Improving Thermo-Oxidative Stability of Nitrile Rubber Composites by Functional Graphene Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GO–PPD
2.3. Preparation of NBR/GO–PPD Composites
2.4. NBR and NBR/GO–PPD Composites Aging Experiment
2.5. Instrumentation
3. Results and Discussion
3.1. Characterization of Filler Particles
3.2. Tensile Properties before and after Aging
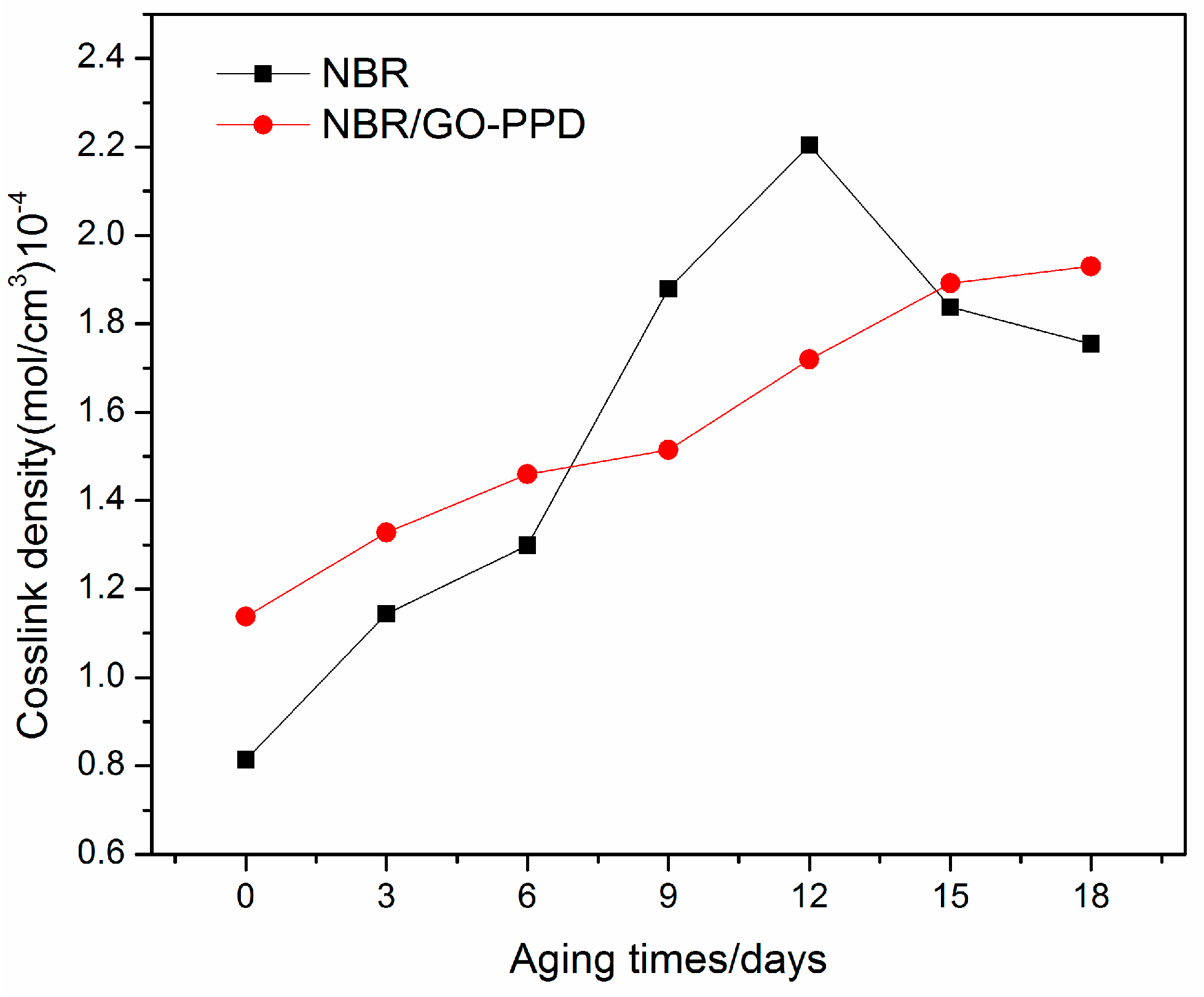
3.3. Crosslink Densities of NBR Composites
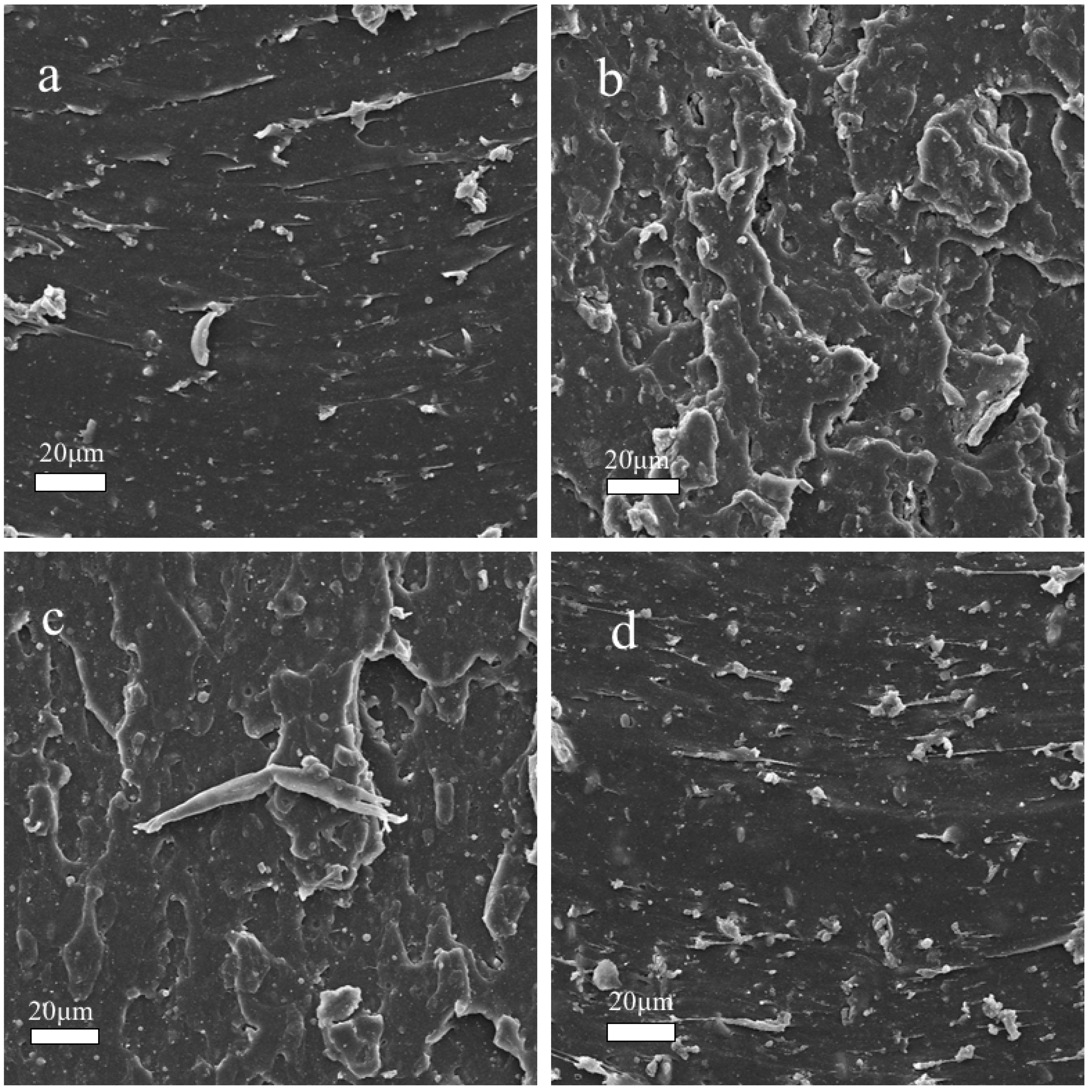
3.4. Microstructure Analyzes of before and after Aged NBR Composites
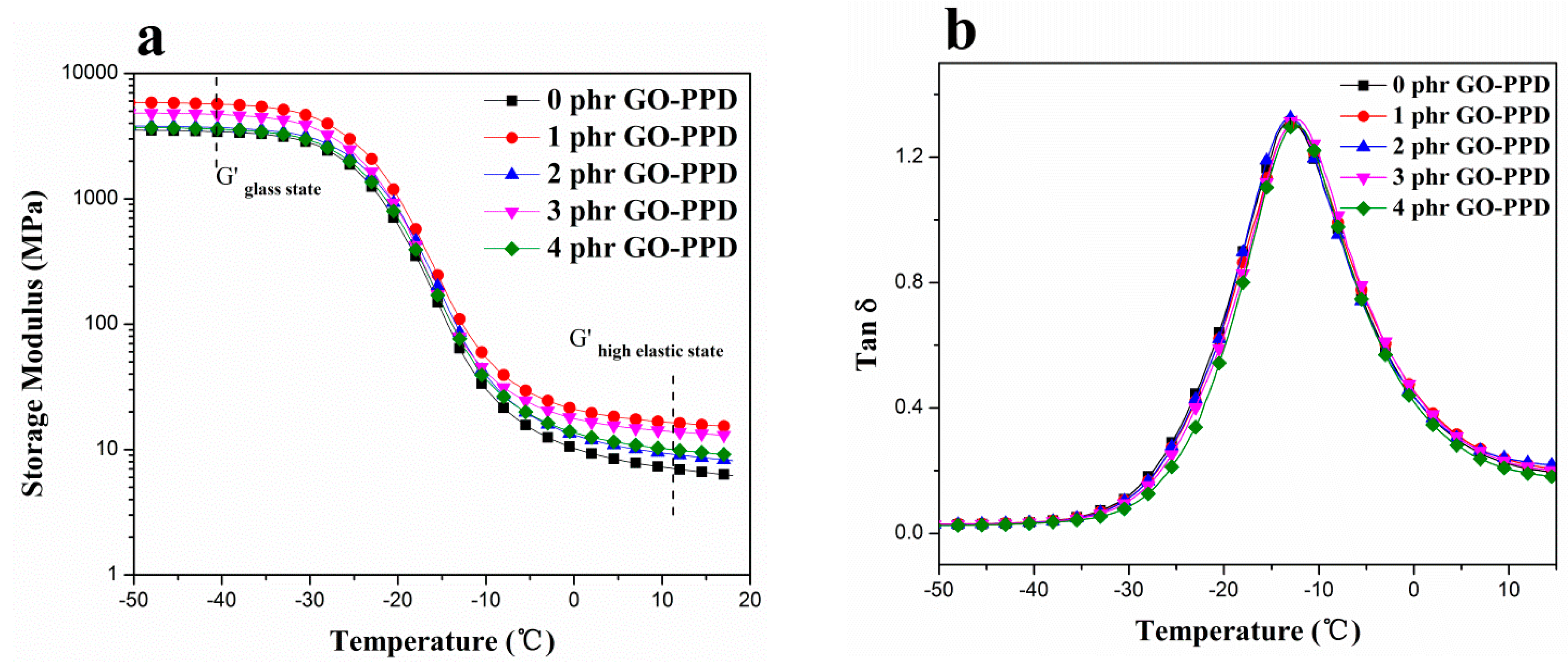
3.5. The Dynamic Mechanical of Different Contents of Composites
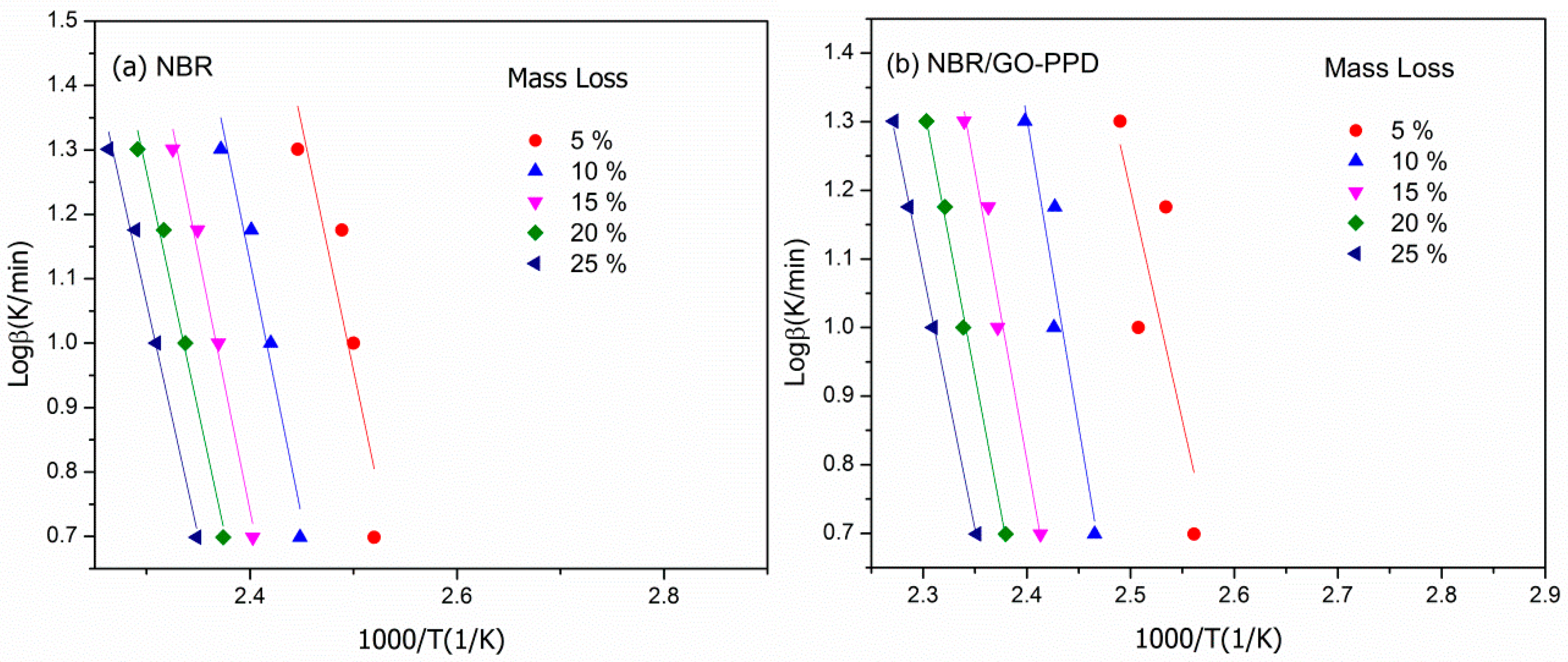
3.6. Degradation Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Romero-Flores, M.; Becerra-Lucatero, L.M.; Salmón-Folgueras, R.; Lopez-Salinas, J.L.; Bremer-Bremer, M.H.; Montesinos-Castellanos, A. Thermal performance of scrap tire blocks as roof insulator. Energy Build. 2017, 149, 384–390. [Google Scholar] [CrossRef]
- Hwang, S.J.; Park, J.H.; Son, J.H.; Choi, J.H.; Seo, H.; Park, M.; Kim, J.; Moon, G.D.; Hyun, D.C. Thermal annealing-driven surface sealing of polymeric bowl. Polymer 2017, 135, 338–347. [Google Scholar] [CrossRef]
