Preparation of a Porous, Sintered and Reaction-Bonded Si3N4 (SRBSN) Planar Membrane for Filtration of an Oil-in-Water Emulsion with High Flux Performance
Abstract
:1. Introduction
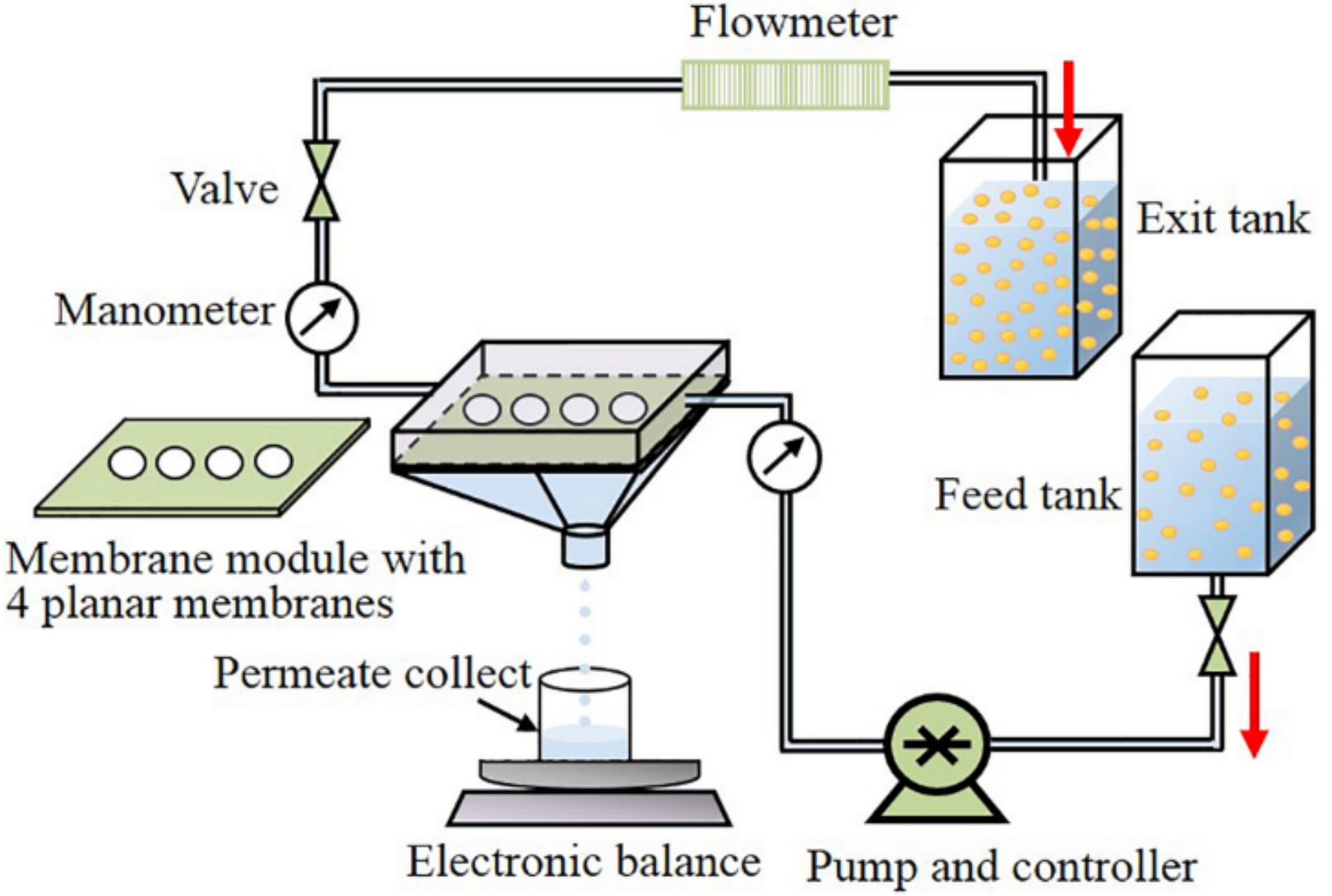
2. Materials and Methods
3. Results
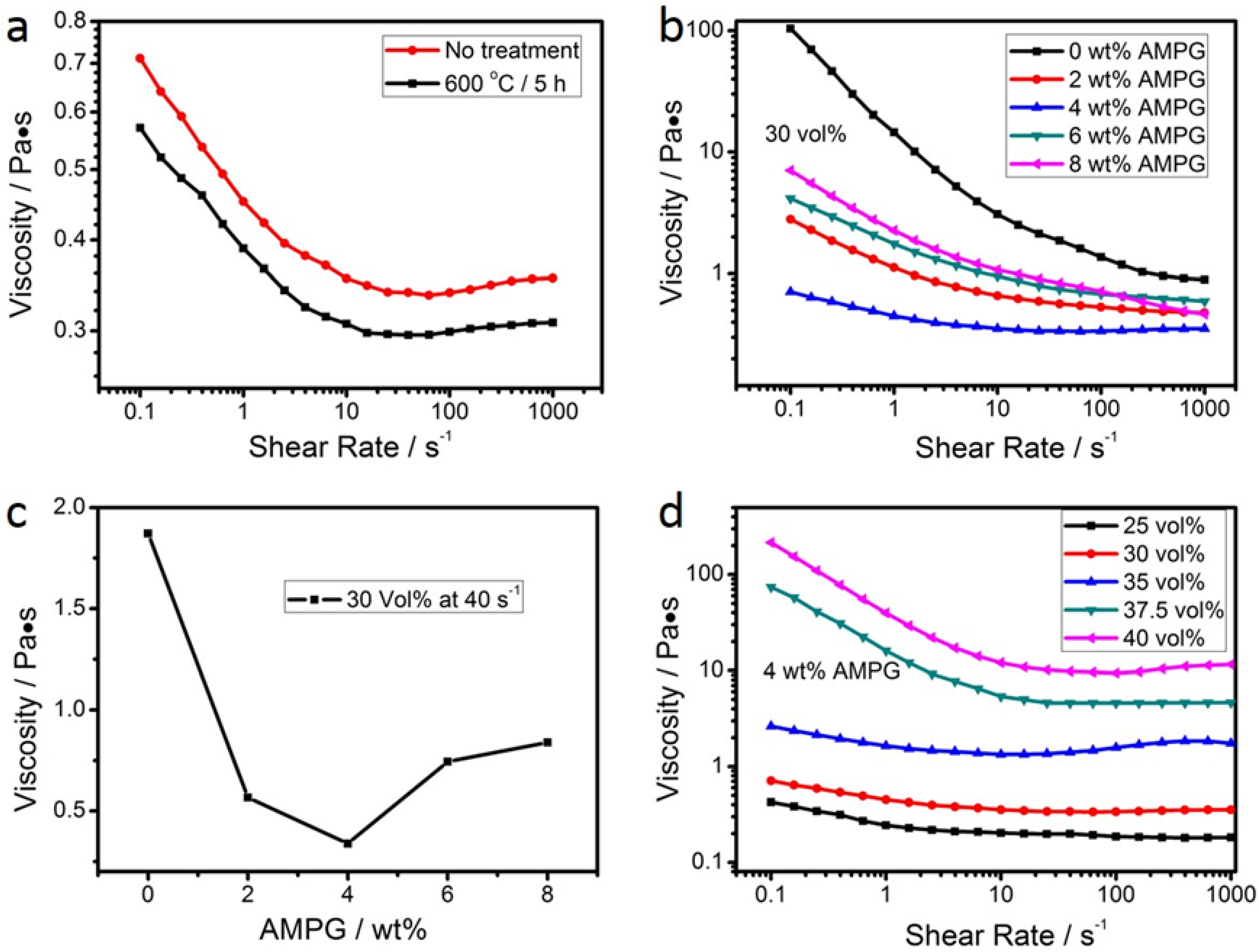
3.1. Rheological Properties of the Suspensions
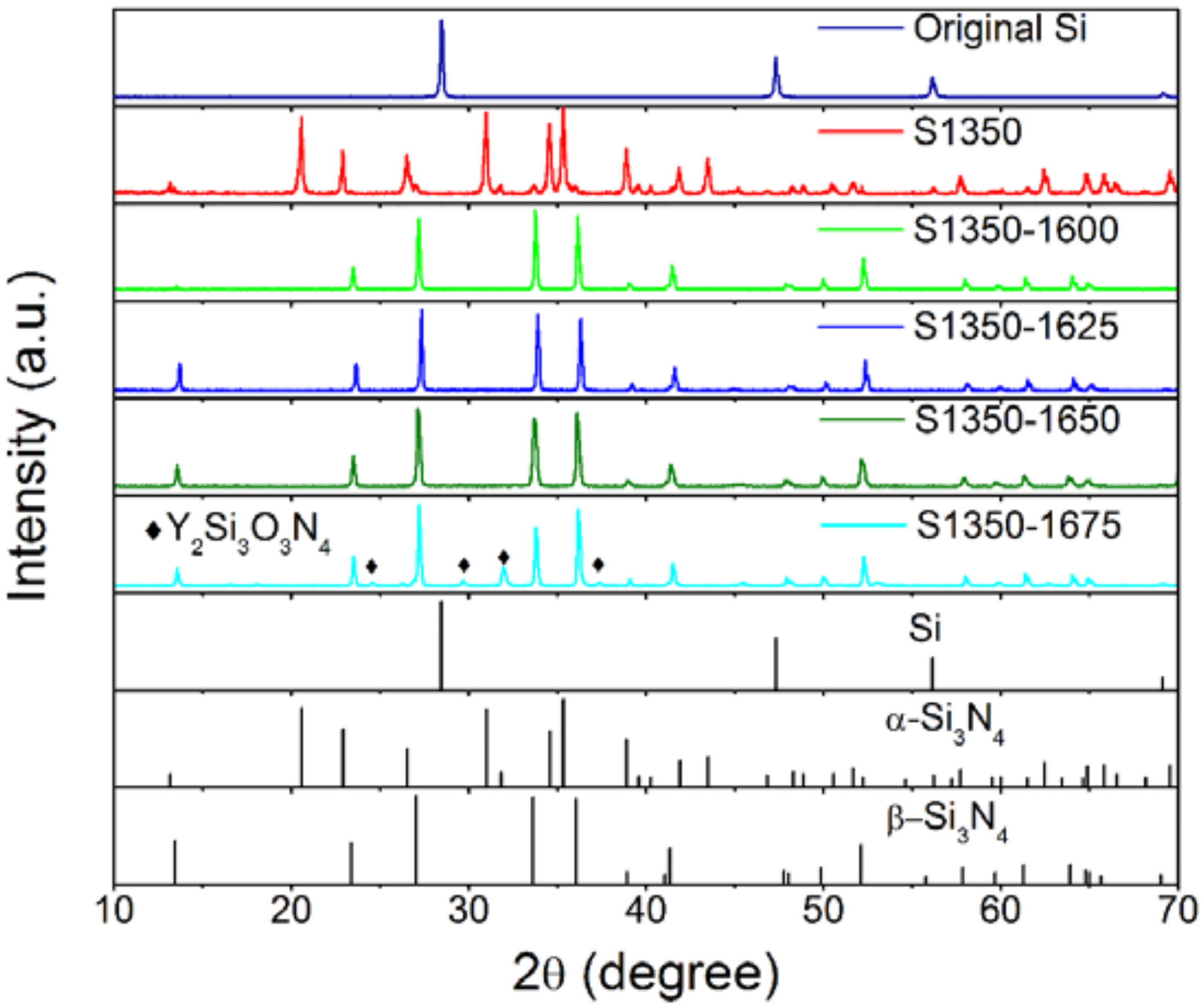
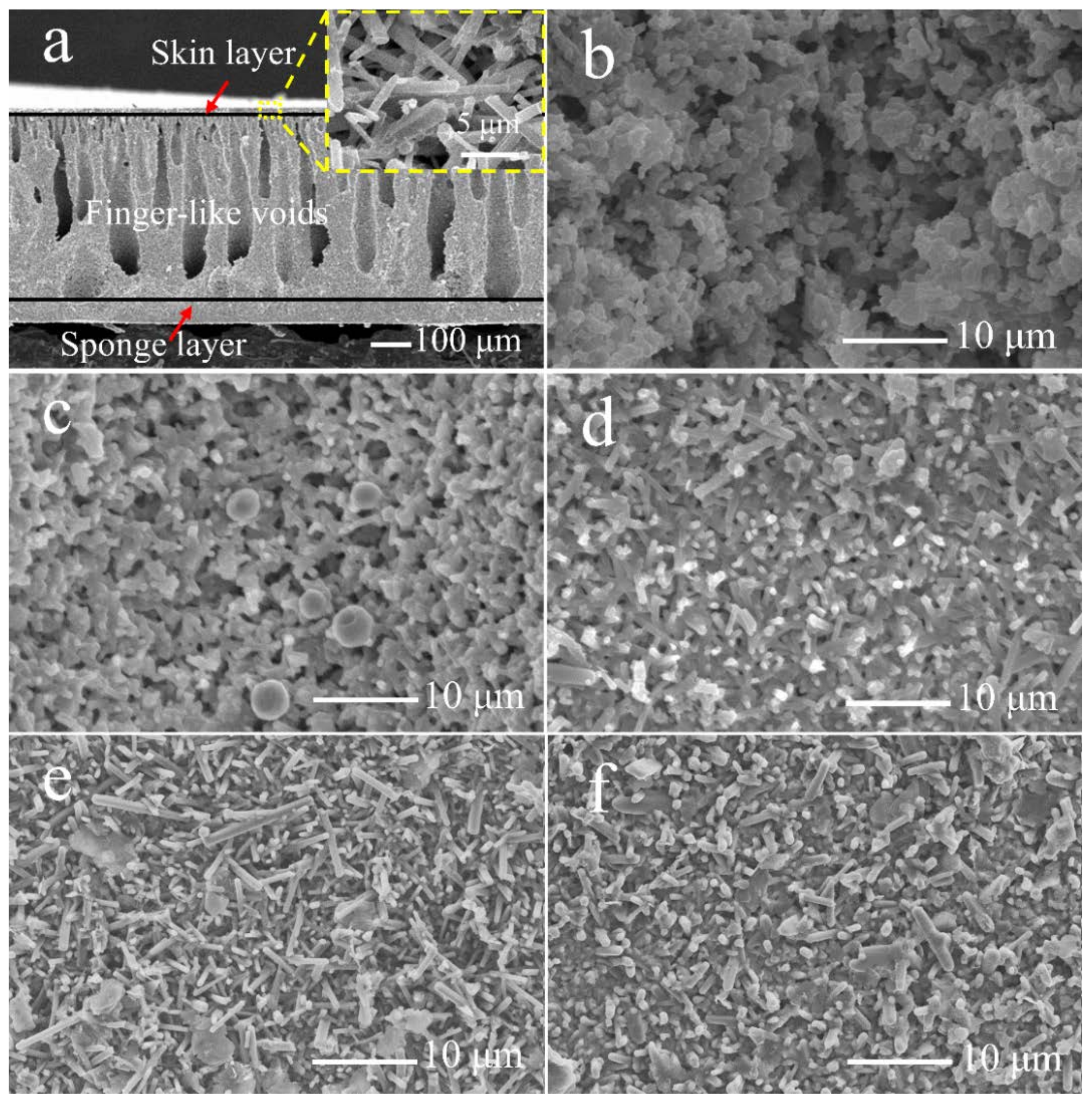
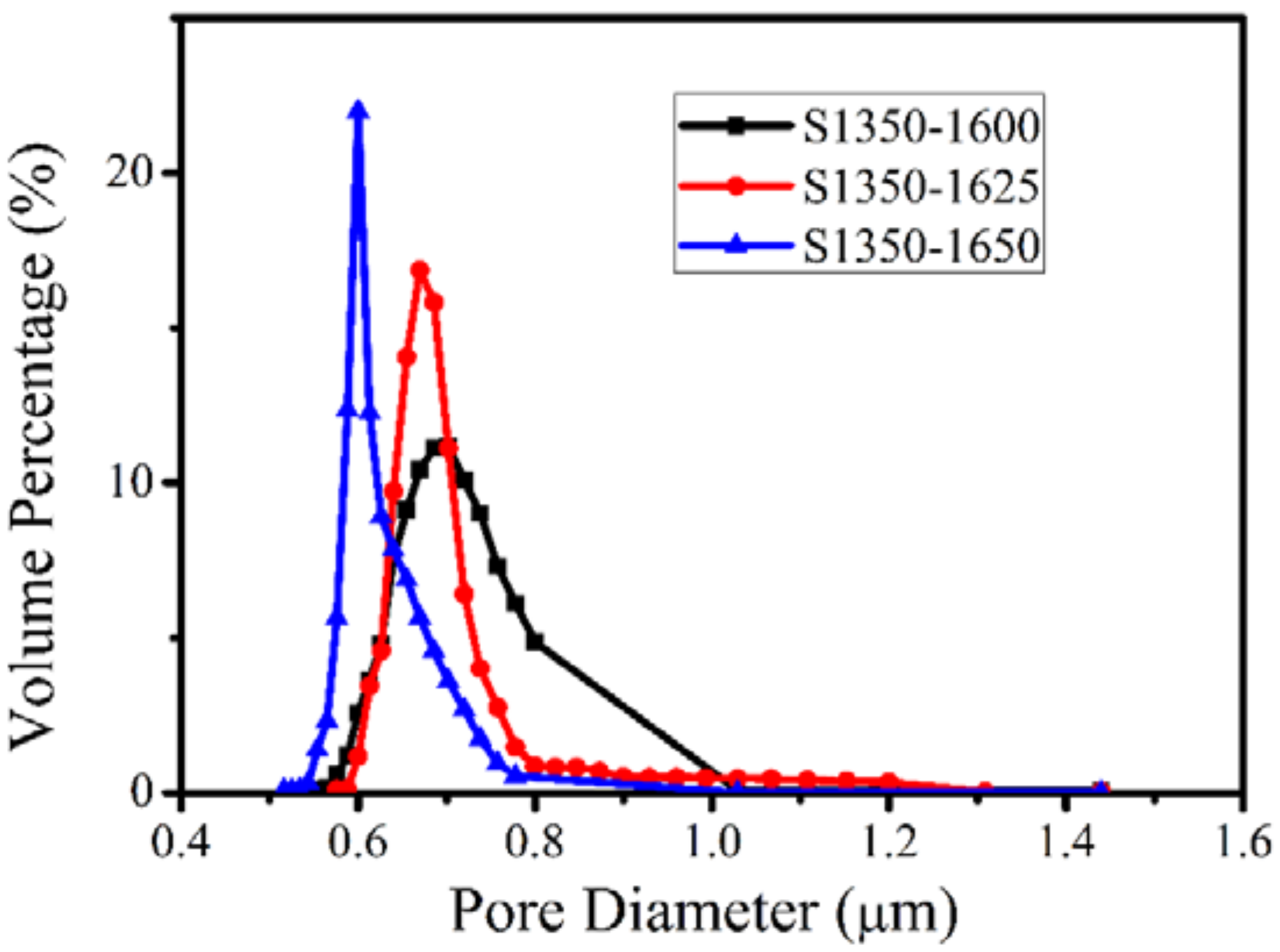
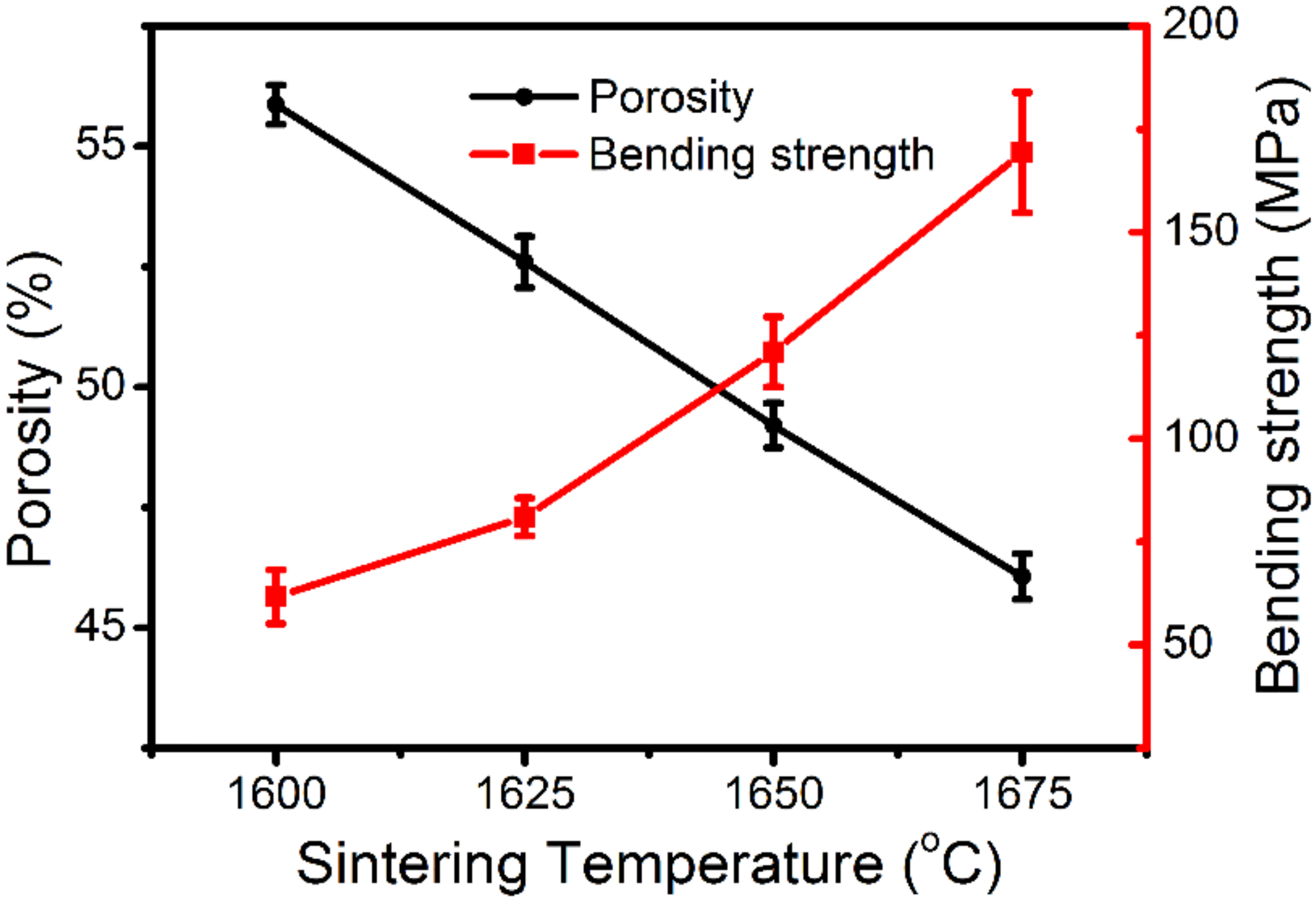
3.2. Characterization of the Membranes
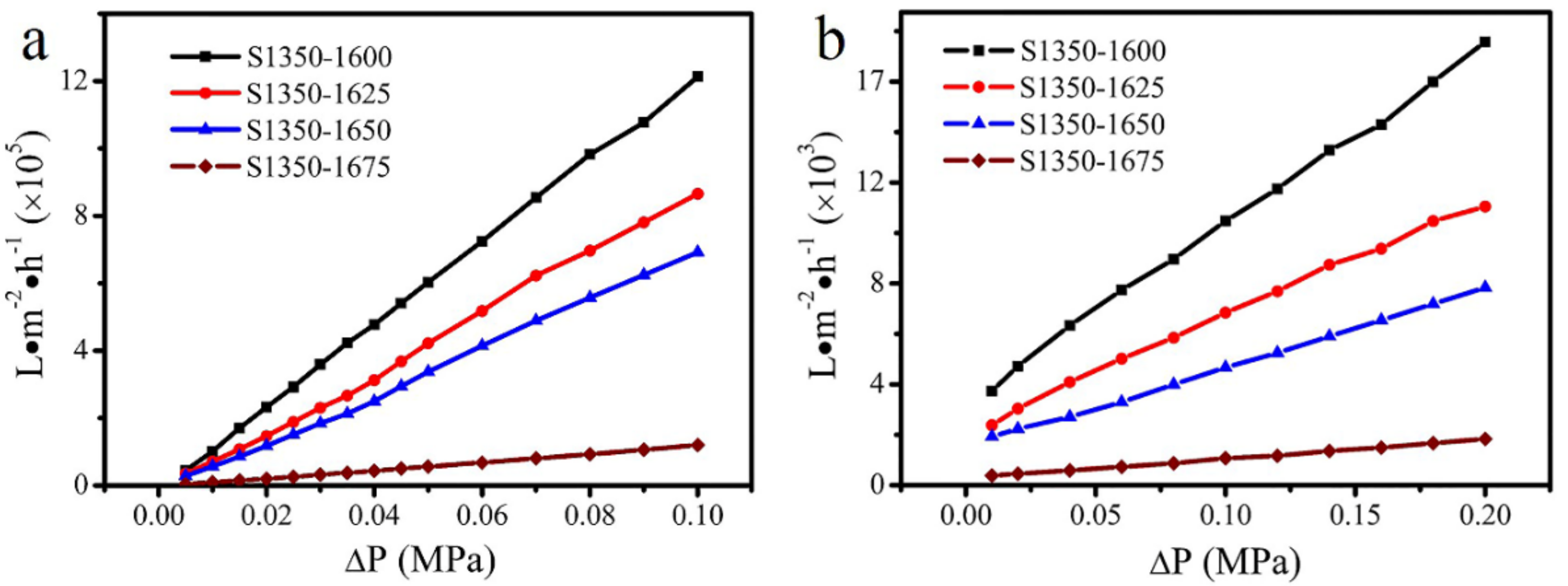
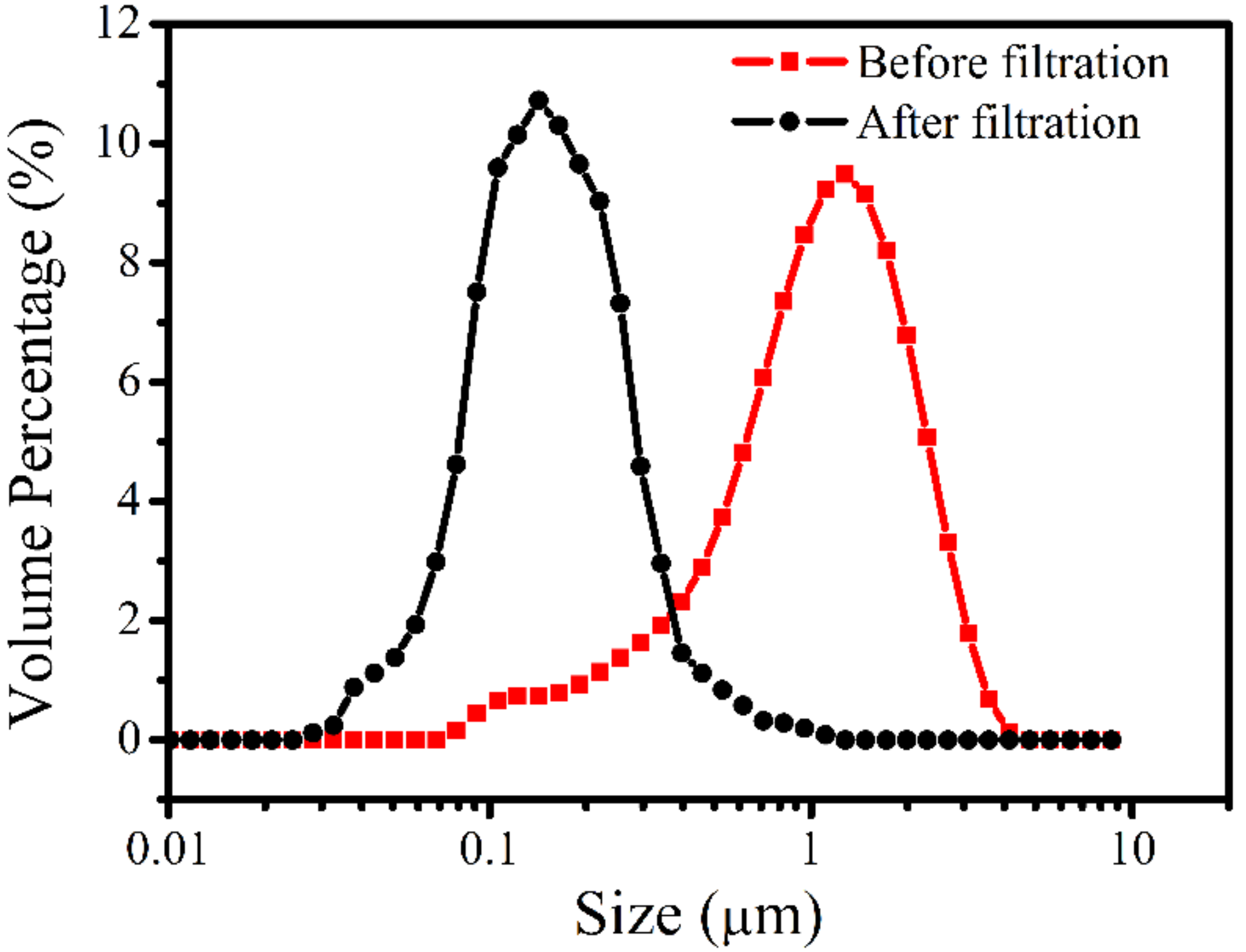
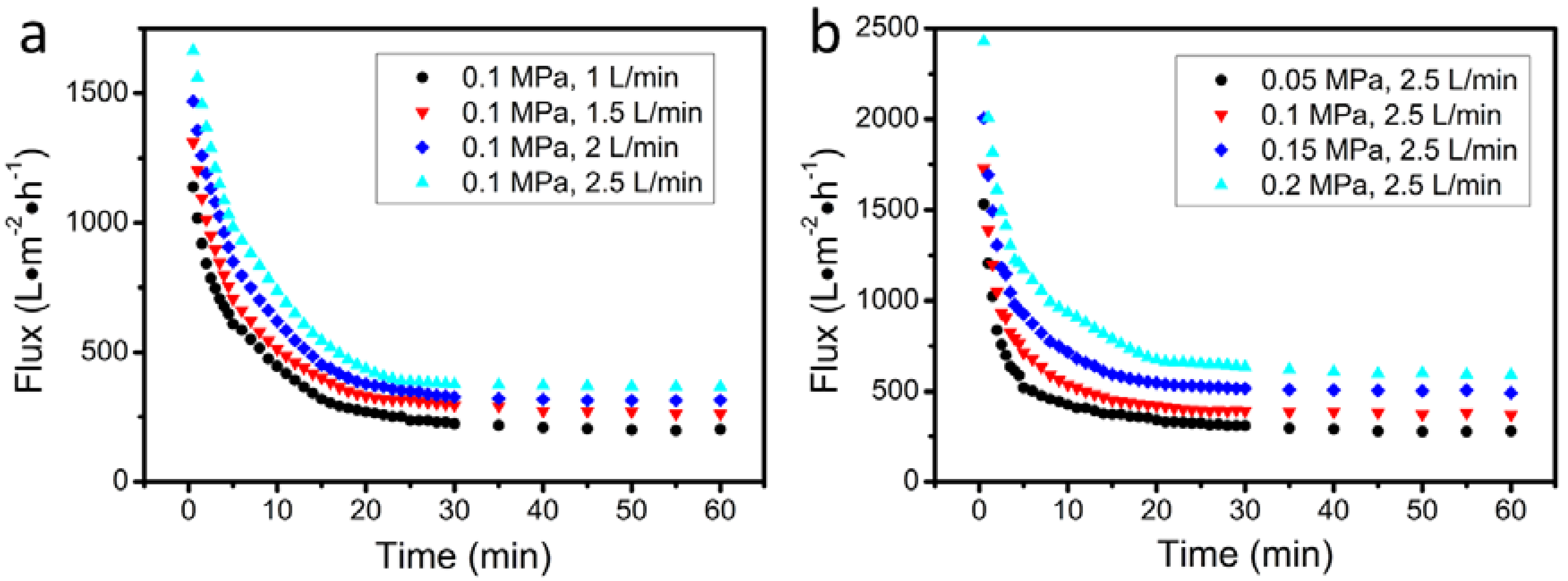
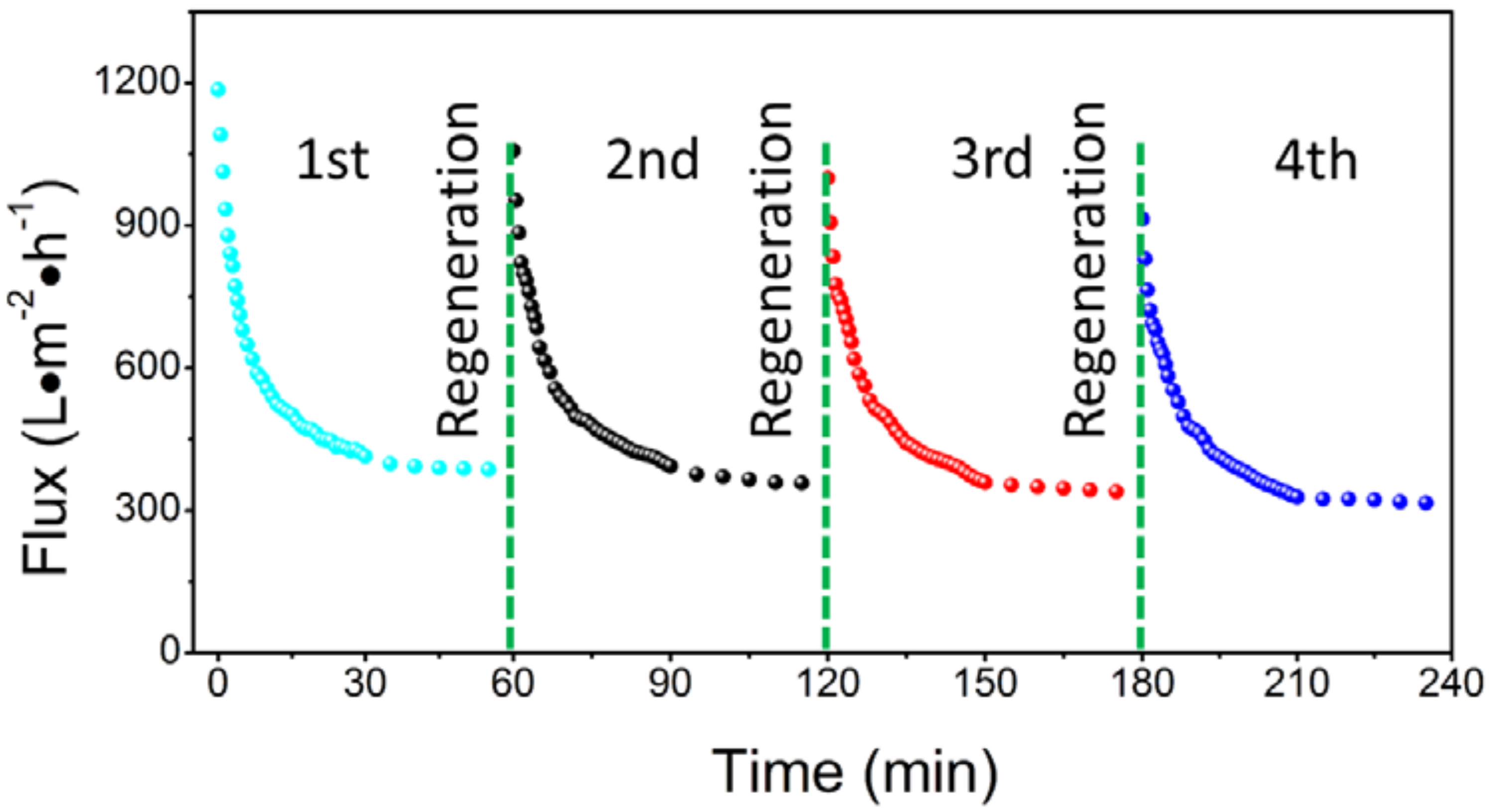
3.3. Filtration of Oil-in-Water Emulsion
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Das, B.; Chakrabarty, B.; Barkakati, P. Separation of oil from oily wastewater using low cost ceramic membrane. Korean J. Chem. Eng. 2017, 34, 2559–2569. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation: A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Garmsiri, E.; Rasouli, Y.; Abbasi, M.; Izadpanah, A.A. Chemical cleaning of mullite ceramic microfiltration membranes which are fouled during oily wastewater treatment. J. Water Process Eng. 2017, 19, 81–95. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, M.; Dong, Y.; Tang, C.Y.; Huang, A.; Li, L. A low-cost mullite-titania composite ceramic hollow fiber microfiltration membrane for highly efficient separation of oil-in-water emulsion. Water Res. 2016, 90, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-E.; Chang, Q.; Wang, Y.; Wang, J.; Meng, G. Separation of stable oil–water emulsion by the hydrophilic nano-sized ZrO2 modified Al2O3 microfiltration membrane. Sep. Purif. Technol. 2010, 75, 243–248. [Google Scholar] [CrossRef]
