4.1. Cast Alloy
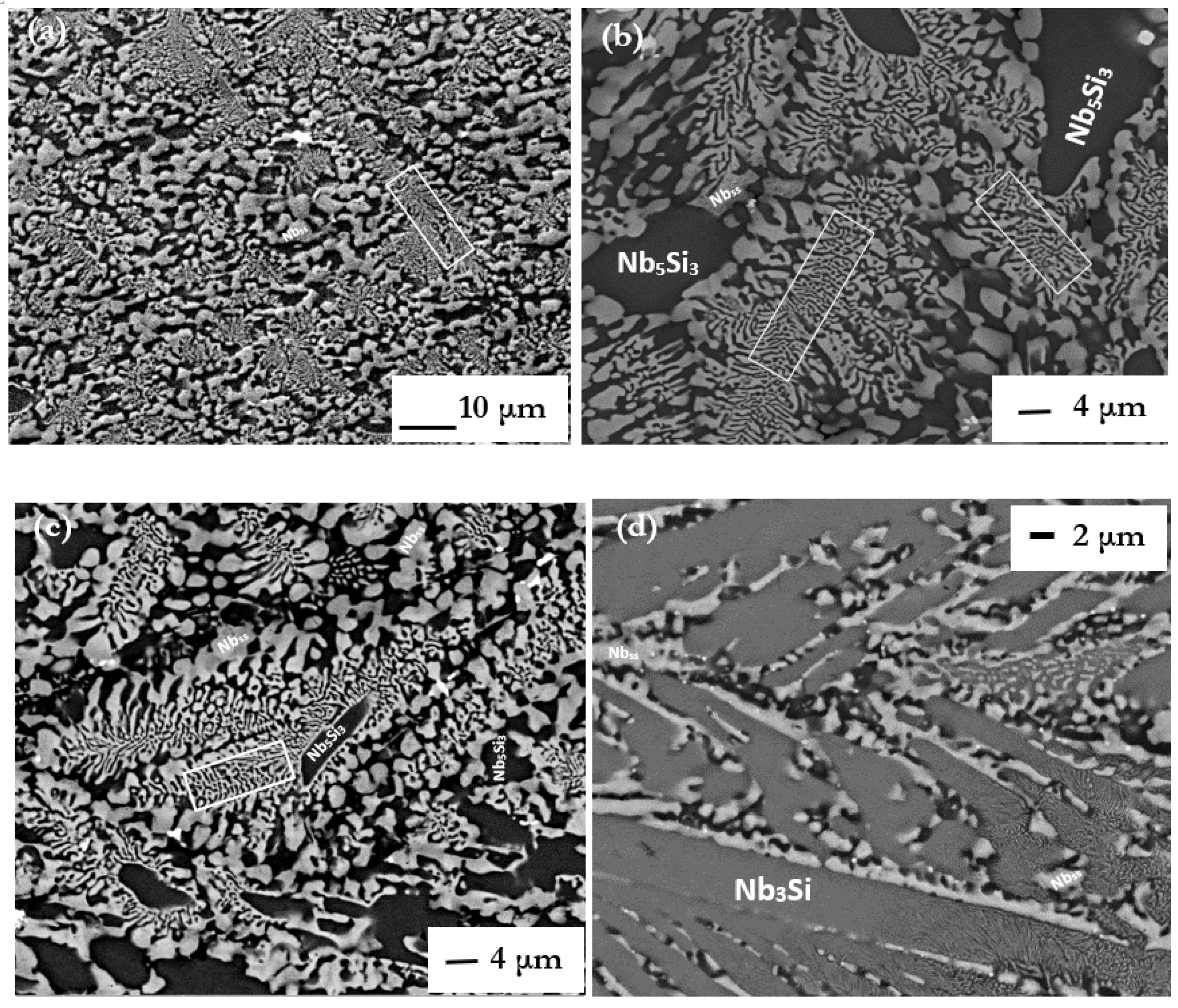
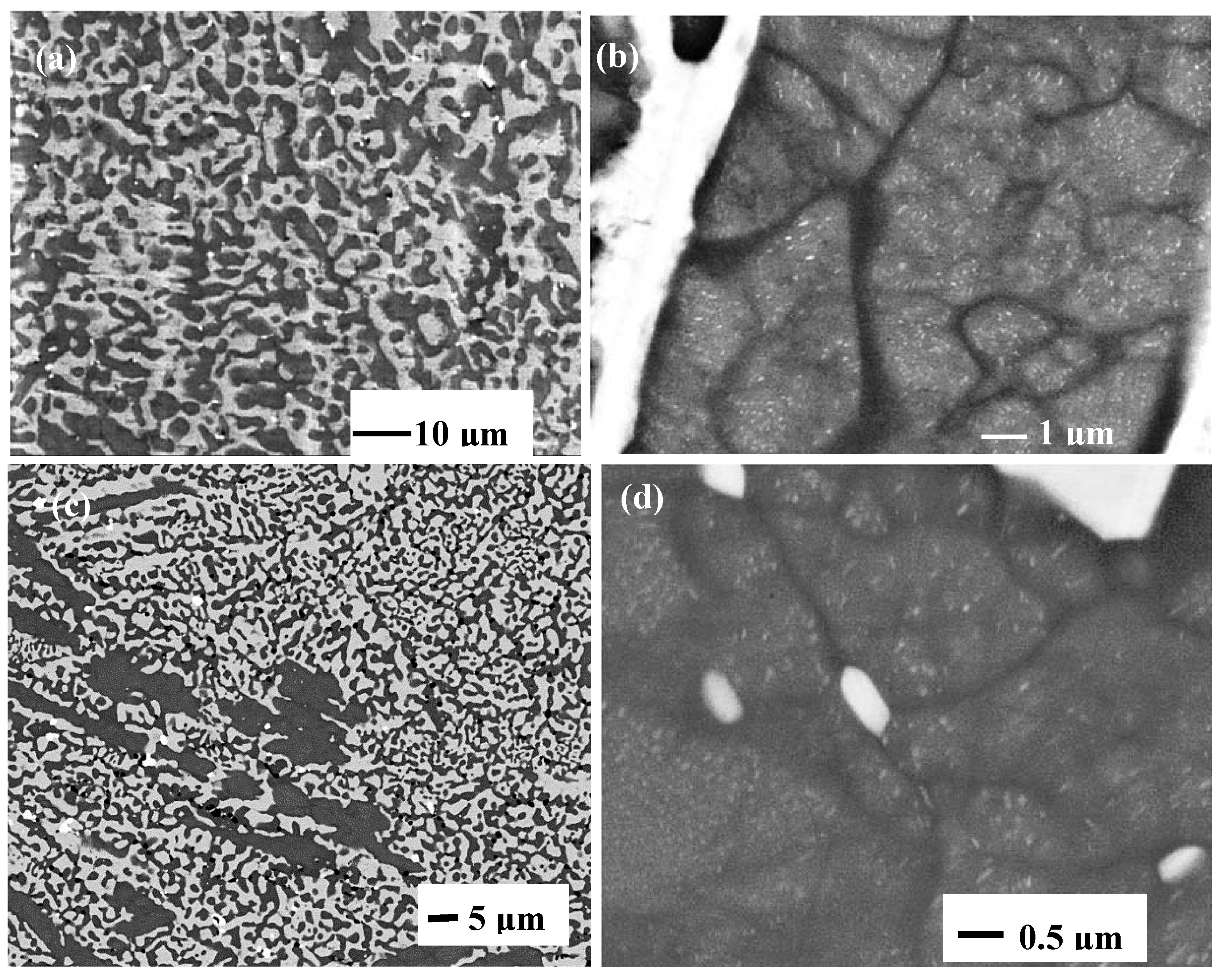
The alloy of this research exhibited a variety of microstructures, most of which comprised the Nbss and Nb5Si3, the latter being either the high temperature βNb5Si3 or the low temperature αNb5Si3. The Nb3Si and the fine products of its eutectoid decomposition were also observed in CM1-8mm, in an area where the suction-cast bar had experienced high cooling rate during solidification. Characteristic features of eutectics and/or eutectoids were observed in lamellar microstructures. The latter were formed around dendrites of Nb5Si3, and in most cases Nbss haloes were formed in-between Nb5Si3 grains and lamellar microstructure.
