Microstructure and Properties of Thermal Electrode Material Si3N4–MoSi2 Composite Ceramics
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
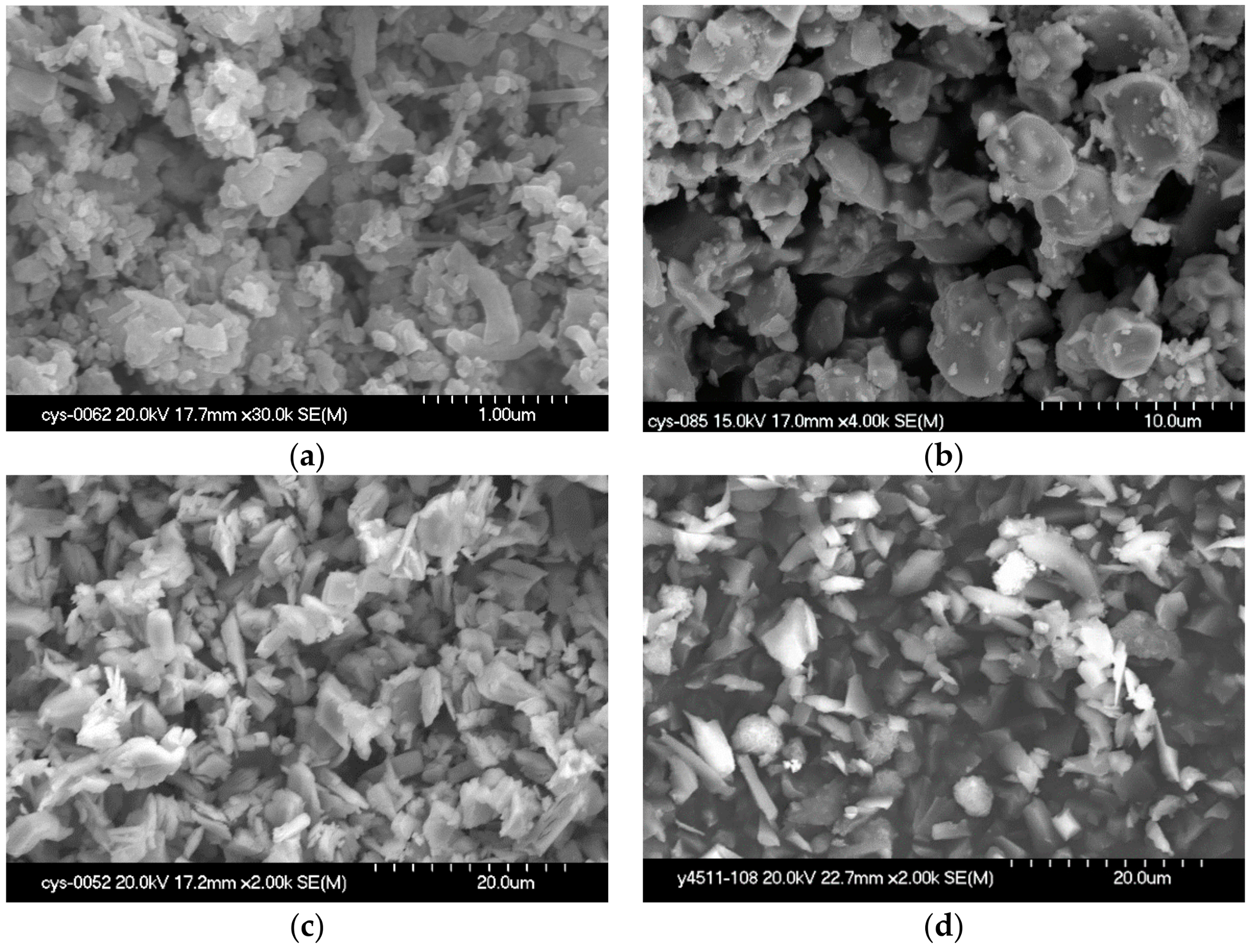
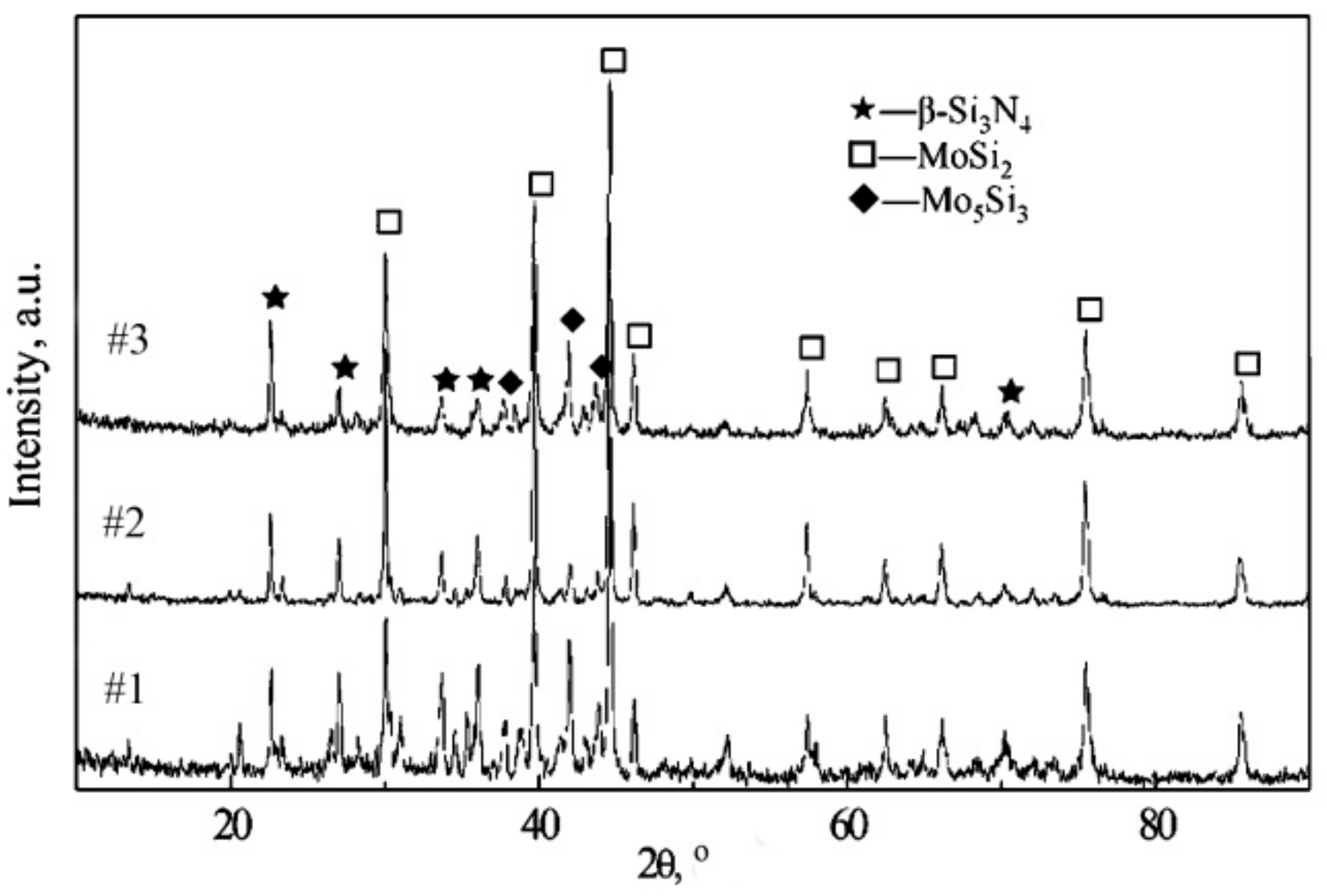
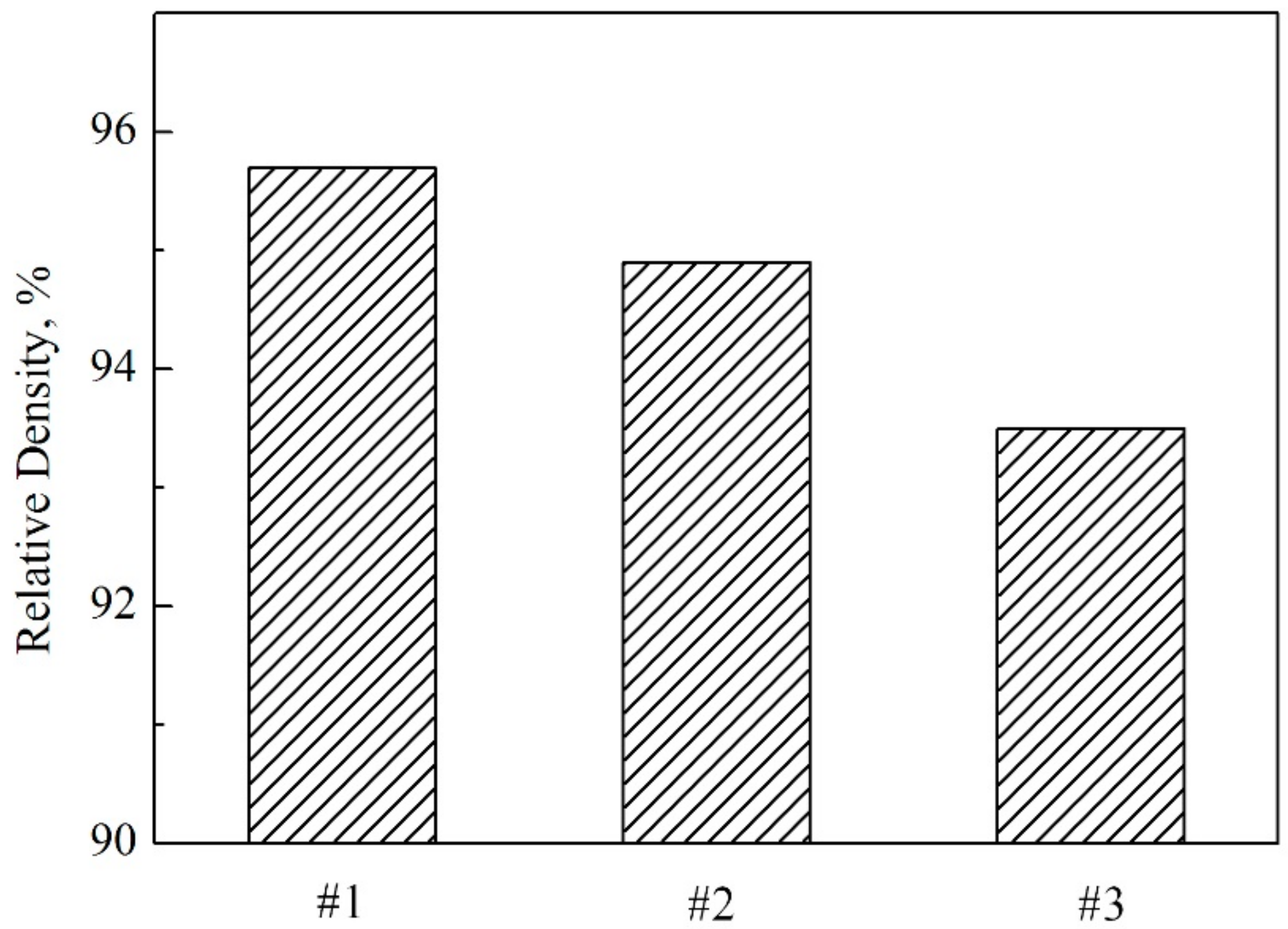
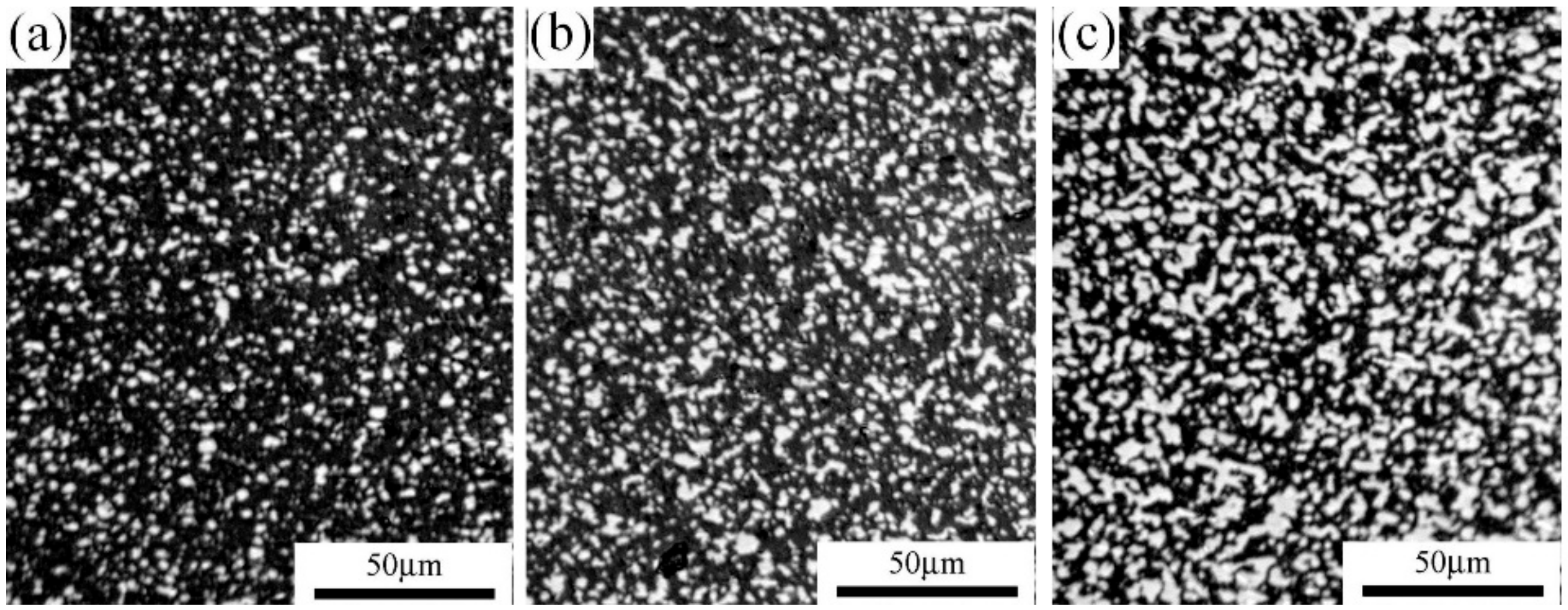
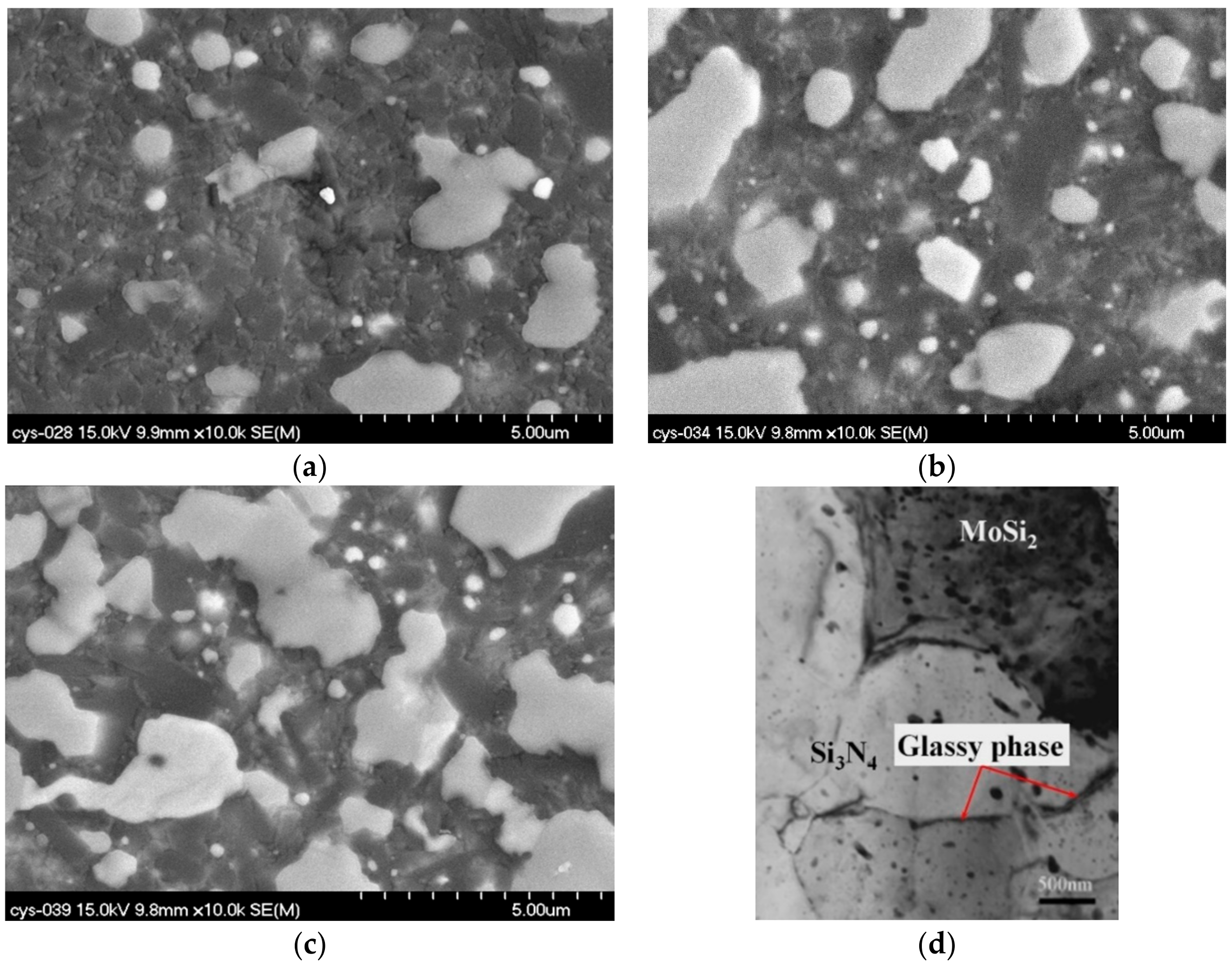
3.1. XRD Results and Optical Microstructure
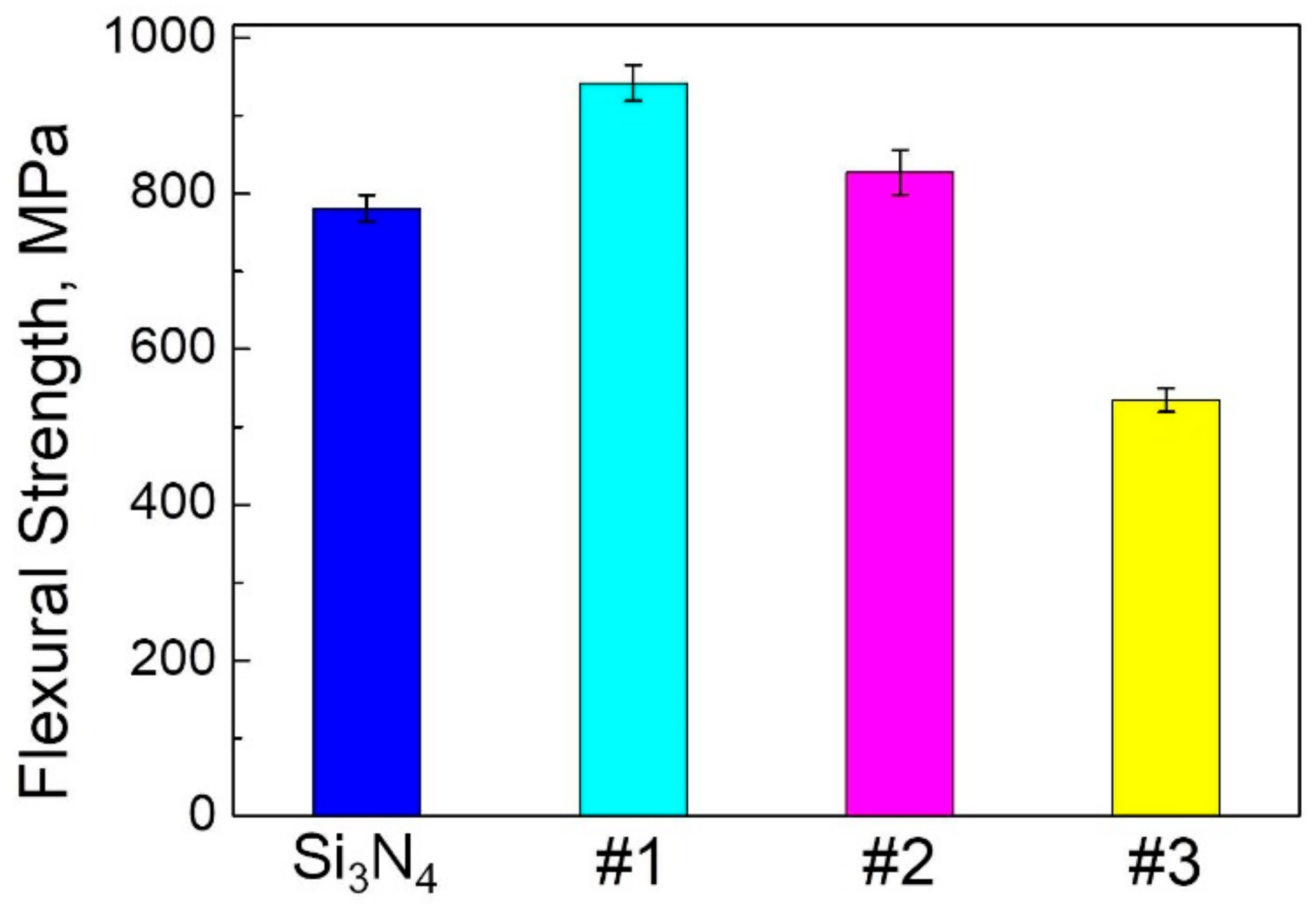
3.2. Flexural Strength
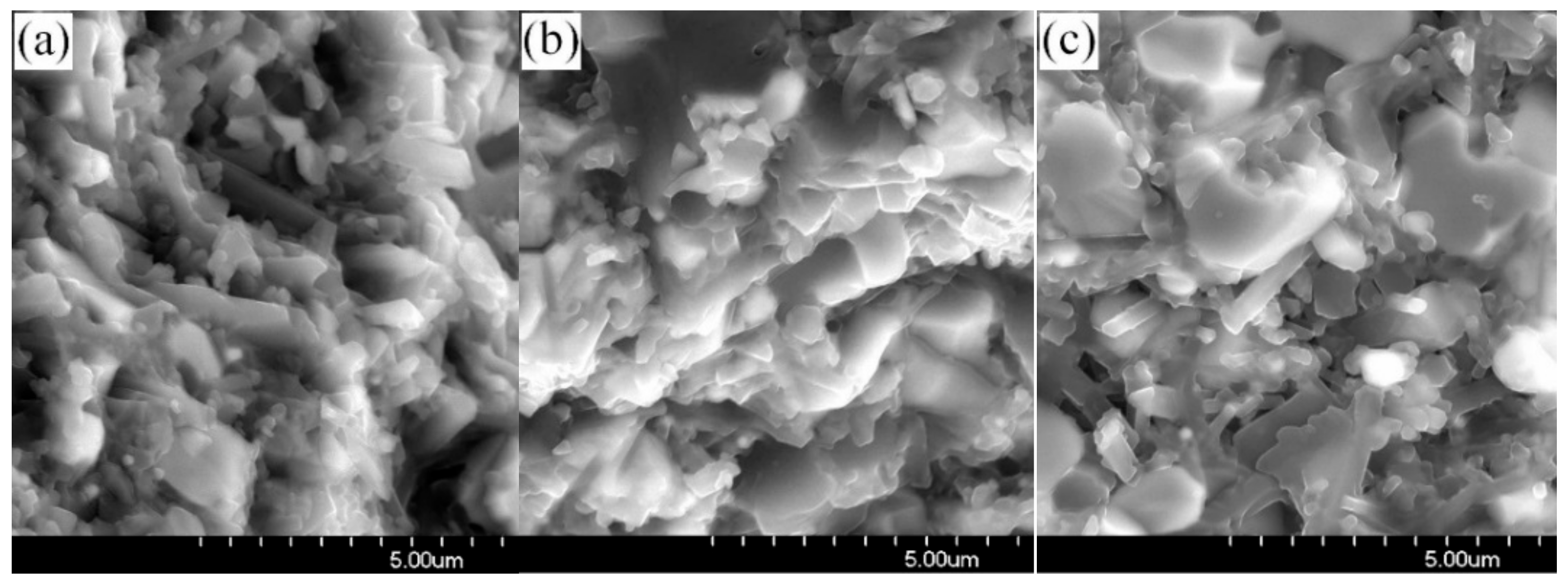
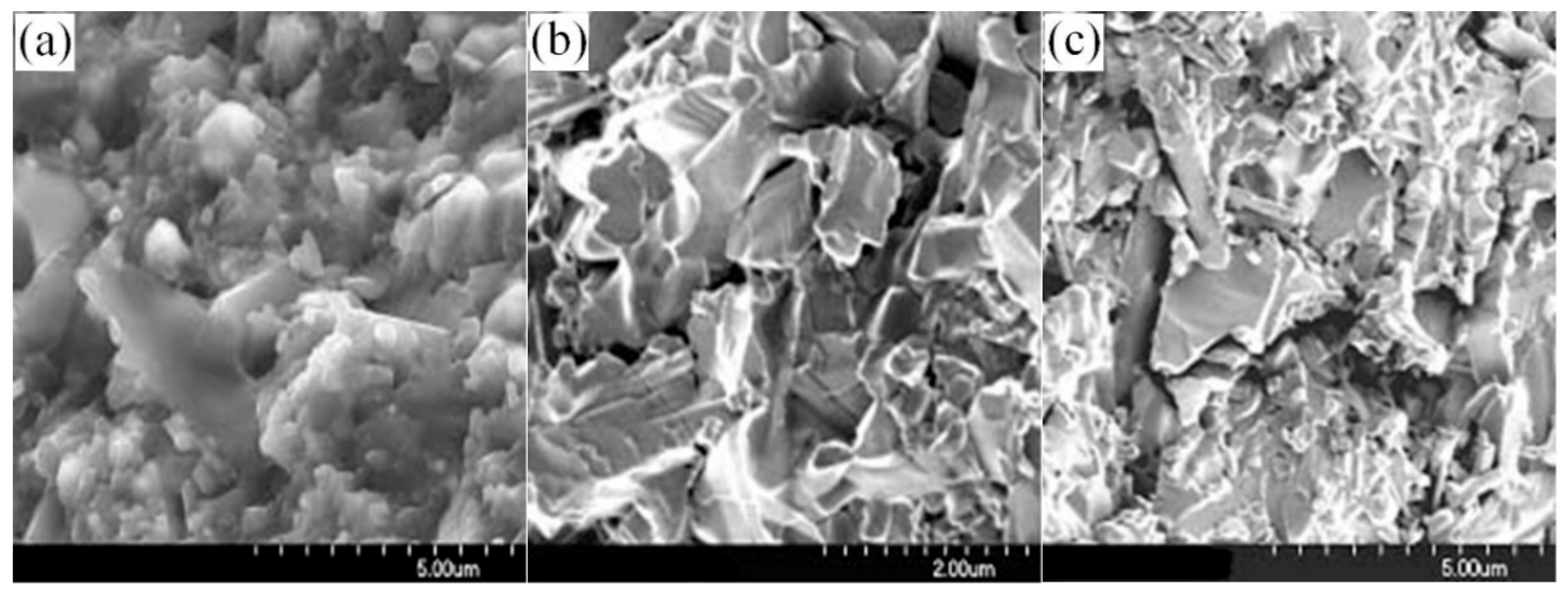
3.3. Thermal Shock Resistance
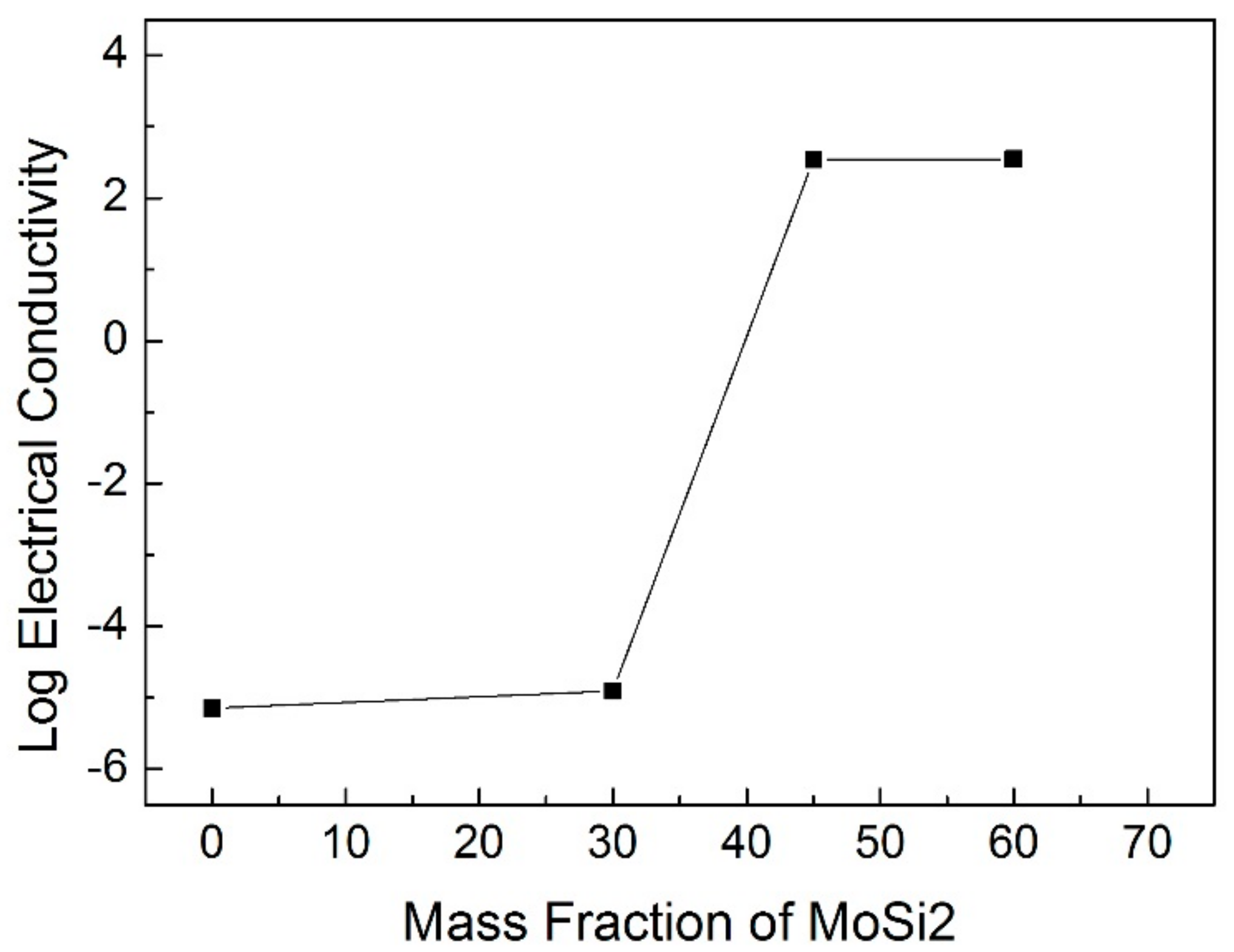
3.4. Electrical Conductivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kerlin, T.W. Practical Thermocouple Thermometry; Instrument Society of America: Research Triangle Park, NC, USA, 1999. [Google Scholar]