- Vasanth, D.; Pugazhenthi, G.; Uppaluri, R. Cross-flow microfiltration of oil-in-water emulsions using low cost ceramic membranes. Desalination 2013, 320, 86–95. [Google Scholar] [CrossRef]
- Zhong, J.; Sun, X.; Wang, C. Treatment of oily wastewater produced from refinery processes using flocculation and ceramic membrane filtration. Sep. Purif. Technol. 2003, 32, 93–98. [Google Scholar] [CrossRef]
- Nandi, B.K.; Moparthi, A.; Uppaluri, R.; Purkait, M.K. Treatment of oily wastewater using low cost ceramic membrane: Comparative assessment of pore blocking and artificial neural network models. Chem. Eng. Res. Des. 2010, 88, 881–892. [Google Scholar] [CrossRef]
- Yi, G.; Chen, S.; Quan, X.; Wei, G.; Fan, X.; Yu, H. Enhanced separation performance of carbon nanotube–polyvinyl alcohol composite membranes for emulsified oily wastewater treatment under electrical assistance. Sep. Purif. Technol. 2018, 197, 107–115. [Google Scholar] [CrossRef]
- Ge, J.; Jin, Q.; Zong, D.; Yu, J.; Ding, B. Biomimetic multilayer nanofibrous membranes with elaborated superwettability for effective purification of emulsified oily wastewater. ACS Appl. Mater. Interfaces 2018, 10, 16183–16192. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; McCutcheon, J.R.; Rahaman, M.S. A high flux polyvinyl acetate-coated electrospun nylon 6/SiO2 composite microfiltration membrane for the separation of oil-in-water emulsion with improved antifouling performance. J. Membr. Sci. 2017, 537, 297–309. [Google Scholar] [CrossRef]
- Liao, Y.; Tian, M.; Wang, R. A high-performance and robust membrane with switchable super-wettability for oil/water separation under ultralow pressure. J. Membr. Sci. 2017, 543, 123–132. [Google Scholar] [CrossRef]
