Self-Assembly of Free-Standing LiMn2O4-Graphene Flexible Film for High-Performance Rechargeable Hybrid Aqueous Battery
Abstract
:1. Introduction
2. Materials and Methods
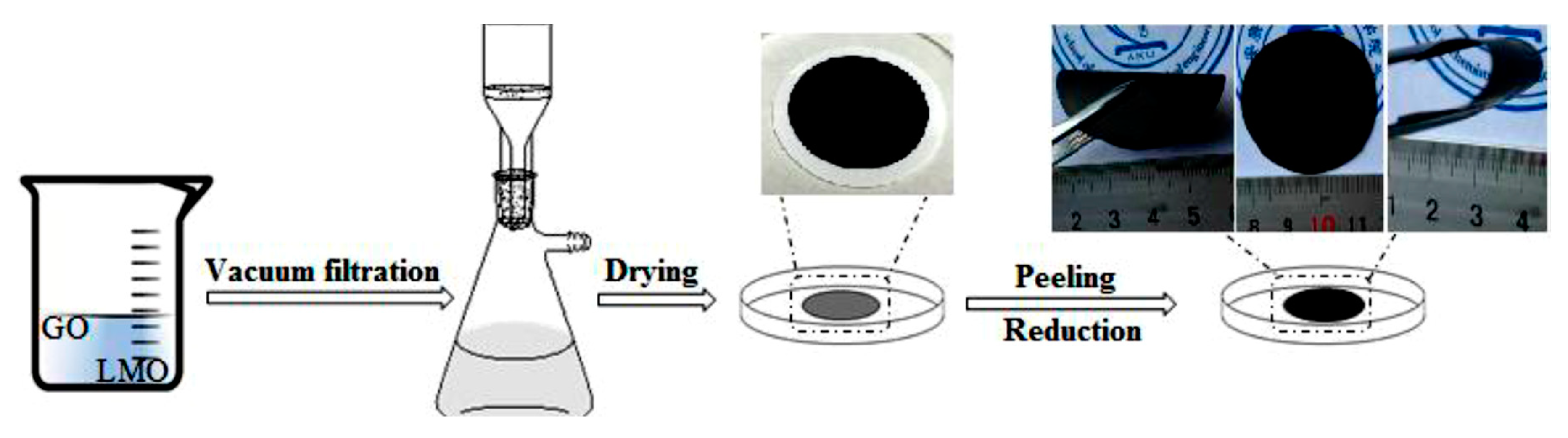
2.1. Materials Preparation
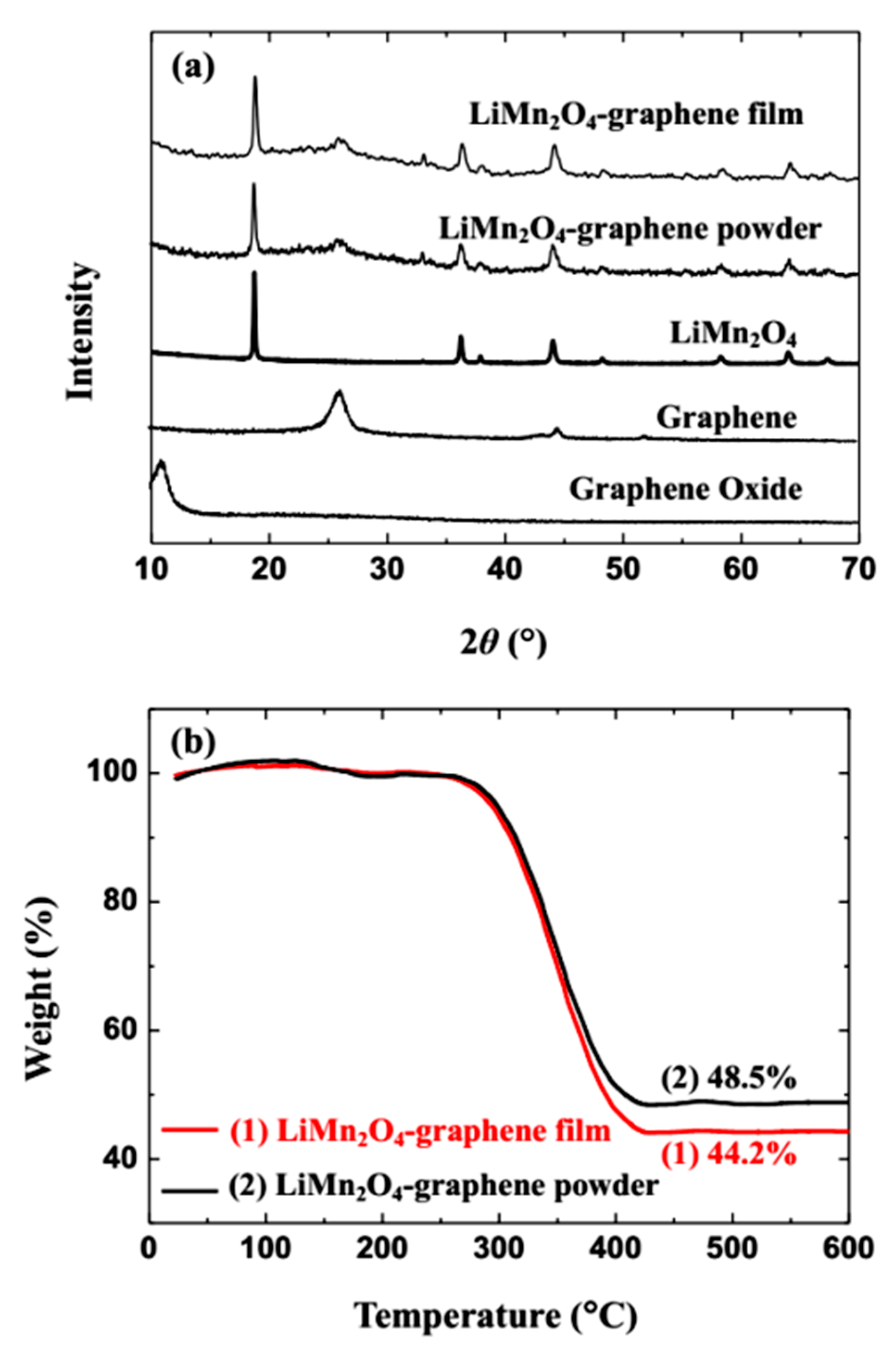
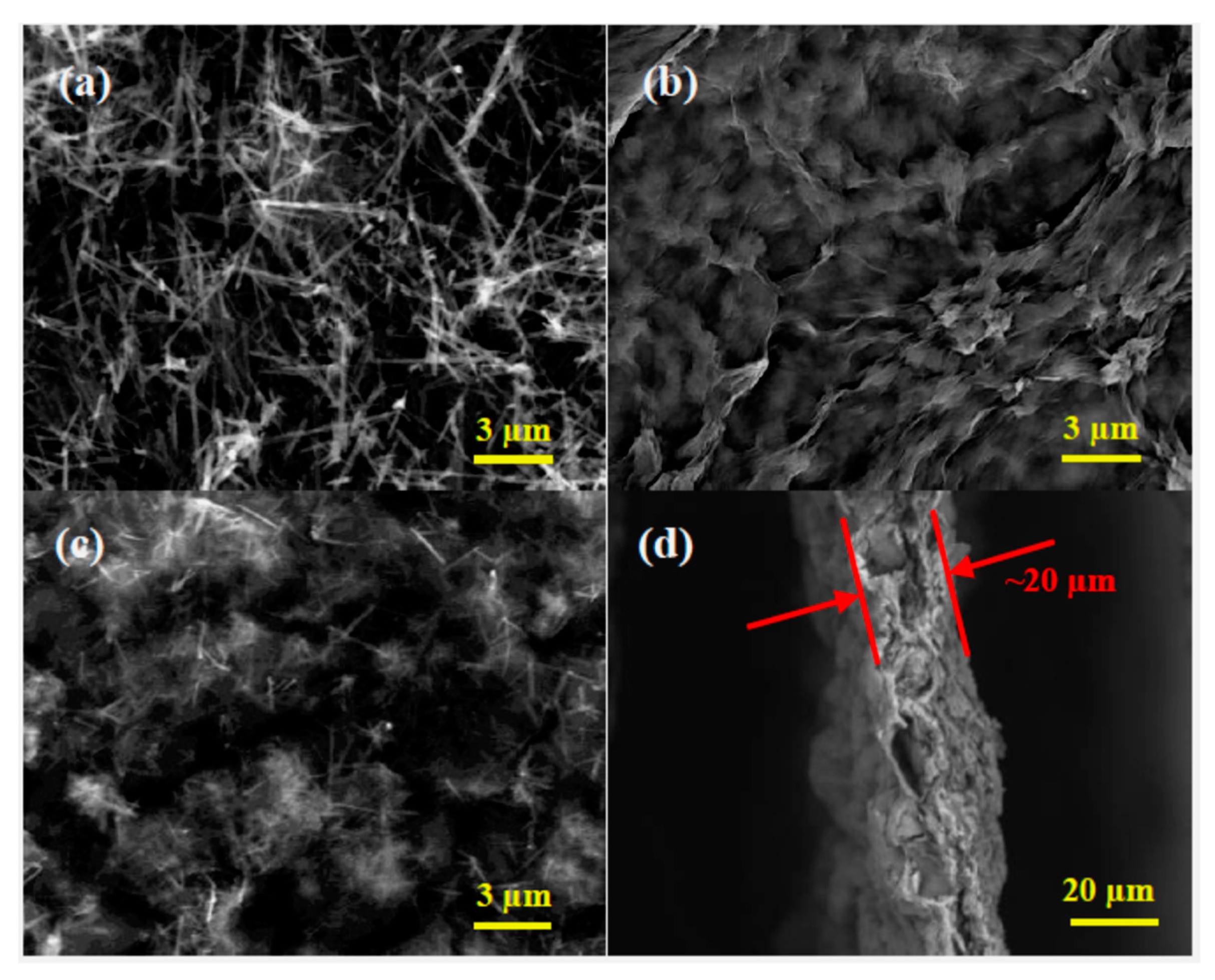
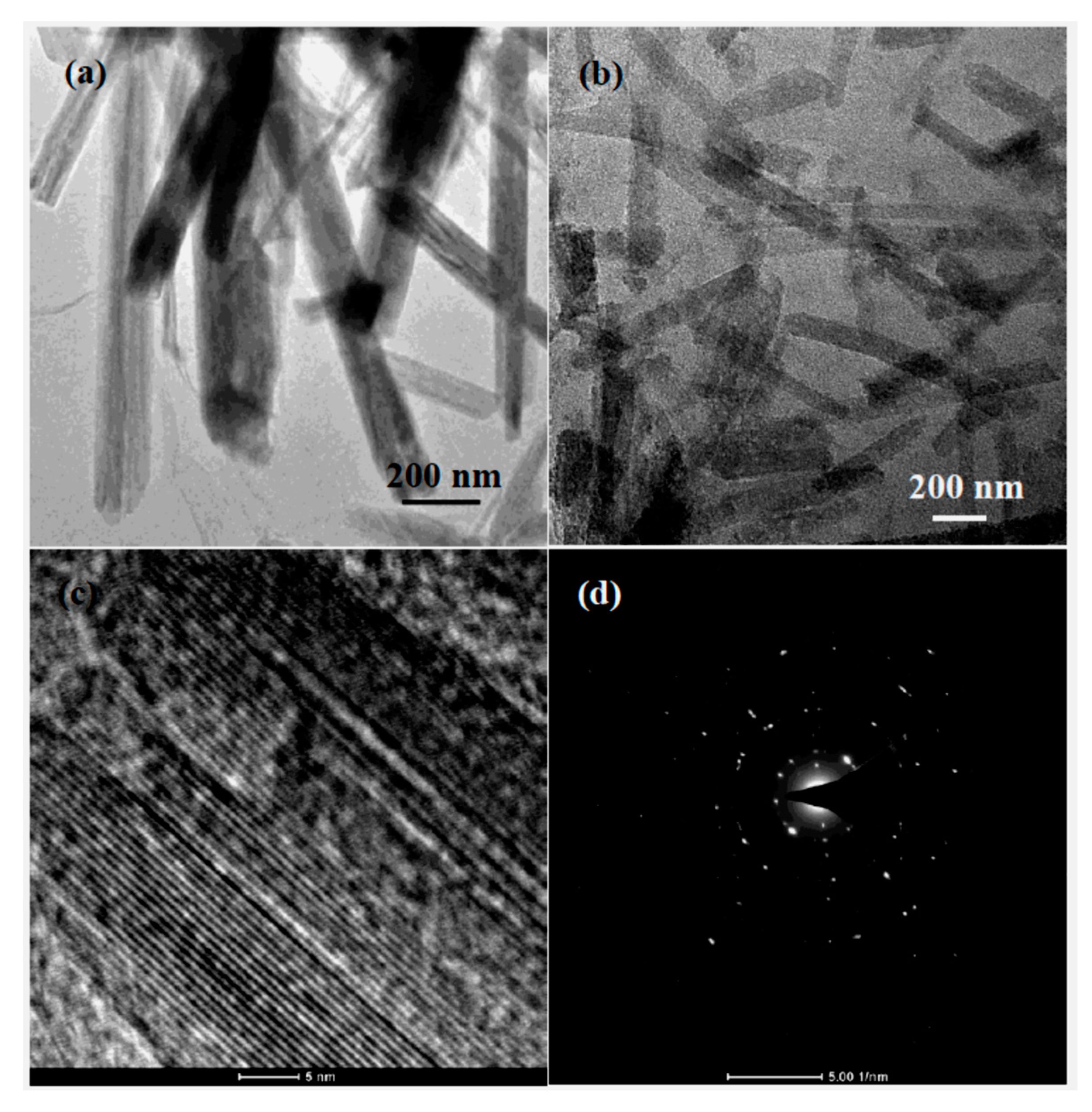
2.2. Material Characterization
2.3. Electrochemical Measurements
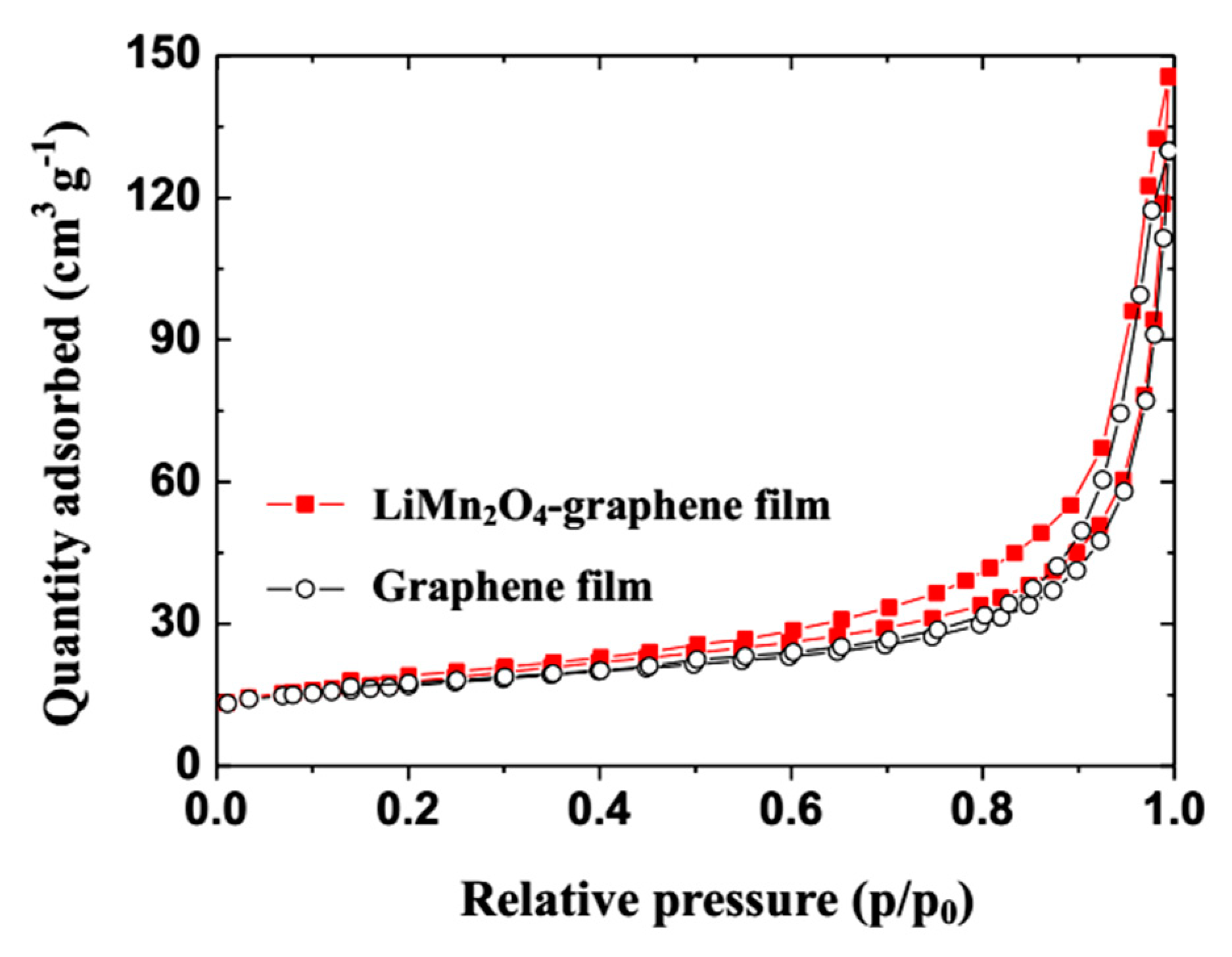
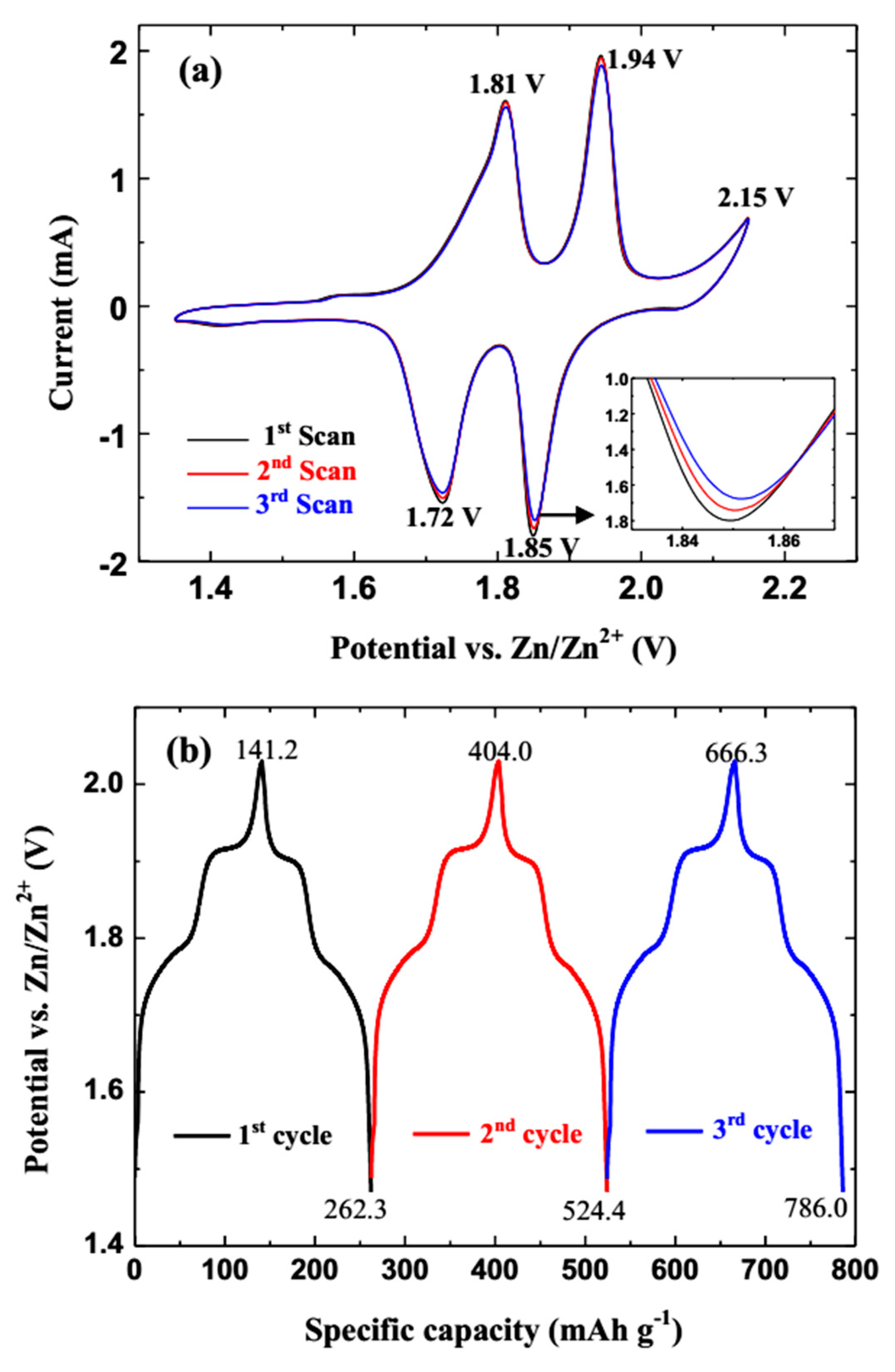
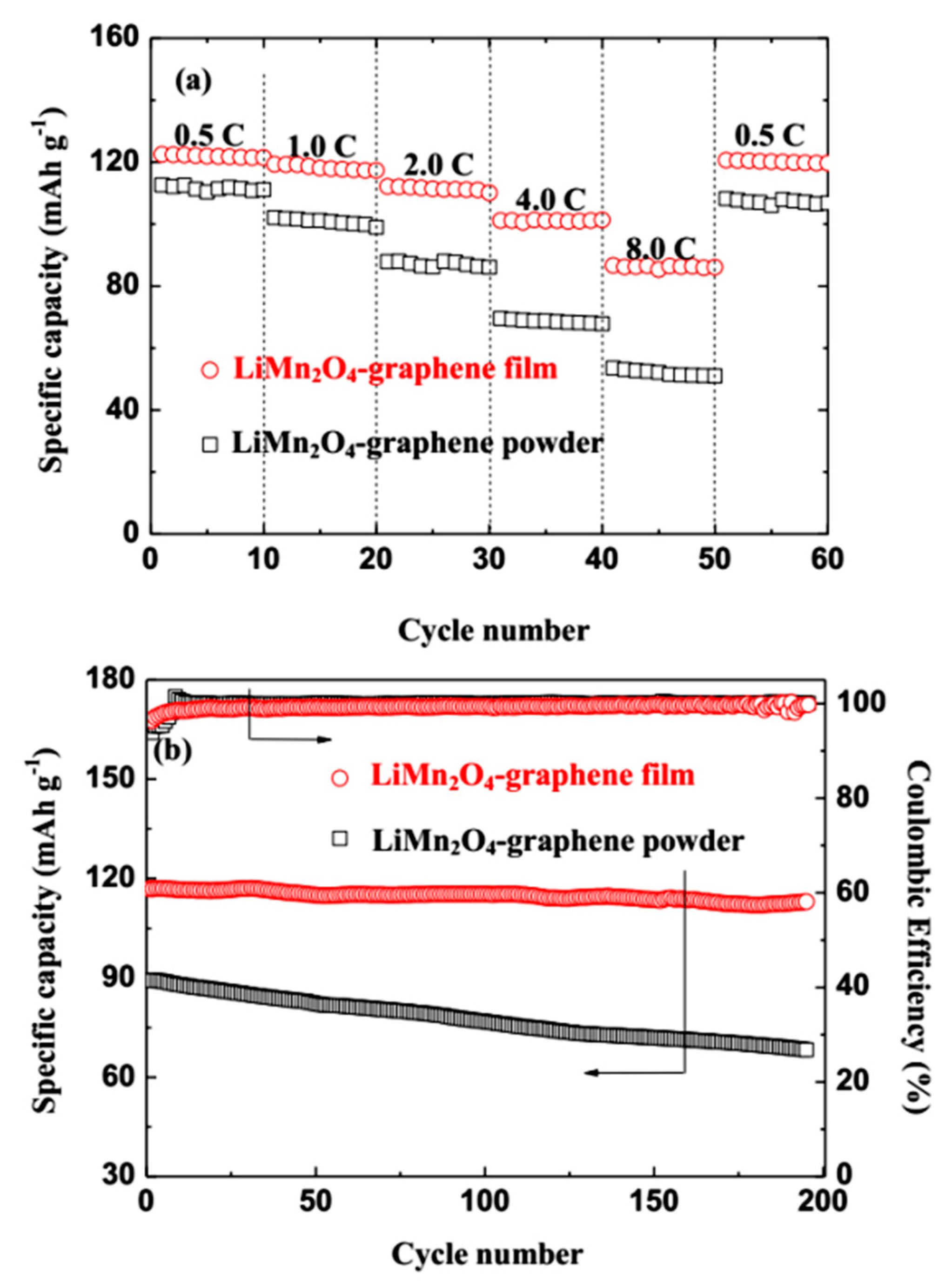
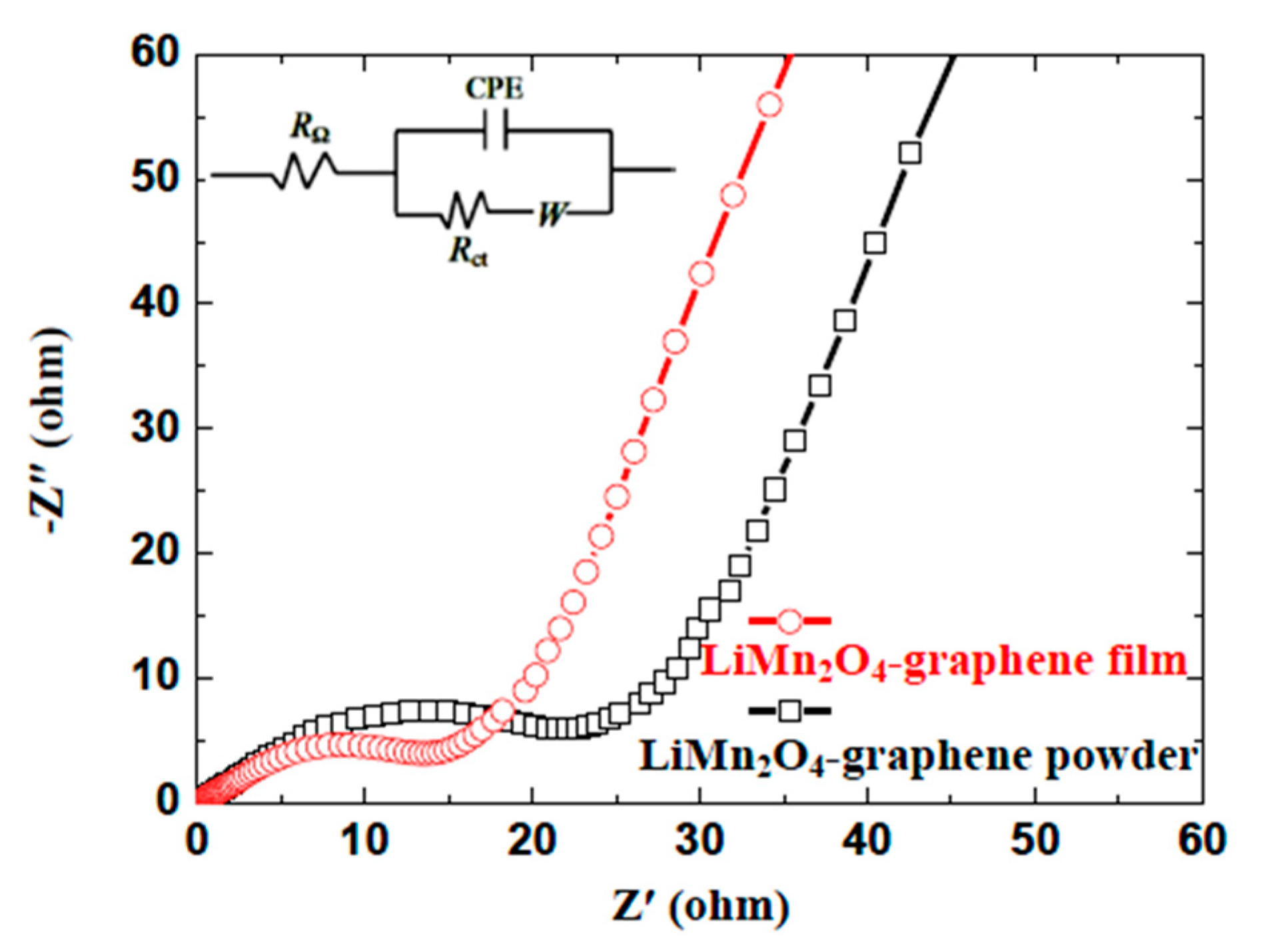
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, J.; Chen, Z.; Ma, Z.; Pan, F.; Curtiss, L.A.; Amine, K. The role of nanotechnology in the development ofbattery materials for electric vehicles. Nat. Nanotechnol. 2016, 11, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.H.; Chao, D.L.; Zhang, Y.Q.; Shen, Z.X.; Fan, H.J. Three-dimensional graphene and their integrated electrodes. Nano Today 2014, 9, 785. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Dell’Era, A.; Pasquali, M.; Bauer, E.M.; Vecchio Ciprioti, S.; Scaramuzzo, F.A.; Lupi, C. Synthesis, characterization, and electrochemical behavior of LiMnxFe(1−x)PO4 composites obtained from phenylphosphonate-based organic-inorganic hybrids. Materials 2017, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yuan, X.; Yu, J.; Liu, J.; Wang, F.; Fu, L.; Wu, Y. A high-capacity dual core–shell structured MWCNTs@S@PPy nanocomposite anode for advanced aqueous rechargeable lithium batteries. Nanoscale 2017, 31, 11004–11011. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, J.; Liu, H.; Bakenov, Z.; Gosselink, D.; Chen, P. Rechargeable hybrid aqueous batteries. J. Power Sour. 2012, 216, 222–226. [Google Scholar] [CrossRef]
- Yuan, G.; Bai, J.; Doan, T.N.L.; Chen, P. Synthesis and electrochemical investigation of nanosized LiMn2O4 as cathode material for rechargeable hybrid aqueous batteries. Mater. Lett. 2014, 137, 311–314. [Google Scholar] [CrossRef]
- Zhao, S.; Han, B.; Zhang, D.; Huang, Q.; Xiao, L.; Chen, L.; Ivey, D.G.; Deng, Y.; Wei, W. Unravelling the reaction chemistry and degradation mechanism in aqueous Zn/MnO2 rechargeable batteries. J. Mater. Chem. A 2018. [Google Scholar] [CrossRef]
- Tang, W.; Hou, Y.; Wang, F.; Liu, L.; Wu, Y.; Zhu, K. LiMn2O4 nanotube as cathode material of second-level charge capability for aqueous rechargeable batteries. Nano Lett. 2013, 13, 2036–2040. [Google Scholar] [CrossRef] [PubMed]
- Zhi, J.; Bertens, K.; Yazdi, A.Z.; Chen, P. Acrylonitrile copolymer/graphene skinned cathode for long cycle life rechargeable hybrid aqueous batteries at high-temperature. Electrochim. Acta 2018, 268, 248–255. [Google Scholar] [CrossRef]
- Zhu, X.; Hoang, T.K.A.; Chen, P. Novel carbon materials in the cathode formulation for high rate rechargeable hybrid aqueous batteries. Energies 2017, 10, 1844. [Google Scholar] [CrossRef]
