Synthesis of Various TiO2 Micro-/Nano-Structures and Their Photocatalytic Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
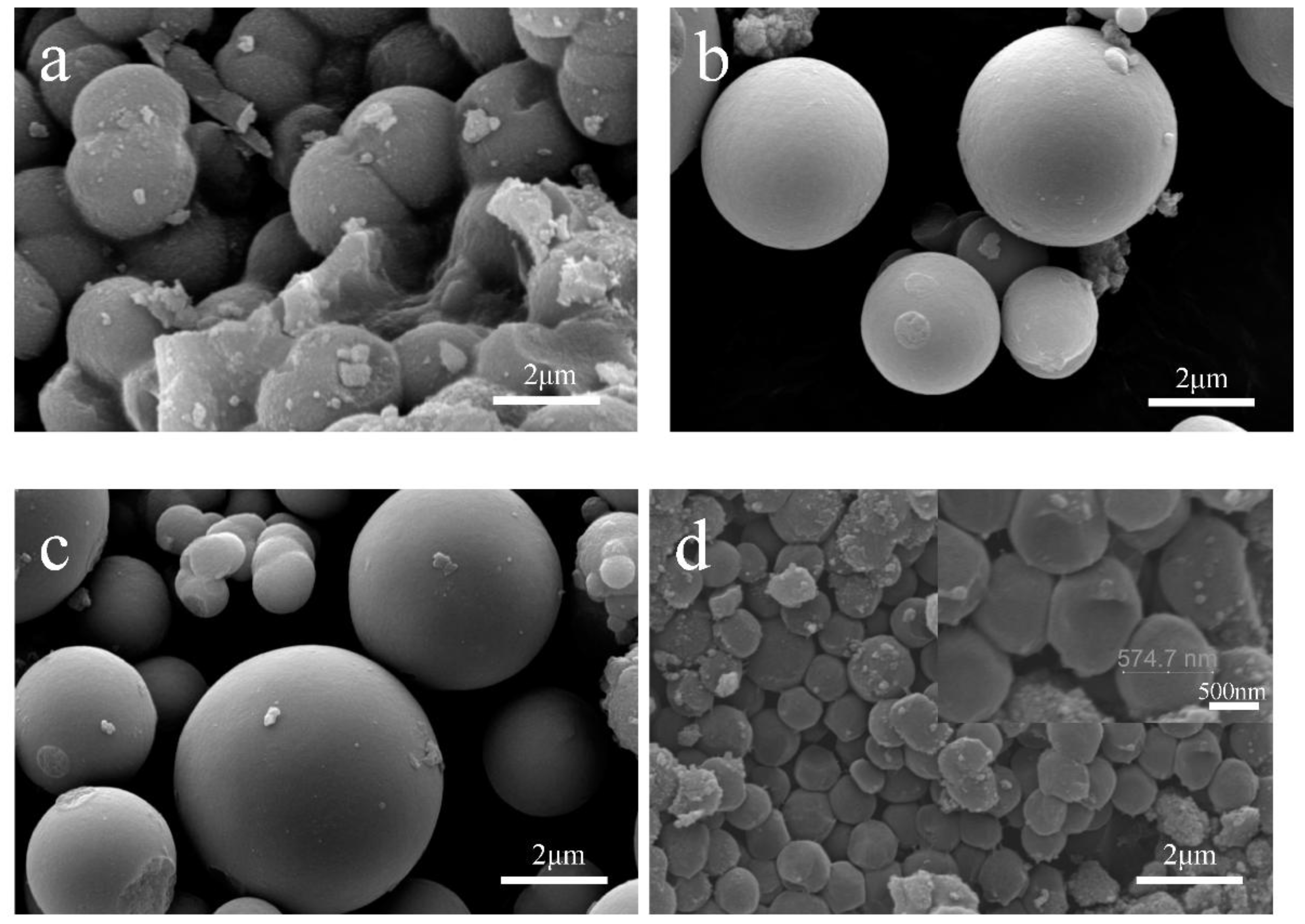
2.2. Preparation of TiO2 Microspheres
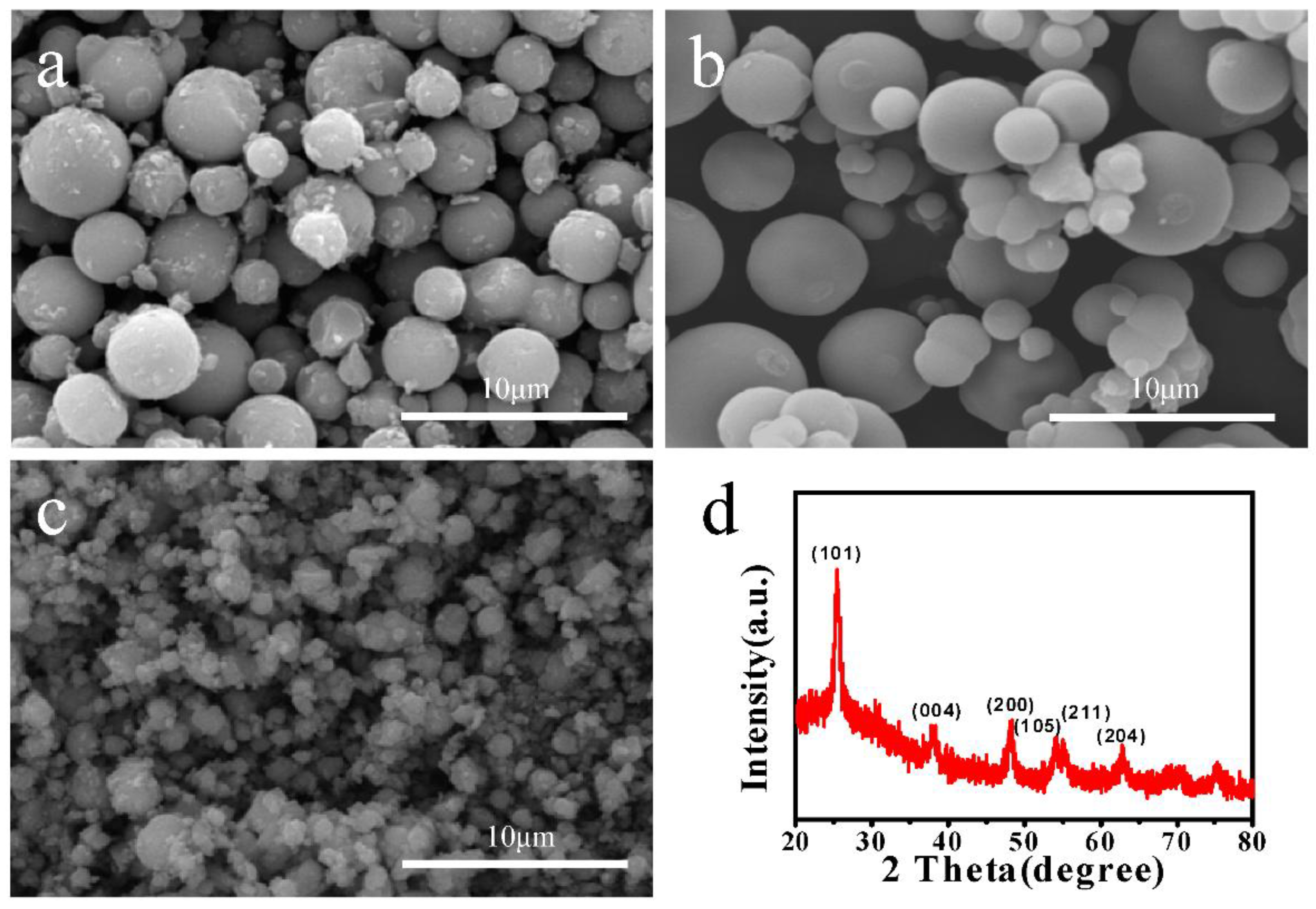
2.3. Preparation of TiO2 Flower Clusters Assembled with Nanorods
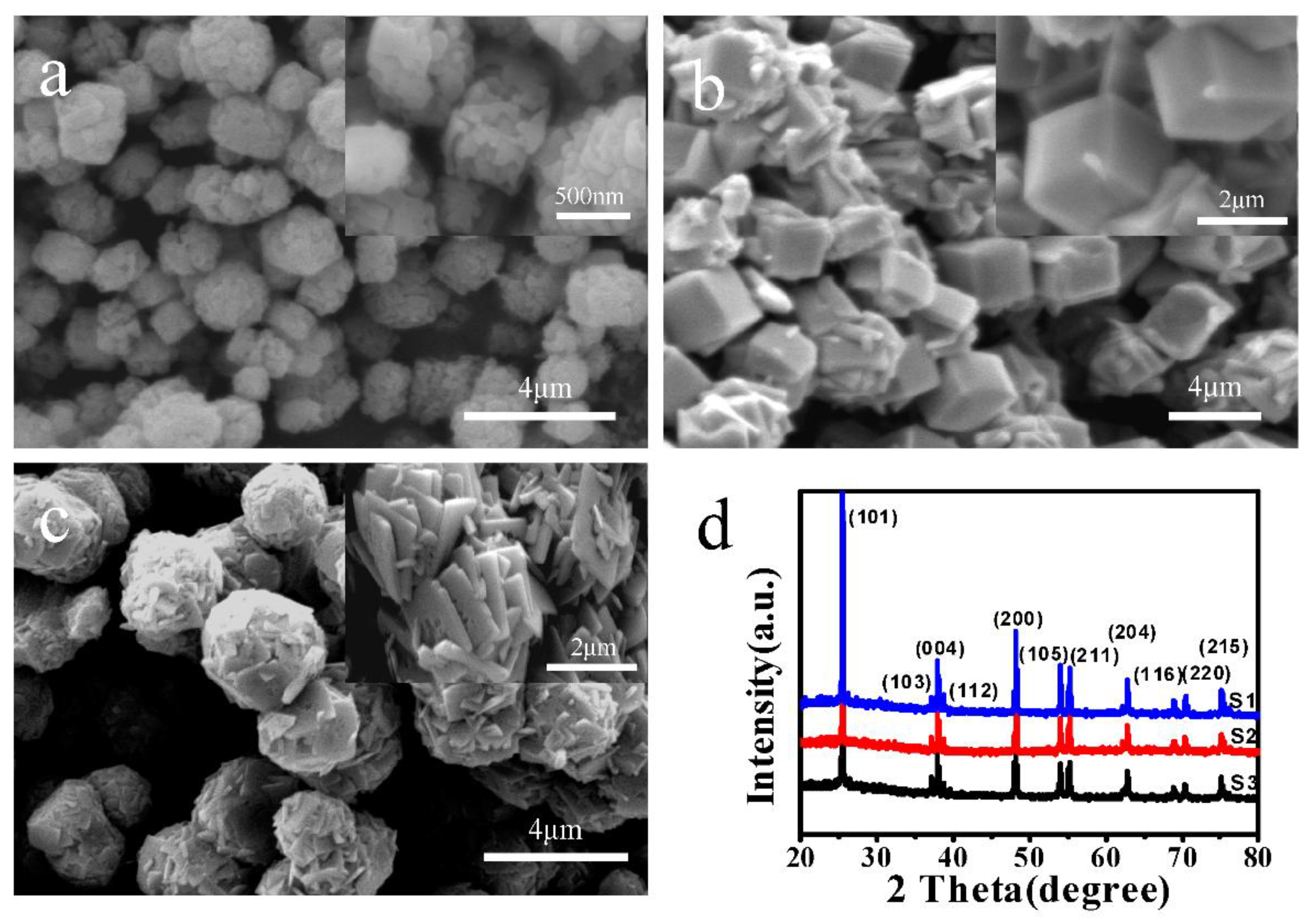
2.4. Preparation of TiO2 Hexahedral and Nanosheet Structures with Different Morphologies
2.5. Characterization
2.6. Photocatalysis Measurements
3. Results and Discussion
3.1. Characterization of TiO2 Microspheres
3.2. Characterization of TiO2 Flower Clusters Assembled with Nanorods
3.3. Characterization of TiO2 Hexahedral and Nanosheet Structures with Different Morphologies
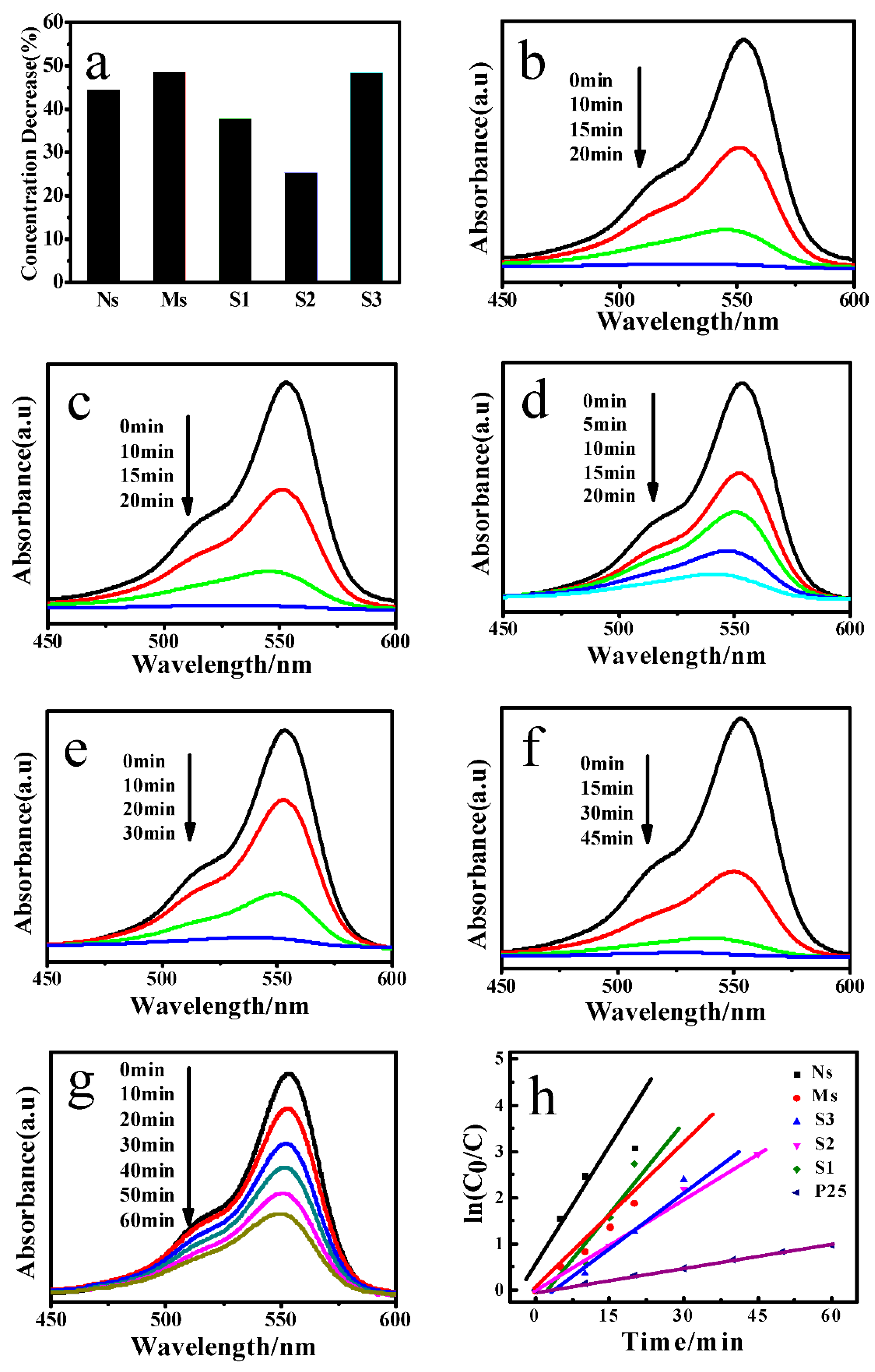
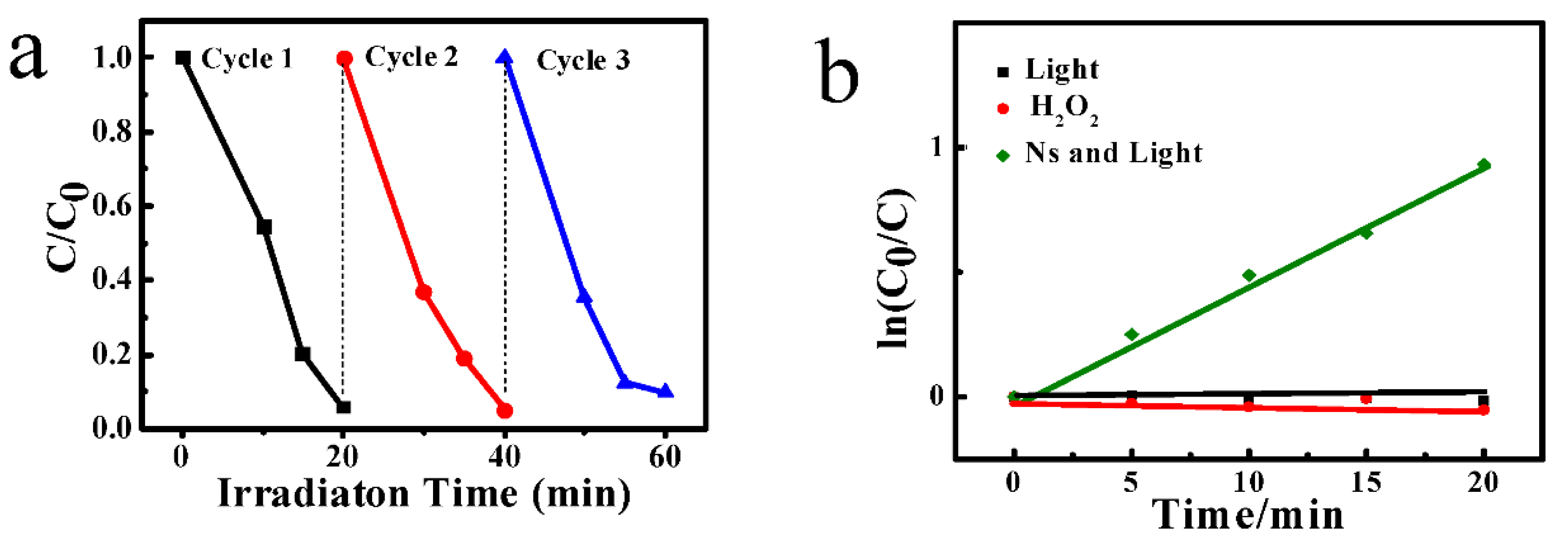
3.4. Photocatalysis Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luo, L.; Qiao, H.; Chen, K.; Fei, Y.Q.; Wei, Q.F. Fabrication of electrospun ZnMn2O4 nanofibers as anode material for lithium-ion batteries. Electrochim. Acta 2015, 177, 283–289. [Google Scholar] [CrossRef]
- Liu, H.; Li, W.; Shen, D.K.; Zhao, D.Y.; Wang, G.X. Graphitic carbon conformal coating of mesoporous TiO2 hollow spheres for high-performance lithium ion battery anodes. J. Am. Chem. Soc. 2015, 137, 13161–13166. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.G.; Zheng, D.P.; Zhang, S.P.; Cui, H.Z.; Li, D.X. Effect of nano-SiO2 on the hydration and microstructure of Portland cement. Nanomaterials 2016, 6, 241. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, Y.; Zhang, L.Z. Bismuth oxyhalide nanomaterials: Layered structures meet photocatalysis. Nanoscale 2014, 6, 8473–8488. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.M.; Li, W.L.; Pham, V.H.; Wang, L.J.; Sheng, P.T.; Cai, Q.Y.; Grimes, C. A CdS/ZnSe/TiO2 nanotube array and its visible light photocatalytic activities. J. Colloid Interface Sci. 2016, 462, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.S.; Dong, S.S.; Zhou, D.D.; Zhou, X.; Ma, D.M.; Du, Y.L. Synthesis of Er3+: Al2O3-doped and rutile-dominant TiO2 composite with increased responsive wavelength range and enhanced photocatalytic performance under visible light irradiation. J. Mol. Catal. A Chem. 2015, 407, 38–46. [Google Scholar] [CrossRef]
- Chen, X.B.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Grover, I.S.; Singh, S.; Pal, B. The preparation, surface structure, zeta potential, surface charge density and photocatalytic activity of TiO2 nanostructures of different shapes. Appl. Surf. Sci. 2013, 280, 366–372. [Google Scholar] [CrossRef]