- Ou, R.; Wei, J.; Jiang, L.; Simon, G.; Wang, H. Robust thermoresponsive polymer composite membrane with switchable superhydrophilicity and superhydrophobicity for efficient oil-water separation. Environ. Sci. Technol. 2016, 50, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, C.; Liu, H.; Huang, Q.; Hao, J.; Fu, H. Poly(vinylidene fluoride-hexafluoropropylene) porous membrane with controllable structure and applications in efficient oil/water separation. Materials 2018, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic membranes: Preparation and application for water treatment and desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, M.; Mirfendereski, M.; Nikbakht, M.; Golshenas, M.; Mohammadi, T. Performance study of mullite and mullite–alumina ceramic MF membranes for oily wastewaters treatment. Desalination 2010, 259, 169–178. [Google Scholar] [CrossRef]
- Atallah, C.; Tremblay, A.Y.; Mortazavi, S. Silane surface modified ceramic membranes for the treatment and recycling of SAGD produced water. J. Pet. Sci. Eng. 2017, 157, 349–358. [Google Scholar] [CrossRef]
- Das, B.; Chakrabarty, B.; Barkakati, P. Preparation and characterization of novel ceramic membranes for micro-filtration applications. Ceram. Int. 2016, 42, 14326–14333. [Google Scholar] [CrossRef]
- Masuda, T.; Asoh, H.; Haraguchi, S.; Ono, S. Fabrication and characterization of single phase alpha-alumina membranes with tunable pore diameters. Materials 2015, 8, 1350–1368. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, O.A.; Mujtaba Khan, M.; Özgür, C. Improving the performance of silica-based crossflow membranes by surface crystallization for treatment of oily wastewater. J. Aust. Ceram. Soc. 2017, 53, 883–894. [Google Scholar] [CrossRef]
- Kim, S.C.; Yeom, H.-J.; Kim, Y.-W.; Song, I.-H.; Ha, J.-H. Processing of alumina-coated glass-bonded silicon carbide membranes for oily wastewater treatment. Int. J. Appl. Ceram. Technol. 2017, 14, 692–702. [Google Scholar] [CrossRef]
