Mechanical and Morphological Effect of Plant Based Antimicrobial Solutions on Maxillofacial Silicone Elastomer
Abstract
:1. Introduction
1.1. Degradation by Environment
1.2. Degradation by Cleaning/Handling
2. Results
2.1. Mechanical Testing
2.2. Morphological Testing
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.1.1. Silicone Elastomer
4.1.2. Antimicrobial Disinfectant
4.1.3. Bacterial Preparation
4.1.4. Antimicrobial Disinfectant Minimum Inhibitory Concentration
4.1.5. Conditioning of Samples for Mechanical Testing
4.1.6. Conditioning Time Periods with Antimicrobial Solution
4.2. Mechanical (Quantitative) Testing
4.2.1. Hardness
4.2.2. Tensile Strength
4.2.3. Tear Strength Test
4.3. Morphology Testing
4.3.1. Visual Observation
4.3.2. Scanning Electron Microscopy Testing
4.3.3. Characterization of Test Samples
4.3.4. Sample Preparation for Scanning
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ATCC | American Type Culture Collection |
| Fb | the force recorded at break |
| °C | degrees Celsius |
| SD | Standard deviation |
| SEM | Scanning electron microscopy |
| Si | silicone (control sample) |
| SiAu | silicone autoclaved |
| SiMO | silicone with Manuka oil |
| SiMOBAu | silicone with manuka oil, staphylococcus epidermidis and autoclaved |
| SiTTO | silicone with tea tree oil |
| SiTTOBAu | silicone with tea tree oil, staphylococcus epidermidis and autoclaved |
| UV | Ultraviolet |
References
- Pigno, M.A.; Goldschmidt, M.C.; Lemon, J.C. The efficacy of antifungal agents incorporated into a facial prosthetic silicone elastomer. J. Prosthet. Dent. 1994, 71, 295–300. [Google Scholar] [CrossRef]
- Micheline Dos Santos, D.; Goiato, M.C.; Moreno, A.; Pesqueira, A.A.; Dekon, S.F.D.C.; Guiotti, A.M. Effect of addition of pigments and opacifier on the hardness, absorption, solubility and surface degradation of facial silicone after artificial ageing. Polym. Degrad. Stab. 2012, 97, 1249–1253. [Google Scholar] [CrossRef]
- Hatamleh, M.M.; Watts, D.C. Bonding of maxillofacial silicone elastomers to an acrylic substrate. Dent. Mater. 2010, 26, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, F.A.; Ayad, N.M.; Saber, M.A.; Arrejaie, A.S.; Morgano, S.M. Mechanical behavior and color change of facial prosthetic elastomers after outdoor weathering in a hot and humid climate. J. Prosthet. Dent. 2015, 113, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhao, Y.; Xie, C.; Powers, J.M.; Kiat-Amnuay, S. Color stability of pigmented maxillofacial silicone elastomer: Effects of nano-oxides as opacifiers. J. Dent. 2010, 38 (Suppl. 2), e100–e105. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kiat-amnuay, S.; Powers, J.M.; Zhao, Y. Effect of nano-oxide concentration on the mechanical properties of a maxillofacial silicone elastomer. J. Prosthet. Dent. 2008, 100, 465–473. [Google Scholar] [CrossRef]
- Guiotti, A.M.; Cunha, B.G.; Paulini, M.B.; Goiato, M.C.; dos Santos, D.M.; Duque, C.; Caiaffa, K.S.; Brandini, D.A.; Narciso de Oliveira, D.T.; Brizzotti, N.S.; et al. Antimicrobial activity of conventional and plant-extract disinfectant solutions on microbial biofilms on a maxillofacial polymer surface. J. Prosthet. Dent. 2016, 116, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ariani, N.; Vissink, A.; van Oort, R.P.; Kusdhany, L.; Djais, A.; Rahardjo, T.B.W.; van der Mei, H.C.; Krom, B.P. Microbial biofilms on facial prostheses. Biofouling 2012, 28, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Karakoca, S.; Aydin, C.; Yilmaz, H.; Bal, B.T. Retrospective study of treatment outcomes with implant-retained extraoral prostheses: Survival rates and prosthetic complications. J. Prosthet. Dent. 2010, 103, 118–126. [Google Scholar] [CrossRef]
- Visser, A.; Raghoebar, D.M.D.G.M.; Oort, R.P.; Van Vissink, A. Fate of Implant-Retained Craniofacial Prostheses: Life Span and Aftercare. Int. J. Oral Maxillofac. Implants 2008, 23, 89–98. [Google Scholar] [PubMed]
