Enhancement on the Surface Hydrophobicity and Oleophobicity of an Organosilicon Film by Conformity Deposition and Surface Fluorination Etching
Abstract
1. Introduction
2. Material Preparation and Experimental Procedure
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Uhlmann, P.; Frenzel, R.; Voit, B.; Mock, U.; Szyszka, B.; Schmidt, B.; Ondratschek, D.; Gochermann, J.; Roths, K. Research agenda surface technology: Future demands for research in the field of coatings materials. Prog. Org. Coat. 2007, 58, 122–126. [Google Scholar] [CrossRef]
- Wu, L.Y.L.; Nigian, S.K.; Chen, Z.; Xuan, D.T.T. Quantitative test method for evaluation of anti-fingerprint property of coated surface. Appl. Surf. Sci. 2011, 257, 2965–2969. [Google Scholar] [CrossRef]
- Yin, L.; Yang, J.; Tang, Y.; Chen, L.; Liu, C.; Tang, H.; Li, C. Mechanical durability of superhydrophobic and oleophobic copper meshes. Appl. Surf. Sci. 2014, 316, 259–263. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Yang, L.; Song, Y. Superoleophilic and superhydrophobic inverse opals for oil sensors. Adv. Funct. Mater. 2008, 18, 3258–3264. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, J.; Su, B.; Shi, L.; Wang, J.; Chen, S.; Wang, L.; Zi, J.; Song, Y.; Jiang, L. Collodial Photonic Crystals with narrow stopbands assembled from low-adhesive superhydrophobic substrates. J. Am. Chem. Soc. 2012, 134, 17053–17058. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J. Silicone hydrophobocity and oleophilicity. Silicon 2017, 9, 651–655. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Zhu, L.; Hao, G.; Chen, Y.; Chen, Y. Investigation on hydrophobic film from a hydrophobic powder. Appl. Surf. Sci. 2012, 261, 863–867. [Google Scholar] [CrossRef]
- Xu, K.; Hu, J.; Jiang, X.; Meng, W.; Lan, B.; Shu, L. Anti-icing performance of hydrophobic silicone-acrylate resin coatings on wind blades. Materials 2018, 8, 15. [Google Scholar] [CrossRef]