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Smith, S.C.; Zou, J.; Cheng, H.M.; Lu, G.Q. Solvothermal synthesis and photoreactivity of anatase TiO2 nanosheets with dominant {001} facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.D.; Fu, N.Q.; Fang, Y.Y.; Li, X.; Liu, Q.; Zhou, X.; Lin, Y. Synthesis and formation mechanism of mesoporous TiO2 microspheres for scattering layer in dye-sensitized solar cells. Electrochim. Acta 2013, 113, 109–116. [Google Scholar] [CrossRef]
- Li, Z.Q.; Ding, Y.; Mo, L.E.; Hu, L.H.; Wu, J.H.; Dai, S.Y. Fine tuning of nanocrystal and pore sizes of TiO2 submicrospheres toward high performance dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 22277–22283. [Google Scholar] [CrossRef] [PubMed]
- Dozzi, M.V.; Selli, E. Specific facets-dominated anatase TiO2: Fluorine-mediated synthesis and photoactivity. Catalysts 2013, 3, 455–485. [Google Scholar] [CrossRef]
- Shalan, A.E.; Rashad, M.M.; Yu, Y.H.; Lira-Cantú, M.; Abdel-Mottaleb, M.S.A. A facile low temperature synthesis of TiO2 nanorods for high efficiency dye sensitized solar cells. Appl. Phys. A 2013, 110, 111–122. [Google Scholar] [CrossRef]
- Li, H.Y.; Lu, W.B.; Tian, J.Q.; Luo, Y.L.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Synthesis and study of plasmon-induced carrier behavior at Ag/TiO2 nanowires. Chem. Eur. J. 2012, 18, 8508–8514. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.Y.; Su, B.L. Titanium oxide nanotubes, nanofibers and nanowires. Colloids Surf. A Physiconchem. Eng. Asp. 2004, 241, 173–183. [Google Scholar] [CrossRef]
