Investigation of the Influence of Pre-Charged Hydrogen on Fracture Toughness of As-Received 2.25Cr1Mo0.25V Steel and Weld
Abstract
1. Introduction
2. Experimental Details
3. Results and Discussion
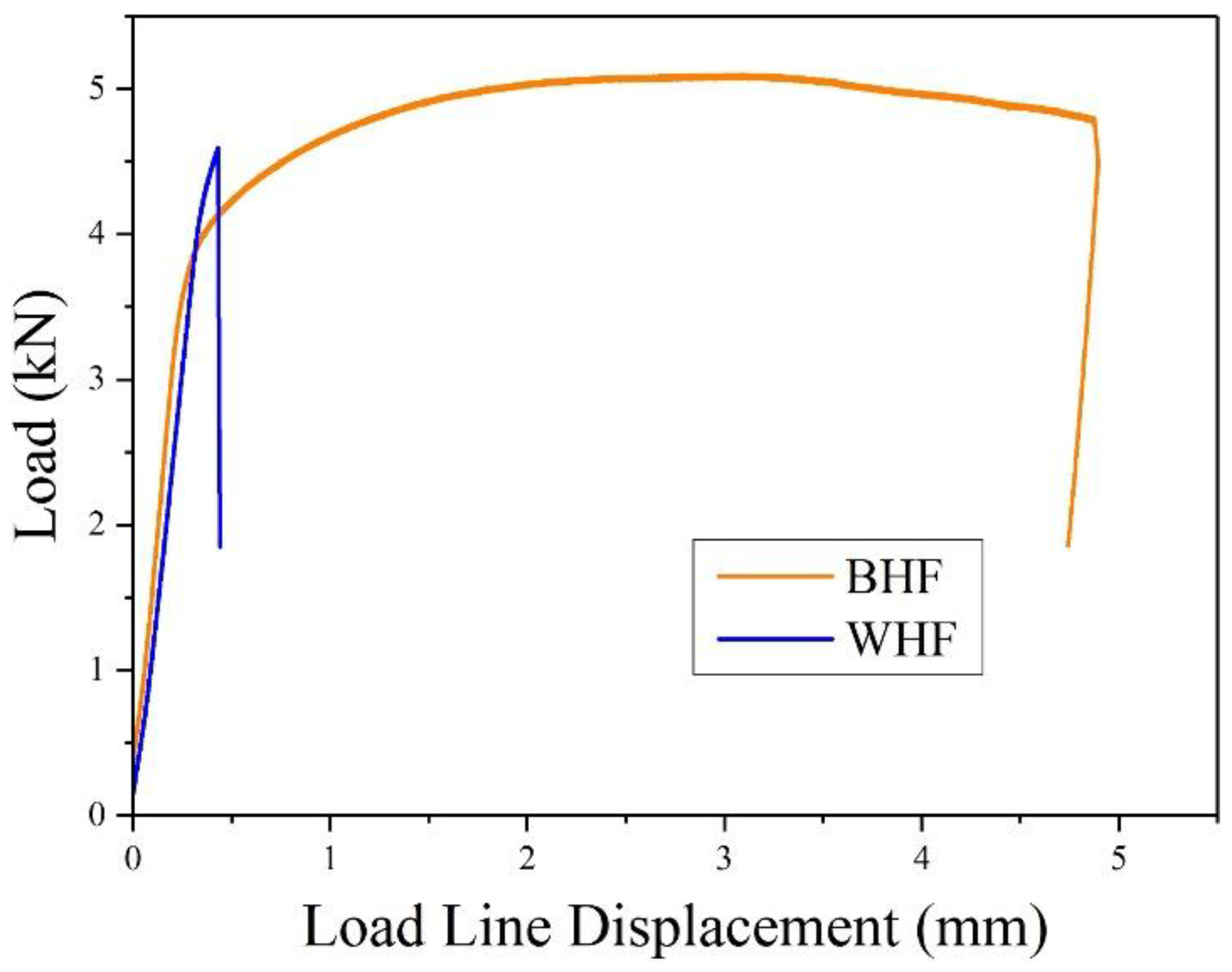
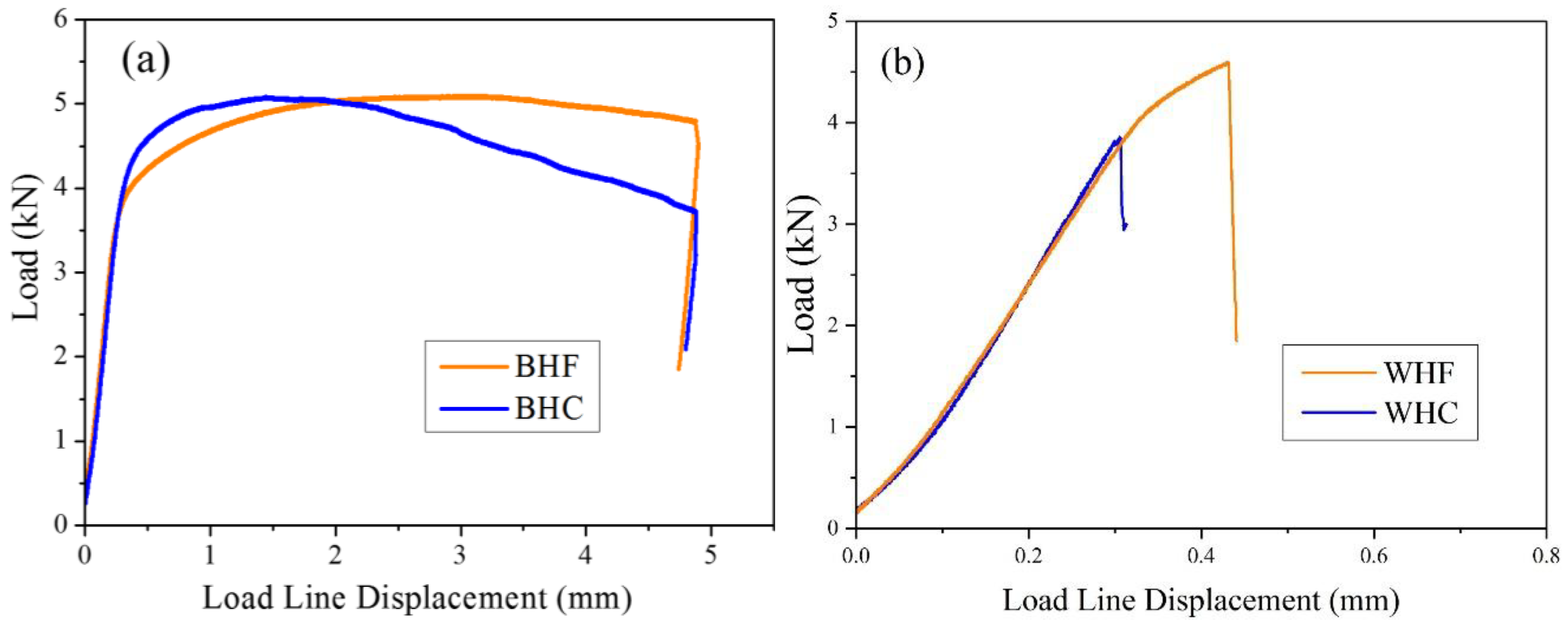
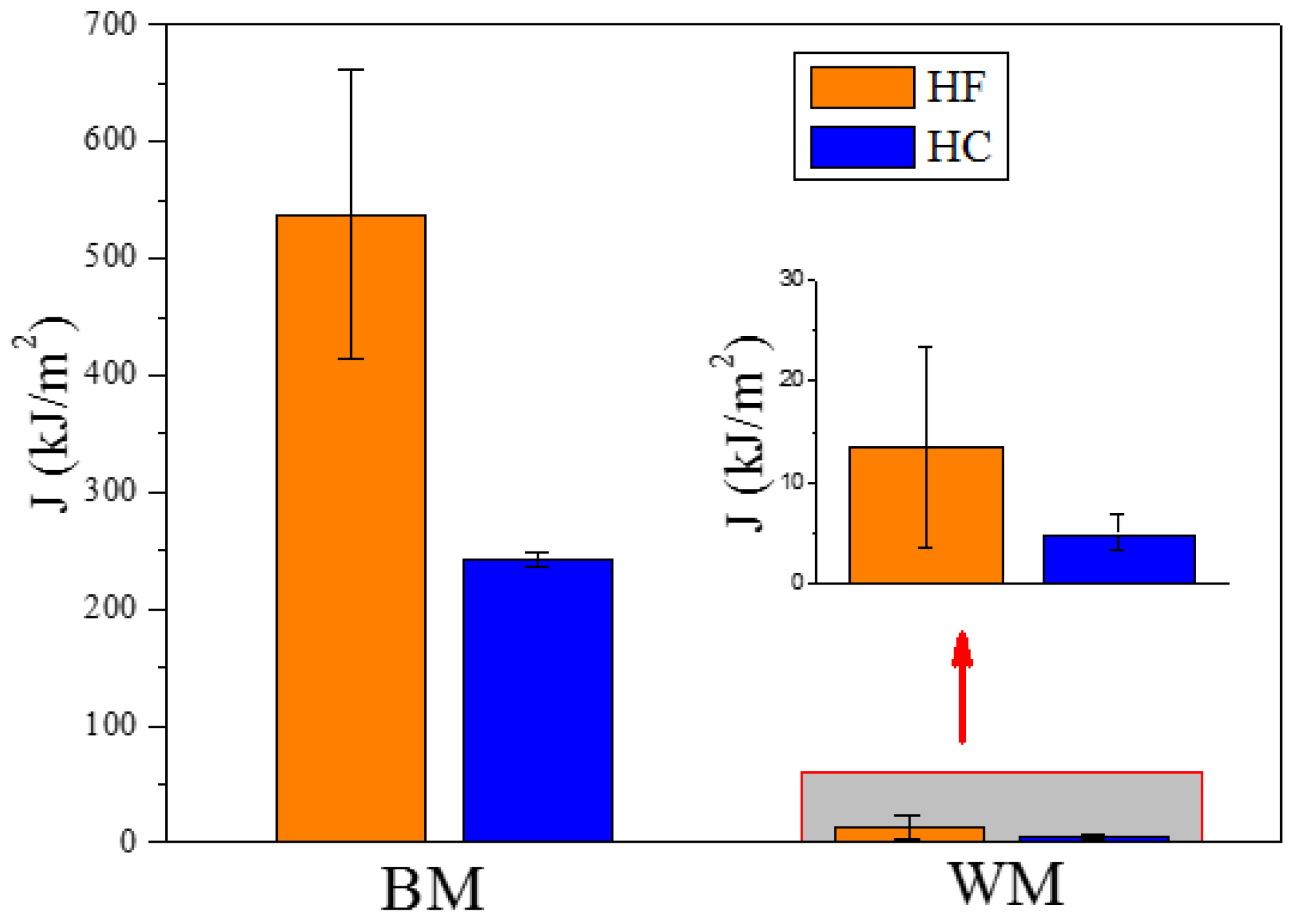
3.1. Estimation of Fracture Toughness
3.2. Fracture Appearance
3.3. Mechanisms of HE
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, C.Y.; Fu, R.D.; Zhou, W.H.; Zhang, W.H.; Zheng, Y.Z. Effect of reheating processes on grain boundary heritance for 2.25Cr-1Mo-0.25V steel. Mater. Sci. Eng. A 2006, 438–440, 1135–1138. [Google Scholar] [CrossRef]
- Fu, R.D.; Yang, Y.Q.; Wang, C.Y.; Zhang, W.H. Effects of weld thermal cycles and post-welding tempering on second phase particles in 2.25Cr-1Mo-0.25V steels. Sci. Technol. Weld. Join. 2008, 13, 349–356. [Google Scholar] [CrossRef]