- Kujawa, J.; Al-Gharabli, S.; Kujawski, W.; Knozowska, K. Molecular grafting of fluorinated and nanofluorinated Alkylsiloxanes on various ceramic membrane surface for the removal of volatile organic compounds applying vacuum membrane distillation. ACS Appl. Mater. Interface 2017, 9, 6571–6590. [Google Scholar] [CrossRef] [PubMed]
- Al-Gharabli, S.; Hamad, E.; Saket, M.; El-Rub, Z.A.; Arafat, H.; Kujawski, W.; Kujawa, J. Advanced material-ordered nanotubular ceramic membranes covalently capped with single-wall carbon nanotubes. Materials 2018, 11, 739. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Chen, W.C.; Liu, D.S. Surface modification layer deposition on flexibility substrates by plasma-enhanced chemical vapour deposition using tertamethylsilane-oxygen gas mixture. J. Phys. D Appl. Phys. 2008, 41, 225305. [Google Scholar] [CrossRef]
- Liu, D.S.; Wu, C.Y. Adhesion enhancement of hard coatings deposited on flexible plastic substrates using an interfacial buffer layer. J. Phys. D Appl. Phys. 2010, 43, 175301. [Google Scholar] [CrossRef]
- Öner, D.; McCarthy, T.J. Ultrahydrophobic surface. Effects of topography length scales on wettability. Langmuir 2000, 16, 7777–7782. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; Mchale, G.; Newton, M.I.; Perry, C.C. Intrinsically superhydrophobic organosilica sol-gel foams. Langmir 2003, 19, 5626–5631. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional surface structures of plants: An inspiration for biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Wu, F.L.; Chen, W.Y. Super water- and oil-repellences form silica-based nanocoatings. Surf. Coat. Technol. 2009, 203, 3377–3384. [Google Scholar] [CrossRef]
- Cansoy, C.E.; Erbil, H.Y.; Akar, O.; Akin, T. Effect of pattern size and geometry on the use of Cassie-Baxter equation for superhydrophobic surfaces. Colloid Surf. A-Physicochem. Eng. Asp. 2011, 386, 116–124. [Google Scholar] [CrossRef]