- Suresh, K.; Pugazhenthi, G. Cross flow microfiltration of oil-water emulsions using clay based ceramic membrane support and TiO2 composite membrane. Egypt. J. Pet. 2017, 26, 679–694. [Google Scholar] [CrossRef]
- Chao, Y.; Guosheng, Z.; Nanping, X.; Jun, S. Preparation and application in oil-water separation of ZrO2/α-Al2O3 MF membrane. J. Membr. Sci. 1998, 142, 235–243. [Google Scholar]
- Hubadillah, S.K.; Othman, M.H.D.; Harun, Z.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; MuniraJamil, S.; Mohtor, N.H. Superhydrophilic, low cost kaolin-based hollow fibre membranes for efficient oily-wastewater separation. Mater. Lett. 2017, 191, 119–122. [Google Scholar] [CrossRef]
- Mohammadi, F.; Mohammadi, T. Optimal conditions of porous ceramic membrane synthesis based on alkali activated blast furnace slag using Taguchi method. Ceram. Int. 2017, 43, 14369–14379. [Google Scholar] [CrossRef]
- Rasouli, Y.; Abbasi, M.; Hashemifard, S.A. Investigation of in-line coagulation-MF hybrid process for oily wastewater treatment by using novel ceramic membranes. J. Clean. Product. 2017, 161, 545–559. [Google Scholar] [CrossRef]
- Da Silva Biron, D.; Zeni, M.; Bergmann, C.P.; dos Santos, V. Analysis of composite membranes in the separation of emulsions sunflower oil/water. Mater. Res. 2017, 20, 843–852. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Nanofibrous metal–organic framework composite membrane for selective efficient oil/water emulsion separation. J. Membr. Sci. 2017, 543, 10–17. [Google Scholar] [CrossRef]
- Wang, K.; Yiming, W.; Saththasivam, J.; Liu, Z. A flexible, robust and antifouling asymmetric membrane based on ultra-long ceramic/polymeric fibers for high-efficiency separation of oil/water emulsions. Nanoscale 2017, 9, 9018–9025. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.F.; Rodrigues, M.I.; Trindade, T.; Daniel-da-Silva, A.L. Chitosan-silica hybrid nanosorbents for oil removal from water. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 305–313. [Google Scholar] [CrossRef]