- Tavares, S.S.M.; Bastos, I.N.; Pardal, J.M.; Montenegro, T.R.; Silva, M.R.D. Slow strain rate tensile test results of new multiphase 17% Cr stainless steel under hydrogen cathodic charging. Int. J. Hydrog. Energy 2015, 40, 16992–16999. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Wu, Y. Effect of lattice matching degree and intermetallic compound on the properties of mg/al dissimilar material welded joints. Sci. Technol. Weld. Join. 2017, 22, 719–725. [Google Scholar] [CrossRef]
- Niu, P.; Li, W.; Yang, X.; Vairis, A. Effects of microstructural asymmetries across friction stir welded aa2024 joints on mechanical properties. Sci. Technol. Weld. Join. 2018, 23, 58–62. [Google Scholar] [CrossRef]
- Pereira, P.A.S.; Franco, C.S.G.; Filho, J.L.M.G.; Santos, D.S.D. Hydrogen effects on the microstructure of a 2.25cr–1mo–0.25v steel welded joint. Int. J. Hydrog. Energy 2015, 40, 17136–17143. [Google Scholar] [CrossRef]
- Guo, Q.; Lu, F.; Liu, X.; Yang, R.; Cui, H.; Gao, Y. Correlation of microstructure and fracture toughness of advanced 9Cr/CrMoV dissimilarly welded joint. Mater. Sci. Eng. A 2015, 638, 240–250. [Google Scholar] [CrossRef]
- García, T.E.; Rodríguez, C.; Belzunce, F.J.; Cuesta, I.I. Effect of hydrogen embrittlement on the tensile properties of CrMoV steels by means of the small punch test. Mater. Sci. Eng. A 2016, 664, 165–176. [Google Scholar] [CrossRef]
- Song, Y.; Chai, M.; Wu, W.; Liu, Y.; Qin, M.; Cheng, G. Experimental investigation of the effect of hydrogen on fracture toughness of 2.25Cr-1Mo-0.25V steel and welds after annealing. Materials 2018, 11, 499. [Google Scholar] [CrossRef] [PubMed]
- ISO 12135: 2016 Metallic materials—unified method of test for the determination of quasistatic fracture toughness. International Organization for Standardization: Geneva, Switzerland, 2016.