- Tian, D.; Song, Y.; Jiang, L. Patterning of controllable surface wettability for printing techniques. Chem. Soc. Rev. 2013, 42, 5184–5209. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.T.; Chen, J.M.; Kuo, R.R.; Lin, T.S.; Wu, C.F. Influence of surface roughness on water- and oil-repellent surfaces coated with nanoparticles. Appl. Surf. Sci. 2005, 240, 318–326. [Google Scholar] [CrossRef]
- Basu, B.J.; Kumar, V.D.; Anandan, C. Surface studies on superhydrophobic and oleophobic polydimethylsiloxane–silica nanocomposite coating system. Appl. Surf. Sci. 2012, 261, 807–814. [Google Scholar] [CrossRef]
- Aminayi, P.; Abidi, N. Ultra-oleophobic cotton fabric prepared using molecular and nanoparticle vapor deposition methods. Surf. Coat. Technol. 2015, 276, 636–644. [Google Scholar] [CrossRef]
- Durrent, J.; Frolet, N.; Gourgon, C. Hydrophobicity and anti-icing performances of nanoimprinted and roughened fluoropolymers film under overcooled temperature. Microelectron. Eng. 2016, 155, 1–6. [Google Scholar] [CrossRef]
- Gingery, D.; Bühlmann, P. Formation of gold nanoparticles on multiwalled carbon nanotubes by thermal evaporation. Carbon 2008, 46, 1966–1972. [Google Scholar] [CrossRef]
- Franc, J.; Bastl, Z. Nickel evaporation in high vacuum and formation of nickel oxide nanoparticles on highly oriented pyrolytic graphite. X-ray photoelectron spectroscopy an atomic force microscopy study. Thin Solid Films 2008, 516, 6095–6103. [Google Scholar] [CrossRef]
- Abou El-Nour, K.M.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Gromov, D.G.; Pavlova, L.M.; Savitsky, A.I.; Yu, T.A. Nucleation and growth of Ag nanoparticles on amorphous carbon surface from vapor phase formed by vacuum evaporation. Appl. Phys. A 2015, 118, 1297–1303. [Google Scholar] [CrossRef]