- Eleni, P.N.; Katsavou, I.; Krokida, M.K.; Polyzois, G.L.; Gettleman, L. Mechanical behavior of facial prosthetic elastomers after outdoor weathering. Dent. Mater. 2009, 25, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Choudhary, S.; Garg, H.; Jagadeesh, H.G. Maxillofacial prosthetic materials—An inclination towards silicones. J. Clin. Diagn. Res. 2014, 8, ZE08–ZE13. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.F. Prosthetic Rehabilitation; Quintessence Publishing Co., Ltd.: London, UK, 1994. [Google Scholar]
- Eggbeer, D. The Computer Aided Design and Fabrication of Facial Prostheses. Ph.D. Thesis, University of Wales Institute, Cardiff, Wales, UK, 2008. [Google Scholar]
- Eleni, P.N.; Krokida, M.K.; Polyzois, G.L. The effect of artificial accelerated weathering on the mechanical properties of maxillofacial polymers PDMS and CPE. Biomed. Mater. 2009, 4, 035001. [Google Scholar] [CrossRef] [PubMed]
- Eleni, P.N.; Krokida, M.K.; Polyzois, G.L.; Charitidis, C.A.; Koumoulos, E.P.; Tsikourkitoudi, V.P.; Ziomas, I. Mechanical behaviour of a poydimethylsiloxane elastomer after outdoor weathering in two different weathering locations. Polym. Degrad. Stab. 2011, 96, 470–476. [Google Scholar] [CrossRef]
- Leow, E.L.; Pereira, B.P.; Kour, A.K.; Pho, R.W. Lifelikeness in multilayered digital prostheses. Prosthet. Orthot. Int. 1997, 21, 40–51. [Google Scholar] [PubMed]
- Vojdani, M.; Zibaei, M.; Aar, K.; Zomorodian, K.; Ma, R.; Boshehri, S. In-vitro Study of the Effect of Clotrimazole Incorporation into Silicone Soft Liner on Fungal Colonization. J. Dent. 2009, 9, 19–23. [Google Scholar]
- Ploux, L.; Beckendorff, S.; Nardin, M.; Neunlist, S. Quantitative and morphological analysis of biofilm formation on self-assembled monolayers. Colloids Surf. B Biointerfaces 2007, 57, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Ariani, N.; Visser, A.; Teulings, M.R.I.M.; Dijk, M.; Rahardjo, T.B.W.; Vissink, A.; van der Mei, H.C. Efficacy of cleansing agents in killing microorganisms in mixed species biofilms present on silicone facial prostheses—An in vitro study. Clin. Oral Investig. 2015, 19, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Ariani, N. Microbial Biofilms on Silicone Facial Prostheses. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2015. [Google Scholar]
- Beumer, J.; Curtis, T.A.; Firtell, D.N. Maxillofacial Rehabilitation. J. Prosthet. Dent. 1979, 333–364. [Google Scholar] [CrossRef]
- Pesqueira, A.A.; Goiato, M.C.; dos Santos, D.M.; Haddad, M.F.; do Prado Ribeiro, P.; Coelho Sinhoreti, M.A.; Marçal Mazza Sundefeld, M.L. Effect of Disinfection and Accelerated Aging on Color Stability of Colorless and Pigmented Facial Silicone. J. Prosthodont. 2011, 20, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; Pesqueira, A.A.; Ramos da Silva, C.; Filho, H.G.; Micheline dos Santos, D. Patient satisfaction with maxillofacial prosthesis. Literature review. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, D.J.; Habakuk, S.W. Hygiene procedures for implant-retained facial prostheses. J. Prosthet. Dent. 1995, 74, 499–502. [Google Scholar] [CrossRef]
- Goiato, M.C.; Pesqueira, A.A.; Moreno, A.; Dos Santos, D.M.; Haddad, M.F.; Bannwart, L.C. Effects of pigment, disinfection, and accelerated aging on the hardness and deterioration of a facial silicone elastomer. Polym. Degrad. Stab. 2012, 97, 1577–1580. [Google Scholar] [CrossRef]
- Markt, J.C.; Lemon, J.C. Extraoral maxillofacial prosthetic rehabilitation at the MD Anderson Cancer Center: A survey of patient attitudes and opinions. J. Prosthet. Dent. 2001, 85, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; Haddad, M.F.; Sinhoreti, M.A.C.; dos Santos, D.M.; Pesqueira, A.A.; Moreno, A. Influence of opacifiers on dimensional stability and detail reproduction of maxillofacial silicone elastomer. Biomed. Eng. 2010, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Áurea, M.; Ferreira, F.; Pereira-cenci, T. Efficacy of denture cleansers on denture liners contaminated with Candida species. Clin. Oral Investig. 2009, 13, 237–242. [Google Scholar]