The discussion will consider first the eutectic between Nb
ss and βNb
5Si
3 (metastable eutectic in the Nb-Si binary). This was observed in the cast CM1-10g and CM1-6mm (
Figure 1a,b), where only the presence of βNb
5Si
3 was confirmed by XRD (
Figures S1 and S2 in supplemental data), and in CM1-8mm where the XRD confirmed only the βNb
5Si
3 (
Figure 1c and
Figure S2 in supplemental data). However, in an area of CM1-8mm that had solidified under high cooling rates there was evidence for Nb
3Si and its eutectoid transformation to Nb
ss and αNb
5Si
3 (
Figure 1d,e) (the case for CM1-600g and CM1-OFZ will be discussed below separately). The Si concentration in CM1-10g, CM1-6mm and CM1-8mm (respectively 22.6, 23.4 and 21.8 at.%) was above the eutectic composition in the Nb-Si binary (reported to be in the range 15.3 to 18.7 at.% [
14]) and the Si concentration of the eutectic was in the range of values for the metastable Nb
ss and βNb
5Si
3 eutectic in the binary [
30].
In eutectic solidification the coupled zone gives the range of compositions and growth temperatures for which the coupled eutectic morphology leads the growth front. The Nb-Si binary has eutectic coupled zones skewed towards the silicides [
36]. The coupled zone depends on the relative change of the interface (growth) temperature of different morphological forms (eutectic, dendrites) as a function of composition and growth rate. Under a set of given growth conditions, the growth form (eutectic, dendrite) with the highest interface temperature will lead the growth front.
Figure 7 shows a schematic diagram of the skewed eutectic zone for the metastable eutectic in the Nb-Si binary, based on [
37]. From constitutional supercooling theory the maximum velocity of the solid-liquid interface V
S/L for coupled Nb + βNb
5Si
3 eutectic will be V
S/LCS = G
L D
L [m
βNb5Si3 (C
o − C
Eu)] where m
βNb5Si3 is the slope of the liquidus of βNb
5Si
3. When V
S/L exceeds V
S/LCS (V
2 > V
S/LCS in
Figure 7), for an alloy of composition C
o the growth temperature of the βNb
5Si
3 is above that of T
Eu(V2). The liquid composition between the primary βNb
5Si
3 and the coupled eutectic S/L interface will be in the range ∆C
E = C
EuNb − C
EuNb5Si3. At a low growth rate V
1 < V
S/LCS the growth temperature T
Eu(V1) of the coupled Nb + βNb
5Si
3 eutectic can be higher than the growth temperatures of each phase, namely T
Nb(V1) and T
Nb5Si3(V1) (note that the growth temperatures T
i (i = Nb, βNb
5Si
3) at the growth rate V
1 are not shown in
Figure 7) and thus according to the criterion of maximum growth temperature the alloy of composition C
o will solidify as coupled Nb + βNb
5Si
3 eutectic.
The microstructures seen in CM1-10g, CM1-6mm and CM1-8mm that formed at different growth rates (growth temperatures) under conditions of positive temperature gradient G
L in the melt (G
L > 0) can be explained with the help of
Figure 7. As the solidification conditions changed coupled Nb + βNb
5Si
3 eutectic with/out primary βNb
5Si
3 formed.
Next, the formation of Nb
3Si in CM1-8mm will be considered. There was no macrosegregation in CM1-8mm. Its Si concentration was lower than that of CM1-6mm and close to the liquid concentration (C
Lp) of the L + βNb
5Si
3 → Nb
3Si peritectic in the Nb-Si binary, for which the highest reported value is 21.1 at.% Si [
14]. It is highly likely that the melt of CM1-8mm was either to the left (primary phase Nb
3Si) or to the right (primary phase βNb
5Si
3) of C
Lp.
With Nb
3Si as the primary phase (alloy composition C
o < C
Lp) the solidification path in the Nb-Si binary is L → L + (Nb
3Si)
primary → (Nb
3Si)
primary + (Nb + Nb
3Si)
eutectic. In this case, no βNb
5Si
3 is formed. If all the Nb
3Si transforms, there will be the fine mixture of eutectoid Nb and αNb
5Si
3, if not, there will also be some remnant of Nb
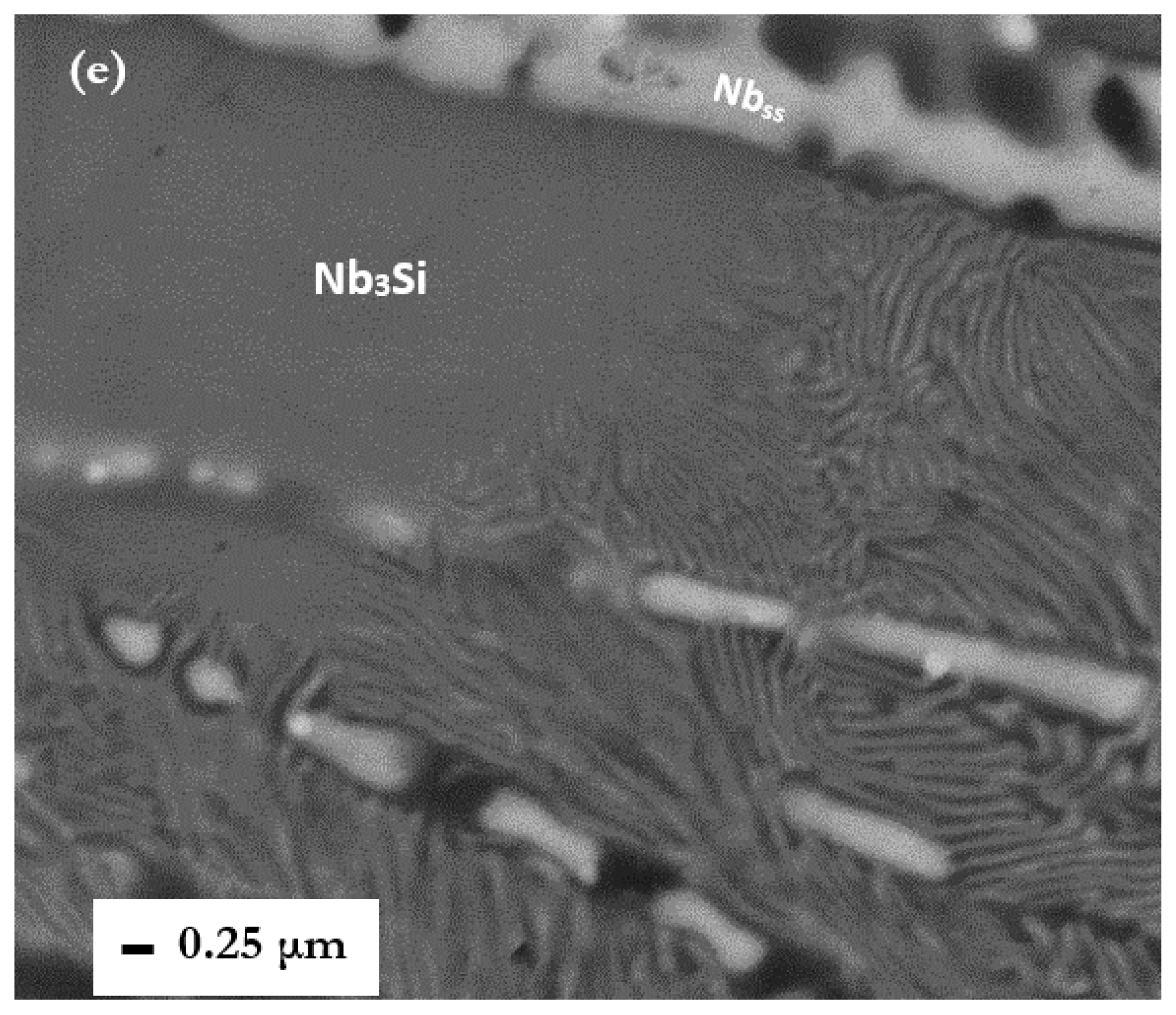
3Si. The evidence in the bottom of CM1-8mm (
Figure 1d,e) is for the latter.