- Irazu, L.; Elejabarrieta, M.J. A novel hybrid sandwich structure: Viscoelastic and eddy current damping. Mater. Des. 2018, 140, 460–472. [Google Scholar] [CrossRef]
- He, X.; Li, T.; Shi, Z.; Wang, X.; Xue, F.; Wu, Z.; Chen, Q. Thermo-oxidative aging behavior of nitrile-butadiene rubber/functional LDHs composites. Polym. Degrad. Stabil. 2016, 133, 219–226. [Google Scholar] [CrossRef]
- Akhlaghi, S.; Gedde, U.W.; Hedenqvist, M.S.; Brana, M.T.C.; Bellander, M. Deterioration of acrylonitrile butadiene rubber in rapeseed biodiesel. Polym. Degrad. Stab. 2015, 111, 211–222. [Google Scholar] [CrossRef]
- Akhlaghi, S.; Pourrahimi, A.M.; Hedenqvist, M.S.; Sjöstedt, C.; Bellander, M.; Gedde, U.W. Degradation of carbon-black-filled acrylonitrile butadiene rubber in alternative fuels: Transesterified and hydrotreated vegetable oils. Polym. Degrad. Stab. 2016, 123, 69–79. [Google Scholar] [CrossRef]
- Marković, G.; Marinović-Cincović, M.S.; Jovanović, V.; Samaržija-Jovanović, S.; Budinski-Simendić, J. Gamma irradiation aging of NBR/CSM rubber nanocomposites. Compos. Part B Eng. 2012, 43, 609–615. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, Y.; Zhang, P.; Cai, Z.; Li, Y.; Fan, H. Preparation of high performance NBR/HNTs nanocomposites using an electron transferring interaction method. Appl. Surf. Sci. 2017, 425, 758–764. [Google Scholar] [CrossRef]