- Yuan, G.; Bai, J.; Doan, T.N.L.; Chen, P. Synthesis and electrochemical properties of LiFePO4/graphene composite as a novel cathode material for rechargeable hybrid aqueous battery. Mater. Lett. 2015, 158, 248–251. [Google Scholar] [CrossRef]
- Pyun, M.H.; Park, Y.J. Graphene/LiMn2O4 nanocomposites for enhanced lithium ion batteries with high rate capability. J. Alloy. Comp. 2015, 643, S90–S94. [Google Scholar] [CrossRef]
- Lin, B.; Yin, Q.; Hu, H.; Lu, F.; Xia, H. LiMn2O4 nanoparticles anchored on graphene nanosheets as high-performance cathode material for lithium-ion batteries. J. Solid State Chem. 2014, 209, 23–28. [Google Scholar] [CrossRef]
- Chen, L.; Chen, L.; Ai, Q.; Li, D.; Si, P.; Feng, J.; Ci, L. Flexible all-solid-state supercapacitors based on freestanding, binder-free carbon nanofibers@polypyrrole@graphene film. Chem. Eng. J. 2018, 334, 184–190. [Google Scholar] [CrossRef]
- Wang, B.; Ryu, J.; Choi, S.; Song, G.; Hong, D.; Hwang, C.; Chen, X.; Wang, B.; Li, W.; Song, H.K.; et al. Folding graphene film yields high areal energy storage in lithium-ion batteries. ACS Nano 2018, 12, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, D.; Oh, I.K. Multilayered graphene-carbon nanotube-iron oxide three-dimensional heterostructure for flexible electromagnetic interference shielding film. Carbon 2017, 111, 248–257. [Google Scholar] [CrossRef]
- Zhao, J.; Pei, S.; Ren, W.; Gao, L.; Cheng, H.M. Efficient preparation of large-area graphene oxide sheets for transparent conductive films. ACS Nano 2010, 4, 5245–5252. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Takeuchi, K.; Hiroka, T.; Furuta, T.; Kasai, T.; Sun, X.; Kiang, C.H.; Dresselhaus, M.S. Stacking nature of graphene layers in carbon nanotubes and nanofibres. J. Phys. Chem. Solids 1997, 58, 1707–1713. [Google Scholar] [CrossRef]
- Qu, Q.; Fu, L.; Zhan, X.; Samuelis, D.; Maier, J.; Li, L.; Tian, S.; Li, Z.; Wu, Y. Porous LiMn2O4 as cathode material with high power and excellent cycling for aqueous rechargeable lithium batteries. Energy Environ. Sci. 2011, 4, 3985–3990. [Google Scholar] [CrossRef]
- Zhang, M.G.; Smith, A.; Gorski, W. Carbon nanotube-chitosan system for electrochemical sensing based on dehydrogenase enzymes. Anal. Chem. 2004, 76, 5045–5050. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, K.; Wang, G.; Liu, X.; Wang, H. Rational design of three-dimensional graphene encapsulated with hollow FeP@carbon nanocomposite as outstanding anode material for lithium ion and sodium ion batteries. ACS Nano 2017, 11, 11602–11616. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, G.; Lv, Z.; Wang, H. In situ synthesis of hierarchical CoFe2O4 nanoclusters/graphene aerogels and their high performance for lithium-ion batteries. Phys. Chem. Chem. Phys. 2015, 17, 27109–27117. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kowal, K.; Farrington, G.C. Mechanism of the electrochemical insertion of lithium into LiMn2O4 spinels. J. Electrochem. Soc. 1998, 145, 459–465. [Google Scholar] [CrossRef]
- Lu, D.; Li, W.; Zuo, X.; Yuan, Z.; Huang, Q. Study on electrode kinetics of Li+ insertion in LixMn2O4 (0 ≤ x ≤ 1) by electrochemical impedance spectroscopy. J. Phys. Chem. C 2007, 111, 12067–12074. [Google Scholar] [CrossRef]