- Kang, C.Y.; Chao, C.H.; Shiu, S.C. Formation of self-organized platinum nanoparticles and their microphotoluminescence enhancement in the visible light region. J. Appl. Phys. 2007, 102, 073508. [Google Scholar] [CrossRef]
- Schmiitt, J.; Hajiw, S.; Lecchi, A.; Degrouard, J.; Salonen, A.; Impéror-Clerc, M.; Pansu, B. Formation of superlattices of gold nanoparticles using Ostwald ripening in emulsions: transition for fcc to bcc structure. J. Phys. Chem. B 2016, 120, 5759–5766. [Google Scholar] [CrossRef] [PubMed]
- Igathinathane, C.; Pordesimo, L.O.; Columbus, E.P.; Batchelor, W.D.; Methuku, S.R. Shape identification and particles size distribution from basic shape parameters using Image. J. Comput. Electron. Agric. 2008, 63, 168–182. [Google Scholar] [CrossRef]
- Maier, S.A.; Atwater, H.A. Plasmonics: Localization and guiding of electromagnetic energy in metal/dielectric structures. J. Appl. Phys. 2005, 98, 011101. [Google Scholar] [CrossRef]
- Willets, K.A.; Wan Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.F.; Chin, C.C.; Hung, D.W.; Wei, P.K. Transparent electrode for organic solar cells using multilayer structures with nanoporous silver film. Sol. Energy Mater. Sol. Cells 2013, 118, 81–89. [Google Scholar] [CrossRef]
- Zhang, S.G.; Zhang, X.W.; Yin, Z.G.; Wang, J.X.; Si, F.T.; Gao, H.L.; Dong, J.J.; Liu, X. Optimization of electroluminescence from n-ZnO/AlN/p-GaN light-emitting diodes by tailoring Ag localizer surface plasmon. J. Appl. Phys. 2012, 112, 013112. [Google Scholar] [CrossRef]
- Mogensen, K.B.; Kneipp, K. Size-dependent shifts of plasmon resonance in silver nanoparticle films using controlled dissolution: Monitoring the onset of the surface screening effects. J. Phys. Chem. C 2014, 118, 28075–28083. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surface to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Hong, B.S.; Han, J.H.; Kim, S.T.; Cho, Y.J.; Park, M.S.; Dolukhanyan, T.; Sung, C. Endurable water-repellent glass for automobiles. Thin Solid Films 1999, 351, 274–278. [Google Scholar] [CrossRef]