- Eleni, P.N.; Perivoliotis, D.; Dragatogiannis, D.A.; Krokida, M.K.; Polyzois, G.L.; Charitidis, C.A.; Ziomas, I.; Gettleman, L. Tensile and microindentation properties of maxillofacial elastomers after different disinfecting procedures. J. Mech. Behav. Biomed. Mater. 2013, 28, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Hatamleh, M.M.; Watts, D.C. Effect of Extraoral Aging Conditions on Color Stability of Maxillofacial Silicone Elastomer. J. Prosthodont. 2010, 19, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Peralta, S.L.; Carvalho, P.H.A.; van de Sande, F.H.; Pereira, C.M.P.; Piva, E.; Lund, R.G. Self-etching dental adhesive containing a natural essential oil: Anti-biofouling performance and mechanical properties. Biofouling 2013, 29, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H. Temperature and humidity effect on aging of silicone rubbers as sealing materials for proton exchange membrane fuel cell applications. Appl. Therm. Eng. 2016, 104, 472–478. [Google Scholar]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (Tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A.; Riley, T.V.; Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Kwieciński, J.; Eick, S.; Wójcik, K. Effects of tea tree (Melaleuca alternifolia) oil on Staphylococcus aureus in biofilms and stationary growth phase. Int. J. Antimicrob. Agents 2009, 33, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Brand, C.; Carson, C.F.; Riley, T.V.; Prager, R.H.; Finlay-Jones, J.J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. 2000, 49, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Brand, C.; Ferrante, A.; Prager, R.H.; Riley, T.V.; Carson, C.F.; Finlay-Jones, J.J.; Hart, P.H. The water-soluble components of the essential oil of Melaleuca alternifolia (tea tree oil) suppress the production of superoxide by human monocytes, but not neutrophils, activated in vitro. Inflamm. Res. 2001, 50, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Yan, S.H.; Yen, M.Y.; Wu, P.F.; Liao, W.T.; Huang, T.S.; Wen, Z.H.; David Wang, H.M. Investigations of kanuka and manuka essential oils for in vitro treatment of disease and cellular inflammation caused by infectious microorganisms. J. Microbiol. Immunol. Infect. 2016, 49, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, S.F.; Docrat, Y.; Kamatou, G.P.P.; Viljoen, A.M. Essential oil composition and antimicrobial interactions of understudied tea tree species. S. Afr. J. Bot. 2014, 92, 7–14. [Google Scholar] [CrossRef]
- Lauten, J.D.; Boyd, L.; Hanson, M.B.; Lillie, D.; Gullion, C.; Madden, T.E. A clinical study: Melaleuca, Manuka, Calendula and green tea mouth rinse. Phyther. Res. 2005, 19, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Christoph, F.; Kaulfers, P.-M.; Stahl-Biskup, E. In vitro Evaluation of the Antibacterial Activity of β-Triketones Admixed to Melaleuca Oils. Planta Med. 2001, 67, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Douglas, M.H.; Van Klink, J.W.; Smallfield, B.M.; Perry, N.B.; Anderson, R.E.; Johnstone, P.; Weavers, R.T. Essential oils from New Zealand manuka: Triketone and other chemotypes of Leptospermum scoparium. Phytochemistry 2004, 65, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Hatamleh, M.M.; Haylock, C.; Watson, J.; Watts, D.C. Maxillofacial prosthetic rehabilitation in the UK: A survey of maxillofacial prosthetists’ and technologists’ attitudes and opinions. Int. J. Oral Maxillofac. Surg. 2010, 39, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; Haddad, M.F.; Santos, D.M.; Pesqueira, A.A.; Moreno, A. Hardness evaluation of prosthetic silicones containing opacifiers following chemical disinfection and accelerated aging. Braz. Oral Res. 2010, 24, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Bibars, A.R.M.; Al-Hourani, Z.; Khader, Y.; Waters, M. Effect of thixotropic agents as additives on the mechanical properties of maxillofacial silicone elastomers. J. Prosthet. Dent. 2018, 119, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Standard Test Method for Rubber Property—Durometer Hardness; ASTM D2240-15; ASTM International: West Conshohocken, PA, USA, 2012.
- Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties; ISO 37; International Organization for Standardization: Geneva, Switzerland, 2017.
- Rubber, Vulcanized or Thermoplastic—Determination of Tear Strength—Part 1; ISO 34-1; International Organization for Standardization: Geneva, Switzerland, 2010.
- Zayed, S.M.; Alshimy, A.M.; Fahmy, A.E. Effect of surface treated silicon dioxide nanoparticles on some mechanical properties of maxillofacial silicone elastomer. Int. J. Biomater. 2014, 2014, 14–15. [Google Scholar] [CrossRef] [PubMed]
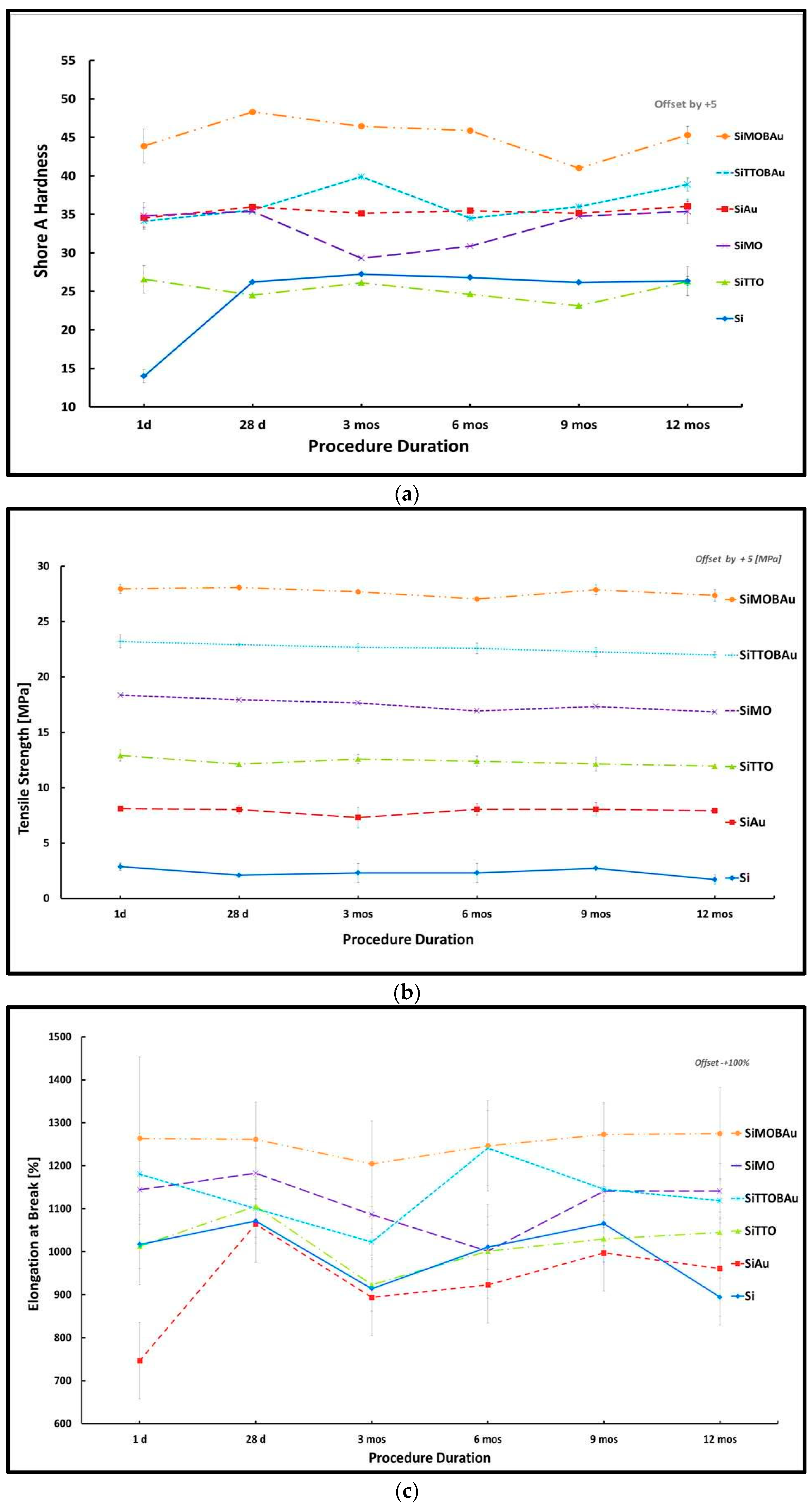
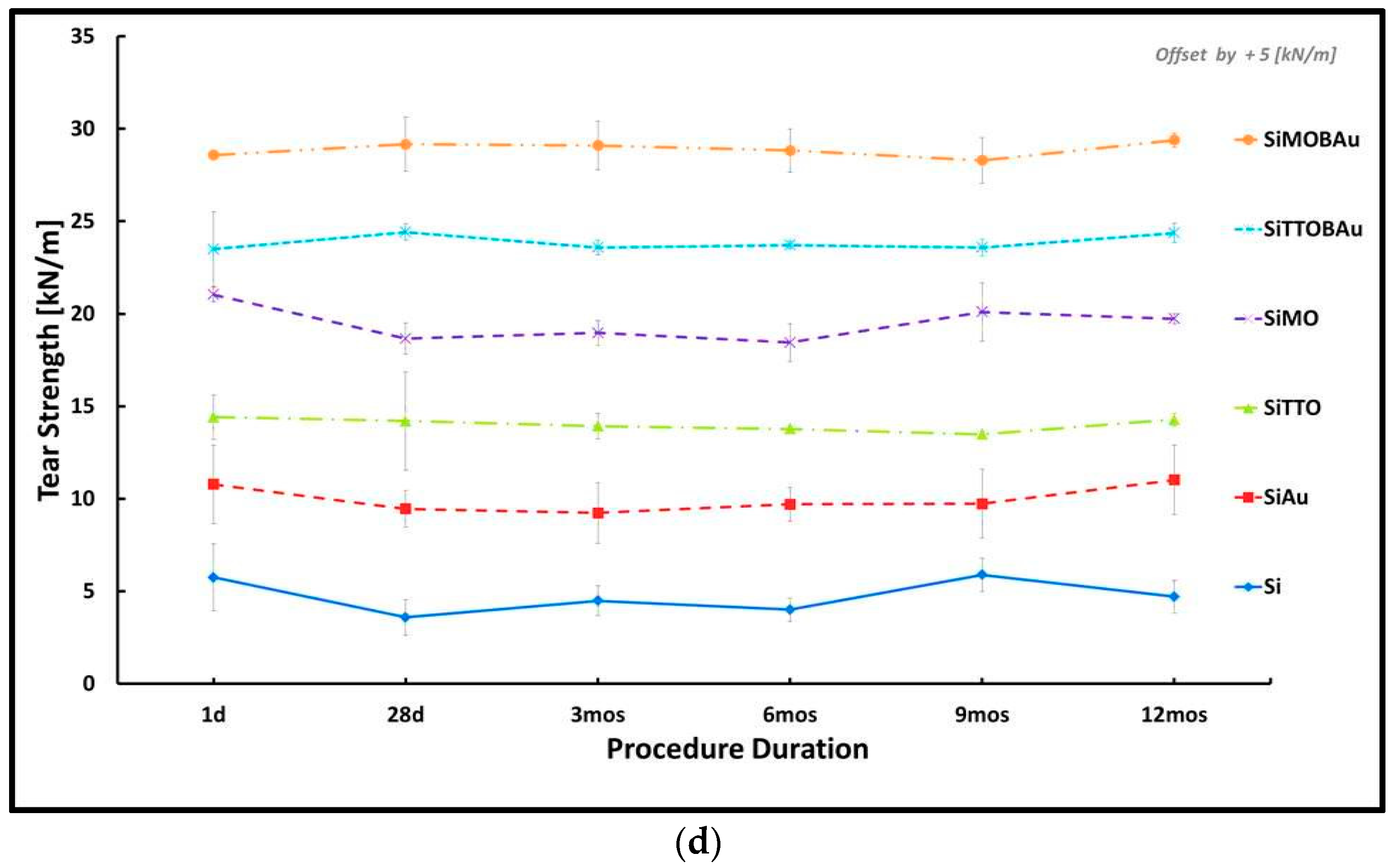
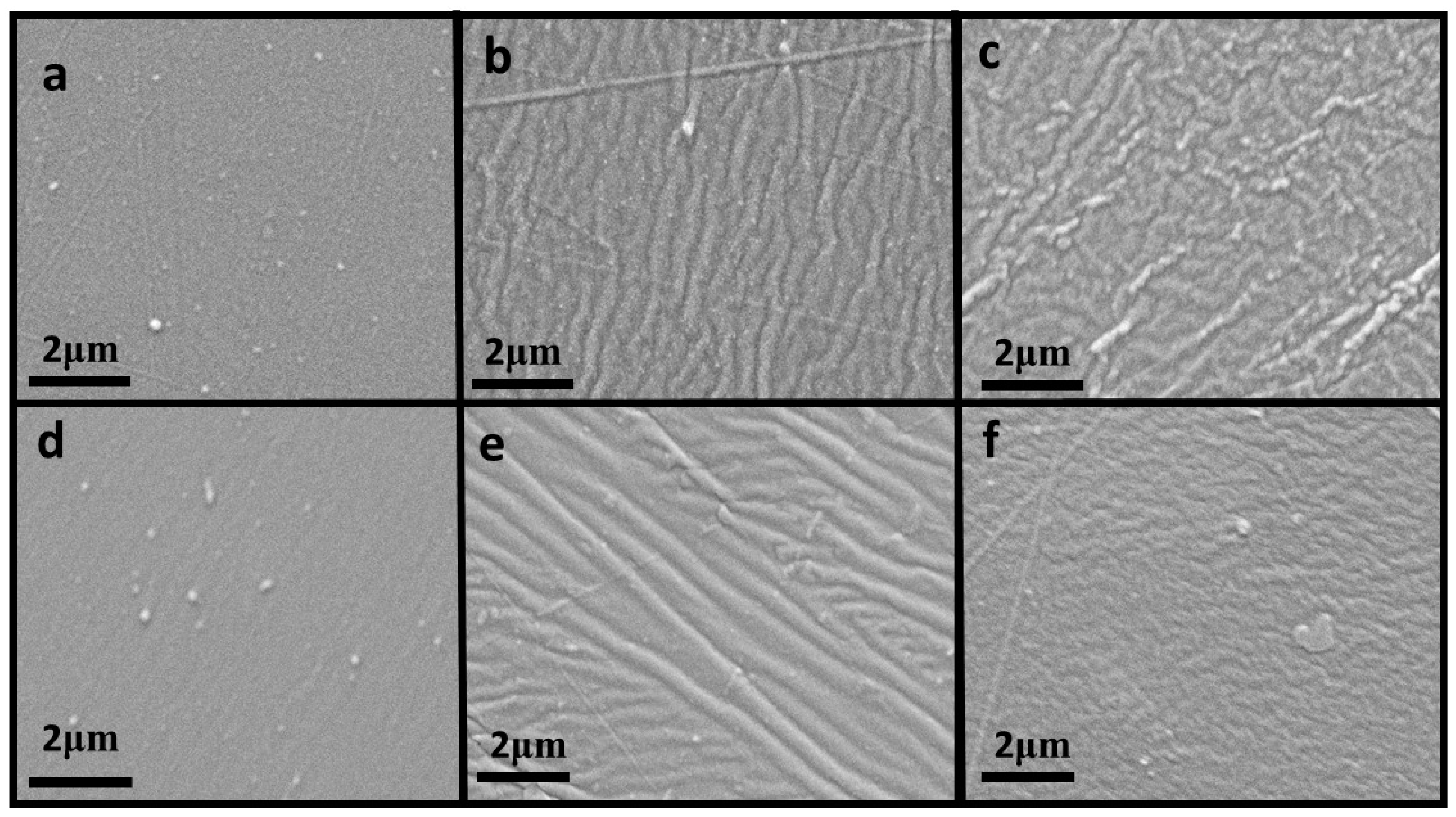
| Mechanical Parameters | R | R2 | Adjusted R Square | F | df2 | Sig. F Change |
|---|---|---|---|---|---|---|
| Hardness | 0.772 | 0.596 | 0.537 | 10.082 | 41 | 0.000 |
| Tensile | 0.772 | 0.596 | 0.353 | 2.457 | 10 | 0.100 |