The data in
Table 1 and
Table S1 in supplemental data would suggest that in CM1-600g and CM1-OFZ at all three growth rates the αNb
5Si
3 was the product of the βNb
5Si
3 → αNb
5Si
3 transformation that occurred under slow cooling and that the lamellar microstructures in the large button and OFZ bars were indeed prior Nb
ss + βNb
5Si
3 eutectics (formed as discussed above for CM1-10g, CM1-6mm, and CM1-8mm, see
Figure 7) in which the βNb
5Si
3 silicide had transformed to αNb
5Si
3. Considering current knowledge of the Nb-Si binary, there is no metastable eutectic between Nb
ss and αNb
5Si
3. The fact that the Nb
3Si and its eutectoid transformation were observed in the area of high cooling rate in CM1-8mm is attributed to the solidification path of the melt in that area (see above). The high cooling rate allowed us to “catch” the formation of Nb
3Si and its early stages of eutectoid decomposition during solid state cooling.
To summarize, in the alloy CM1 the primary phase was the βNb5Si3, the Nbss + βNb5Si3 eutectic formed and in the large button (i.e., CM1-600 g) and CM1-OFZ, both of which cooled slower compared with the CM1-10g, CM1-6mm and CM1-8mm, the βNb5Si3 transformed to αNb5Si3 during solid state cooling. Please note that if only the large button or OFZ bars grown at each of the three growth rates of this study had been investigated, the conclusion that αNb5Si3 was the primary phase would be misleading.
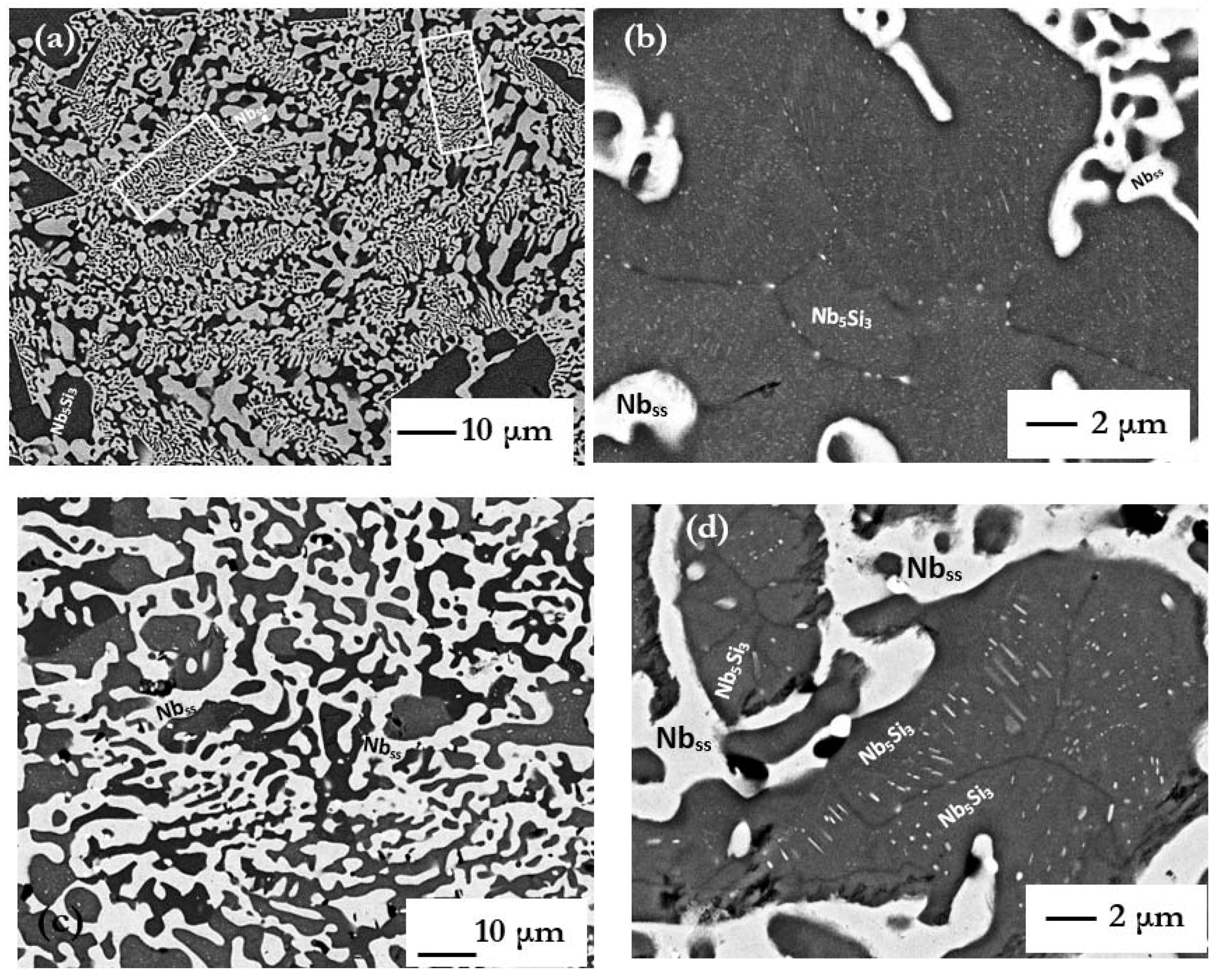
The microstructures in CM1-600g and CM1-OFZ were similar to those observed in the cast CM1-10g (
Figure 1a and
Figure 2a) but the XRD data of the former two confirmed the presence of Nb
ss and αNb
5Si
3 (
Figures S1 and S3 in supplemental data). The other difference between the large and small buttons (i.e., CM1-600g and CM1-10g) was the subgrain formation with fine precipitates of a second phase exhibiting same contrast as the Nb
ss in the large button,
Figure 2b. The difference between CM1-600g and CM1-OFZ was that in the latter no subgrains were observed in the αNb
5Si
3 grains with fine precipitates. In CM1-10g, CM1-6mm and CM1-8mm the βNb
5Si
3 had no subgrains and no fine second phase precipitates of a phase with contrast similar to that of the Nb
ss, but subgrains and fine precipitates formed in αNb
5Si
3 in CM1-10g, CM1-6mm and CM1-8mm after the heat treatment (
Figure 5).
Table S1 in supplemental data summarizes the phases observed in the cast alloy CM1.
The subgrain formation in CM1-600g is related to the βNb5Si3 to αNb5Si3 transformation in the cast CM1-600g and could be attributed to “recovery” of the 5-3 silicide during solid state cooling driven by the strain energy arising from differences in thermal contraction between Nbss and Nb5Si3. First, subgrain formation in Nb5Si3 will be considered using the Nb-Si binary (i.e., ignoring for the time being the role of the other solutes).
First-principles calculations performed in our group showed that the Young’s moduli of Nb, βNb
5Si
3 and αNb
5Si
3 respectively are 105, 269 and 291 GPa [
38]. The CTE (coefficient of thermal expansion) of Nb is 7.3 to 7.6 10
−6 K
−1 depending on purity. The βNb
5Si
3 and αNb
5Si
3 exhibit anisotropy in CTE and the ratio of CTE values α
a/α
c along the a and c axes of their lattices (i.e., the CTE anisotropy ratio) is different for each phase, namely 0.783 and 0.658 for αNb
5Si
3 and βNb
5Si
3, respectively [
19,
39]. The CTE values of βNb
5Si
3 and αNb
5Si
3 along their a-axes are very close (8.961 10
−6 K
−1 and 8.777 10
−6 K
−1) but those along their c-axes are different, namely 11.095 10
−6 K
−1 and 13.331 10
−6 K
−1, respectively [
19]. All the aforementioned values are for the unalloyed phases and are expected to change as they become alloyed (see below and [
39]).
