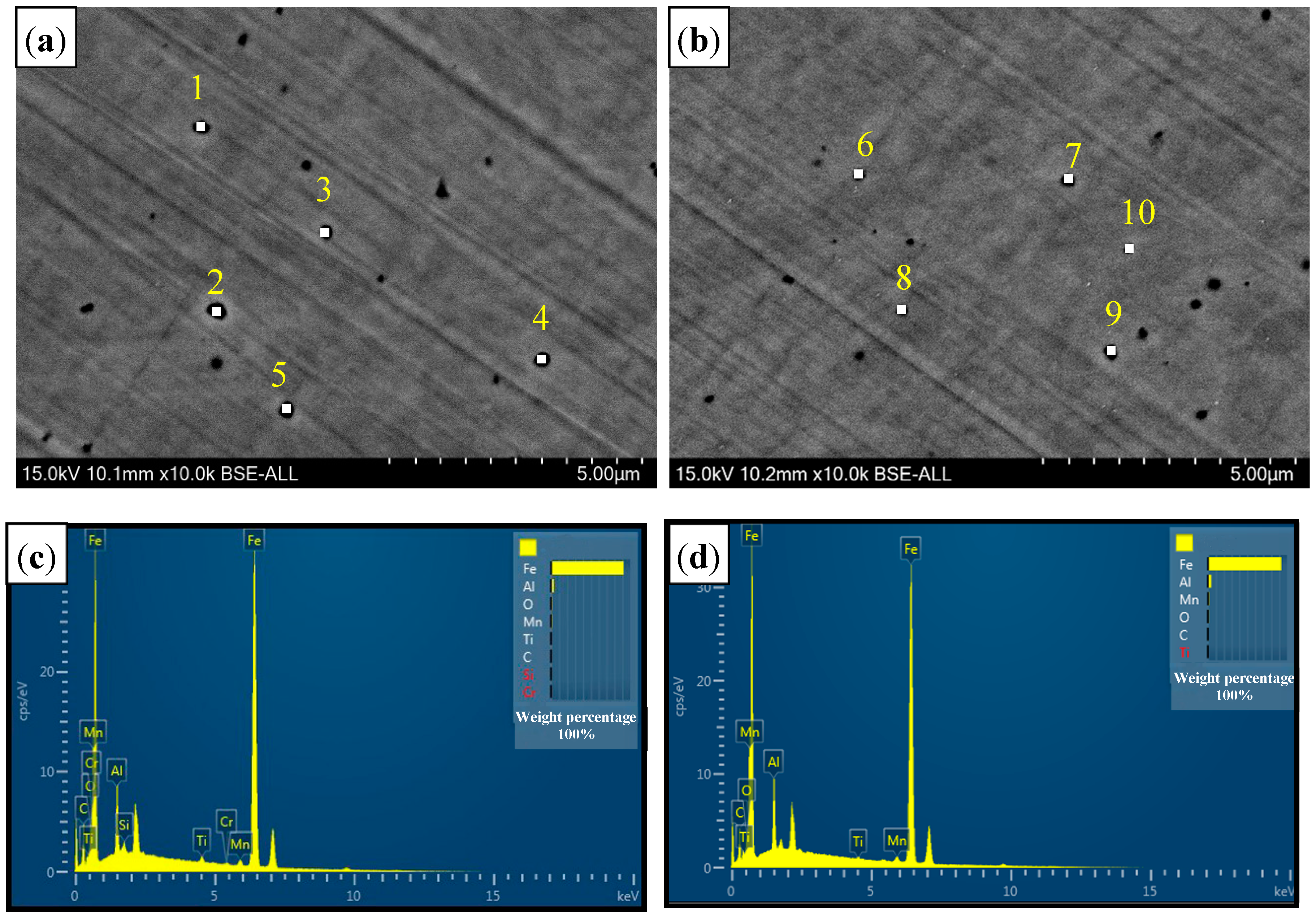
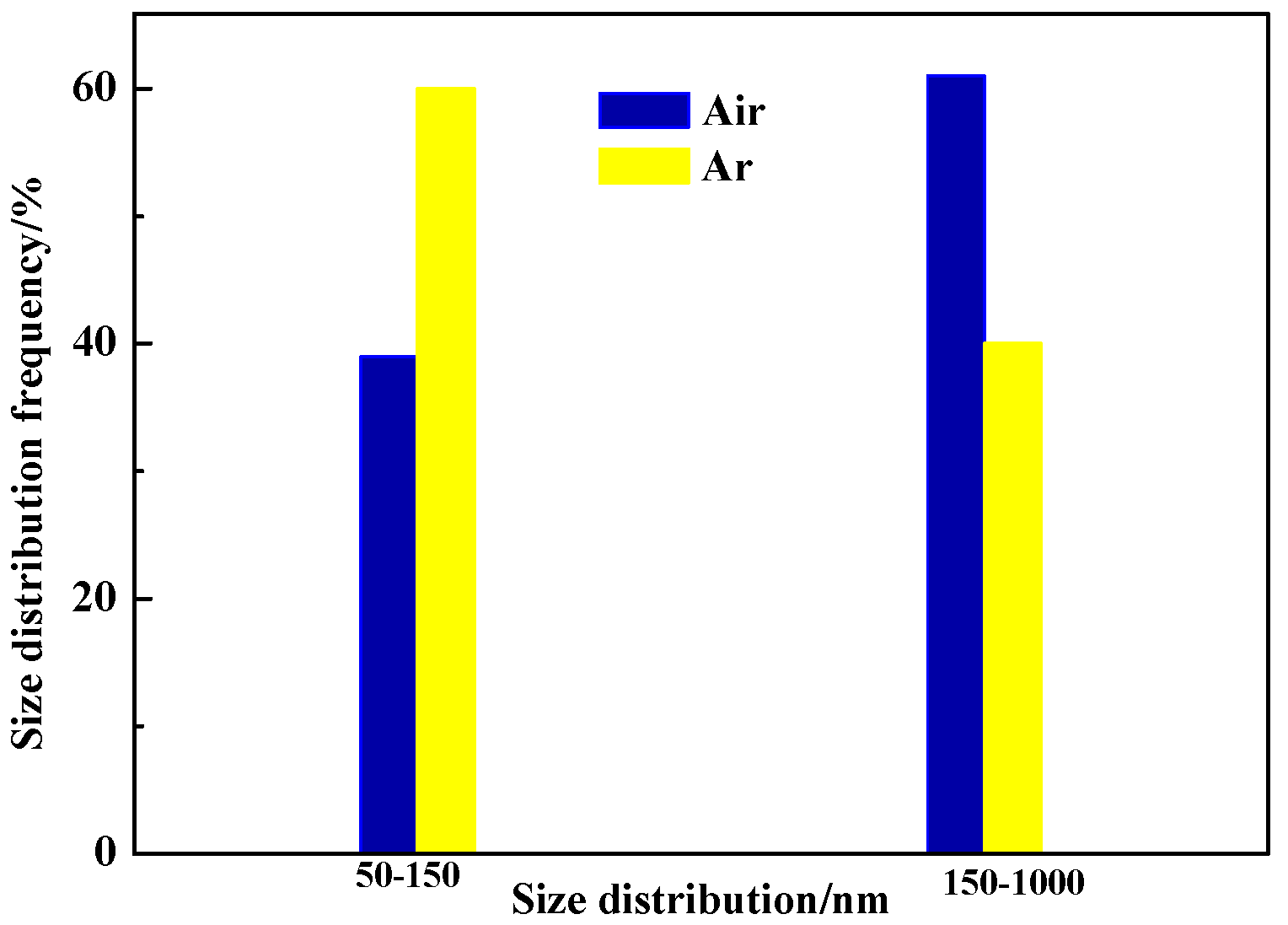
| C | Si | Mn | P | S | N | Al | Ti | B | Cr |
|---|---|---|---|---|---|---|---|---|---|
| 0.23 | 0.27 | 1.13 | 0.013 | 0.001 | 0.0042 | 0.037 | 0.038 | 0.0025 | 0.16 |
| Ar | Air | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Al | 1.92 | 1.98 | 1.99 | 2.14 | 2.00 | 1.80 | 1.57 | 1.62 | 1.74 | 1.67 |
| Si | 0.31 | 0.35 | 0.56 | 0.47 | 0.54 | 0.38 | 0.30 | 0.33 | 0.38 | 0.36 |
| Fe | 96.77 | 95.14 | 96.27 | 94.76 | 95.29 | 95.11 | 95.80 | 95.68 | 95.46 | 95.54 |
| Others | Balance | |||||||||
| Ar | Air | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Al | 3.58 | 3.91 | 3.16 | 2.50 | 2.64 | 4.13 | 3.93 | 2.86 | 3.53 | 3.63 |
| Si | 0.36 | 0.37 | 0.33 | 0.41 | 0.39 | 0.33 | 0.34 | 0.32 | 0.37 | 0.40 |
| Fe | 91.74 | 92.12 | 88.86 | 94.55 | 93.96 | 92.71 | 93.38 | 90.08 | 93.29 | 94.24 |
| O | 1.46 | 0.59 | 0.74 | 0.50 | 0.46 | 1.11 | 0.42 | 0.64 | 0.45 | 0.61 |
| Others | Balance | |||||||||
| Location | Atmospheres | Al | Si | Mn |
|---|---|---|---|---|
| I zone | Air | 4.84 | 0.88 | 0.81 |
| Ar | 5.7 | 0.88 | 1.08 | |