- Liu, C.-T.; Su, P.-K.; Hu, C.-C.; Lai, J.-Y.; Liu, Y.-L. Surface modification of porous substrates for oil/water separation using crosslinkable polybenzoxazine as an agent. J. Membr. Sci. 2018, 546, 100–109. [Google Scholar] [CrossRef]
- Wu, J.-M.; Zhang, X.-Y.; Xu, J.; Gan, K.; Li, J.-L.; Li, C.-H.; Yang, J.L.; Shi, Y.S. Preparation of porous Si3N4 ceramics via tailoring solid loading of Si3N4 slurry and Si3N4 poly-hollow microsphere content. J. Adv. Ceram. 2015, 4, 260–266. [Google Scholar] [CrossRef]
- Riley, F.L. Silicon nitride and related materials. J. Am. Ceram. Soc. 2000, 83, 245–265. [Google Scholar] [CrossRef]
- Neumann, A.; Reske, T.; Held, M.; Jahnke, K. Comparative investigation of the biocompatibility of various silicon nitride ceramic qualities in vitro. J. Mater. Sci. Mater. Med. 2004, 15, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Dion, I.; Bordenave, L.; Lefebvre, F.; Bareille, R.; Baquey, C. Physico-chemistry and cytotoxicity of ceramics. J. Mater. Sci. Mater. Med. 1994, 5, 18–24. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Fang, H.; Hao, L.-Y.; Xu, X.; Chen, C.-S. Preparation of silicon nitride hollow fibre membrane for desalination. Mater. Lett. 2012, 68, 457–459. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Fang, H.; Wang, J.-W.; Hao, L.-Y.; Xu, X.; Chen, C.-S. Preparation and characterization of silicon nitride hollow fiber membranes for seawater desalination. J. Membr. Sci. 2014, 450, 197–206. [Google Scholar] [CrossRef]
- Wang, J.-W.; Li, L.; Zhang, J.-W.; Xu, X.; Chen, C.-S. β-Sialon ceramic hollow fiber membranes with high strength and low thermal conductivity for membrane distillation. J. Eur. Ceram. Soc. 2016, 36, 59–65. [Google Scholar] [CrossRef]