- Hunold, K. Thermocouple for High Temperatures. Adv. Mater. Process. 1986, 2, 4–5. [Google Scholar]
- Farrell, D.M.; Parmar, J.; Robbins, B.J. The Development of Ceramic-Based Thermocouples for Application in Gas Turbines. In Proceedings of the Turbo Expo 2001: ASME Turbo Expo 2001, New Orleans, LA, USA, 4–7 June 2001; pp. 1–5. [Google Scholar]
- Klein, R.; Medri, V.; Desmaison-Brut, M.; Bellosi, A.; Desmaison, J. Influence of Additives Content on the High Temperature Oxidation of Silicon Nitride based Composites. J. Eur. Ceram. Soc. 2003, 23, 603–611. [Google Scholar] [CrossRef]
- Taguchi, S.P.; Ribeiro, S. Silicon Nitride Oxidation Behaviour at 1000 and 1200 °C. J. Mater. Process. Technol. 2004, 147, 336–342. [Google Scholar] [CrossRef]
- Cook, J.; Khan, A.; Lee, E.; Mahapatra, M. Oxidation of MoSi2-based Composites. Mater. Sci. Eng. A 1992, 155, 183–198. [Google Scholar] [CrossRef]
- Berztiss, D.; Cerchiara, R.R. Oxidation of MoSi2 and Comparison with Other Silicide Materials. Mater. Sci. Eng. A 1992, 155, 165–181. [Google Scholar] [CrossRef]
- Tan, W.; Lin, X.P.; Ma, J.T.; Zhang, B.Q. Study on the Conductive Property of Si3N4-MoSi2 Composite Ceramics. Rare Met. Mater. Eng. 2011, 40, 196–198. [Google Scholar]
- Huang, Y.; Lin, J.; Zhang, H. Effect of Si3N4 Content on Microstructures and Antioxidant Properties of MoSi2/Si3N4 Composite Coatings on Mo Substrate. Ceram. Int. 2015, 41, 13903–13907. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, J.; Wu, Y.; Gu, S.; Huang, Y.; Chen, Y. Oxidation Behavior of (Mo, W) Si2–Si3N4 Composite Coating on Molybdenum Substrate at 1600 °C. Ceram. Int. 2015, 41, 14890–14895. [Google Scholar] [CrossRef]