- Bao, Z.Q.; Xie, H.X.; Zhu, Q.; Qian, J.S.; Ruan, P.; Zhou, X.F. Microsphere assembly of TiO2 with tube-in-tube nanostructures: Anisotropic etching and photovoltaic enhancement. CrystEngComm 2013, 15, 8972–8976. [Google Scholar] [CrossRef]
- Ahirwar, D.; Bano, M.; Khan, F. Synthesis of mesoporous TiO2 and its role as a photocatalyst in degradation of indigo carmine dye. J. Sol-Gel Sci. Technol. 2016, 79, 228–237. [Google Scholar] [CrossRef]
- Yu, J.G.; Yu, H.G.; Cheng, B.; Zhao, X.J.; Zhang, Q.J. Preparation and photocatalytic activity of mesoporous anatase TiO2 nanofibers by a hydrothermal method. J. Photochem. Photobiol. A 2006, 182, 121–127. [Google Scholar] [CrossRef]
- Bian, Z.F.; Zhu, J.; Cao, F.L.; Huo, Y.N.; Lu, Y.F.; Li, H.X. Solvothermal synthesis of well-defined TiO2 mesoporous nanotubes with enhanced photocatalytic activity. Chem. Commun. 2010, 46, 8451–8453. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, P.W.; Mao, Y.; Xing, M.Y.; Zhang, J.L. Preparation of homogeneous nitrogen-doped mesoporous TiO2 spheres with enhanced visible-light photocatalysis. Appl. Catal. B Environ. 2015, 164, 352–359. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.X.; Wang, J.X.; Elzatahry, A.A.; Zhao, D.Y. A perspective on mesoporous TiO2 materials. Chem. Mater. 2013, 26, 287–298. [Google Scholar] [CrossRef]
