Versatile Poly(Diallyl Dimethyl Ammonium Chloride)-Layered Nanocomposites for Removal of Cesium in Water Purification
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization
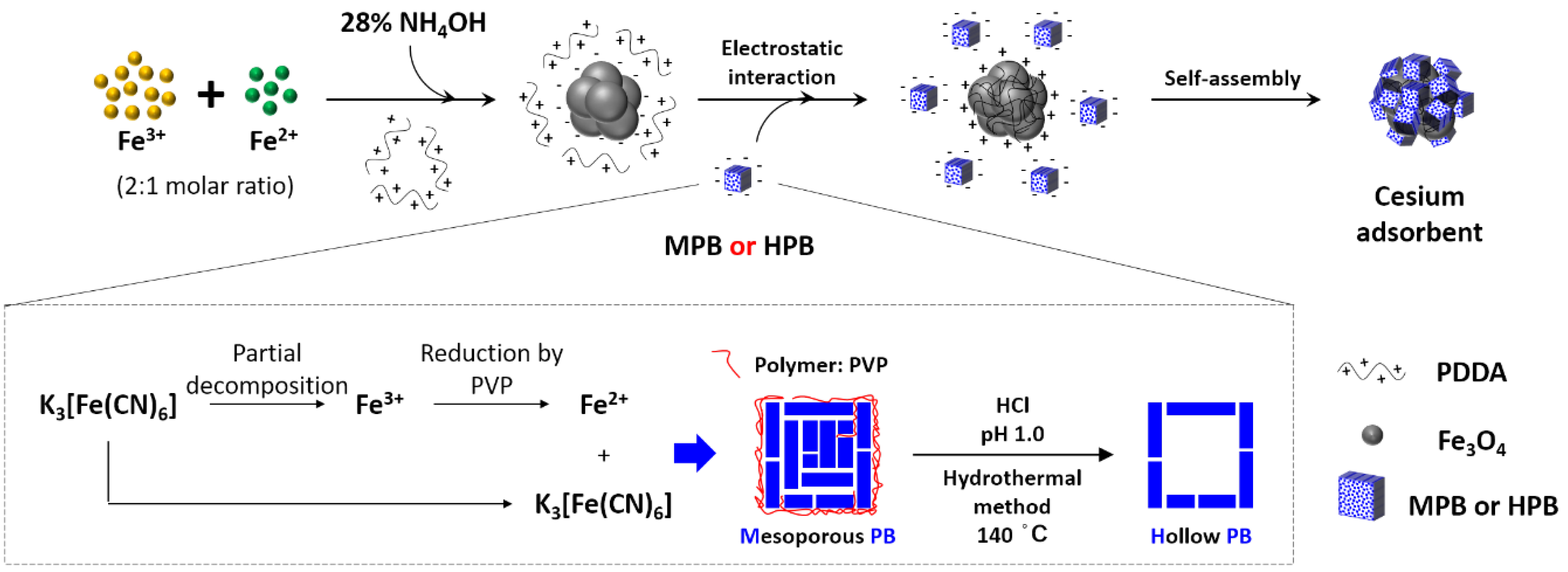
2.2. Preparation of Magnetic PDDA@Fe3O4
2.3. Synthesis of Mesocrystal and Hollow PB Particles
2.4. Synthesis of PB@PDDA@Fe3O4 Composite
2.5. Adsorption Experiments
2.6. Radioactive 137Cs Decontamination
2.7. The Influence of pH on the Removal of 137Cs
3. Results and Discussion
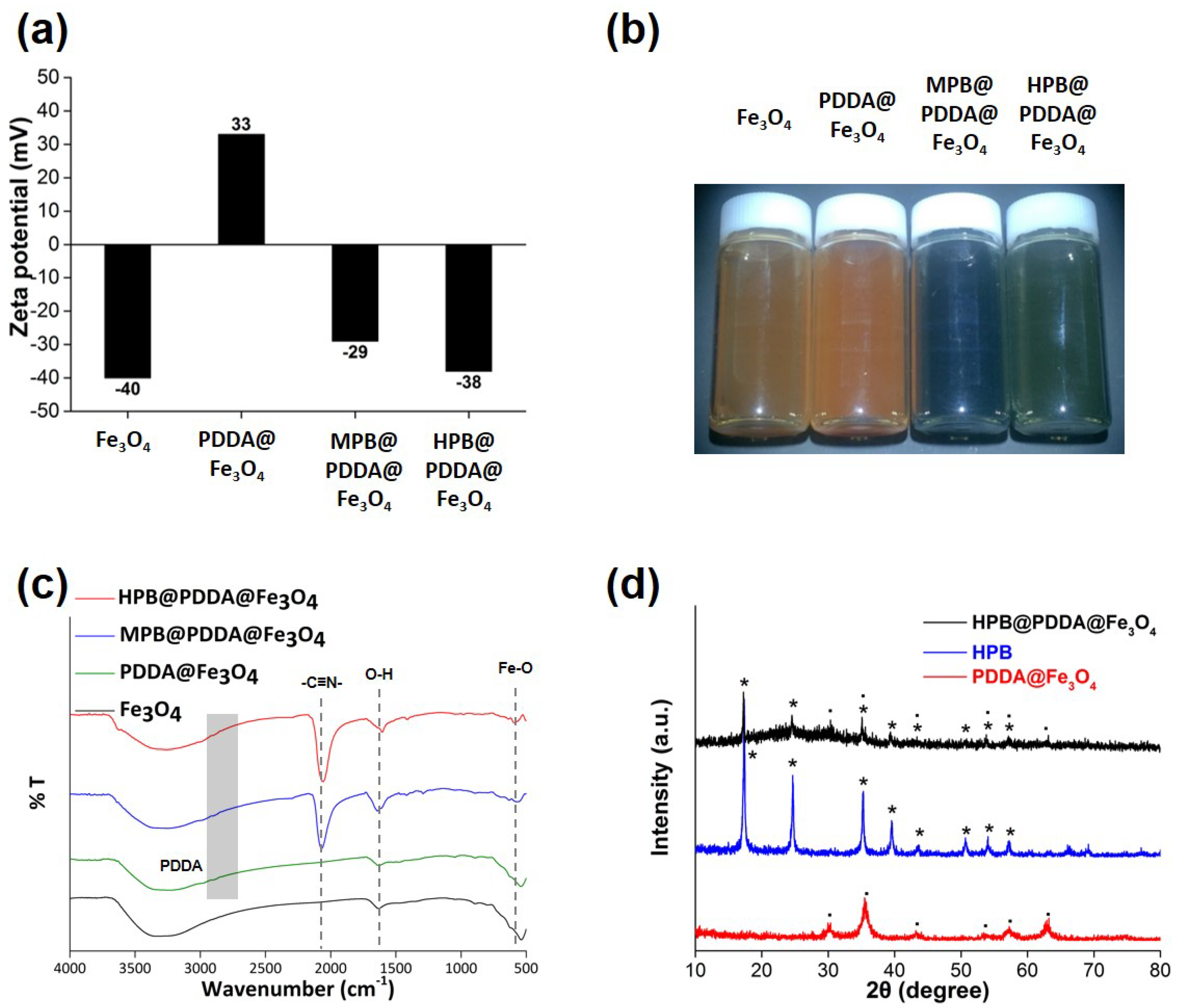
3.1. Fabrication of the Magnetic Adsorbent
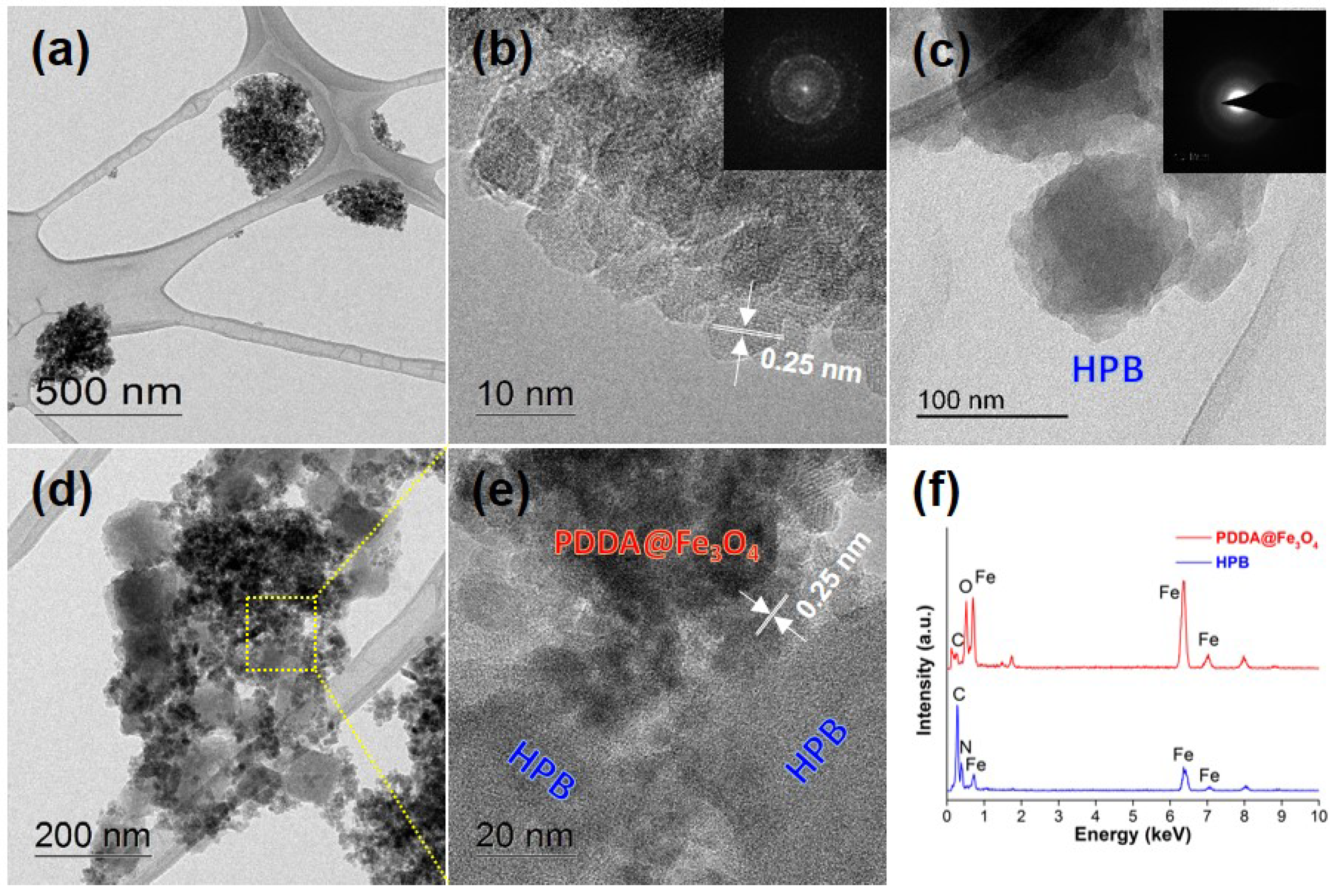
3.2. Morphological and Surface Studies
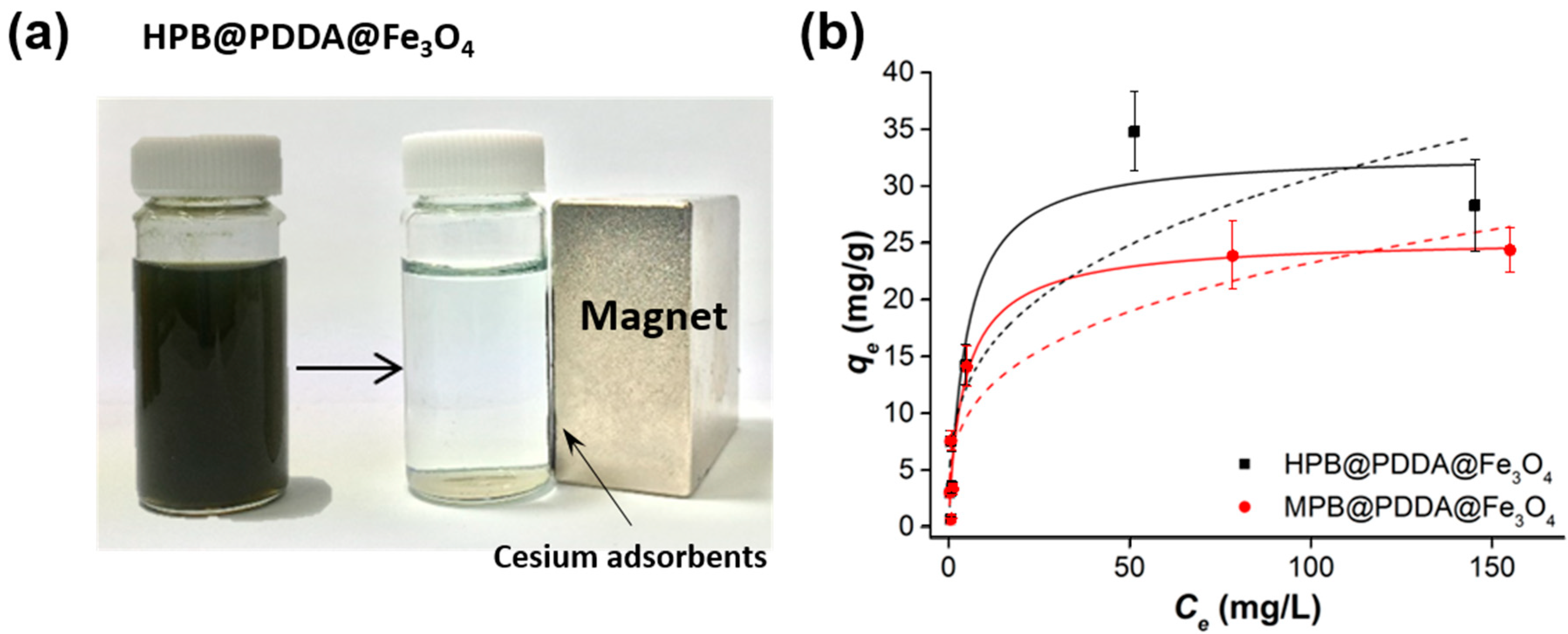
3.3. Performance Evaluation of Cesium Removal
3.4. Radioactive Cesium Adsorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Buesseler, K.O.; Jayne, S.R.; Fisher, N.S.; Rypina, I.I.; Baumann, H.; Baumann, Z.; Breier, C.F.; Douglass, E.M.; George, J.; Macdonald, A.M. Fukushima-derived radionuclides in the ocean and biota off Japan. Proc. Natl. Acad. Sci. USA 2012, 109, 5984–5988. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Yasutaka, T.; Kawabe, Y.; Onishi, T.; Komai, T. Distribution of dissolved and particulate radiocesium concentrations along rivers and the relations between radiocesium concentration and deposition after the nuclear power plant accident in Fukushima. Water Res. 2014, 60, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Hayano, R.S.; Tsubokura, M.; Miyazaki, M.; Satou, H.; Sato, K.; Masaki, S.; Sakuma, Y. Comprehensive whole-body counter surveys of Miharu-town school children for three consecutive years after the Fukushima NPP accident. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2014, 90, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Montaña, M.; Camacho, A.; Devesa, R.; Vallés, I.; Céspedes, R.; Serrano, I.; Blázquez, S.; Barjola, V. The presence of radionuclides in wastewater treatment plants in Spain and their effect on human health. J. Clean. Prod. 2013, 60, 77–82. [Google Scholar] [CrossRef]
- Steinhauser, G.; Schauer, V.; Shozugawa, K. Concentration of strontium-90 at selected hot spots in Japan. PLoS ONE 2013, 8, e57760. [Google Scholar] [CrossRef] [PubMed]
- Leppänen, A.-P.; Mattila, A.; Kettunen, M.; Kontro, R. Artificial radionuclides in surface air in Finland following the Fukushima dai-ichi nuclear power plant accident. J. Environ. Radioact. 2013, 126, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Sangvanich, T.; Sukwarotwat, V.; Wiacek, R.J.; Grudzien, R.M.; Fryxell, G.E.; Addleman, R.S.; Timchalk, C.; Yantasee, W. Selective capture of cesium and thallium from natural waters and simulated wastes with copper ferrocyanide functionalized mesoporous silica. J. Hazard. Mater. 2010, 182, 225–231. [Google Scholar] [CrossRef] [PubMed]
- García, O.; Mandina, T. DNA damage evaluated by the comet assay in lymphocytes of children with 137Cs internal contamination caused by the Chernobyl accident. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2005, 565, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, W.F.; Cullings, H. Use of the individual data of the a-bomb survivors for biologically based cancer models. Radiat. Environ. Biophys. 