- Essabir, H.; Raji, M.; Essassi, E.M.; Rodrigue, D.; Bouhfid, R.; el kacem Qaiss, A. Morphological, thermal, mechanical, electrical and magnetic properties of ABS/PA6/SBR blends with Fe3O4, nano-particles. J. Mater. Sci. Mater. Electron. 2017, 28, 17120–17130. [Google Scholar] [CrossRef]
- Jovanović, V.; Budinski-Simendić, J.; Milić, J.; Aroguz, A.; Ristić, I.; Prendzov, S.; Korugic-Karasz, L. The Effect of Filler Particles on the Properties of Elastomeric Materials Based on Different Network Precursors. Contemp. Sci. Polym. Mater. 2010, 1061, 167–193. [Google Scholar]
- Zhang, Z.; He, X.; Zhang, J.; Lu, X.; Yang, C.; Liu, T.; Wang, X.; Zhang, R. Influence of graphene/ferriferrous oxide hybrid particles on the properties of nitrile rubber. RSC Adv. 2016, 6, 91798–91805. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X.; Wang, X.; Rodrigues, A.M.; Zhang, R. Reinforcement of the mechanical properties in nitrile rubber by adding graphene oxide/silicon dioxide hybrid nanoparticles. J. Appl. Polym. Sci. 2018, 135, 46091–46099. [Google Scholar] [CrossRef]
- Aydemir, D.; Kiziltas, A.; Kiziltas, E.E.; Gardner, D.J.; Gunduz, G. Heat treated wood–nylon 6 composites. Compos. Part B Eng. 2015, 68, 414–423. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Yan, H.; Chevali, V.S.; Wang, H. Synergistic flame retardancy effect of graphene nanosheets and traditional retardants on epoxy resin. Compos. Part A Appl. Sci. Manuf. 