| Tear Strength | 0.821 | 0.675 | 0.480 | 3.459 | 10 | 0.041 |
| Elongation | 0.918 | 0.842 | 0.747 | 8.876 | 10 | 0.002 |
| Mechanical Parameters | Source | df | SS | MS | F | p |
|---|---|---|---|---|---|---|
| Hardness | Time (within) | 2.893 | 183.105 | 63.287 | 12.72 | 0.000 |
| Time × Silicone (within) | 14.466 | 797.509 | 55.129 | 11.080 | 0.000 | |
| Tensile | Time (within) | 5 | 9.484 | 1.897 | 10.093 | 0.000 |
| Time × Silicone (within) | 25 | 7.484 | 0.299 | 1.593 | 0.076 | |
| Elongation at break | Time (within) | 1.00 | 249,062.363 | 249,062.363 | 3.909 | 0.074 |
| Time × Silicone (within) | 12.072 | 378,233.869 | 31,330.260 | 1.187 | 0.341 | |
| Tear Strength | Time (within) | 5 | 7,620,067.608 | 1,524,012.521 | 1.049 | 0.389 |
| Time × Silicone (within) | 25 | 21,850,848.090 | 874,033.924 | 0.601 | 0.917 |
| Materials | Manufacturer | Batch/Lot Number |
|---|---|---|
| M511 Platinum Silicone Part A | Technovent, Bridgend, Wales, UK | B17D/B17AH |