Allowing for the strain energy in Nb5Si3 to arise from the CTE anisotropy, meaning the lower the αa/αc ratio the higher the anisotropy, the subgrain formation as the βNb5Si3 transformed to αNb5Si3 would be accompanied by a reduction in strain energy (the “driving force”), the latter driving “recovery” and subgrain formation in the silicide.
In CM1-OFZ and for all growth rates the αNb
5Si
3 was present in the cast microstructures (
Figure S3 in supplemental data) but no subgrains were observed in the 5-3 silicide grains. Given that thermal stresses in the “grown” solid can arise during solidification (see below) and that the sign and magnitude of these stresses depends on changes in cross section during OFZ and transport phenomena (heat and mass transfer) in the melt zone (see below), the absence of subgrains in CM1-OFZ was attributed to reduced strain energy due to the thermal stresses arising during OFZ counter-balancing stresses arising from the anisotropy of CTE of the 5-3 silicide (see below).
Subgrain formation was not observed in CM1-10g, CM1-6mm and CM1-8mm owing to the faster cooling during solid state cooling which did not give enough time for the transformation of the βNb5Si3 to αNb5Si3 and for “recovery” processes to occur. To our knowledge, subgrain formation in Nb5Si3 in binary Nb-Si alloys has not been reported in the literature.
Next, we consider the results of this research in the context of the available data in the literature about Nb-Si-Mo, Nb-Si-W, Nb-Ti-Si and Mo-Si-W ternary phase equilibria and the properties of α(Nb,Ti)5Si3 and β(Nb,Ti)5Si3 (i.e., alloyed 5-3 silicide). Below, alloy compositions are given in at.%.
The Nb
3Si was destabilized by small Mo additions in Nb-Si-Mo alloys [
40]. In Nb-16Si-xMo alloys with x ≤ 2 the primary phase was the Nb
ss and for x > 2 the β(Nb,Mo)
5Si
3 + Nb
ss eutectic replaced the (Nb,Mo)
3Si + Nb
ss eutectic. In Nb-19Si-xMo alloys the primary phase was the (Nb,Mo)
3Si for x ≤ 2 and the β(Nb,Mo)
5Si
3 for x > 2. In the latter alloys only the β(Nb,Mo)
5Si
3 + Nb
ss eutectic was stable for x ≥ 4 but both the β(Nb,Mo)
5Si
3 + Nb
ss and (Nb,Mo)
3Si + Nb
ss eutectics were formed for x = 3. In Nb-20Si-xMo alloys the primary phase was the β(Nb,Mo)
5Si
3, and peritectic (Nb,Mo)
3Si and (Nb,Mo)
3Si + Nb
ss eutectic formed for x = 1 and only β(Nb,Mo)
5Si
3 + Nb
ss eutectic for x ≥ 3 [
41].
The composition of βNb
5Si
3 in the alloy Nb-19Si-3Mo was Nb-38.6Si-0.8Mo according to Ma et al. [
41]. Sekido et al. [
42] reported higher Mo solubilities in Nb
5Si
3, namely 3.6 at.% and 5.2 at.% in αNb
5Si
3 and βNb
5Si
3, respectively. Solubilities of 2.5 at.% Mo and 0.6 at.% Mo in βNb
5Si
3 and αNb
5Si
3 were reported respectively for the cast and heat-treated conditions of the alloy Nb-18Si-5Al-5Cr-5Mo and slightly lower Mo concentration in the βNb
5Si
3 in the cast alloy Nb-24Ti-18Si-5Al-5Cr-5Mo [
43]. In other words, the data in the literature shows that the solubility of Mo is different in βNb
5Si
3 and αNb
5Si
3, depends on the presence or not of Ti in the alloy and also on the concentration of Mo in the alloy.
Sekido et al. [
42] reported that Mo stabilized the βNb
5Si
3 and that the transformation βNb
5Si
3 → αNb
5Si
3 (i) did not occur when the concentration of Mo in Nb
5Si
3 exceeded 6 at.% and (ii) depended on the cooling rate. After heat treatment at 1400 °C for 100 h a mixture of both βNb
5Si
3 and αNb
5Si
3 was observed when the Mo concentration in Nb
5Si
3 was 4 or 5 at.%. Also, they reported that a two phase Nb
ss + αNb
5Si
3 or Nb
ss + βNb
5Si
3 phase equilibria could be attained in the Nb-Si-Mo ternary at 1700 °C.
In the Nb-Si-W ternary phase equilibria the W has the same effect as Mo, namely it destabilizes the Nb
3Si and stabilizes the Nb
ss and βNb
5Si
3 eutectic when the W concentration is above ≈ 3 at.% [
40]. Addition of 10 at.% W allowed formation of amorphous Nb-Si-W alloys [
44], which would suggest that W enhances the undercool-ability of Nb-Si-W alloys. Continuous solid solutions between βNb
5Si
3 and W
5Si
3 have been reported in cast ternary alloys [
45] where the transformation of βNb
5Si
3 to αNb
5Si
3 did not allow the continuous solid solutions to persist to lower temperatures. The W solubility in αNb
5Si
3 was about 1 at.% [
40]. Mo and W form continuous solid solutions and Mo
5Si
3 and W
5Si
3 (and βNb
5Si
3) have the same prototype.
The early data that supported the construction of the Nb-Ti-Si liquidus projection(s) did not identify the structure of 5-3 silicides in the studied alloys (i.e., did not clarify which Nb
5Si
3 polymorph was formed) and the liquidus projection gave a Nb
5Si
3 area, without specifying whether this was the βNb
5Si
3 or the αNb
5Si
3 or the hexagonal γNb
5Si
3 [
21]. Geng et al. [
23] proposed a liquidus projection with a large αNb
5Si
3 area. Li et al. [
25] revised the Nb-Ti-Si liquidus projection and proposed only a very narrow area for the αNb
5Si
3 in the center of the projection. A small area of αNb
5Si
3 in the center of the projection has also been proposed by Gigolotti et al. [
26]. The Scheil solidification path for Nb-19Si-5Hf was given as L → L + Nb(Hf)
3Si → (Nb,Hf)
3Si + (Nb,Hf,Si)
ss [
46].
Sekido et al. [
42] studied arc melted Nb-xMo-36Si and Nb-xMo-37.5Si (at.%, x = 0 to 10) alloys in the as cast and heat-treated conditions. They reported that Nb
ss precipitates formed in both βNb
5Si
3 and αNb
5Si
3 after heat treatment at 1300 °C for 20 h. Precipitation of Nb
ss in βNb
5Si
3 was not observed in the cast condition but after heat treatment at 1500 °C for 100 h. Plate shaped Nb
ss precipitates were formed in βNb
5Si
3 and the orientation relationship {
01}
Nb //{2
0}
βNb5Si3, <111> // <121>
βNb5Si3 was observed. In the αNb
5Si
3 that formed from the βNb
5Si
3 → αNb
5Si
3 transformation after heat treatment at 1500 °C for 100 h there was Nb
ss precipitation in αNb
5Si
3 and for these precipitates two orientation relationships were observed, namely (01
)
Nbss // (12
)
αNb5Si3, [133]
Nbss // [111]
αNb5Si3 and (
12)
Nbss // (1
0)
αNb5Si3, [110]
Nbss // [110]
αNb5Si3, which agreed with the orientation relationships reported for eutectoid Nb
ss/αNb
5Si
3 lamellae by Sekido et al. [
47] and Miura et al. [
48]. Sekido et al. [
42] suggested that the βNb
5Si
3 → αNb
5Si
3 transformation occurred before the Nb
ss precipitated or simultaneously and attributed the precipitation of Nb
ss to the βNb
5Si
3 exhibiting temperature dependent solubility owing to anti-site substitution of Nb and Mo atoms for Si sites. The off-stoichiometry towards the Si rich side of the phase diagram was suggested to be attained by substitutional, i.e., anti-site defects and not by vacancies on Nb sites.