- Wang, J.-W.; Li, X.-Z.; Fan, M.; Gu, J.-Q.; Hao, L.-Y.; Xu, X.; Chen, C.-S.; Wang, C.-M.; Hao, Y.-Z.; Agathopoulos, S. Porous β-Sialon planar membrane with a robust polymer-derived hydrophobic ceramic surface. J. Membr. Sci. 2017, 535, 63–69. [Google Scholar] [CrossRef]
- Alem, A.; Drew, R.A.L.; Pugh, M.D. Sintered reaction-bonded silicon nitride foams with a high level of interconnected porosity. J. Mater. Sci. 2014, 50, 570–576. [Google Scholar] [CrossRef]
- Golla, B.R.; Ko, J.W.; Kim, J.-M.; Kim, H.-D. Effect of particle size and oxygen content of Si on processing, microstructure and thermal conductivity of sintered reaction bonded Si3N4. J. Alloys Compd. 2014, 595, 60–66. [Google Scholar] [CrossRef]
- Yao, D.; Xia, Y.; Zuo, K.-H.; Jiang, D.; Günster, J.; Zeng, Y.-P.; Heinrich, J.G. The effect of fabrication parameters on the mechanical properties of sintered reaction bonded porous Si3N4 ceramics. J. Eur. Ceram. Soc. 2014, 34, 3461–3467. [Google Scholar] [CrossRef]
- Tuyen, D.-V.; Park, Y.-J.; Kim, H.-D.; Lee, B.-T. Formation of rod-like Si3N4 grains in porous SRBSN bodies using 6Y2O3–2MgO sintering additives. Ceram. Int. 2009, 35, 2305–2310. [Google Scholar] [CrossRef]
- Ren, C.; Fang, H.; Gu, J.; Winnubst, L.; Chen, C. Preparation and characterization of hydrophobic alumina planar membranes for water desalination. J. Eur. Ceram. Soc. 2015, 35, 723–730. [Google Scholar] [CrossRef]
- Gu, J.; Ren, C.; Zong, X.; Chen, C.; Winnubst, L. Preparation of alumina membranes comprising a thin separation layer and a support with straight open pores for water desalination. Ceram. Int. 2016, 42, 12427–12434. [Google Scholar] [CrossRef]