- Petrovic, J.J. Toughening Strategies for MoSi2-Based High Temperature Structural Silicides. Intermetallics 2000, 8, 1175–1182. [Google Scholar] [CrossRef]
- Petrovic, J.J.; Pena, M.I.; Kung, H.H. Fabrication and Microstructures of MoSi2 Reinforced-Si3N4 Matrix Composites. J. Am. Ceram. Soc. 1997, 80, 1111–1116. [Google Scholar] [CrossRef]
- Kirkpatrick, S. Percolation and Conduction. Rev. Mod. Phys. 1973, 45, 574–588. [Google Scholar] [CrossRef]
- Balat, M.; Czerniak, M.; Berjoan, R. Oxidation of Silicon Nitride under Standard Air or Microwave-Excited Air at High Temperature and Low Pressure. J. Mater. Sci. 1997, 32, 1187–1193. [Google Scholar] [CrossRef]
- Sheehan, J.E. Passive and Active Oxidation of Hot-pressed Silicon Nitride Materials with Two Magnesia Contents. J. Am. Ceram. Soc. 1982, 65, C111–C113. [Google Scholar] [CrossRef]
- Hebsur, M.G. Development and Characterization of SiC(f)/MoSi2-Si3N4(p) Hybrid Composites. Mater. Sci. Eng. A 1999, 216, 24–37. [Google Scholar] [CrossRef]
- Sadananda, K.; Feng, C.R.; Mitra, R.; Deevi, S.C. Creep and Fatigue Properties of High Temperature Silicides and Their Composites. Mater. Sci. Eng. A 1999, 216, 223–238. [Google Scholar] [CrossRef]
- Petrovic, J.J.; Pena, M.I.; Reimnis, I.E.; Sandlin, M.S.; Conzone, S.D.; Kung, H.H.; Butt, D.P. Mechanical Behavior of MoSi2 Reinforced-Si3N4 Matrix Composites. J. Am. Ceram. Soc. 1997, 80, 3070–3076. [Google Scholar] [CrossRef]
- Xie, N.; Shao, W.Z.; Zhen, L.; Feng, L.C. Mechanical and Physical Properties of Cu2O−xCu Cermet. In Mechanical Properties and Performance of Engineering Ceramics II: Ceramic Engineering and Science Proceedings; John Wiley & Sons: Hoboken, NJ, USA, 2007; Volume 27, pp. 423–434. [Google Scholar]