- Matsuoka, S.; Tanaka, H.; Homma, N.; Murakami, Y. Influence of hydrogen and frequency on fatigue crack growth behavior of cr-mo steel. Int. J. Fract. 2010, 168, 101–112. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, G.; Qin, M.; Li, Q.; Zhang, Z.; Chen, K.; Li, Y.; Hu, H.; Wu, W.; Zhang, J. Effect of high temperature deformation on the microstructure, mechanical properties and hydrogen embrittlement of 2.25Cr-1Mo-0.25V steel. Int. J. Hydrog. Energy 2017, 42, 24549–24559. [Google Scholar] [CrossRef]
- Chai, M.; Zhang, Z.; Duan, Q.; Song, Y. Assessment of fatigue crack growth in 316ln stainless steel based on acoustic emission entropy. Int. J. Fatigue 2018, 109, 145–156. [Google Scholar] [CrossRef]
- Martínez-Pañeda, E.; García, T.E.; Rodríguez, C. Fracture toughness characterization through notched small punch test specimens. Mater. Sci. Eng. A 2016, 657, 422–430. [Google Scholar] [CrossRef]
- Chai, M.; Duan, Q.; Hou, X.; Zhang, Z.; Li, L. Fracture toughness evaluation of 316ln stainless steel and weld using acoustic emission technique. ISIJ Int. 2016, 56, 875–882. [Google Scholar] [CrossRef]
- Djukic, M.; Bakic, G.; Lasseigne, A.N.; Jackson, J.E. Hydrogen embrittlement of industrial components: Prediction, prevention, and models. Corrosion 2016, 72, 943–961. [Google Scholar] [CrossRef]
- Dadfarnia, M.; Nagao, A.; Wang, S.; Martin, M.L.; Somerday, B.P.; Sofronis, P. Recent advances on hydrogen embrittlement of structural materials. Int. J. Fract. 2015, 196, 1–21. [Google Scholar] [CrossRef]
- Troiano, A.R. The role of hydrogen and other interstitials in the mechanical behavior of metals. Metallogr. Microstruct. Anal. 2016, 5, 557–569. [Google Scholar] [CrossRef]
- Lynch, S.P. Interpreting hydrogen-induced fracture surfaces in terms of deformation processes: A new approach. Scr. Mater. 2011, 65, 851–854. [Google Scholar] [CrossRef]