| M511 Platinum Silicone Part B | Technovent, Bridgend, Wales, UK | B16C/B17D |
| Manuka Oil | Essential Oils Direct, Oldham, UK | 8583/9124 |
| Tea Tree Oil | Essential Oils Direct, Oldham, UK | 9100 |
| Volume Used | Volume/Volume Percent Solution (v/v) % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % | 0.05 | 0.1 | 0.2 | 0.4 | 1.0 | 2.0 | 4.0 | 8.0 | 16.0 | |
| Tea Tree | µL | 15 | 30 | 60 | 120 | 300 | 600 | 1200 | - | - |
| Manuka | - | - | 2 | 4 | 10 | 20 | 40 | 80 | 160 | |
| Test Agent | Staphylococcus Epidermidis |
|---|---|
| Tea Tree Oil | 0.2% (v/v) |
| Manuka Oil | 0.4% (v/v) |
| Procedure Duration Utilised for Conditioning Samples | ||||||
|---|---|---|---|---|---|---|
| Simulated Time (m—months; d—days) | 12 m | 9 m | 6 m | 3 m | 28 d | 1 d |
| Procedure Time (hours) | 30 | 22.5 | 15 | 7.5 | 2.5 | 0.083 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetteh, S.; Bibb, R.J.; Martin, S.J. Mechanical and Morphological Effect of Plant Based Antimicrobial Solutions on Maxillofacial Silicone Elastomer. Materials 2018, 11, 925. https://doi.org/10.3390/ma11060925
Tetteh S, Bibb RJ, Martin SJ. Mechanical and Morphological Effect of Plant Based Antimicrobial Solutions on Maxillofacial Silicone Elastomer. Materials. 2018; 11(6):925. https://doi.org/10.3390/ma11060925
Chicago/Turabian StyleTetteh, Sophia, Richard J. Bibb, and Simon J. Martin. 2018. "Mechanical and Morphological Effect of Plant Based Antimicrobial Solutions on Maxillofacial Silicone Elastomer" Materials 11, no. 6: 925. https://doi.org/10.3390/ma11060925
APA StyleTetteh, S., Bibb, R. J., & Martin, S. J. (2018). Mechanical and Morphological Effect of Plant Based Antimicrobial Solutions on Maxillofacial Silicone Elastomer. Materials, 11(6), 925. https://doi.org/10.3390/ma11060925