In the alloy CM1, the formation of primary βNb
5Si
3 is consistent with the Nb-Si-Mo and Nb-Si-W liquidus projections. In the Nb-Ti-Si liquidus projection by Li et al. [
25] the primary phase in the alloy CM1 is the βNb
5Si
3 when Nb, Mo, Hf and W are considered as equivalent, meaning the alloy CM1 is considered to be an (Nb,Mo,Hf,W)-Ti-Si alloy. The suppression of Nb
3Si and the formation of the Nb
ss + βNb
5Si
3 eutectic is also consistent with the liquidus projections for the Nb-Si-Mo and Nb-Si-W ternaries. The solidification of the Nb
ss + βNb
5Si
3 eutectic can be explained with the help of
Figure 7. The formation of Nb
3Si in CM1-8mm can be explained as discussed above but also can be related to variations in Si and Mo concentration of the melt and the data from [
41] for Nb-19Si-xMo and Nb-20Si-xMo alloys. The presence of αNb
5Si
3 in CM1-600g can be attributed to the βNb
5Si
3 → αNb
5Si
3 transformation during solid state cooling that depends on the Mo concentration in the βNb
5Si
3 and on cooling rate. Phase equilibria data for 1700 °C for Nb-Si-Mo [
40] and Nb-Si-W [
41] shows that the αNb
5Si
3 is in equilibrium with Nb
ss (in the Nb-Si binary the αNb
5Si
3 is stable below about 1920 °C). The observation of αNb
5Si
3 only in the cast CM1-600g and not in the cast CM1-10g, CM1-6mm or CM1-8mm would suggest that the slower cooling of the larger ingot was enough for the βNb
5Si
3 → αNb
5Si
3 transformation to occur, consistent with the observations of Sekido et al. [
42].
Subgrains were observed in the αNb
5Si
3 in CM1-600g. First-principles calculations have been performed in our group to show the effect of Ti substituting Nb in the βNb
5Si
3 and αNb
5Si
3 on the moduli of elasticity of β(Nb,Ti)
5Si
3 and α(Nb,Ti)
5Si
3 and their CTE values [
19]. The Young’s modulus (E) of αNb
5Si
3 increased with Ti substituting Nb but that of βNb
5Si
3 decreased. For 12.5 at.% Ti in each of the two silicides the E values were 314 and 244 GPa, respectively. The CTE of the βNb
5Si
3 and αNb
5Si
3 also changed and continued to exhibit anisotropy and the ratio α
a/α
c (i.e., the CTE anisotropy ratio) was different for each phase, namely 0.797 and 0.611 for αNb
5Si
3 and βNb
5Si
3, respectively, when 12.5 at.% Ti substituted Nb in Nb
5Si
3. The CTE values of βNb
5Si
3 and αNb
5Si
3 along their c-axes were very close (10.682 10
−6 K
−1 and 10.980 10
−6 K
−1) but those along their a-axes were different, namely 8.510 10
−6 K
−1 and 6.709 10
−6 K
−1. In other words, the substitution of Nb by Ti in the tetragonal Nb
5Si
3 makes the low temperature αNb
5Si
3 polymorph more isotropic (the ratio of CTE values α
a/α
c increases) and the high temperature βNb
5Si
3 polymorph more anisotropic.
Considering strain energy in alloyed βNb5Si3 to arise from the CTE anisotropy, meaning the lower the αa/αc ratio the higher the anisotropy, the subgrain formation as the β(Nb,Ti)5Si3 transformed to α(Nb,Ti)5Si3 would be accompanied by a reduction in strain energy (the “driving force” for recovery and subgrain formation).
The above discussion has ignored the role of Mo substituting Nb atoms in Nb5Si3 because no data is available, but pointed to some special role played by Ti and Mo in the alloy CM1 (owing to the formation of subgrains and fine Nbss precipitates in αNb5Si3 in CM1-600g). We shall expand on this point below where the case for CM1-OFZ will be considered.
OFZ
The floating zone process is containerless and surface tension forces “hold” the melt. The upper limit of the length of the melt zone (meaning the length beyond which the zone becomes unstable and collapses) is proportional to (γ
LV/ρg)
1/2 where γ
LV is the surface tension of the melt, ρ is melt density and g is the gravitational acceleration [
49]. For elemental Si, Nb, Mo the (γ
LV/ρg)
1/2 is large (respectively 0.17, 0.16 and 0.15) which means that necking in the melt zone is not severe. For Nb silicide-based alloys with densities in the range 6.5 < ρ < 7.5 g/cm
3 [
28,
29,
33,
50,
51] this ratio is in the range 0.167 to 0.18 (not accounting for changes in γ
LV).
In gravity conditions, convection in the liquid can arise from surface tension gradients, thermo-capillary convection (owing to temperature and concentration gradients) or from buoyancy (natural convection). In OFZ, convection in the liquid zone can have an effect on liquid zone length, the shape of the S/L interface or on zone stability. Forced convection can be introduced by rotations. Uniform (axisymmetric) heating is possible with slow rotation rates.
In the OFZ process there is a liquid-vapour (L-V) interface (often referred to as the liquid surface) with an imposed temperature gradient. The surface tension is a function of temperature. Thus, the temperature gradient will produce motion in the liquid (convection). The shape of the solid (S) depends on the L-V interface at the S-L-V junction.
The surface tension of hot liquid is lower compared with that of colder liquid. Thus, the system will attempt to lower its free energy via movement of the liquid from areas of low surface tension to areas of high surface tension. The resulting convection is referred to as surface driven flow, thermocapillary flow or Marangoni flow or Marangoni convection in temperature gradient (MCT). This is described by the thermal Marangoni number Ma(T) = PrM, where Pr is the Prandtl number (see below) and M is the surface tension parameter (see below). Gradients in composition will also produce such a flow that is referred to as Marangoni convection in concentration gradient (MCC). The buoyancy driven convection arises from interaction of density variations in the liquid (due to temperature variations) with gravity.
The Marangoni flow has an effect on transport phenomena (i.e., mass and heat transfer) during OFZ. The shape of the S/L interface and the distribution of solute (i.e., the development or not of chemical inhomogeneities) depend on the mass and heat transfer. The homogeneity of the solid is influenced by mass transfer and heat transfer, due to their effect on the shape of the S/L interface, and the shape of the solid and the stability of the floating zone are influenced by heat transfer. For Pr << 1, laminar flow has a small influence on the heat transfer.
Dimensionless numbers that are important for understanding OFZ processing include the surface tension parameter M, and the Prandtl Pr and Schmidt Sc numbers. The surface tension parameter is M = ρ(T
o − T
m)(−∂γ
LV/∂T)α/μ
2 and is used to characterize thermocapillary flow in OFZ. In this equation ρ is the density of the liquid, α is the radius of the zone, μ is the liquid viscosity, T
o is the temperature of the liquid surface at the center of the zone (K) and T
m is the interface temperature taken to be the melting point (K). For elemental Nb the term − ρ(∂γ
LV/∂T)/μ
2 is ≈ 1380 (the data for Nb is from [
52]), compared with M ≈ 14,000 for Si [
52]. Thus, for elemental Nb and α = 5 mm (the diameter of the solid rods used for OFZ in this research was 10 mm) the value of M is 690, 345 and 34.5 for T
o − T
m equal to 1, 0.5 and 0.05 K, respectively. According to Chang and Wilcox [
52] buoyancy driven convection in enclosed spaces is oscillatory for T
o − T
m above 0.1 K and turbulent above 2 K.