2016, 89, 26–32. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Lai, X.; Wu, W.; Zeng, X. Hindered phenol functionalized graphene oxide for natural rubber. Mater. Lett. 2017, 210, 239–242. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, P.; Wang, J.; Qu, J.; Wang, L.; Wang, X.; Guan, C.; Pan, K. Membranes with Selective Laminar Nanochannels of Modified Reduced Graphene Oxide for Water Purification. Carbon 2016, 103, 94–100. [Google Scholar] [CrossRef]
- Jiang, P.; Yang, C.; He, X.; Rodrigues, A.M.; Zhang, R. Viscoelastic changes in chlorinated butyl rubber modified with graphene oxide. Iran. Polym. J. 2017, 26, 861–870. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Liang, B.; Liu, Y.; Xu, T.; Wang, L.; Cao, B.; Pan, K. Graphene oxide as effective barrier on a porous nanofibrous membrane for water treatment. ACS Appl. Mater. Interface 2016, 8, 6211–6218. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, X.; Jia, H.; Yin, Q.; Wang, J.; Yin, B.; Xu, Z. High mechanical properties, thermal conductivity and solvent resistance in graphene oxide/styrene-butadiene rubber nanocomposites by engineering carboxylated acrylonitrile-butadiene rubber. Compos. Part B Eng. 2017, 130, 257–266. [Google Scholar] [CrossRef]
