Magnesium Potassium Phosphate Compound for Immobilization of Radioactive Waste Containing Actinide and Rare Earth Elements
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Phase Composition and Structure of MKP Compounds
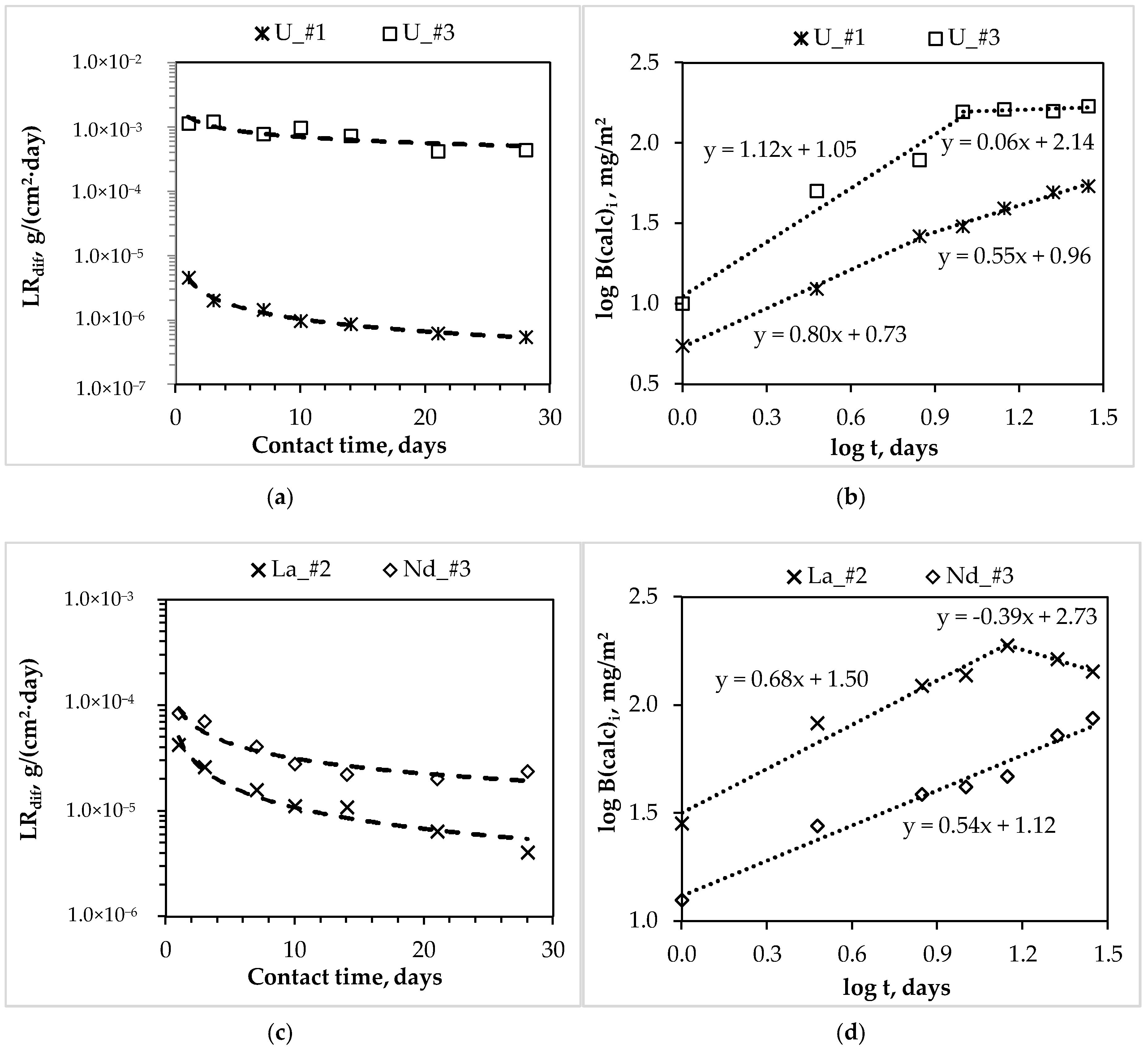
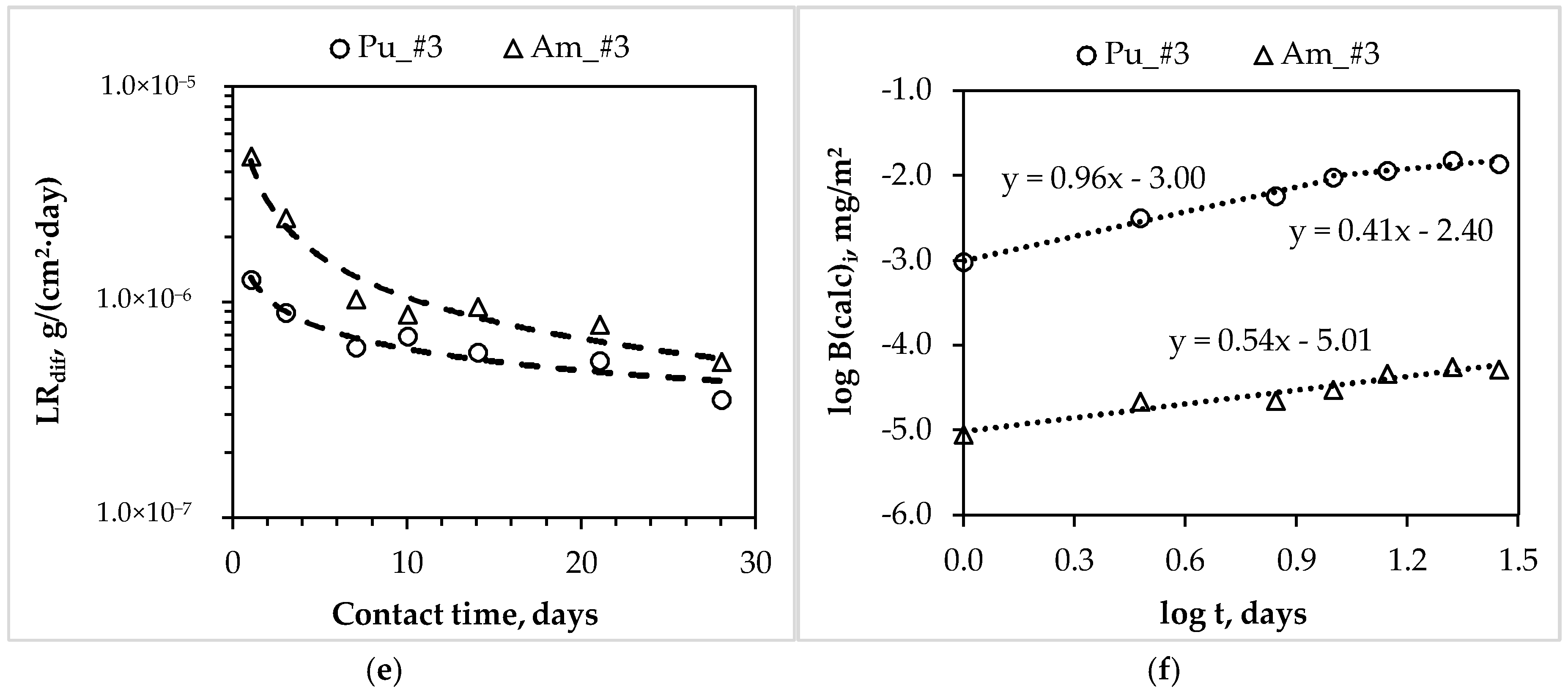
4.2. The Leaching Rate and Mechanism of Actinides and REE from MKP Compounds
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ojovan, M.I.; Lee, W.E. An Introduction to Nuclear Waste Immobilisation, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–362. ISBN 978-0-08-099392-8. [Google Scholar]
- Stefanovsky, S.V.; Yudintsev, S.V.; Vinokurov, S.E.; Myasoedov, B.F. Chemical-technological and mineralogical-geochemical aspects of the radioactive waste management. Geochem. Int. 2016, 54, 1136–1156. [Google Scholar] [CrossRef]
- Stefanovsky, S.V.; Stefanovskaya, O.I.; Vinokurov, S.E.; Danilov, S.S.; Myasoedov, B.F. Phase composition, structure, and hydrolytic durability of glasses in the Na2O-Al2O3-(Fe2O3)-P2O5 system at replacement of Al2O3 by Fe2O3. Radiochemistry 2015, 57, 348–355. [Google Scholar] [CrossRef]