The Prandtl (Pr) number (=C
p μ/k) is the ratio of momentum diffusivity (μ/ρ = kinematic viscosity) to thermal diffusivity (k/ρC
P) where C
P is the specific heat at constant pressure and k is the thermal conductivity. The Schmidt (Sc) number (=μ/ρD) is the ratio of momentum diffusivity to diffusion coefficient, where D is the diffusion coefficient. For elemental Nb the values are Pr ≈ 0.026 (compared with Pr ≈ 0.023 for Si and Pr ≈ 0.025 for Mo) and Sc ≈ 145 (compared with Sc ≈ 5 for Si) (the data for elemental Nb is from [
52]). The Prandtl number Pr is important for the shape of the melt zone. When the power input increases, the shape of the S/L interface changes from convex towards the liquid to flat to concave [
53].
Transport by convection becomes more significant compared with transport by conduction or diffusion as Pr or Sc increases. Liquid metals are low Pr number materials. Small value of the Prandtl number, Pr << 1, means the thermal diffusivity dominates and large Prandtl number, Pr >> 1, means the momentum diffusivity dominates. The Pr values for elemental Si and Nb indicate that the heat conduction is more significant compared with convection and that thermal diffusivity is dominant. Thus, if these elements were to be grown using OFZ, it is most likely that they will exhibit thermocapillary flow. The latter can introduce considerable inhomogeneities even at gentle flow. Thermocapillary flow instability in the liquid may appear before instability from the L-V interface sets in. The above could explain the chemical inhomogeneity between the bulk and edge of the OFZ bars grown at the three different growth rates that was observed in CM1-OFZ.
Louchev et al. [
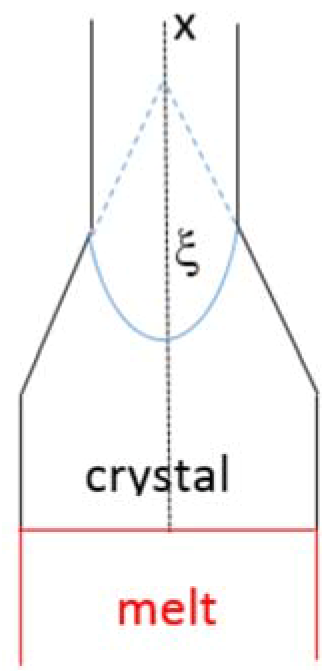
54] used an 1D heat transfer problem to study the thermal stresses in the initial stages of crystal growth from the melt for the geometry shown in
Figure 8, and considered the effect of change in cross sectional area with distance from the growth interface using the angle ξ. They proposed that the thermal stress is given by the equation σ
thermal ≈ αEw
2(d
2T/dx
2) where α is the linear CTE, E is the Young’s modulus, w = 0.2R (R is the diameter of the crystal) and dT/dx is the temperature gradient in the crystal. Tensile stress (σ
thermal > 0) would arise from the heat dissipation from the lateral surface and compressive stress (σ
thermal < 0) from the radiation heat flux incoming to the crystal surface and from the geometry of the growing crystal (described using the angle ξ in
Figure 8). For small ξ (<30°) the thermal stress is tensile. The temperature gradient in the solid (dT/dx)
S decreases with increasing ξ and the highest temperature gradient occurs for ξ = 0. During the OFZ processing of the alloy CM1 and at each growth rate, thermal stresses could have resulted from small or large change(s) in cross sectional area that could have occurred for any of the reasons discussed above. These thermal stresses would have interacted with any stresses that had resulted from CTE anisotropy of the alloyed Nb
5Si
3 (note that the surfaces of CM1-OFZ bars were not absolutely smooth cylindrical surfaces, see results).
In OFZ processing the formation of constitutionally undercooled liquid and therefore the “breakdown” of the interface is most likely at the centre rather than at the periphery of the solidifying solid. At low M values, if the growth rate is not low there will be radial variations in solute concentration. At intermediate M values oscillations and formation of striations parallel to the S/L interface are likely.
Consider an alloy of composition C
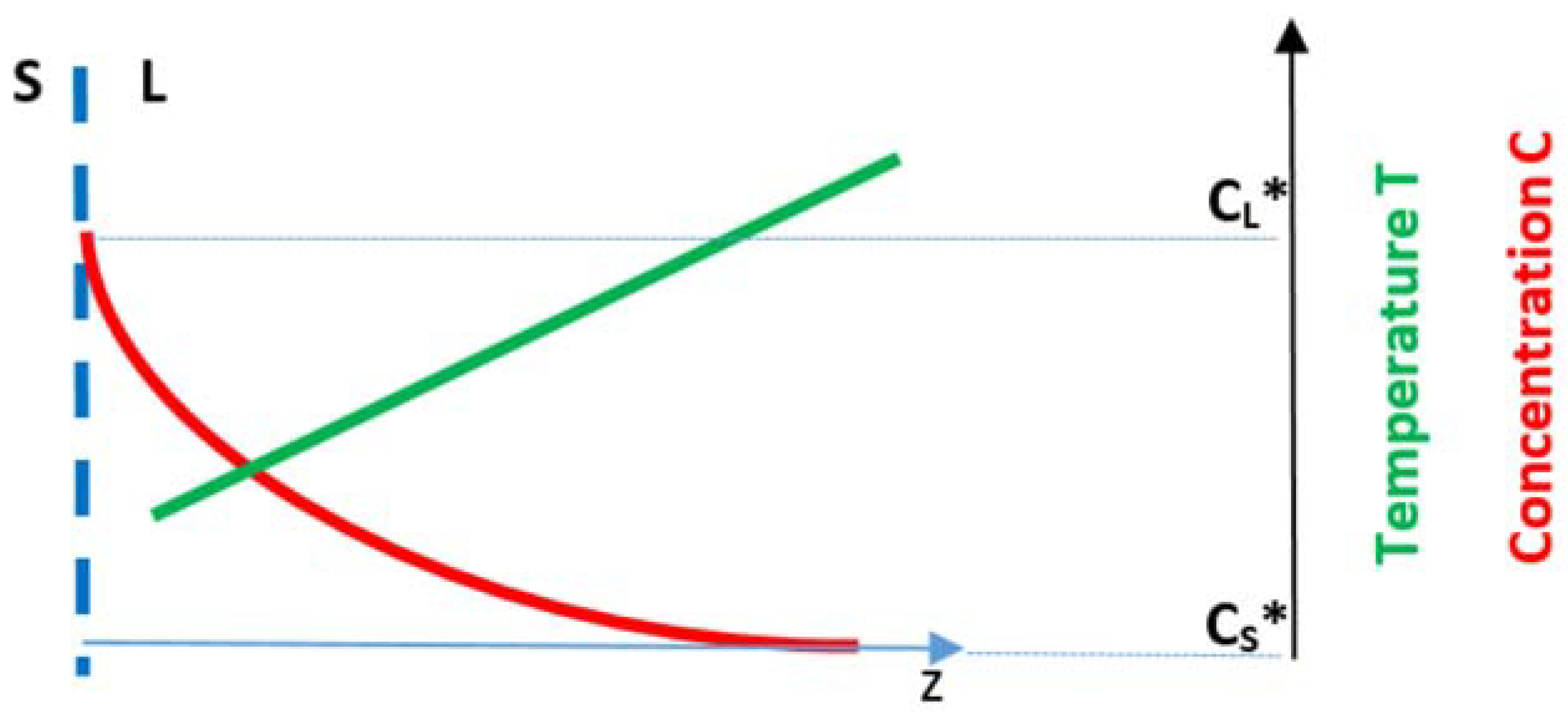
o with solute having partition coefficient k < 1 and reducing the surface tension of the melt. A solute boundary layer is formed ahead of the S/L interface. The
Figure 9 shows the position of the S/L interface at T = T* with the vertical dashed line (with the compositions of the solid and liquid at this temperature as C
S* and C
L*, respectively, which can be read on the axis on the right hand side), the solute rich boundary layer ahead of the interface (red curve) and the temperature gradient in the melt (green line). Assume that the alloy is processed using OFZ and allow for oscillations of temperature in the liquid.