- Tan, I.H.; da Silva, M.L.P.; Demarquette, N.R. Paper surface modification by plasma deposition of double layers of organic silicon compounds. J. Mater. Chem. 2001, 11, 1019–1025. [Google Scholar] [CrossRef]
- Teshima, K.; Sugimura, H.; Inoue, Y.; Takai, O. Gas barrier performance of surface-modified silica films with grafted organosilane molecules. Langmuir 2003, 19, 8331–8334. [Google Scholar] [CrossRef]
- Kim, J.D.; Lee, K.Y.; Kim, K.Y.; Sugimura, H.; Takai, O.; Wu, Y.; Inoue, Y. Characteristics and high water-repellency of a-C:H films deposited by r.f. PECVD. Surf. Coat. Technol. 2003, 162, 135–139. [Google Scholar] [CrossRef]
- Wu, C.Y.; Liao, R.M.; Lai, L.W.; Jeng, M.S.; Liu, D.S. Organosilicon/silicon oxide gas barrier structure encapsulated flexible plastic substrate by using plasma-enhanced chemical vapor deposition. Surf. Coat. Technol. 2012, 206, 4685–4691. [Google Scholar] [CrossRef]
- Nakagawa, T.; Hiwatashi, T. Water-repellent thin films from mixtures of fluoroalkylmethoxysilane and bis-(trialkoxysilyl)alkanes of various carbon-chain lengths using the sol–gel method and the fluoroalkylmethoxysilane dispersion mechanism. J. Non-Cryst. Solids 2003, 316, 228–237. [Google Scholar] [CrossRef]
- Kang, G.S.; Ko, H.J.; Choi, C.K. Chemical bond structure of a-C:F films with a low dielectric constant deposited by using CH4/CF4 ICPCVD. J. Korean Phys. Soc. 2003, 42, 676–681. [Google Scholar]
- Chen, G.; Zhang, J.; Yang, S. Fabrication of hydrophobic fluorinated amorphous carbon thin films by an electrochemical route. Electrochem. Commun. 2008, 10, 7–11. [Google Scholar] [CrossRef]
- Choi, W.K.; Ong, T.Y.; Tan, L.S.; Loh, F.C.; Tan, K.L. Infrared and x-ray photoelectron spectroscopy studies of as-prepared and furnace-annealed radio-frequency sputtered amorphous silicon carbide films. J. Appl. Phys. 1998, 83, 4968–4973. [Google Scholar] [CrossRef]