- Birnbaum, H.K.; Sofronis, P. Hydrogen-enhanced localized plasticity—a mechanism for hydrogen-related fracture. Mater. Sci. Eng. A 1994, 176, 191–202. [Google Scholar] [CrossRef]
- Depover, T.; Verbeken, K. The detrimental effect of hydrogen at dislocations on the hydrogen embrittlement susceptibility of Fe-C-X alloys: An experimental proof of the help mechanism. Int. J. Hydrog. Energy 2018, 43, 3050–3061. [Google Scholar] [CrossRef]
- Kumar, B.S.; Kain, V.; Singh, M.; Vishwanadh, B. Influence of hydrogen on mechanical properties and fracture of tempered 13 wt.% Cr martensitic stainless steel. Mater. Sci. Eng. A 2017, 700, 140–151. [Google Scholar] [CrossRef]
- Chandler, M.Q.; Horstemeyer, M.F.; Baskes, M.I.; Gullett, P.M.; Wagner, G.J.; Jelinek, B. Hydrogen effects on nanovoid nucleation in face-centered cubic single-crystals. Acta Mater. 2008, 56, 95–104. [Google Scholar] [CrossRef]
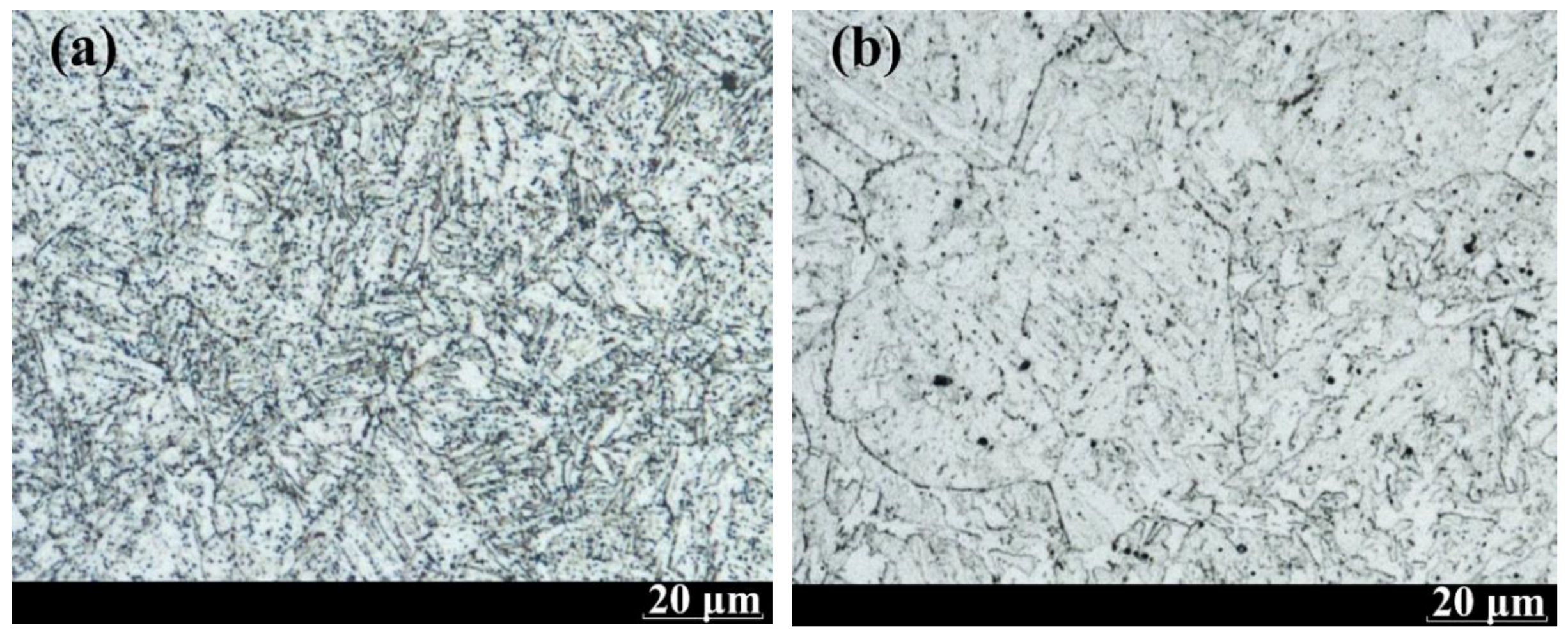

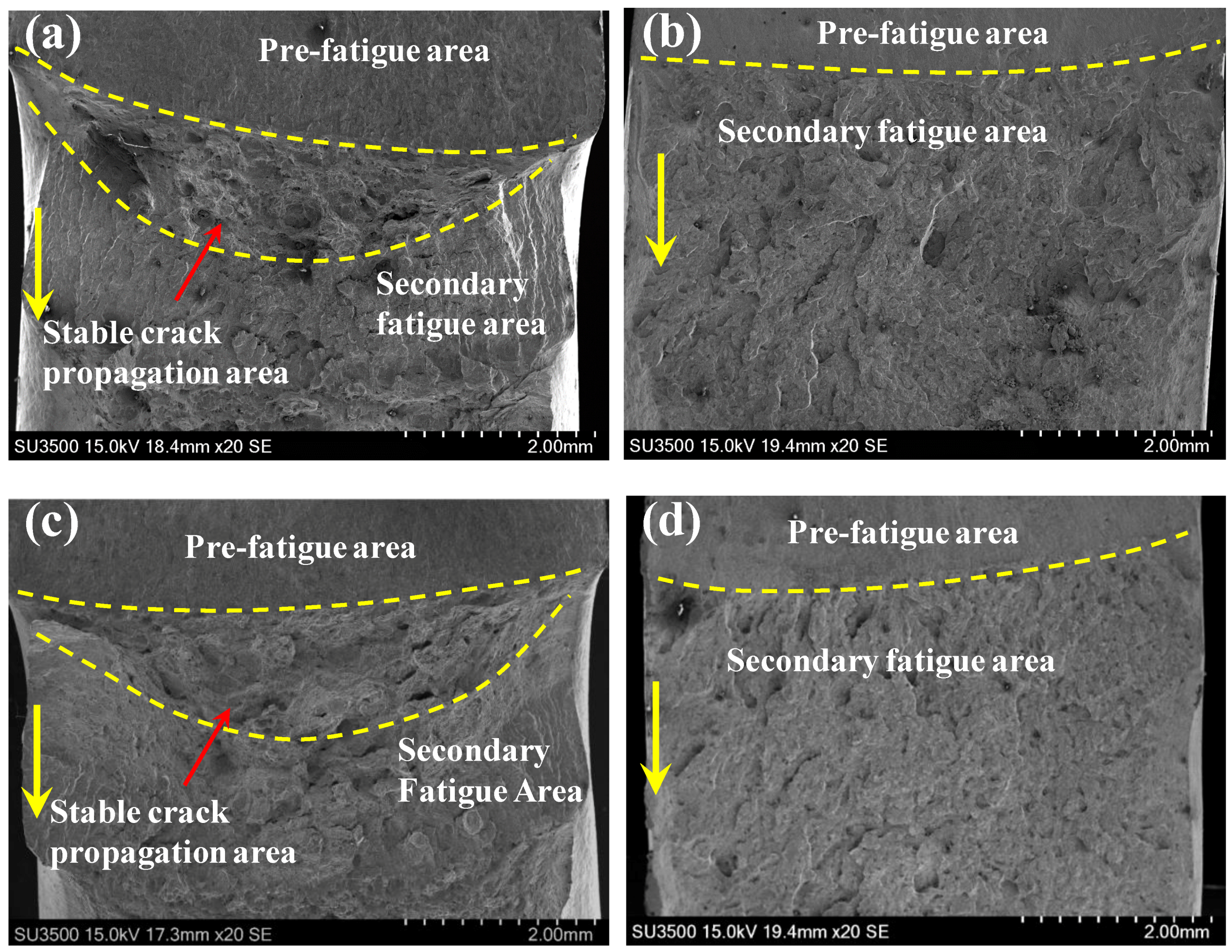