- Bakierska, M.; Świętosławski, M.; Dziembaj, R.; Molenda, M. Nature of the electrochemical properties of sulphur substituted LiMn2O4 spinel cathode material studied by electrochemical impedance spectroscopy. Materials 2016, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Punckt, C.; Muckel, F.; Wolff, S.; Aksay, I.A.; Chavarin, C.A.; Bacher, G.; Mertin, W. The effect of degree of reduction on the electrical properties of functionalized graphene sheets. App. Phys. Lett. 2013, 102, 023114. [Google Scholar] [CrossRef]
- Li, K.; Lin, S.; Shua, F.; Zhang, J.; Chen, K.; Xue, D. A rapid combustion route to synthesize high-performance nanocrystalline cathode materials for Li-ion batteries. Cryst. Eng. Comm. 2014, 16, 10969–10976. [Google Scholar] [CrossRef]
- Aurbach, D. Electrode–solution interactions in Li-ion batteries: a short summary and new insights. J. Power Sour. 2003, 119, 497–503. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, G.; Huang, T.; Kou, Y.; Ji, Z.; Zhao, Y. Self-Assembly of Free-Standing LiMn2O4-Graphene Flexible Film for High-Performance Rechargeable Hybrid Aqueous Battery. Materials 2018, 11, 1056. https://doi.org/10.3390/ma11071056
Yuan G, Huang T, Kou Y, Ji Z, Zhao Y. Self-Assembly of Free-Standing LiMn2O4-Graphene Flexible Film for High-Performance Rechargeable Hybrid Aqueous Battery. Materials. 2018; 11(7):1056. https://doi.org/10.3390/ma11071056
Chicago/Turabian StyleYuan, Guanghui, Ting Huang, Ying Kou, Zhen Ji, and Yan Zhao. 2018. "Self-Assembly of Free-Standing LiMn2O4-Graphene Flexible Film for High-Performance Rechargeable Hybrid Aqueous Battery" Materials 11, no. 7: 1056. https://doi.org/10.3390/ma11071056
APA StyleYuan, G., Huang, T., Kou, Y., Ji, Z., & Zhao, Y. (2018). Self-Assembly of Free-Standing LiMn2O4-Graphene Flexible Film for High-Performance Rechargeable Hybrid Aqueous Battery. Materials, 11(7), 1056. https://doi.org/10.3390/ma11071056