2010, 49, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B. Surprising results from Hiroshima studies. Med. Sci. 2014, 30, 211–213. [Google Scholar]
- Vasilenko, I. The biological action of nuclear fission products. Their metabolism and acute lesions. Radiobiologiia 1992, 32, 69–78. [Google Scholar] [PubMed]
- Gupta, R.; Dubey, S. Removal of cesium ions from aqueous solution by polyaniline: A radiotracer study. J. Polym. Res. 2005, 12, 31–35. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Park, M.; Kim, J.; Oh, M.; Lee, E.-H.; Kim, K.-W.; Chung, D.-Y.; Moon, J.-K. Equilibrium, kinetic and thermodynamic study of cesium adsorption onto nanocrystalline mordenite from high-salt solution. Chemosphere 2016, 150, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Thammawong, C.; Opaprakasit, P.; Tangboriboonrat, P.; Sreearunothai, P. Prussian blue-coated magnetic nanoparticles for removal of cesium from contaminated environment. J. Nanopart. Res. 2013, 15, 1689. [Google Scholar] [CrossRef]
- Faustino, P.J.; Yang, Y.; Progar, J.J.; Brownell, C.R.; Sadrieh, N.; May, J.C.; Leutzinger, E.; Place, D.A.; Duffy, E.P.; Houn, F. Quantitative determination of cesium binding to ferric hexacyanoferrate: Prussian blue. J. Pharm. Biomed. Anal. 2008, 47, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-C.; Haldorai, Y.; Lee, G.-W.; Hwang, S.-K.; Han, Y.-K.; Roh, C.; Huh, Y.S. Porous three-dimensional graphene foam/prussian blue composite for efficient removal of radioactive 137Cs. Sci. Rep. 2015, 5, 17510. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Ma, J.; He, W.; Hua, D. Facile synthesis of Prussian blue derivate-modified mesoporous material via photoinitiated thiol-ene click reaction for cesium adsorption. Chem. Asian J. 2015, 10, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Delchet, C.; Tokarev, A.; Dumail, X.; Toquer, G.; Barré, Y.; Guari, Y.; Guerin, C.; Larionova, J.; Grandjean, A. Extraction of radioactive cesium using innovative functionalized porous materials. RSC Adv. 2012, 2, 5707–5716. [Google Scholar] [CrossRef]
- Jang, S.-C.; Kang, S.-M.; Haldorai, Y.; Giribabu, K.; Lee, G.-W.; Lee, Y.-C.; Hyun, M.S.; Han, Y.-K.; Roh, C.; Huh, Y.S. Synergistically strengthened 3d micro-scavenger cage adsorbent for selective removal of radioactive cesium. Sci. Rep. 2016, 6, 38384. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-C.; Hong, S.-B.; Yang, H.-M.; Lee, K.-W.; Moon, J.-K.; Seo, B.-K.; Huh, Y.S.; Roh, C. Removal of radioactive cesium using prussian blue magnetic nanoparticles. Nanomaterials 2014, 4, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Furukawa, S.; Ohtani, R.; Sukegawa, H.; Nemoto, Y.; Reboul, J.; Kitagawa, S.; Yamauchi, Y. Synthesis of Prussian blue nanoparticles with a hollow interior by controlled chemical etching. Angew. Chem. 2012, 124, 1008–1012. [Google Scholar] [CrossRef]