- Brinkmann, M.; Chan, V.Z.H.; Thomas, E.L.; Lee, V.Y.; Miller, D.; Hadjichristidis, N.; Avgeropoulos, A. Room-temperature synthesis of a-SiO2 thin films by UV-assisted ozonolysis of a polymer precursor. Chem. Mater. 2001, 13, 967–972. [Google Scholar] [CrossRef]
- Trey, S.M.; Sidenvall, P.; Alavi, K.; Ståhlberg, D.; Johansson, M. Dual cure (UV/thermal) primers for composite substrates—Effect of surface treatment and primer composition on adhesion. Prog. Org. Coat. 2009, 64, 489–496. [Google Scholar] [CrossRef]
- Khung, Y.L.; Nghlim, S.H.; Meda, L.; Narducci, D. Preferential formation of Si-O-C over Si-C linkage upon thermal grafting on hydrogen-terminated silicon (111). Chemistry 2014, 20, 15151–15158. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Bhandavat, R.; Barrera, U.; Singh, G. Silicon oxycarbide glass-graphene composite paper electrode for long cycle lithium-ion batteries. Nat. Commun. 2016, 7, 10998. [Google Scholar] [CrossRef] [PubMed]
- Ferraria, A.M.; da Silva, J.D.L.; do Rego, A.M.B. XPS studied of directly fluorinated HDPE: Problem and solution. Polymer 2003, 44, 7241–7249. [Google Scholar] [CrossRef]
- Wang, C.; Lai, P.C.; Syu, S.H.; Leu, J. Effects of CF4 plasma treatment on the moisture uptake, diffusion, and WVTR of poly(ethylene terephthalate) flexible films. Surf. Coat. Technol. 2011, 206, 318–324. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Bharathidasan, T.; Bera, P.; Basu, B.J. Fabrication of superhydrophobic and oleophobic sol–gel nanocomposite coating. Surf. Coat. Technol. 2012, 206, 3888–3894. [Google Scholar] [CrossRef]
- True, J.E.; Thomas, T.D.; Winter, R.W.; Gard, G.L. Electronegativities from core-ionization energies: Electronegativities of SF5 and CF3. Inorg. Chem. 2003, 42, 4437–4441. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Cai, S.; Wu, L.; Yang, W.; Xie, J.; Wen, W.; Zheng, J.C.; Zhu, Y. Surface modified CFx cathode material for ultrafast discharge and high energy density. J. Mater. Chem. A 2014, 2, 20896–20901. [Google Scholar] [CrossRef]