- Ewing, R.C.; Lutze, W.F. High-level nuclear waste immobilization with ceramics. Ceramics Int. 1991, 17, 287–293. [Google Scholar] [CrossRef]
- Schlenz, H.; Neumeier, S.; Hirsch, A.; Peters, L.; Roth, G. Phosphates as safe containers for radionuclides. In Highlights in Applied Mineralogy; Heuss-Aßbichler, S., Amthauer, G., John, M., Eds.; De Gruyter: Munich, Germany, 2017; pp. 171–196. ISBN 9783110497342. [Google Scholar]
- Schlenz, H.; Heuser, J.; Neumann, A.; SchmitzI, S.; Bosbach, D. Monazite as a suitable actinide waste form. Z. Kristallogr. Cryst. Mater. 2013, 228, 113–123. [Google Scholar] [CrossRef]
- Wagh, A.S. Chemically Bonded Phosphate Ceramics: Twenty-First Century Materials with Diverse Applications, 2nd ed.; Elsevier: Amsterdam, Netherlands, 2016; pp. 1–422. ISBN 978-0-08-100380-0. [Google Scholar]
- Roy, D.M. New Strong Cement Materials: Chemically Bonded Ceramics. Science 1987, 235, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Vinokurov, S.E.; Kulikova, S.A.; Krupskaya, V.V.; Danilov, S.S.; Gromyak, I.N.; Myasoedov, B.F. Investigation of the leaching behavior of components of the magnesium potassium phosphate matrix after high salt radioactive waste immobilization. J. Radioanal. Nucl. Chem. 2018, 315, 481–486. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulikova, S.A.; Krupskaya, V.V.; Myasoedov, B.F. Magnesium Potassium Phosphate Compound for Radioactive Waste Immobilization: Phase Composition, Structure, and Physicochemical and Hydrolytic Durability. Radiochemistry 2018, 60, 70–78. [Google Scholar] [CrossRef]
- Myasoedov, B.F.; Kalmykov, S.N.; Kulyako, Y.M.; Vinokurov, S.E. Nuclear fuel cycle and its impact on the environment. Geochem. Int. 2016, 54, 1156–1167. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulyako, Y.M.; Slyunchev, O.M.; Rovny, S.I.; Myasoedov, B.F. Low-temperature immobilization of actinides and other components of high-level waste in magnesium potassium phosphate matrices. J. Nucl. Mater. 2009, 385, 189–192. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulyako, Y.M.; Slyunchev, O.M.; Rovnyi, S.I.; Wagh, A.S.; Maloney, M.D.; Myasoedov, B.F. Magnesium potassium phosphate matrices for immobilization of high-level liquid wastes. Radiochemistry 2009, 51, 65–72. [Google Scholar] [CrossRef]
- Wagh, A.S.; Sayenko, S.Y.; Shkuropatenko, V.A.; Tarasov, R.V.; Dykiy, M.P.; Svitlychniy, Y.O.; Virych, V.D.; Ulybkina, E.A. Experimental study on cesium immobilization in struvite structures. J. Hazard. Mater. 2016, 302, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Wagh, A.S.; Strain, R.; Jeong, S.Y.; Reed, D.; Kraus, T.; Singh, D. Stabilization of Rocky Flats Pu-contaminated ash within chemically bonded phosphate ceramics. J. Nucl. Mater. 1999, 265, 295–307. [Google Scholar] [CrossRef]
- Singh, D.; Mandalika, V.R.; Parulekar, S.J.; Wagh, A.S. Magnesium potassium phosphate ceramic for 99Tc immobilization. J. Nucl. Mater. 2006, 348, 272–282. [Google Scholar] [CrossRef]
- Graeser, S.; Postl, W.; Bojar, H.-P.; Berlepsch, P.; Armbruster, T.; Raber, T.; Ettinger, K.; Walter, F. Struvite-(K), KMgPO4∙6H2O, the potassium equivalent of struvite – a new mineral. Eur. J. Miner. 2008, 20, 629–633. [Google Scholar] [CrossRef]
- GOST R 52126-2003. Long Time Leach Testing of Solidified Radioactive Waste Forms; Gosstandart of Russia: Moscow, Russia, 2003; pp. 1–8. [Google Scholar]
- De Groot, G.J.; van der Sloot, H.A. Determination of leaching characteristics of waste materials leading to environmental product certification. In Stabilization and Solidification of Hazardous, Radioactive and Mixed Wastes: 2nd Volume; Gilliam, T.M., Wiles, C.C., Eds.; ASTM International: West Conshohocken, PA, USA, 1992; Volume 2, pp. 149–170. [Google Scholar] [CrossRef]