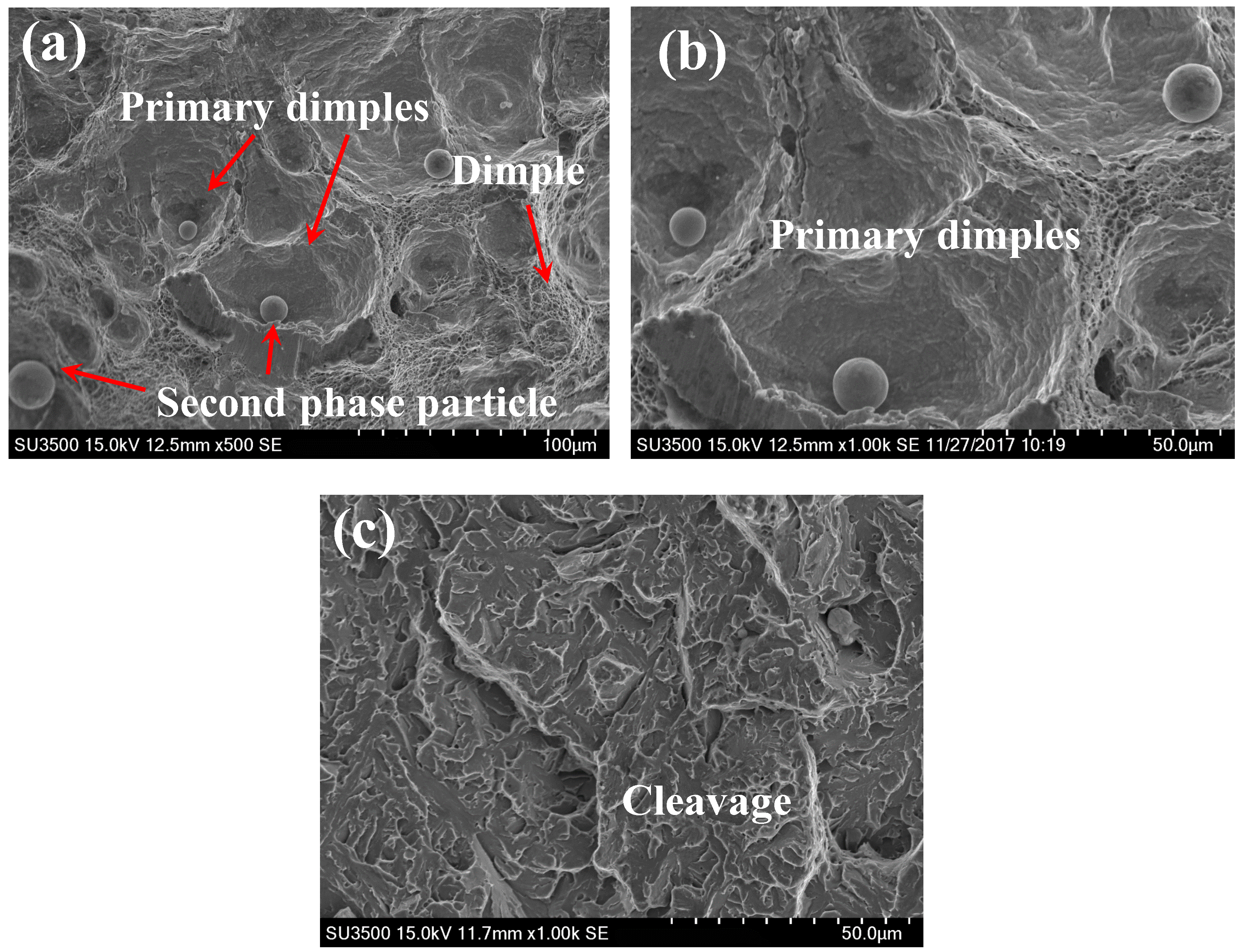

| Element | C | Si | Mn | P | S | Cr | Mo | V | Al | Ni | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BM | 0.15 | 0.1 | 0.54 | 0.009 | 0.01 | 2.3 | 0.98 | 0.3 | 0.05 | - | - |
| WM | 0.12 | 0.22 | 1.07 | 0.004 | 0.004 | 2.45 | 1.03 | 0.42 | - | 0.03 | 0.11 |
| Specimen | Hydrogen Condition | Fracture Toughness (kJ·m−2) | SEM Features | Fracture Mechanisms |
|---|---|---|---|---|
| BM | HF | 538.06 ± 124.32 | dimples | ductile fracture |
| BM | HC | 242.27 ± 6.83 | dimples and quasi-cleavage facets | ductile and brittle fracture |
| WM | HF | 13.55 ± 9.85 | cleavage facets | brittle fracture |
| WM | HC | 5.06 ± 1.77 | cleavage facets | brittle fracture |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Chai, M.; Yang, B.; Han, Z.; Ai, S.; Liu, Y.; Cheng, G.; Li, Y. Investigation of the Influence of Pre-Charged Hydrogen on Fracture Toughness of As-Received 2.25Cr1Mo0.25V Steel and Weld. Materials 2018, 11, 1068. https://doi.org/10.3390/ma11071068
Song Y, Chai M, Yang B, Han Z, Ai S, Liu Y, Cheng G, Li Y. Investigation of the Influence of Pre-Charged Hydrogen on Fracture Toughness of As-Received 2.25Cr1Mo0.25V Steel and Weld. Materials. 2018; 11(7):1068. https://doi.org/10.3390/ma11071068
Chicago/Turabian StyleSong, Yan, Mengyu Chai, Bin Yang, Zelin Han, Song Ai, Yilun Liu, Guangxu Cheng, and Yun Li. 2018. "Investigation of the Influence of Pre-Charged Hydrogen on Fracture Toughness of As-Received 2.25Cr1Mo0.25V Steel and Weld" Materials 11, no. 7: 1068. https://doi.org/10.3390/ma11071068
APA StyleSong, Y., Chai, M., Yang, B., Han, Z., Ai, S., Liu, Y., Cheng, G., & Li, Y. (2018). Investigation of the Influence of Pre-Charged Hydrogen on Fracture Toughness of As-Received 2.25Cr1Mo0.25V Steel and Weld. Materials, 11(7), 1068. https://doi.org/10.3390/ma11071068