- Bai, X.; Wan, C.; Zhang, Y.; Zhai, Y. Reinforcement of hydrogenated carboxylated nitrile-butadiene rubber with exfoliated graphene oxide. Carbon 2011, 49, 1608–1613. [Google Scholar] [CrossRef]
- Amani, J.; Maleki, M.; Khoshroo, A.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M. An electrochemical immunosensor based on poly p-phenylenediamine and graphene nanocomposite for detection of neuron-specific enolase via electrochemically amplified detection. Anal. Biochem. 2018, 548, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Elwakeel, K.Z.; Al-Bogami, A.S. Influence of Mo(VI) immobilization and temperature on As(V) sorption onto magnetic separable poly p-phenylenediamine-thiourea-formaldehyde condensate. J. Hazard. Mater. 2017, 342, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Hashim, A.S.; Kohjiya, S. Curing of epoxidized natural rubber with p-phenylenediamine. J. Polym. Sci. Pol. Chem. 2010, 32, 1149–1157. [Google Scholar] [CrossRef]
- Li, G.Y.; Koenig, J.L. FTIR imaging of oxidation of polyisoprene 2. The role of N-phenyl-N′-dimethyl-butyl-p-phenylenediamine antioxidant. Polym. Degrad. Stab. 2003, 81, 377–385. [Google Scholar] [CrossRef]
- Boochathum, P.; Prajudtake, W. Vulcanization of cis- and trans-polyisoprene and their blends: Cure characteristics and crosslink distribution. Eur. Polym. J. 2001, 37, 417–427. [Google Scholar] [CrossRef]