- Guo, C.S.; Xu, J.; Zhang, Y.; He, Y. Hierarchical mesoporous TiO2 microspheres for the enhanced photocatalytic oxidation of sulfonamides and their mechanism. RSC Adv. 2012, 2, 4720–4727. [Google Scholar] [CrossRef]
- He, F.; Li, J.L.; Li, T.; Li, G.X. Solvothermal synthesis of mesoporous TiO2: The effect of morphology, size and calcination progress on photocatalytic activity in the degradation of gaseous benzene. Chem. Eng. J. 2014, 237, 312–321. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.K.; Hu, Y.Y.; O’Hayre, R.; Shao, Z.P. Facile synthesis of nanocrystalline TiO2 mesoporous microspheres for lithium-ion batteries. J. Phys. Chem. C 2011, 115, 2529–2536. [Google Scholar] [CrossRef]
- Chi, B.; Zhao, L.; Jin, T. One One-step template-free route for synthesis of mesoporous N-doped titania spheres. J. Phys. Chem. C 2007, 111, 6189–6193. [Google Scholar] [CrossRef]
- Crossland, E.J.W.; Noel, N.; Sivaram, V.; Leijtens, T.; Alexander-Webber, J.A.; Snaith, H.J. Mesoporous TiO2 single crystals delivering enhanced mobility and optoelectronic device performance. Nature 2013, 495, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.A.; Shen, S.S.; Zhou, L.; Liu, Z.S.; Feng, P.Z.; Ou, X.M.; Qiang, Y.H. Synthesis and hydrogenation of anatase TiO2 microspheres composed of porous single crystals for significantly improved photocatalytic activity. RSC Adv. 2016, 6, 62907–62910. [Google Scholar] [CrossRef]