- Li, Y.; Veith, G.M.; Browning, K.I.; Chen, J.; Hensley, D.K.; Paranthaman, M.P.; Dai, S.; Sun, X.G. Lithium malonatoborate additives enabled stable cycling of 5 V lithium metal and lithium ion batteries. Nano Energy 2017, 40, 9–19. [Google Scholar] [CrossRef]
- Ma, J.; Guo, X.; Zhang, Y.; Ge, H. Catalytic performance of TiO2@Ag composites prepared by modified photodeposition method. Chem. Eng. J. 2014, 258, 247–253. [Google Scholar] [CrossRef]
- Liu, F.C.; Li, J.Y.; Chen, T.H.; Chang, C.H.; Lee, C.T.; Hsiao, W.H.; Liu, D.S. Effect of Silver Dopants on the ZnO Thin Films Prepared by a Radio Frequency Magnetron Co-Sputtering System. Materials 2017, 10, 797–808. [Google Scholar] [CrossRef] [PubMed]
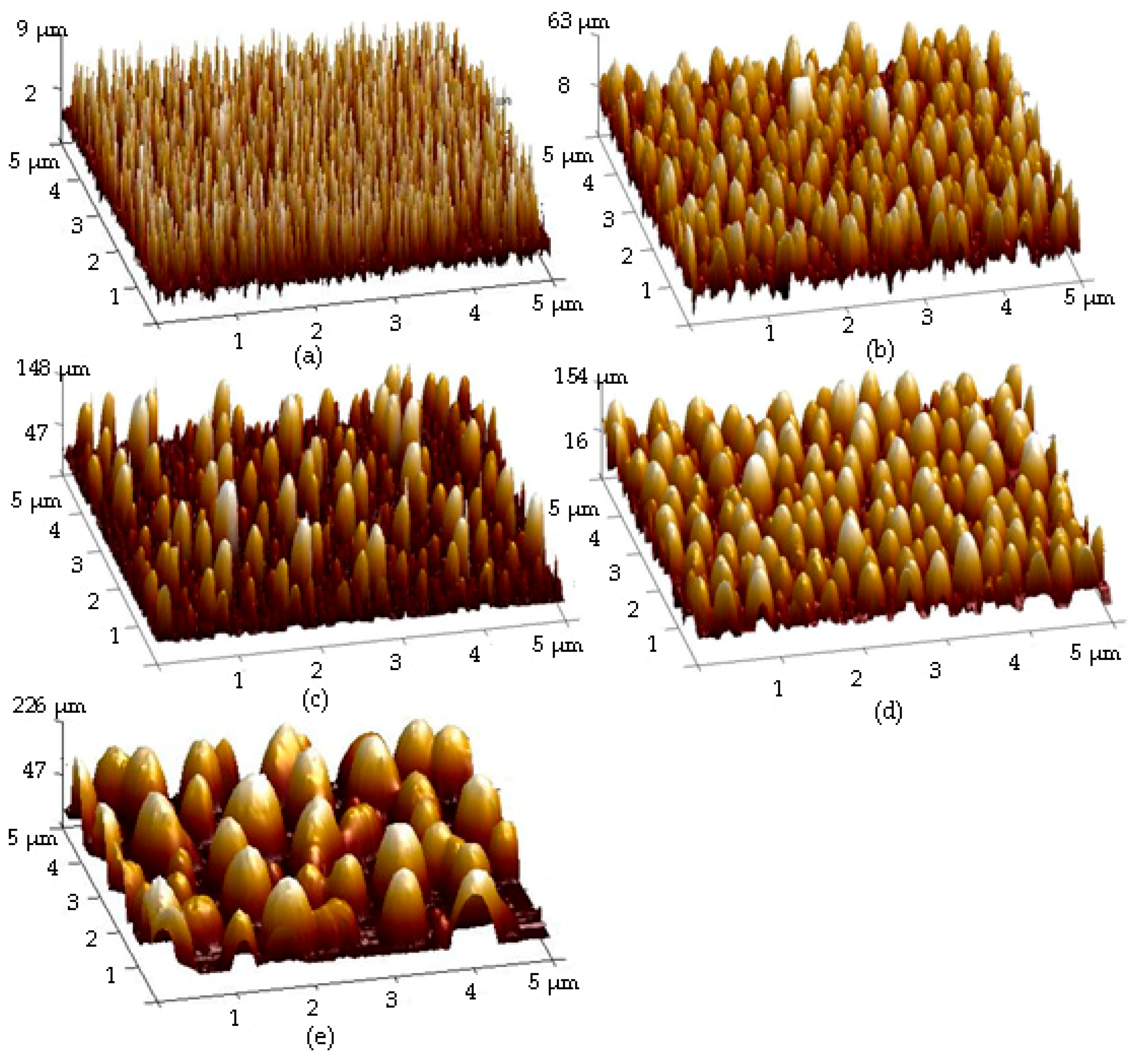
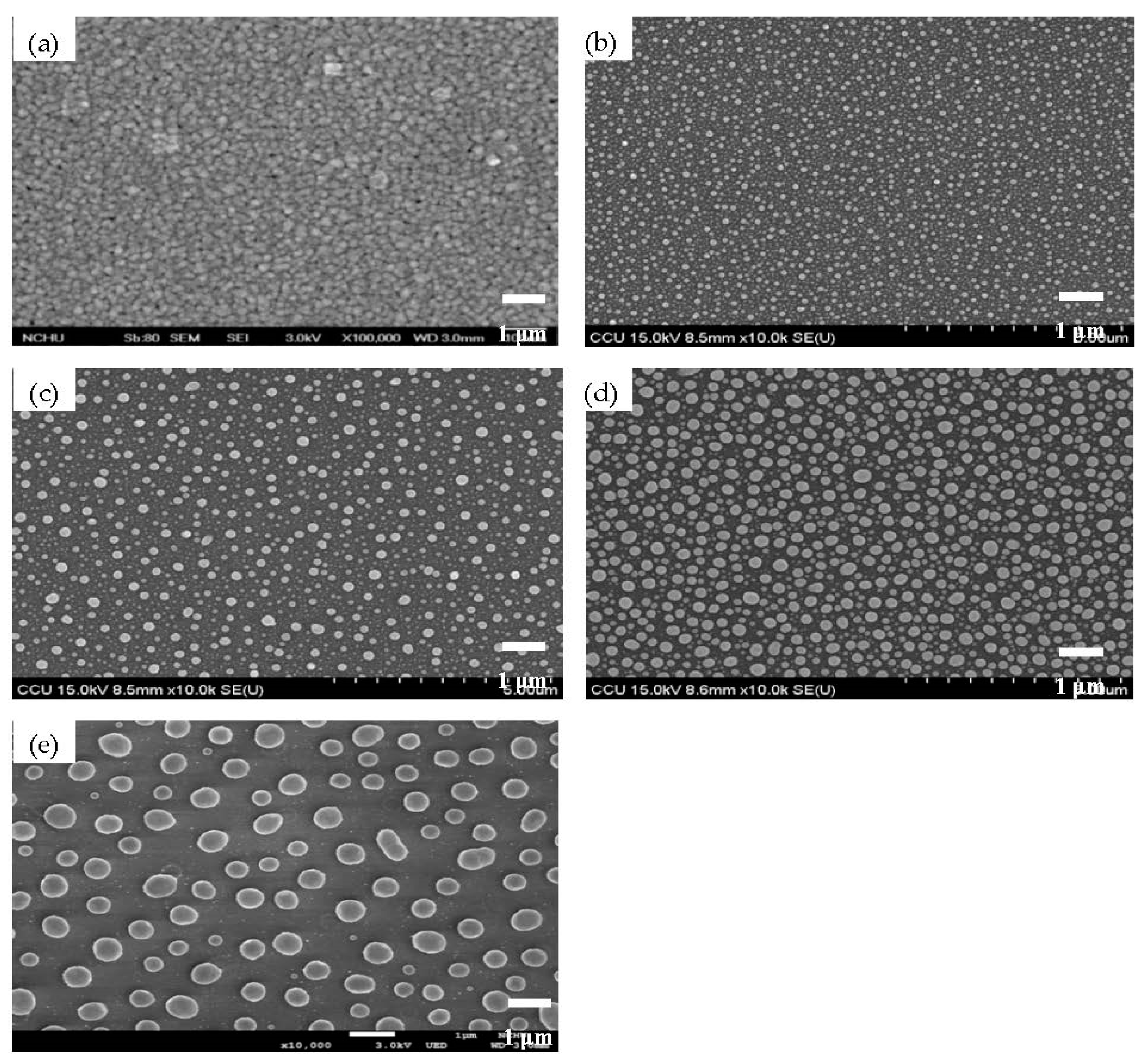
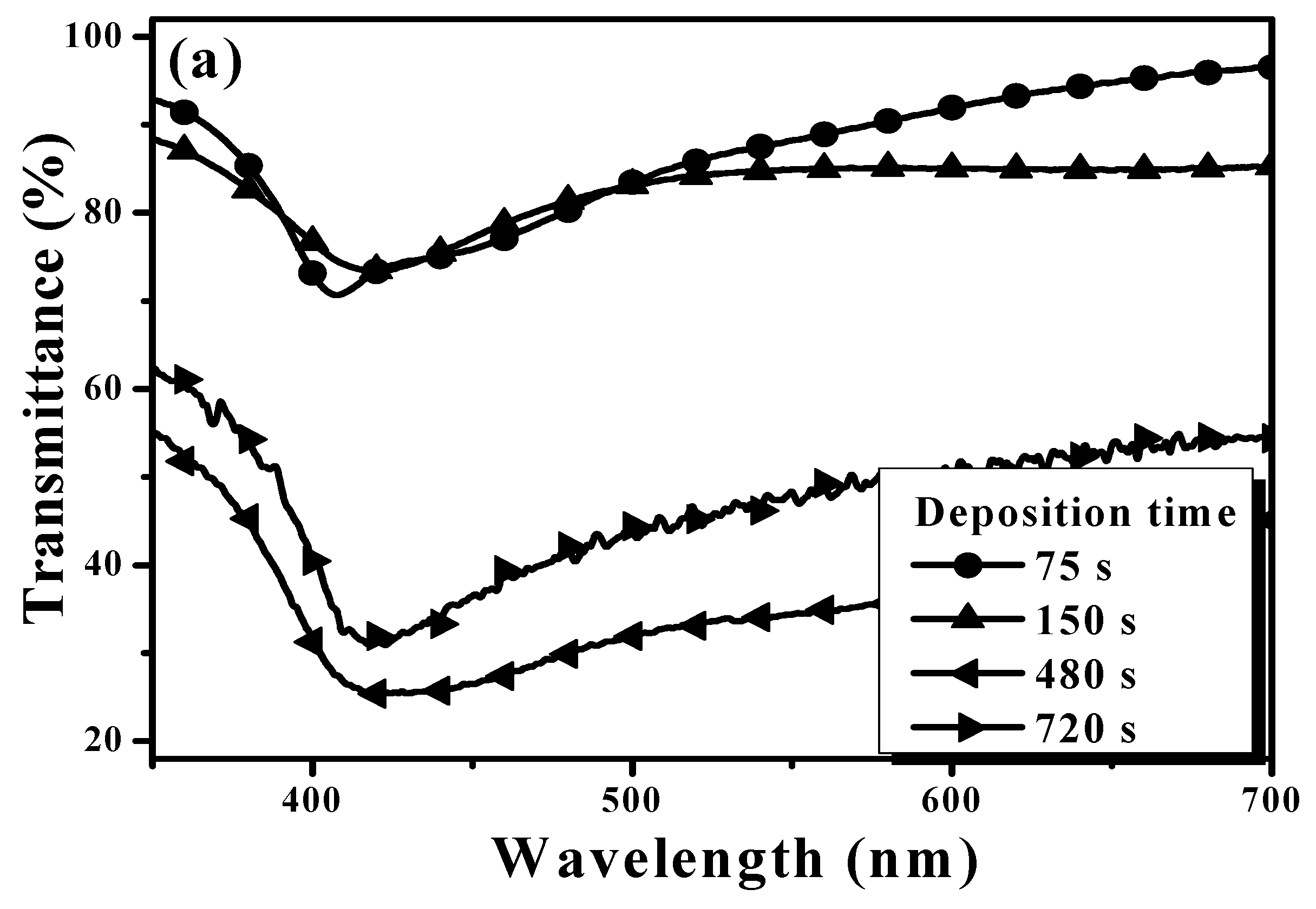
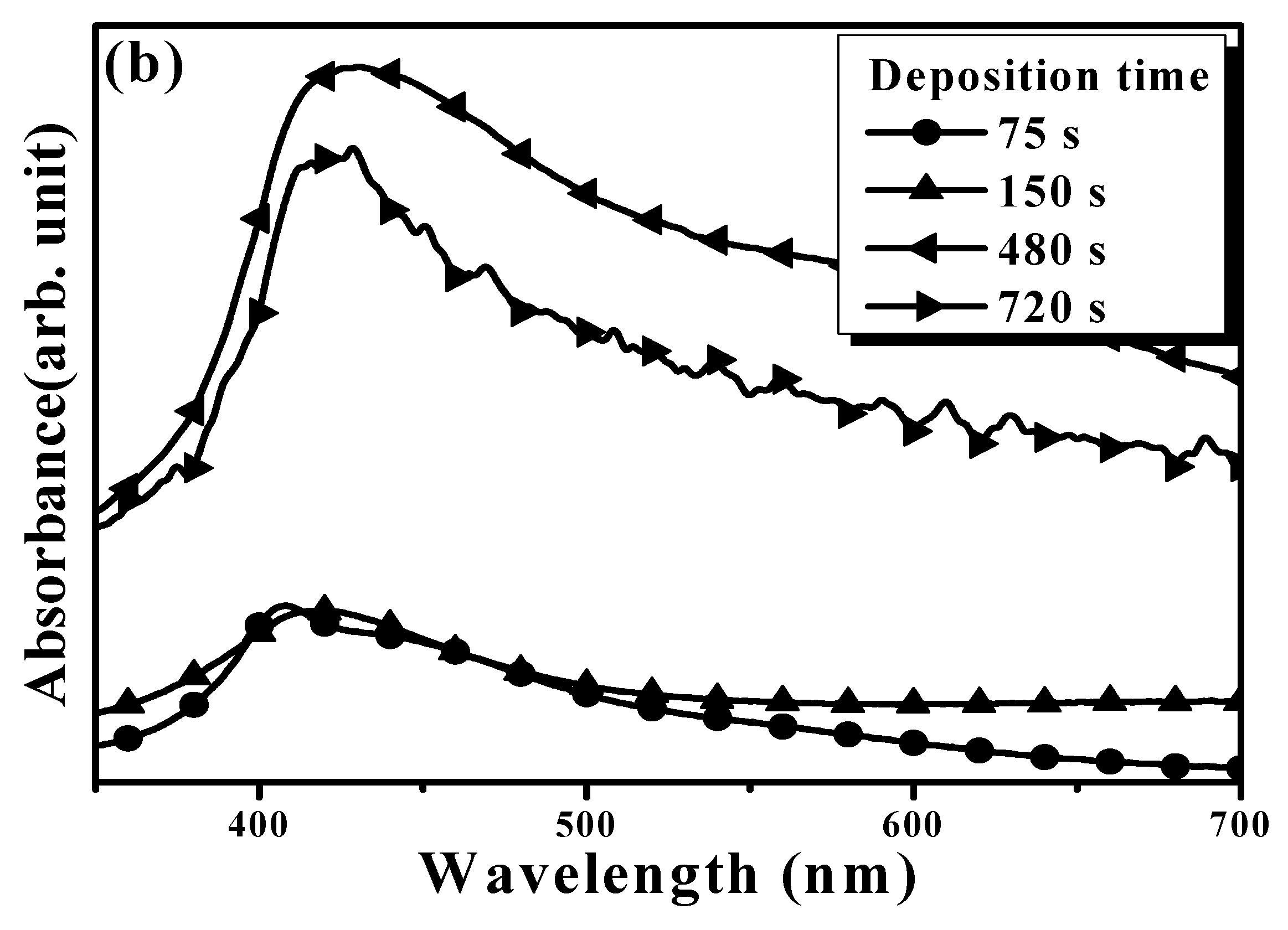
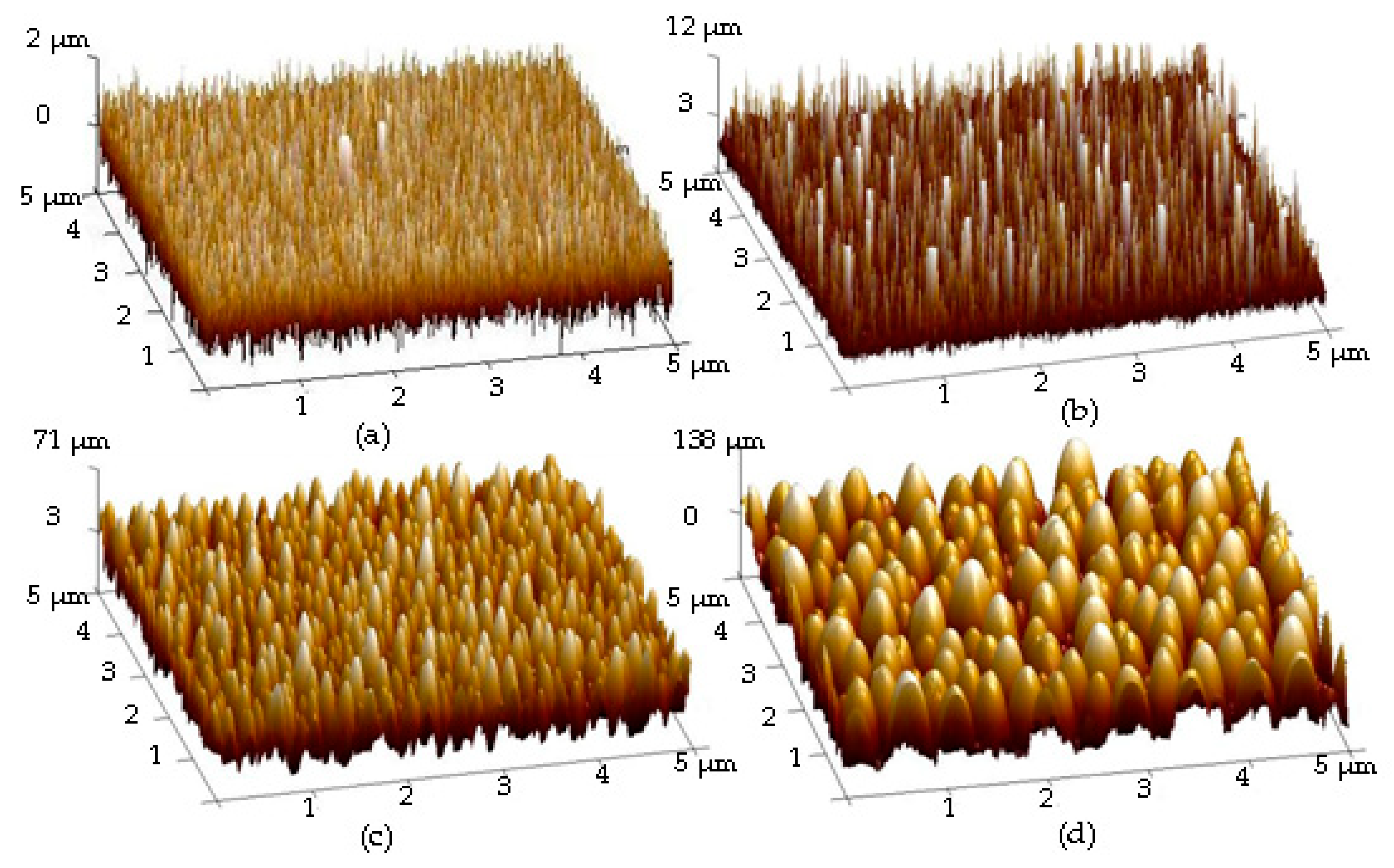
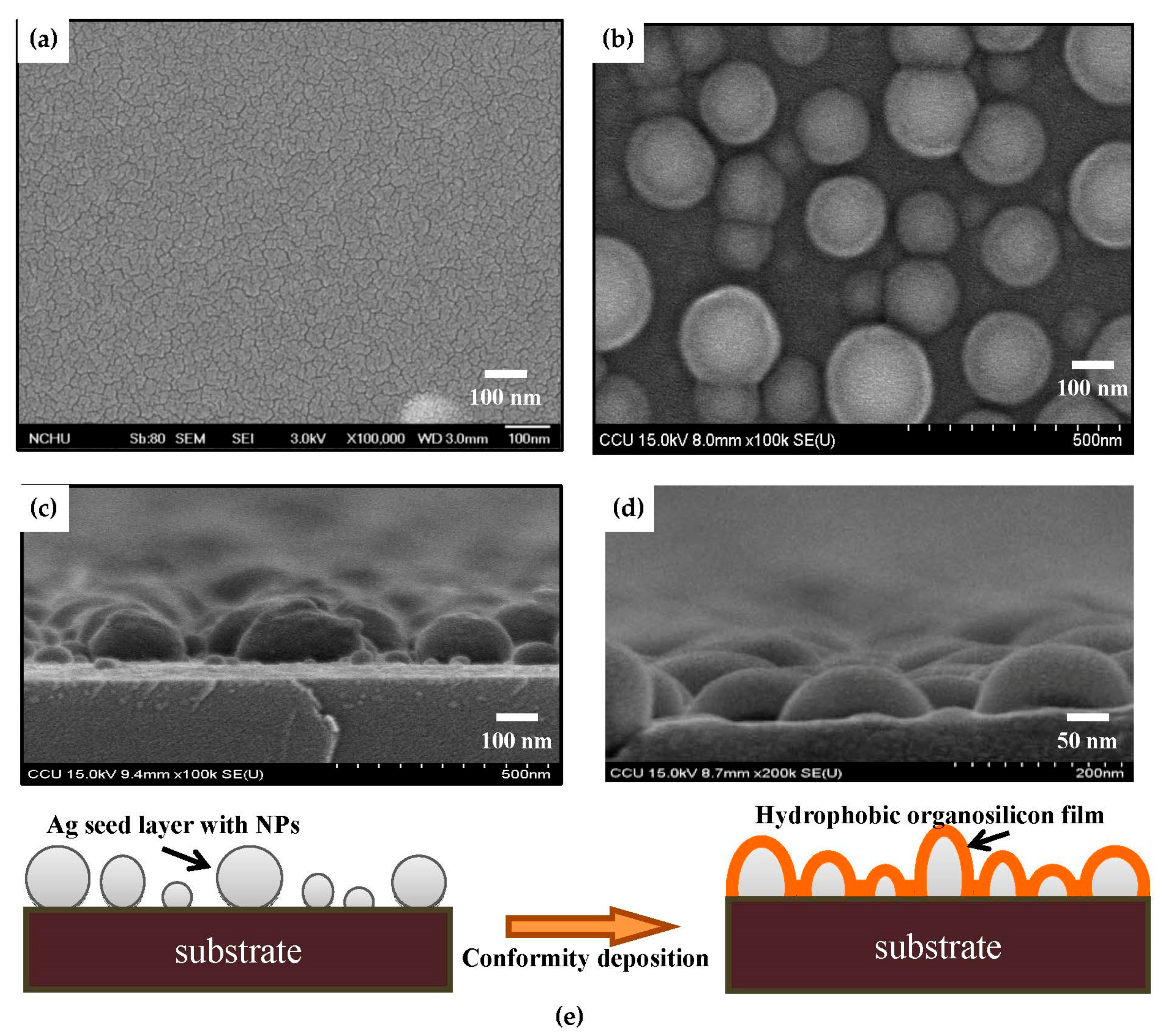
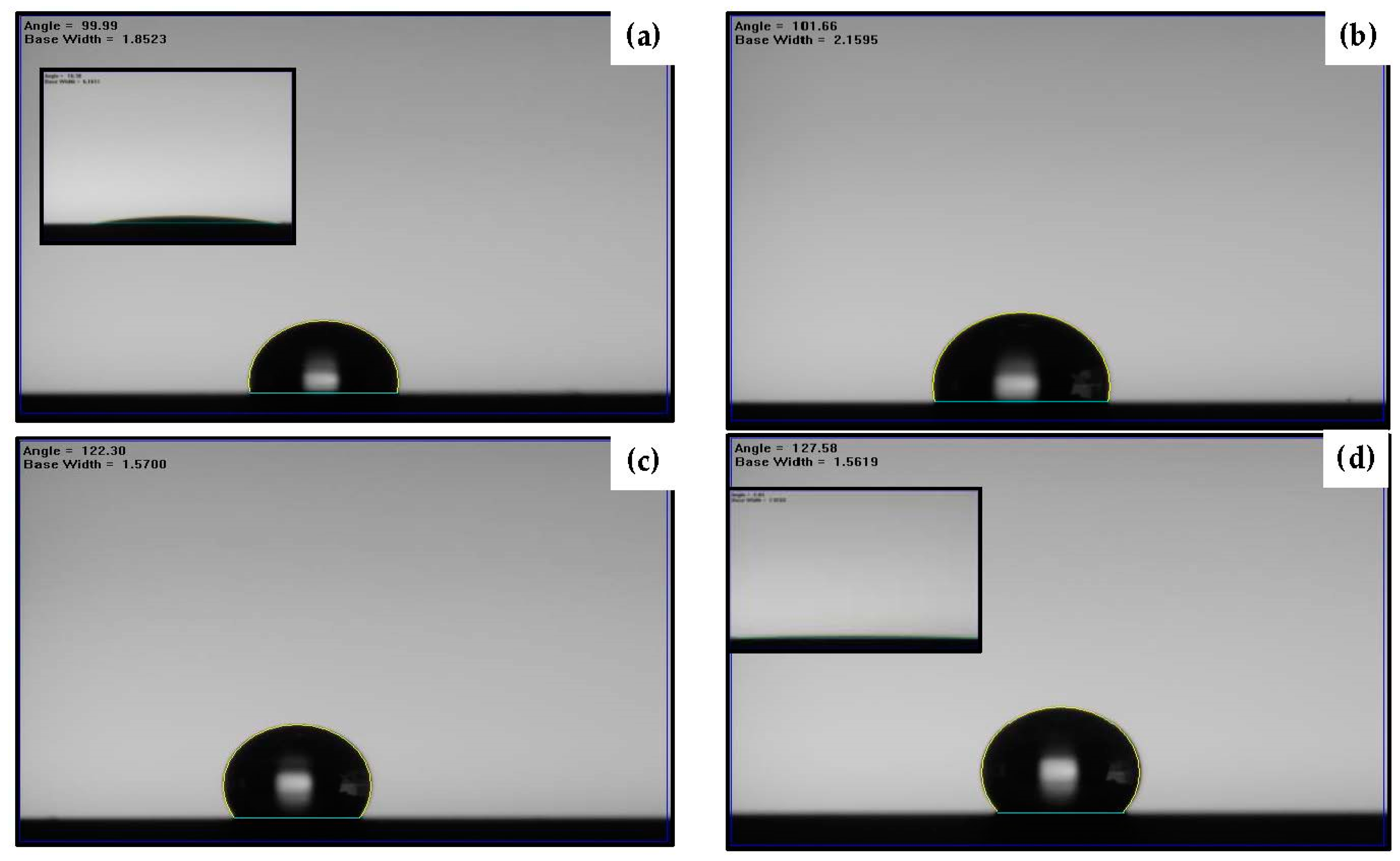
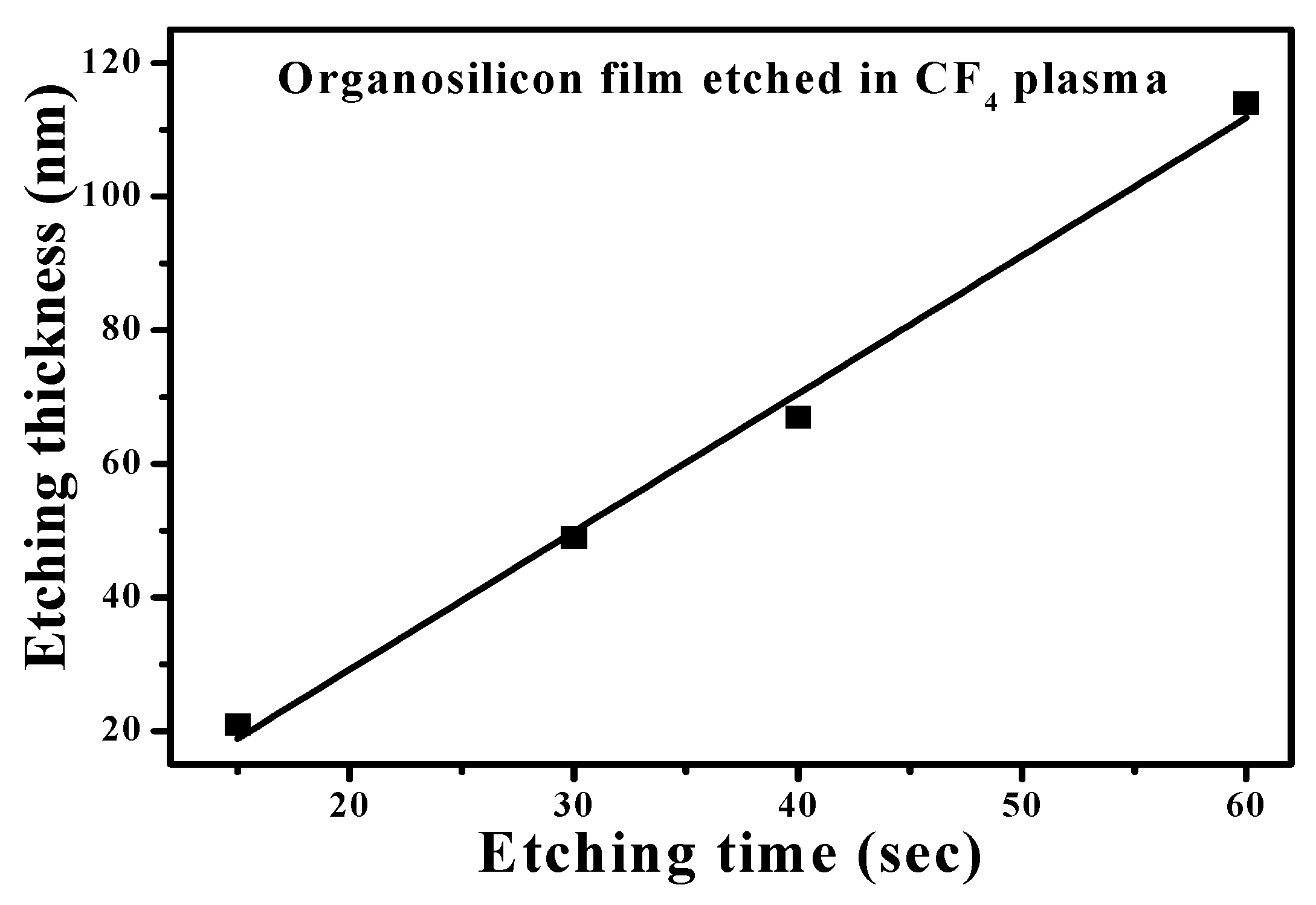
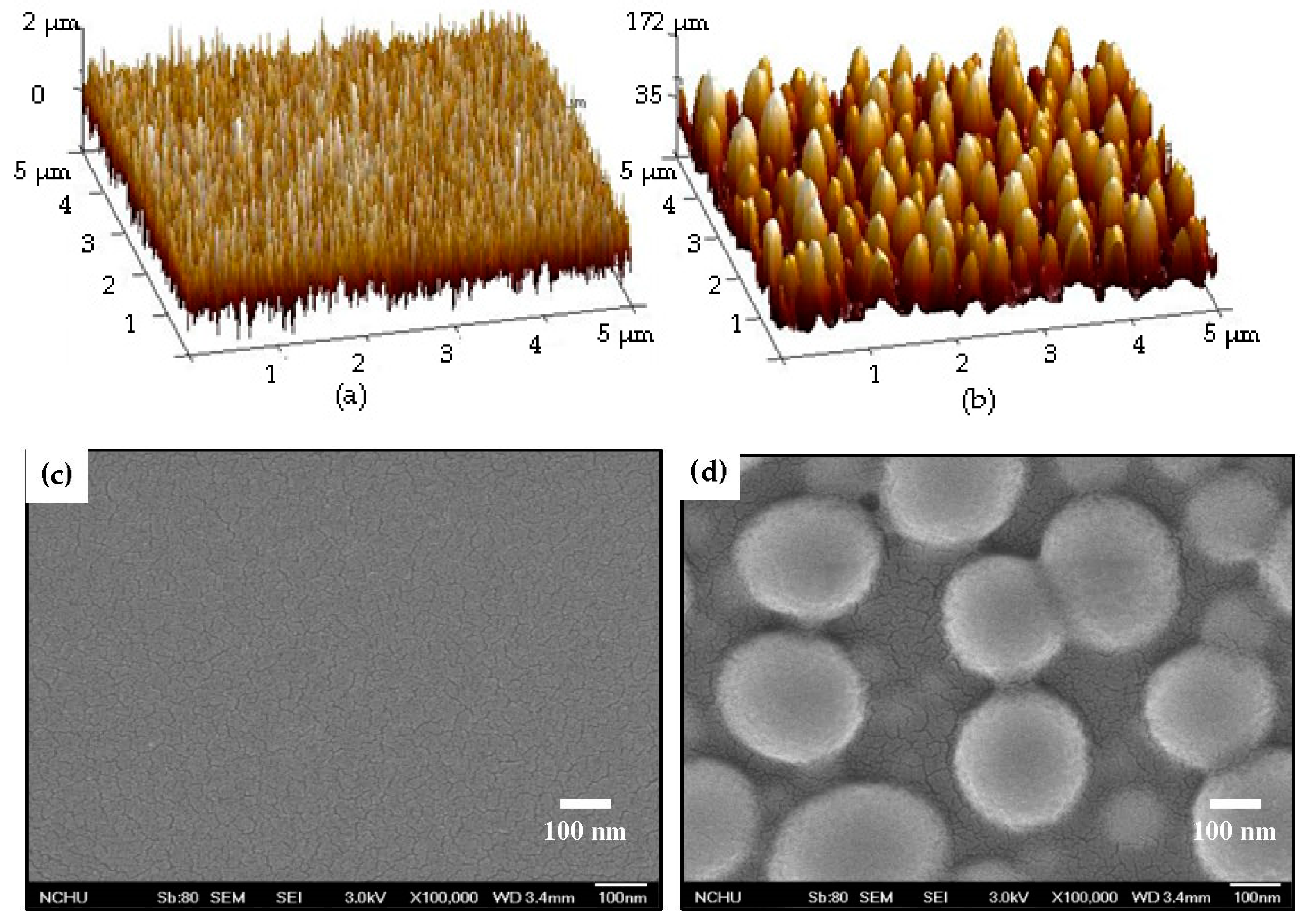
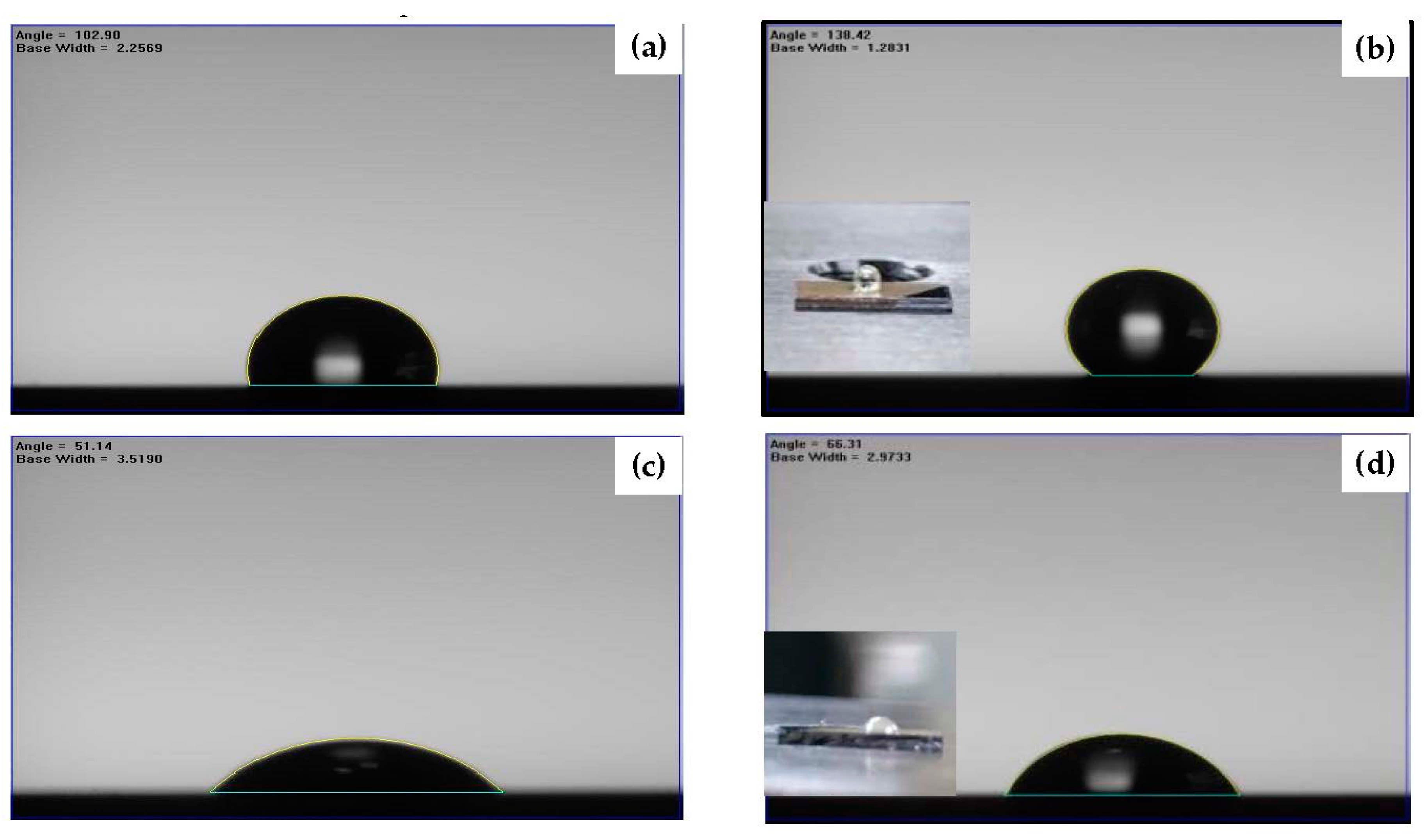


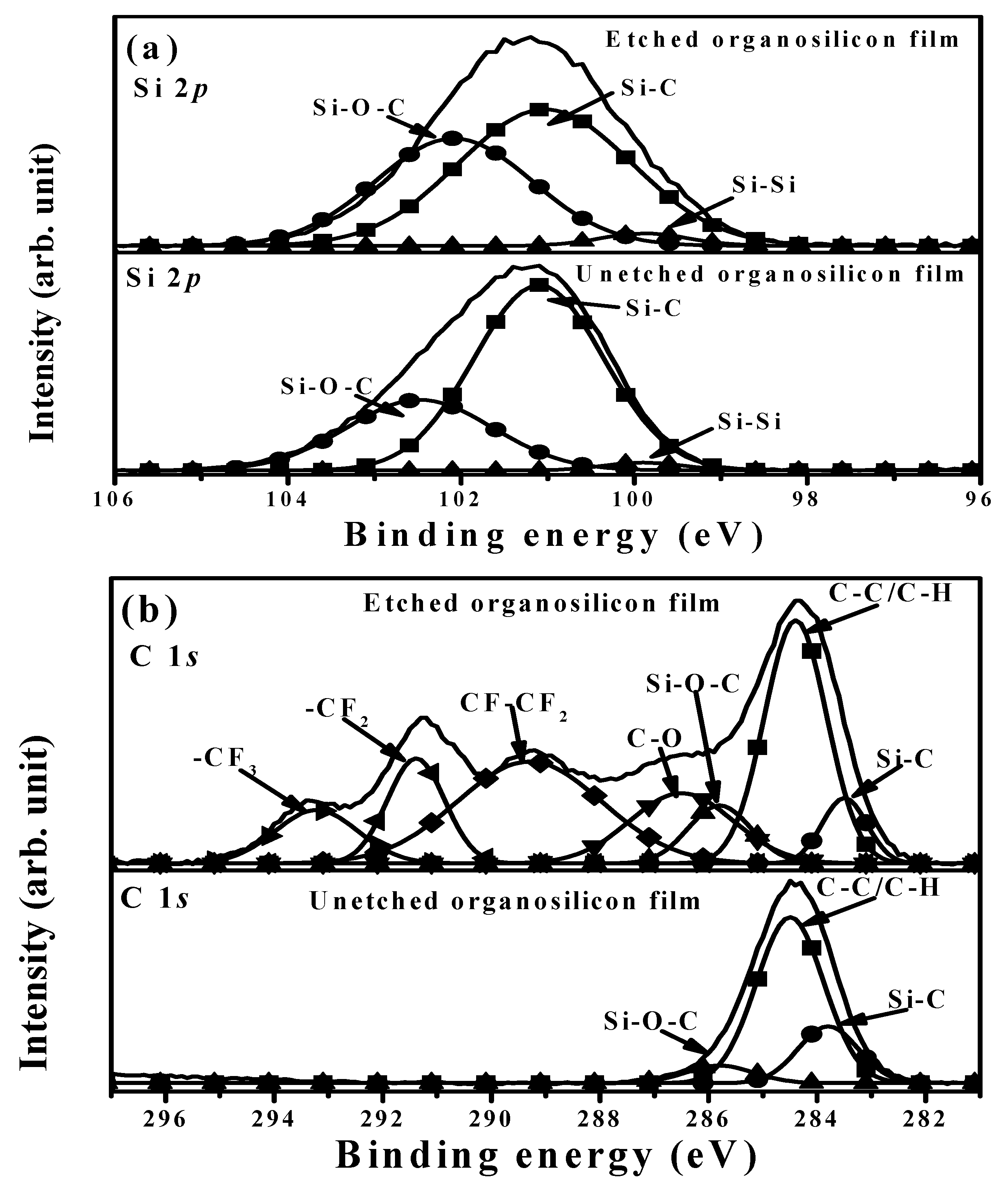
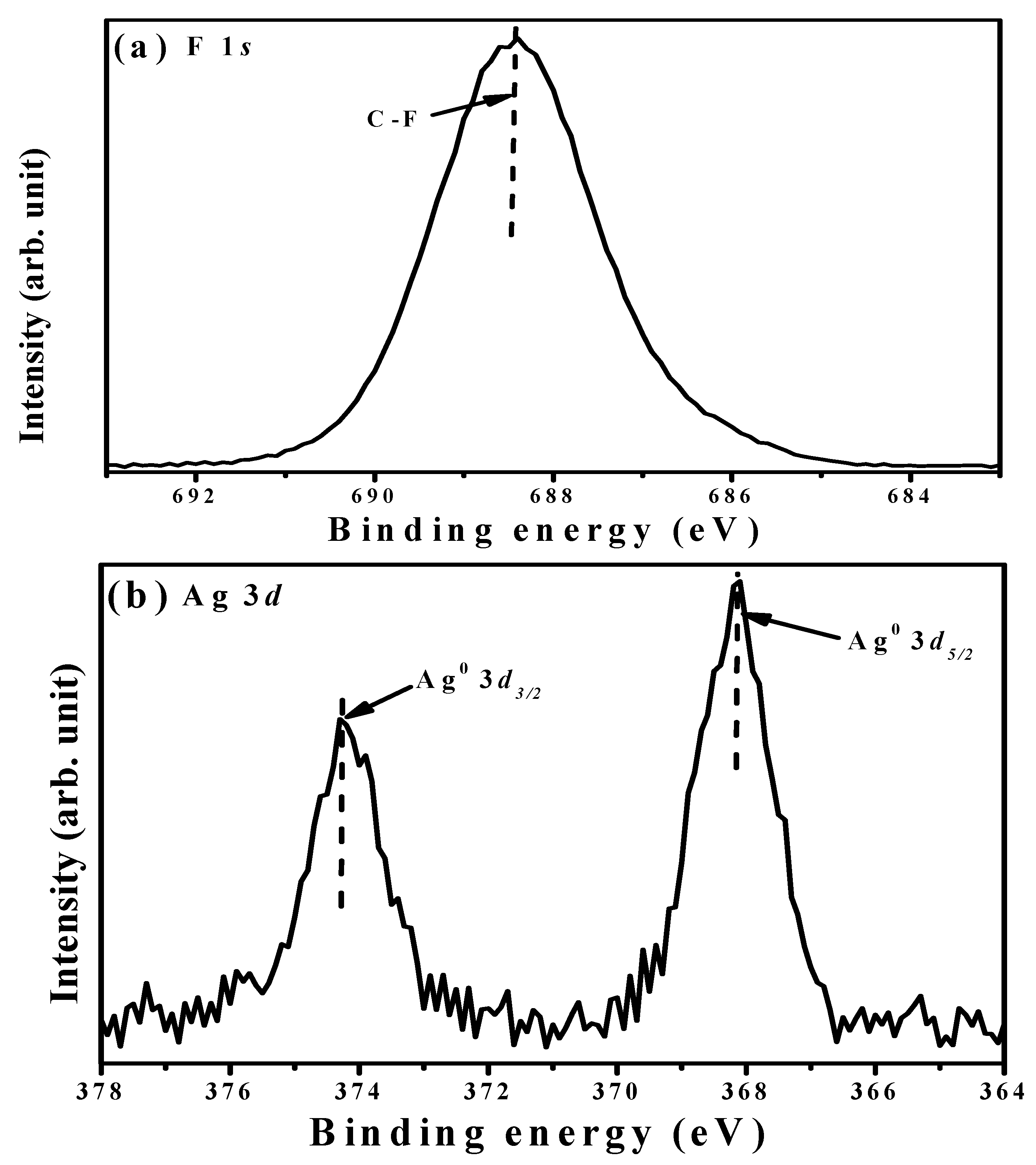
| Sample | Ag Deposition Time (s) | ||||
|---|---|---|---|---|---|
| Parameter | 75 | 150 | 480 | 720 | |
| Rq (nm) | 18.2 | 31.6 | 52.8 | 76.0 | |
| Mean particle size (nm) | 72 ± 44 | 84 ± 66 | 181 ± 97 | 232 ± 138 | |
| Tavg (%) | 86 | 82 | 35 | 46 | |
| Surface coverage (%) | 20.4 | 22.9 | 41.3 | 28.1 | |
| Absorbance peak (nm) | 411 | 417 | 437 | 438 | |
| Sample | Rq (nm) | WCA (°) | OCA (°) |
|---|---|---|---|
| Organosilicon on silicon substrate | 0.4 | 100 ± 0.2 | 11 ± 1.1 |
| Organosilicon on as-deposited Ag seed layer | 1.9 | 103 ± 0.6 | 10 ± 0.4 |