- Varenik, M.; Nadiv, R.; Levy, I.; Vasilyev, G.; Regev, O. Breaking Through the Solid/Liquid Processability Barrier: Thermal Conductivity and Rheology in Hybrid Graphene-Graphite Polymer Composites. ACS Appl. Mater. Interface 2017, 9, 7556–7564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Y.; Hu, X.; Feng, A.; Jiang, F.; Song, L. p-Phenylenediamine strengthened graphene oxide for the fabrication of superhydrophobic surface. Mater. Des. 2017, 127, 22–29. [Google Scholar] [CrossRef]
- Hussein, A.; Sarkar, S.; Lee, K.; Kim, B. Cryogenic fracture behavior of epoxy reinforced by a novel graphene oxide/poly(p-phenylenediamine) hybrid. Compos. Part B Eng. 2017, 129, 133–142. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y.; Li, D.; Yang, H.; Zhang, X.; Lin, B. Novel ternary composites reduced-graphene oxide/zine oxide/poly(p-phenylenediamine) for supercapacitor: Synthesis and properties. J. Alloys Compd. 2017, 708, 787–795. [Google Scholar] [CrossRef]
- Lu, X.; Li, L.; Song, B.; Moon, K.S.; Hu, N.; Liao, G.; Shi, T.; Wong, C. Mechanistic investigation of the graphene functionalization using p-phenylenediamine and its application for supercapacitors. Nano Energy 2015, 17, 160–170. [Google Scholar] [CrossRef]