- Arun, T.; Prakash, K.; Kuppusamy, R.; Joseyphus, R.J. Magnetic properties of Prussian blue modified Fe3O4 nanocubes. J. Phys. Chem. Solids 2013, 74, 1761–1768. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, L.; Du, L.; Yue, L.; Guan, R.; Zhang, Q.; Hou, G.; Shao, R. Layer-by-layer assembly modification to prepare firmly bonded Si–graphene composites for high-performance anodes. RSC Adv. 2016, 6, 4835–4842. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.-C.; Kang, S.-M.; Kim, G.Y.; Rethinasabapathy, M.; Haldorai, Y.; Lee, I.; Han, Y.-K.; Renshaw, J.C.; Roh, C.; Huh, Y.S. Versatile Poly(Diallyl Dimethyl Ammonium Chloride)-Layered Nanocomposites for Removal of Cesium in Water Purification. Materials 2018, 11, 998. https://doi.org/10.3390/ma11060998
Jang S-C, Kang S-M, Kim GY, Rethinasabapathy M, Haldorai Y, Lee I, Han Y-K, Renshaw JC, Roh C, Huh YS. Versatile Poly(Diallyl Dimethyl Ammonium Chloride)-Layered Nanocomposites for Removal of Cesium in Water Purification. Materials. 2018; 11(6):998. https://doi.org/10.3390/ma11060998
Chicago/Turabian StyleJang, Sung-Chan, Sung-Min Kang, Gi Yong Kim, Muruganantham Rethinasabapathy, Yuvaraj Haldorai, Ilsong Lee, Young-Kyu Han, Joanna C. Renshaw, Changhyun Roh, and Yun Suk Huh. 2018. "Versatile Poly(Diallyl Dimethyl Ammonium Chloride)-Layered Nanocomposites for Removal of Cesium in Water Purification" Materials 11, no. 6: 998. https://doi.org/10.3390/ma11060998
APA StyleJang, S.-C., Kang, S.-M., Kim, G. Y., Rethinasabapathy, M., Haldorai, Y., Lee, I., Han, Y.-K., Renshaw, J. C., Roh, C., & Huh, Y. S. (2018). Versatile Poly(Diallyl Dimethyl Ammonium Chloride)-Layered Nanocomposites for Removal of Cesium in Water Purification. Materials, 11(6), 998. https://doi.org/10.3390/ma11060998