- Gu, J.; Wang, J.; Li, Y.; Xu, X.; Chen, C.; Winnubst, L. Engineering durable hydrophobic surfaces on porous alumina ceramics using in-situ formed inorganic-organic hybrid nanoparticles. J. Eur. Ceram. Soc. 2017, 37, 4843–4848. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, L.; Xing, W.; Wang, H.; Xu, N. Preparation of TiO2 hollow fibers using poly(vinylidene fluoride) hollow fiber microfiltration membrane as a template. Mater. Chem. Phys. 2005, 94, 322–327. [Google Scholar] [CrossRef]
- Vinoth Kumar, R.; Kumar Ghoshal, A.; Pugazhenthi, G. Elaboration of novel tubular ceramic membrane from inexpensive raw materials by extrusion method and its performance in microfiltration of synthetic oily wastewater treatment. J. Membr. Sci. 2015, 490, 92–102. [Google Scholar] [CrossRef]
- Emani, S.; Uppaluri, R.; Purkait, M.K. Cross flow microfiltration of oil–water emulsions using kaolin based low cost ceramic membranes. Desalination 2014, 341, 61–71. [Google Scholar] [CrossRef]
- Fang, J.; Qin, G.; Wei, W.; Zhao, X.; Jiang, L. Elaboration of new ceramic membrane from spherical fly ash for microfiltration of rigid particle suspension and oil-in-water emulsion. Desalination 2013, 311, 113–126. [Google Scholar] [CrossRef]

| Sample | Powder Mixture | AMPG | PESf | NMP |
|---|---|---|---|---|
| S2A375 | 50 | 1 | 7.14 | 35.61 |
| S4A375 | 50 | 2 | 7.14 | 35.61 |
| S6A375 | 50 | 3 | 7.14 | 35.61 |
| S8A375 | 50 | 4 | 7.14 | 35.61 |
| S4A35 | 50 | 2 | 7.14 | 39.68 |
| S4A40 | 50 | 2 | 7.14 | 32.05 |
| S4A425 | 50 | 2 | 7.14 | 26.12 |
| Pressing Pressure (MPa) | Flow Velocity (L min−1) | Permeation Flux (L m−2 h−1) | Rejection (%) |
|---|---|---|---|
| 0.1 | 1 | 202 | 81.2 |
| 0.1 | 1.5 | 262 | 83.6 |
| 0.1 | 2 | 316 | 86.3 |
| 0.1 | 2.5 | 367 | 88.6 |
| 0.05 | 2.5 | 279 | 91.4 |
| 0.1 | 2.5 | 367 | 88.6 |
| 0.15 | 2.5 | 492 | 85.8 |
| 0.2 | 2.5 | 592 | 83.5 |
| Material | Pore Size (μm) | Porosity (%) | Oil Concentration (mg/L) | Pressure (kPa) | Permeate Flux (L m−2 h−1) | Oil Rejection (%) | Reference |