According to Schwabe et al., the opposite forces, f
i, acting on the S/L interface due to the temperature and concentration gradients, respectively will be fz(T) = (∂γ/∂T)(∂T/∂z) and fz(C) = (∂γ/∂C)(∂C/∂z), where γ is surface tension [
55]. Consider fz(T) > fz(C). The resultant force means that phenomena will be driven by Marangoni convection in temperature gradient. This MCT will carry hot liquid to the interface and will support the heat transfer to the interface from the liquid ahead of the S/L interface. If there were to be an increase in V
S/L the solute profile in the boundary layer would change and the fz(C) will increase, which means that the resulting force on the interface will be reduced, i.e., the MCT will be reduced and this will result in an increase in V
S/L. If instead there were to be a decrease in V
S/L, the solute profile in the boundary layer would change and the fz(C) will decrease, which means that the resulting force on the interface will be increased, i.e., the MCT will be increased, meaning the MCT carries more hot liquid to the interface making it more morphologically stable. This simple argument shows how growth instabilities could have resulted from MCT and MCC and also provides another “mechanism” for counter-balancing the stresses that arise from the CTE anisotropy of alloyed Nb
5Si
3.
In floating zone melting the temperature gradient in the liquid (dT/dx)
L is linked with that in the solid (dT/dx)
S via the equation k
S(dT/dx)
S = k
L(dT/dx)
L − V
S/L∆Hρ
S where k
i (i = S, L) is thermal conductivity, ∆H is enthalpy and ρ
S is the density of the solid. For growth at a constant rate, a decrease in (dT/dx)
S is linked with a decrease in (dT/dx)
L and thus would affect the conditions for forming constitutionally undercooled liquid ahead of the S/L interface and therefore the morphological stability of the latter. As (dT/dx)
S decreases with increasing ξ (see
Figure 8) a change in cross section of the crystal (i.e., change in ξ), which results to compressive thermal stress in the crystal (see above), will also reduce (dT/dx)
L (=G
L in
Figure 9) and thus encourage the onset of constitutional undercooling [
54]. This simple argument provides another “mechanism” for counter-balancing the stresses that arise from the CTE anisotropy of alloyed Nb
5Si
3.
4.2. Heat-Treated Alloy
After the heat treatment the phases present in the microstructures of CM1-10g, CM1-600g, CM1-6mm, CM1-8mm and CM1-OFZ were the Nb
ss and αNb
5Si
3 and HfO
2. The former two phases (and the absence of Nb
3Si) is in accordance with the ternary Nb-Si-Mo and Nb-Si-W phase equilibria at 1700 °C [
40,
41].
With the exception of CM1-600g, in which there were Nb
ss grains with no Si and grains where the solubility of Si in the Nb
ss was low and at similar concentration to that reported for the Nb
ss in heat-treated Nb-silicide-based alloys [
6,
28,
33], in all other forms of the alloy CM1 the Nb
ss was free of Si (i.e., Nb with no Si, see [
34]), in agreement with other research for arc melted Nb-silicide alloys with refractory metal additions [
34,
35].
Precipitation of second phase with contrast similar to that of Nb
ss has been reported by our group in αNb
5Si
3 in the heat-treated Nb-24Ti-18Si-5Al [
56] and Nb-18Si-5Al-5Cr-5Mo alloys [
43] and in αNb
5Si
3 and/or βNb
5Si
3 in the heat-treated Nb-24Ti-18Si-5Al-5Cr, Nb-24Ti-18Si-6Ta-5Al-5Cr, Nb-24Ti-18Si-8Cr-4Al, Nb-24Ti-18Si-6Ta-8Cr-4Al [
18,
56], Nb-24Ti-18Si-5Al-5Cr-5Mo, Nb-24Ti-18Si-5Al-5Cr-2Mo [
43], Nb-24Ti-18Si-5Al-5Cr-5Hf-5Sn-2Mo [
57] and Nb-18Si-5Al-5Ge and Nb-24Ti-18Si-5Al-5Ge [
33] alloys. Precipitation of Nb
ss in αNb
5Si
3 in Nb-20Ti-18Si-4Hf-5Cr-3Al-1.5Sn also has been reported by Cheng et al. [
58]. In the latter alloy the orientation relationship (222)
Nbss // (002)
αNb5Si3, [1
0]
Nbss // [1
0]
αNb5Si3 was reported, and the interface between Nb
ss and αNb
5Si
3 was found to be enriched in Hf.
For the heat-treated alloy Nb-24Ti-18Si-5Al, Zelenitsas and Tsakiropoulos [
18] suggested that the Nb
ss precipitates were the product of the βNb
5Si
3 → αNb
5Si
3 + Nb
ss phase transformation. The data in [
18,
56] showed that in the heat-treated Nb-24Ti-18Si-5Al, Nb-24Ti-18Si-6Ta-5Al-5Cr, Nb-24Ti-18Si-8Cr-4Al and Nb-24Ti-18Si-6Ta-8Cr-4Al alloys the Nb
ss precipitates did not form in Ti rich areas in the αNb
5Si
3 and/or βNb
5Si
3. The data from our research group [
18,
33,
43,
57] and Cheng et al. [
58] would suggest that precipitation of Nb
ss in βNb
5Si
3 and/or αNb
5Si
3 is a more general phenomenon in Nb-silicide-based alloys and that many solutes contribute to this precipitation.
Precipitation of Nb
ss was observed in the cast alloy CM1 (a) in subgrained αNb
5Si
3 in CM1-600g, and (b) in the non-subgrained αNb
5Si
3 in CM1-OFZ grown at 150 mm/h, 60 mm/h and 12 mm/h. Precipitation of Nb
ss was observed in the heat-treated CM1 (c) in all the subgrained αNb
5Si
3 in the heat-treated CM1-10g, CM1-6mm, CM1-8mm, CM1-600g, and CM1-OFZ (for all three growth rates), see
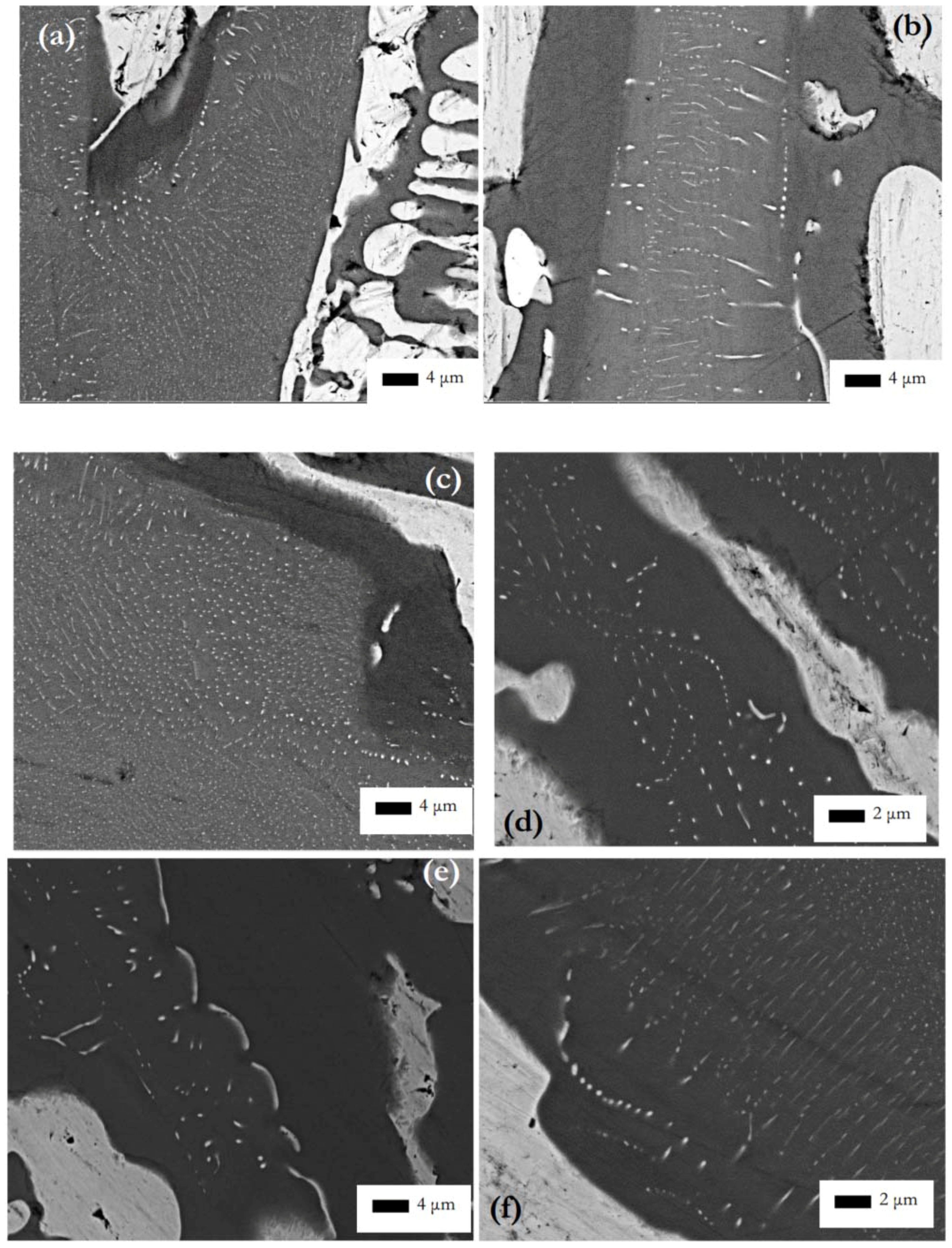
Table S1 in supplemental data.