| II zone | Air | 6.83 | 1.01 | 0.77 |
| Ar | 9.37 | 1.24 | 0.69 | |
| III zone | Air | 3.51 | 1.14 | 0.96 |
| Ar | 4.28 | 0.95 | 0.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Di, H.-S.; Wang, X.-N.; Chen, X.-M.; Qi, X.-N.; Li, J.-P. A Study on Microstructure and Properties of PHS Fiber Laser Welded Joints Obtained in Air Atmospheres. Materials 2018, 11, 1135. https://doi.org/10.3390/ma11071135
Sun Q, Di H-S, Wang X-N, Chen X-M, Qi X-N, Li J-P. A Study on Microstructure and Properties of PHS Fiber Laser Welded Joints Obtained in Air Atmospheres. Materials. 2018; 11(7):1135. https://doi.org/10.3390/ma11071135
Chicago/Turabian StyleSun, Qian, Hong-Shuang Di, Xiao-Nan Wang, Xia-Ming Chen, Xiao-Nan Qi, and Jian-Ping Li. 2018. "A Study on Microstructure and Properties of PHS Fiber Laser Welded Joints Obtained in Air Atmospheres" Materials 11, no. 7: 1135. https://doi.org/10.3390/ma11071135
APA StyleSun, Q., Di, H.-S., Wang, X.-N., Chen, X.-M., Qi, X.-N., & Li, J.-P. (2018). A Study on Microstructure and Properties of PHS Fiber Laser Welded Joints Obtained in Air Atmospheres. Materials, 11(7), 1135. https://doi.org/10.3390/ma11071135