| Organosilicon on annealed Ag seed layer (150 s) | 18.1 | 122 ± 0.5 | 7 ± 0.5 |
| Organosilicon on annealed Ag seed layer (480 s) | 49.8 | 128 ± 0.8 | 3 ± 0.3 |
| Sample | CF4 Plasma Etching Time (s) | |||||
|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 40 | 60 | ||
| Organosilicon on silicon substrate | WCA (o) | 100 ± 0.2 | 104 ± 0.3 | 103 ± 0.2 | 103 ± 0.3 | 103 ± 0.4 |
| OCA (o) | 11 ± 1.1 | 50 ± 1.4 | 51 ± 1.0 | 51 ± 1.6 | 50 ± 0.8 | |
| Organosilicon on annealed Ag seed layer | WCA (o) | 128 ± 0.8 | 132 ± 0.9 | 135 ± 0.8 | 138 ± 0.6 | 70 ± 0.7 |
| OCA (o) | 3 ± 0.3 | 60 ± 0.6 | 62 ± 0.9 | 66 ± 0.7 | 7 ± 0.2 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.-W.; Zhang, Y.-K.; Chen, T.-H.; Chang, J.-H.; Lee, T.-H.; Li, P.-Y.; Liu, D.-S. Enhancement on the Surface Hydrophobicity and Oleophobicity of an Organosilicon Film by Conformity Deposition and Surface Fluorination Etching. Materials 2018, 11, 1089. https://doi.org/10.3390/ma11071089
Xu Z-W, Zhang Y-K, Chen T-H, Chang J-H, Lee T-H, Li P-Y, Liu D-S. Enhancement on the Surface Hydrophobicity and Oleophobicity of an Organosilicon Film by Conformity Deposition and Surface Fluorination Etching. Materials. 2018; 11(7):1089. https://doi.org/10.3390/ma11071089
Chicago/Turabian StyleXu, Zheng-Wen, Yu-Kai Zhang, Tai-Hong Chen, Jin-How Chang, Tsung-Hsin Lee, Pei-Yu Li, and Day-Shan Liu. 2018. "Enhancement on the Surface Hydrophobicity and Oleophobicity of an Organosilicon Film by Conformity Deposition and Surface Fluorination Etching" Materials 11, no. 7: 1089. https://doi.org/10.3390/ma11071089
APA StyleXu, Z.-W., Zhang, Y.-K., Chen, T.-H., Chang, J.-H., Lee, T.-H., Li, P.-Y., & Liu, D.-S. (2018). Enhancement on the Surface Hydrophobicity and Oleophobicity of an Organosilicon Film by Conformity Deposition and Surface Fluorination Etching. Materials, 11(7), 1089. https://doi.org/10.3390/ma11071089