- Niu, J.A.; Shen, S.S.; He, S.F.; Liu, Z.; Feng, P.; Zhang, S.; Ou, X.M.; Qiang, Y.H.; Zhu, Z. Synthesis and photoactivity of anatase porous single crystals with different pore sizes. Ceram. Int. 2015, 41, 11936–11944. [Google Scholar] [CrossRef]
- Ma, S.L.; Zhan, S.H.; Jia, Y.N.; Zhou, Q.X. Superior Antibacterial Activity of Fe3O4-TiO2 Nanosheets under Solar Light. ACS Appl. Mater. Interfaces 2015, 7, 21875–21883. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Zhang, N.; Tang, Z.R.; Xu, Y.J. Synthesis of one-dimensional CdS@TiO2 core–shell nanocomposites photocatalyst for selective redox: The dual role of TiO2 shell. ACS Appl. Mater. Interfaces 2012, 4, 6378–6385. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wu, X.M.; Fan, Y.M.; Zhou, X.Y. Fabrication of hierarchically structured rutile TiO2 nanorods on mica particles and their superhydrophilic coating without UV irridiation. Appl. Surf. Sci. 2014, 289, 281–288. [Google Scholar] [CrossRef]
- Zhu, Z.F.; Yan, Y.; Li, J.Q. One-step synthesis of flower-like WO3/Bi2WO6 heterojunction with enhanced visible light photocatalytic activity. J. Mater. Sci. 2016, 51, 2112–2120. [Google Scholar] [CrossRef]

| TBOT Addition (mL) | HCl Addition (mL) | Absolute Ethanol (mL) | Reaction Time (h) |
|---|---|---|---|
| 1.3 | 0.00 | 9.30 | 4 |
| 1.3 | 0.03 | 9.30 | 4 |
| 1.3 | 0.43 | 9.30 | 4 |
| 1.3 | 1.50 | 9.30 | 4 |
| 1.3 | 0.23 | 4.65 | 4 |
| 1.3 | 0.23 | 9.30 | 4 |
| 1.3 | 0.23 | 18.6 | 4 |