|---|---|---|---|---|---|---|---|
| Si3N4 | 0.63 | 48.7 | 1000 | 100 | 367.0 | 88.6 | This work |
| M, Q, etc. | 1.06 | 26.0 | 100 | 207 | 199.4 | 87.0 | [6] |
| M, Q, etc. | 2.16 | 37.4 | 400 | 207 | 79.7 | 98.5 | [49] |
| M-A | 0.29 | 56.0 | 1000 | 300 | 244.0 | 81.3 | [16] |
| ZrO2/α-Al2O3 | 1.00 | 40.0 | 5500 | 100 | 27.0 | 94.3 | [23] |
| Fly ash | 0.77 | - | 2000 | 100 | 93.0 | 98.2 | [50] |
| TiO2 | 0.90 | 43.3 | 200 | 207 | 186.6 | 99.6 | [22] |
| SiC/Al2O3 | 0.07 | 44.6 | 600 | 101 | 64.1 | 99.9 | [21] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Gao, E.-Z.; Abadikhah, H.; Wang, J.-W.; Hao, L.-Y.; Xu, X.; Agathopoulos, S. Preparation of a Porous, Sintered and Reaction-Bonded Si3N4 (SRBSN) Planar Membrane for Filtration of an Oil-in-Water Emulsion with High Flux Performance. Materials 2018, 11, 990. https://doi.org/10.3390/ma11060990
Li L, Gao E-Z, Abadikhah H, Wang J-W, Hao L-Y, Xu X, Agathopoulos S. Preparation of a Porous, Sintered and Reaction-Bonded Si3N4 (SRBSN) Planar Membrane for Filtration of an Oil-in-Water Emulsion with High Flux Performance. Materials. 2018; 11(6):990. https://doi.org/10.3390/ma11060990
Chicago/Turabian StyleLi, Lin, Er-Ze Gao, Hamidreza Abadikhah, Jun-Wei Wang, Lu-Yuan Hao, Xin Xu, and Simeon Agathopoulos. 2018. "Preparation of a Porous, Sintered and Reaction-Bonded Si3N4 (SRBSN) Planar Membrane for Filtration of an Oil-in-Water Emulsion with High Flux Performance" Materials 11, no. 6: 990. https://doi.org/10.3390/ma11060990
APA StyleLi, L., Gao, E.-Z., Abadikhah, H., Wang, J.-W., Hao, L.-Y., Xu, X., & Agathopoulos, S. (2018). Preparation of a Porous, Sintered and Reaction-Bonded Si3N4 (SRBSN) Planar Membrane for Filtration of an Oil-in-Water Emulsion with High Flux Performance. Materials, 11(6), 990. https://doi.org/10.3390/ma11060990