- Bundschuh, K.; Schüze, M.; Müller, C.; Greil, P.; Heider, W. Selection of Materials for Use at Temperatures Above 1500 °C in Oxidizing Atmospheres. J. Eur. Ceram. Soc. 1998, 18, 2389–2391. [Google Scholar] [CrossRef]
- Natesan, K.; Deevi, S.C. Oxidation Behavior of Molybdenum Silicides and Their Composites. Intermetallics 2000, 8, 1147–1158. [Google Scholar] [CrossRef]
- Kao, M.Y. Properties of Silicon Nitride-Molybdenum Disilicide Particulate Ceramic Composites. J. Am. Ceram. Soc. 1993, 76, 2879–2883. [Google Scholar] [CrossRef]
- Klemm, H.; Tangermann, K.; Schubert, C.; Hermel, W. Influence of Molybdenum Silicide Additions on High-Temperature Oxidation Resistance of Silicon Nitride Materials. J. Am. Ceram. Soc. 1996, 79, 2429–2435. [Google Scholar] [CrossRef]
- Schutze, K.B.M. Materials for Temperatures above 1500 °C in Oxidizing Atmospheres Part I: Basic Considerations on Materials Selection. Mater. Corros. 2001, 52, 204–212. [Google Scholar]
- Monteverde, F.; Bellosi, A. High Oxidation Resistance of Hot Pressed Silicon Nitride Containing Yttria and Lanthania. J. Eur. Ceram. Soc. 1998, 18, 2313–2321. [Google Scholar] [CrossRef]

| α Phase-Si3N4/wt % | MoSi2/wt % | La2O3/wt % | Y2O3/wt % | ||||
|---|---|---|---|---|---|---|---|
| purity | >92 | purity | >99 | purity | >99 | purity | >99 |
| N | >38.5 | Si | >36.5 | REO | >94.5 | REO | >94.5 |
| O | <1.5 | O | <0.85 | CaO | <0.15 | CaO | <0.15 |
| C | <0.1 | Fe | <0.09 | Fe2O3 | <0.10 | Fe2O3 | <0.10 |
| Fe | <200 ppm | C | <0.01 | CeO2 | <0.05 | CeO2 | <0.05 |
| No. | Si3N4/wt % | MoSi2/wt % | La2O3/wt % | Y2O3/wt % |
|---|---|---|---|---|
| #1 | 60 | 30 | 5 | 5 |
| #2 | 45 | 45 | 5 | 5 |
| #3 | 30 | 60 | 5 | 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Guan, P.; Yu, X.; He, Y. Microstructure and Properties of Thermal Electrode Material Si3N4–MoSi2 Composite Ceramics. Materials 2018, 11, 986. https://doi.org/10.3390/ma11060986
Feng L, Guan P, Yu X, He Y. Microstructure and Properties of Thermal Electrode Material Si3N4–MoSi2 Composite Ceramics. Materials. 2018; 11(6):986. https://doi.org/10.3390/ma11060986
Chicago/Turabian StyleFeng, Lichao, Pengfei Guan, Xuemei Yu, and Yiqiang He. 2018. "Microstructure and Properties of Thermal Electrode Material Si3N4–MoSi2 Composite Ceramics" Materials 11, no. 6: 986. https://doi.org/10.3390/ma11060986
APA StyleFeng, L., Guan, P., Yu, X., & He, Y. (2018). Microstructure and Properties of Thermal Electrode Material Si3N4–MoSi2 Composite Ceramics. Materials, 11(6), 986. https://doi.org/10.3390/ma11060986