The formation of subgrains in the αNb5Si3 in the heat-treated CM1-OFZ bars grown at the three different growth rates was attributed to the strain energy reduction via “recovery” phenomena, and the strain energy was attributed to the anisotropy of CTE due to the partitioning of solutes.
Figure 5g shows the microstructure of an αNb
5Si
3 grain with fine precipitates. The subgrain boundary exhibits dark contrast but the bulk of the subgrains exhibits lighter contrast, meaning the latter must be richer in high atomic number elements (Hf, W). In this alloy the Nb
ss in CM1-10g was also rich in W. Please note that near the subgrain boundary some precipitates are larger in size. Overall the Nb
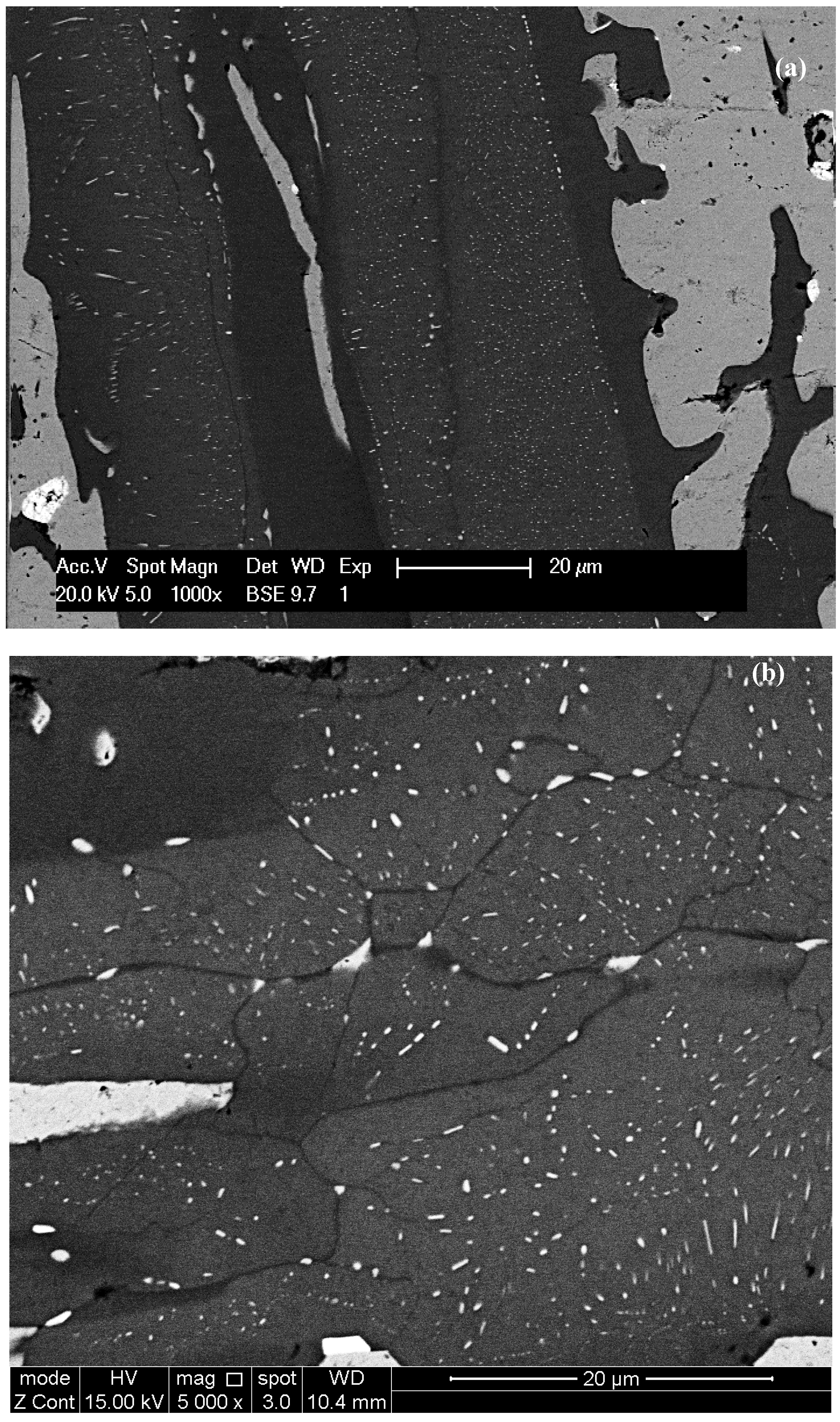
ss precipitates were of nanometer size and the spacing between them was also in the nanometer scale. Precipitates with high aspect ratio together with fine spherical precipitates can be seen in
Figure 2d and
Figure 6b. In
Figure 2d and
Figure 6 it should be noted that there is a precipitate free area (zone) near the interface of Nb
ss and αNb
5Si
3.
In the alloy CM1, in the βNb
5Si
3 the high Ti concentration was associated with low Mo (and W) concentrations (the Hf and W concentrations were very low, practically zero), and Nb
ss precipitates formed in the large silicide grains in areas where X-ray maps (
Figure S5 in supplemental data) indicated low Ti and high Mo content, and there was a “band” of Ti rich and Mo poor silicide that was precipitate free. Such bands were formed next to the Nb
ss surrounding the Nb
5Si
3. In the Nb
ss the high Ti concentration was associated with low Mo and W content, there were Ti rich and Ti poor areas in the Nb
ss after the heat treatment, and the W concentration in the Nb
ss also varied significantly. Given that no precipitates were observed in the Ti rich (and Mo poor) areas (bands) formed at the interface between Nb
ss and Nb
5Si
3 (which according to the literature [
18,
56] are the areas where Ti segregates during the solidification of Nb-silicide-based alloys), the precipitation of Nb
ss occurred in the Ti poor and Mo rich areas in the bulk of the silicide, which is in agreement with previous research (see above) and the role of Mo in the precipitation of Nb
ss according to the work of Sekido et al. [
42]. The
Figure 2d,
Figure 4f,
Figure 5g would suggest that there was some orientation relationship of the Nb
ss precipitates in the silicide grain.