- Torras, J.; Buj, I.; Rovira, M.; de Pablo, J. Semi-dynamic leaching tests of nickel containing wastes stabilized/solidified with magnesium potassium phosphate cements. J. Hazard. Mater. 2011, 186, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Al-Abed, S.R.; Hageman, P.L.; Jegadeesan, G.; Madhavan, N.; Allen, D. Comparative evaluation of short-term leach tests for heavy metal release from mineral processing waste. Sci. Total Environ. 2006, 364, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.H.; Dermatas, D. An evaluation of lead leachability from stabilized/solidified soils under modified semi-dynamic leaching conditions. Eng. Geol. 2006, 85, 67–74. [Google Scholar] [CrossRef]
- Xue, Q.; Wang, P.; Li, J.-S.; Zhang, T.-T.; Wang, S.-Y. Investigation of the leaching behavior of lead in stabilized/solidified waste using a two-year semi-dynamic leaching test. Chemosphere 2017, 166, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, M.J.; Atkin, D. V-Meta-ankoleÏte, hydrated potassium uranyl phosphate. Bull. Geol. Soc. Great Britain. 1966, 25, 49–54. [Google Scholar]
- Fleischer, M. New mineral names. Am. Miner. 1967, 52, 559–564. [Google Scholar]
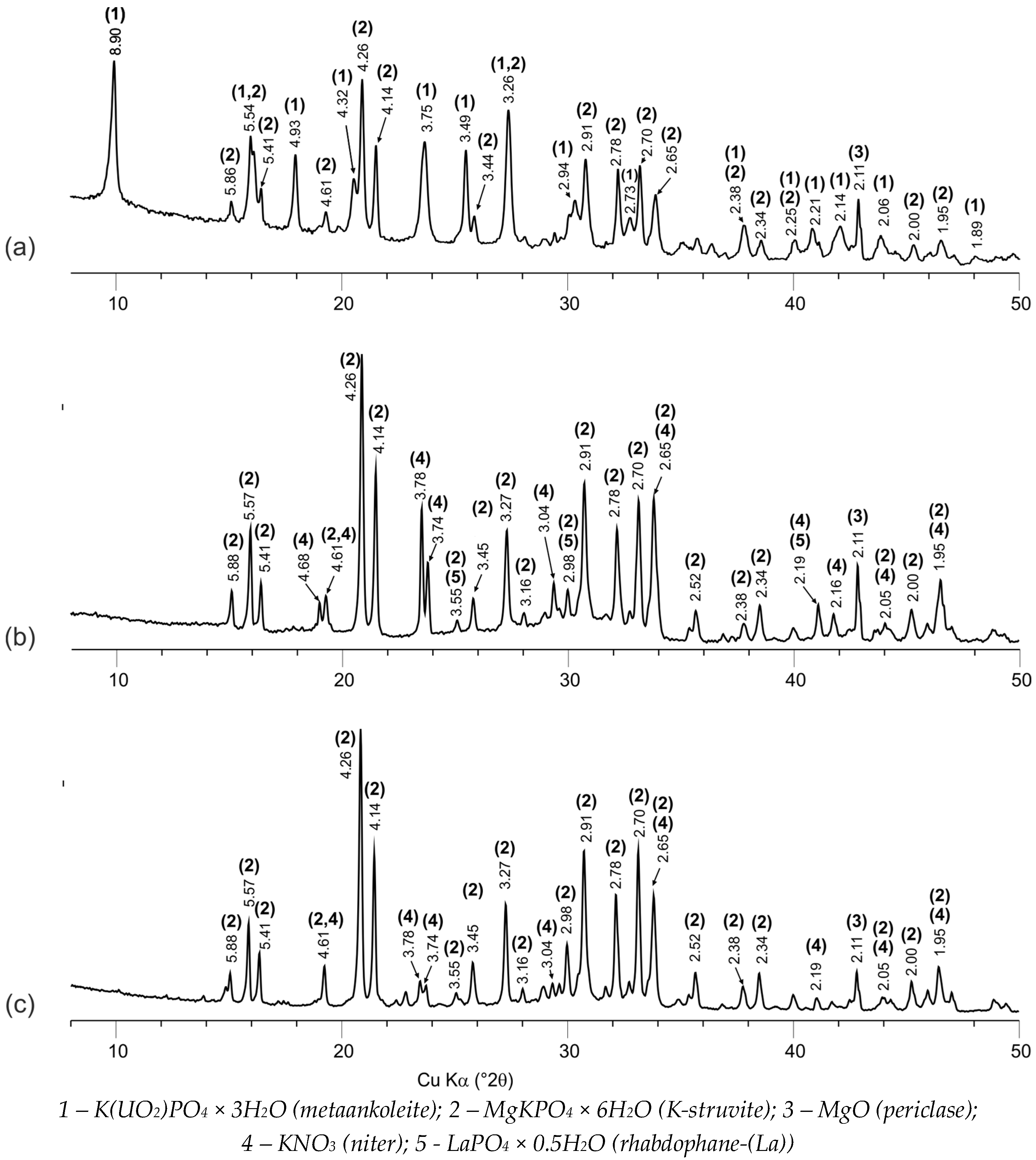
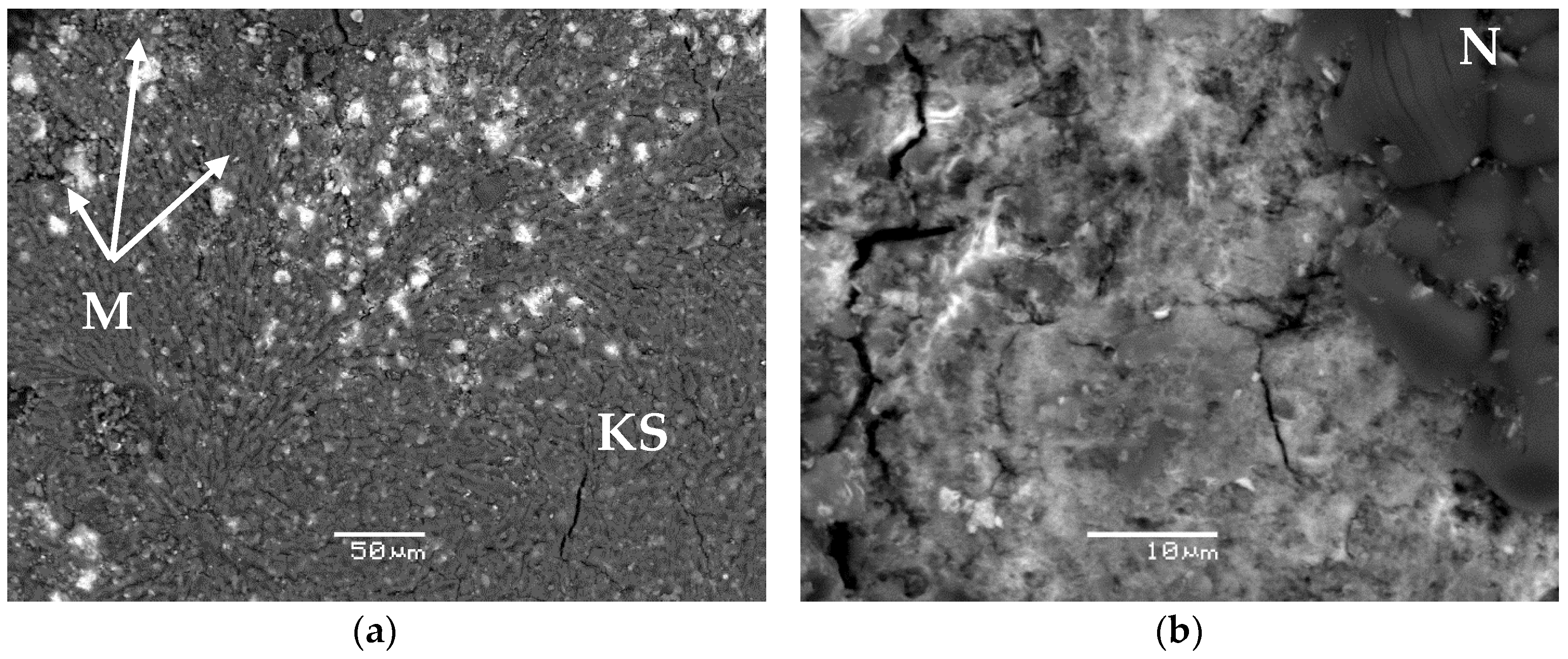
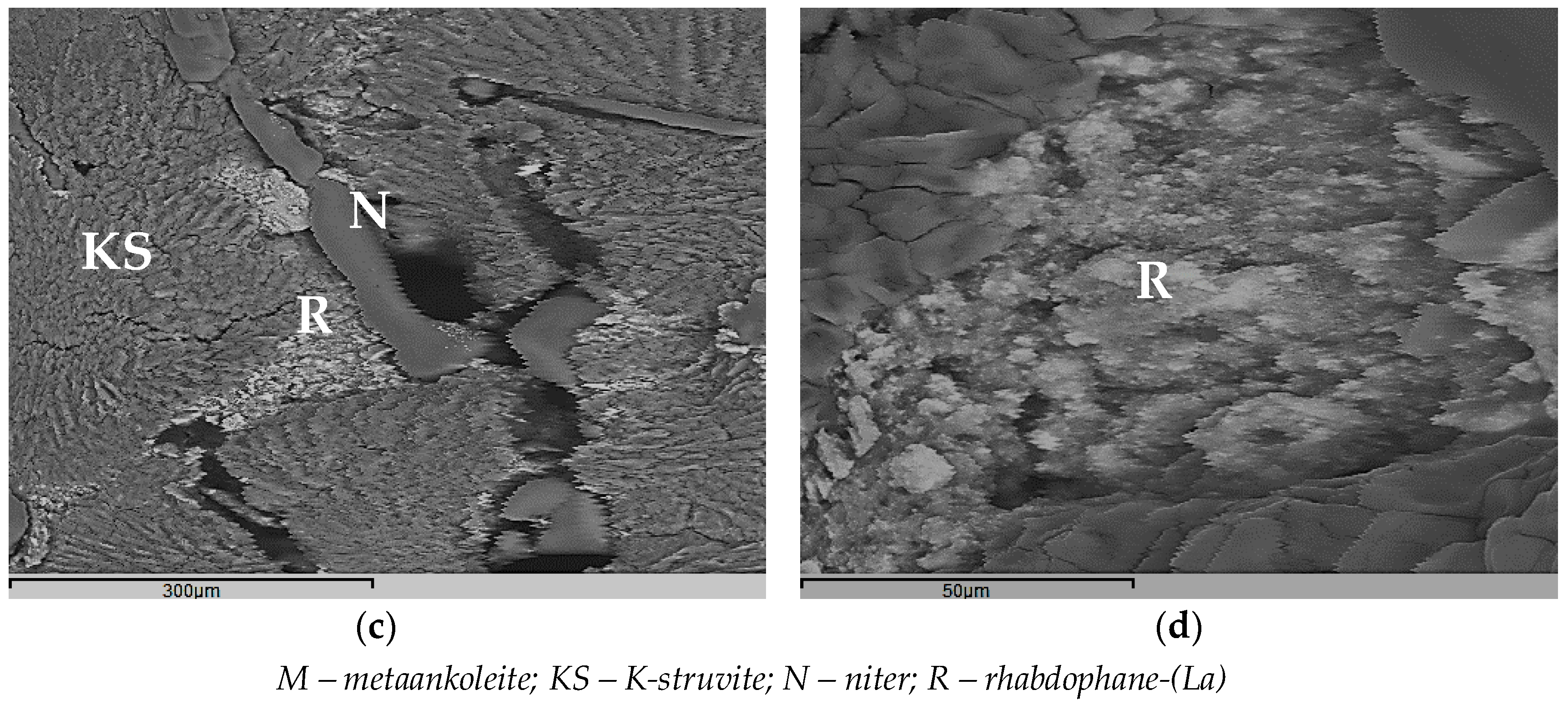
| Specific Activity of Actinides (Bq·L−1) | Metal Content (g·L−1) | HNO3 Content (mol·L−1) | Density (g·L−1) | Salt Content (g·L−1) |
|---|---|---|---|---|
| 239Pu – 3.8 × 108 241Am – 5.2 × 107 | Na – 13.3; Sr – 3.9; Zr – 7.6; Mo – 0.9; Pd – 5.4; Cs – 9.3; Ba – 6.4; Nd – 28.8; Fe – 1.0; Cr – 2.8; Ni – 0.5; U – 3.1 | 3.2 | 1210 | 206.6 |
| Compound | Liquid Waste (wt %) | Binders (wt %) | ||
|---|---|---|---|---|
| KH2PO4 | H3BO3 | MgO | ||
| #1 | 39.5 | 44.2 | 1.5 | 14.8 |
| #2 | 43.4 | 41.3 | 1.5 | 13.8 |
| #3 | 41.5 | 42.9 | 1.3 | 14.3 |
| Components of the MKP Compounds | Correspond Figure | Contact Time of the Samples with Water, Days | Slope of the Lines | Leaching Mechanism |
|---|---|---|---|---|
| U_#1 | 3b | 1–7 7–28 | 0.80 0.55 | dissolution diffusion |
| U_#3 | 3b | 1–10 10–28 | 1.12 0.06 | dissolution depletion |
| La_#2 | 3d | 1–14 14–28 | 0.68 −0.39 | dissolution depletion |