| C6H36O4Ti Addition (mL) | HCl Addition (mL) | H2O (mL) | TiO2 Addition (g) | Reaction Time (h) |
|---|---|---|---|---|
| 0.67 | 16 | 16 | 0.006 | 12 |
| 0.67 | 16 | 16 | 0.006 | 18 |
| 0.67 | 16 | 16 | 0.006 | 24 |
| 0.67 | 16 | 16 | 0.0 | 18 |
| Sample | TiF4 Addition (mmol/L) | HCl Addition (µL) | Ionic Liquid Aqueous Solution (mL) | SiO2 Addition (g) | Reaction Time (h) |
|---|---|---|---|---|---|
| S1 | 40 | 1000 | 1.5 | 0.325 | 20 |
| S2 | 40 | 1000 | 1.5 | 0.0 | 20 |
| S3 | 200 | 20 | 1.5 | 0.0 | 16 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, A.; Zhu, Y.; Guo, X.; Zhou, L.; Jiang, Q. Synthesis of Various TiO2 Micro-/Nano-Structures and Their Photocatalytic Performance. Materials 2018, 11, 995. https://doi.org/10.3390/ma11060995
Deng A, Zhu Y, Guo X, Zhou L, Jiang Q. Synthesis of Various TiO2 Micro-/Nano-Structures and Their Photocatalytic Performance. Materials. 2018; 11(6):995. https://doi.org/10.3390/ma11060995
Chicago/Turabian StyleDeng, Anquan, Yufu Zhu, Xin Guo, Lei Zhou, and Qingsong Jiang. 2018. "Synthesis of Various TiO2 Micro-/Nano-Structures and Their Photocatalytic Performance" Materials 11, no. 6: 995. https://doi.org/10.3390/ma11060995
APA StyleDeng, A., Zhu, Y., Guo, X., Zhou, L., & Jiang, Q. (2018). Synthesis of Various TiO2 Micro-/Nano-Structures and Their Photocatalytic Performance. Materials, 11(6), 995. https://doi.org/10.3390/ma11060995