| Nd_#3 | 3d | 1–28 | 0.54 | diffusion |
| Am_#3 | 3f | 1–28 | 0.54 | diffusion |
| Pu_#3 | 3f | 1–10 10–28 | 0.96 0.41 | dissolution diffusion |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinokurov, S.E.; Kulikova, S.A.; Myasoedov, B.F. Magnesium Potassium Phosphate Compound for Immobilization of Radioactive Waste Containing Actinide and Rare Earth Elements. Materials 2018, 11, 976. https://doi.org/10.3390/ma11060976
Vinokurov SE, Kulikova SA, Myasoedov BF. Magnesium Potassium Phosphate Compound for Immobilization of Radioactive Waste Containing Actinide and Rare Earth Elements. Materials. 2018; 11(6):976. https://doi.org/10.3390/ma11060976
Chicago/Turabian StyleVinokurov, Sergey E., Svetlana A. Kulikova, and Boris F. Myasoedov. 2018. "Magnesium Potassium Phosphate Compound for Immobilization of Radioactive Waste Containing Actinide and Rare Earth Elements" Materials 11, no. 6: 976. https://doi.org/10.3390/ma11060976
APA StyleVinokurov, S. E., Kulikova, S. A., & Myasoedov, B. F. (2018). Magnesium Potassium Phosphate Compound for Immobilization of Radioactive Waste Containing Actinide and Rare Earth Elements. Materials, 11(6), 976. https://doi.org/10.3390/ma11060976