- Dong, C.L.; Yuan, C.Q.; Bai, X.Q.; Yan, X.P.; Peng, Z. Tribological properties of aged nitrile butadiene rubber under dry sliding conditions. Wear 2015, 322, 226–237. [Google Scholar] [CrossRef]
- Xie, C.; Jia, Z.; Jia, D.; Luo, Y.; You, C. The Effect of Dy(III) Complex with 2-Mercaptobenzimidazole on the Thermo-Oxidation Aging Behavior of Natural Rubber Vulcanizates. Int. J. Polym. Mater. Polym. Biomater. 2010, 59, 663–679. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.R.; Rodrigues, A.M.; Guo, Q.P. Softening dynamics of polymer blends and composites investigated by differentia spectra of dynamic mechanical analysis. Adv. Polym. Technol. 2017. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Lai, Z.; Yang, D. Effect of some inorganic particles on the softening dispersion of the dynamics of butyl rubber. Polym. Bull. 2017, 74, 1031–1043. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.R.; Yu, H. Why tanδ of poly (butyl acrylate) and poly (ethyl acrylate) with little double Bounds are becoming asymmetric? Polymer 2014, 55, 4720–4727. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Yu, H.; Chen, K. Detecting the Rouse and sub-Rouse modes in poly (butyl acrylate) and poly (ethyl acrylate) through two-dimensional dynamic mechanical spectra. J. Polym. Sci. Pol. Phys. 2014, 53, 1642–1653. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.R. Crystallization and molecular dynamics of ethylene-vinyl acetate copolymer/butyl rubber blends. RSC Adv. 2015, 5, 130–135. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Yang, D.; Lai, Z. Non-isothermal crystallization kinetics and segmental dynamics of high density polyethylene/butyl rubber blends. Polym. Int. 2015, 64, 1252–1261. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.; Huang, G. A review of the slow relaxation processes in the glass–rubber transition region of amorphous polymers. Phase Transit. 2015, 88, 843–858. [Google Scholar] [CrossRef]
- Zhang, R.; He, X.R.; Huang, G.S. Dynamics of Poly (butyl acrylate) and Poly (ethyl acrylate) with internal double bonds. J. Polym. Res. 2014, 21, 388. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, L.; Zhao, X.; He, J.; Wang, A.; Chan, T.W.; Wu, S. Data for effects of lanthanum complex on the thermo-oxidative aging of natural rubber. Data Brief 2015, 120, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, A.; Zhou, T.; Cao, X.; Xie, Z. A study on thermal oxidation mechanism of styrene–butadiene–styrene block copolymer (SBS). Polym. Degrad. Stabil. 2007, 92, 1682–1691. [Google Scholar] [CrossRef]
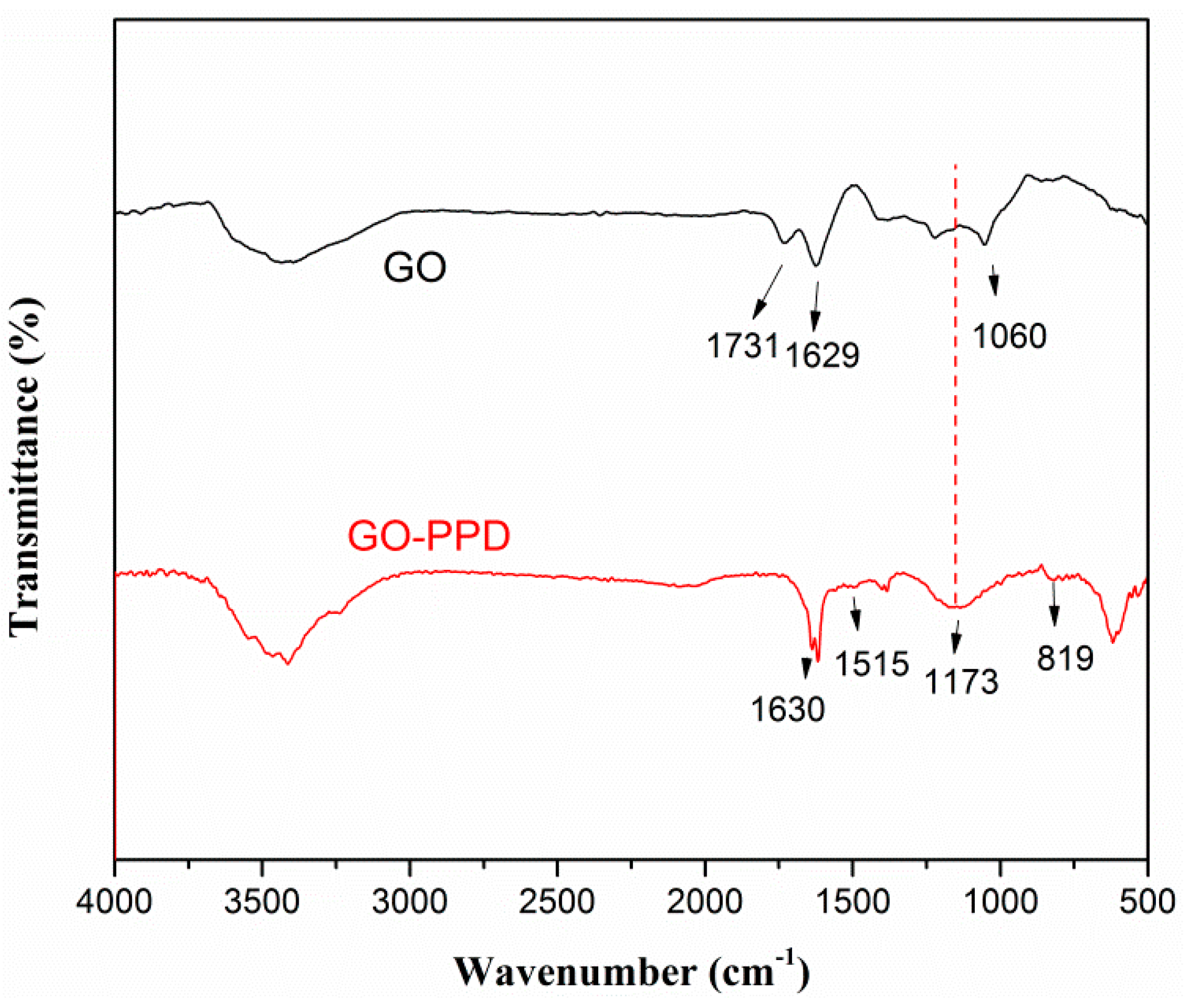

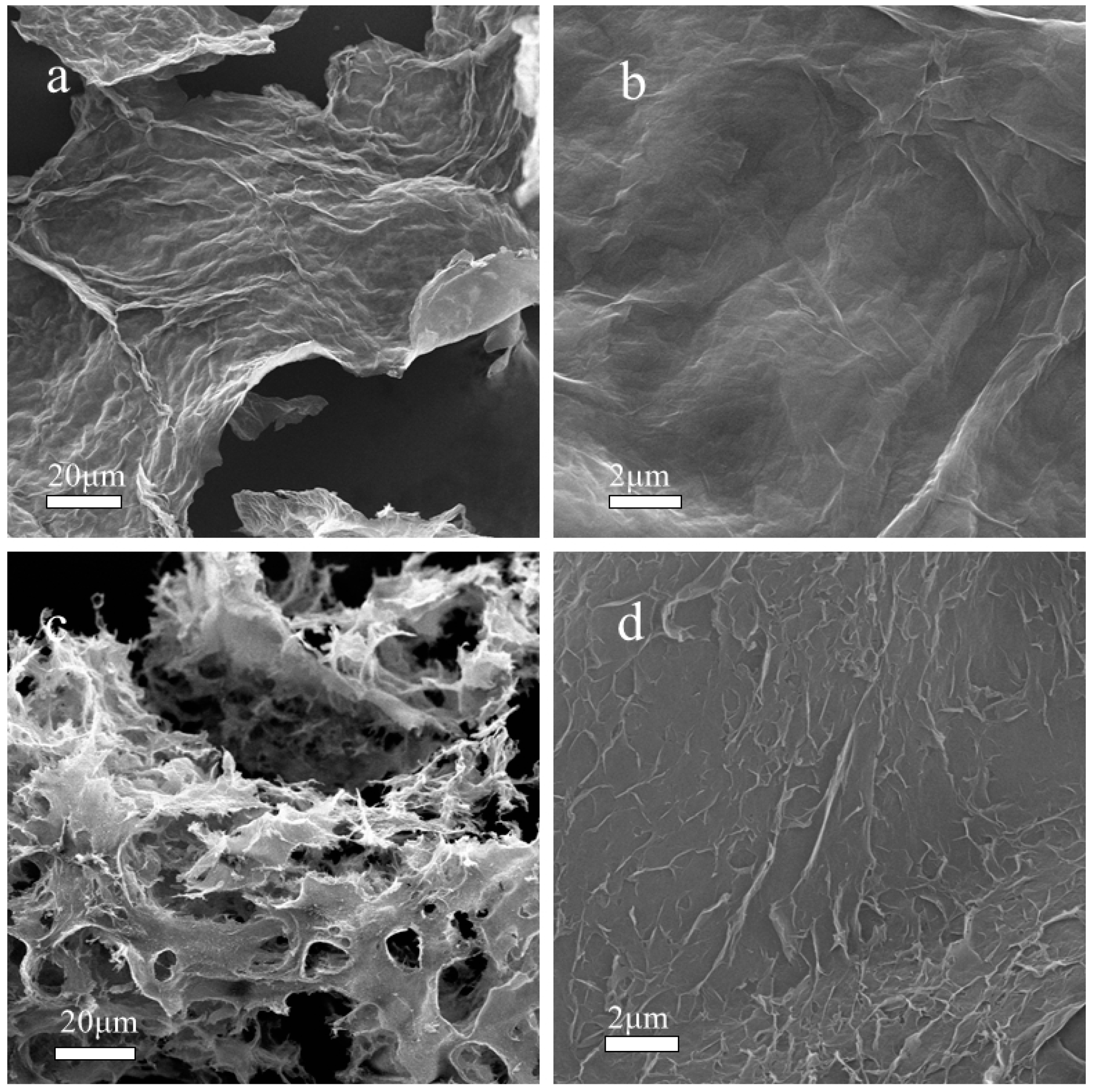
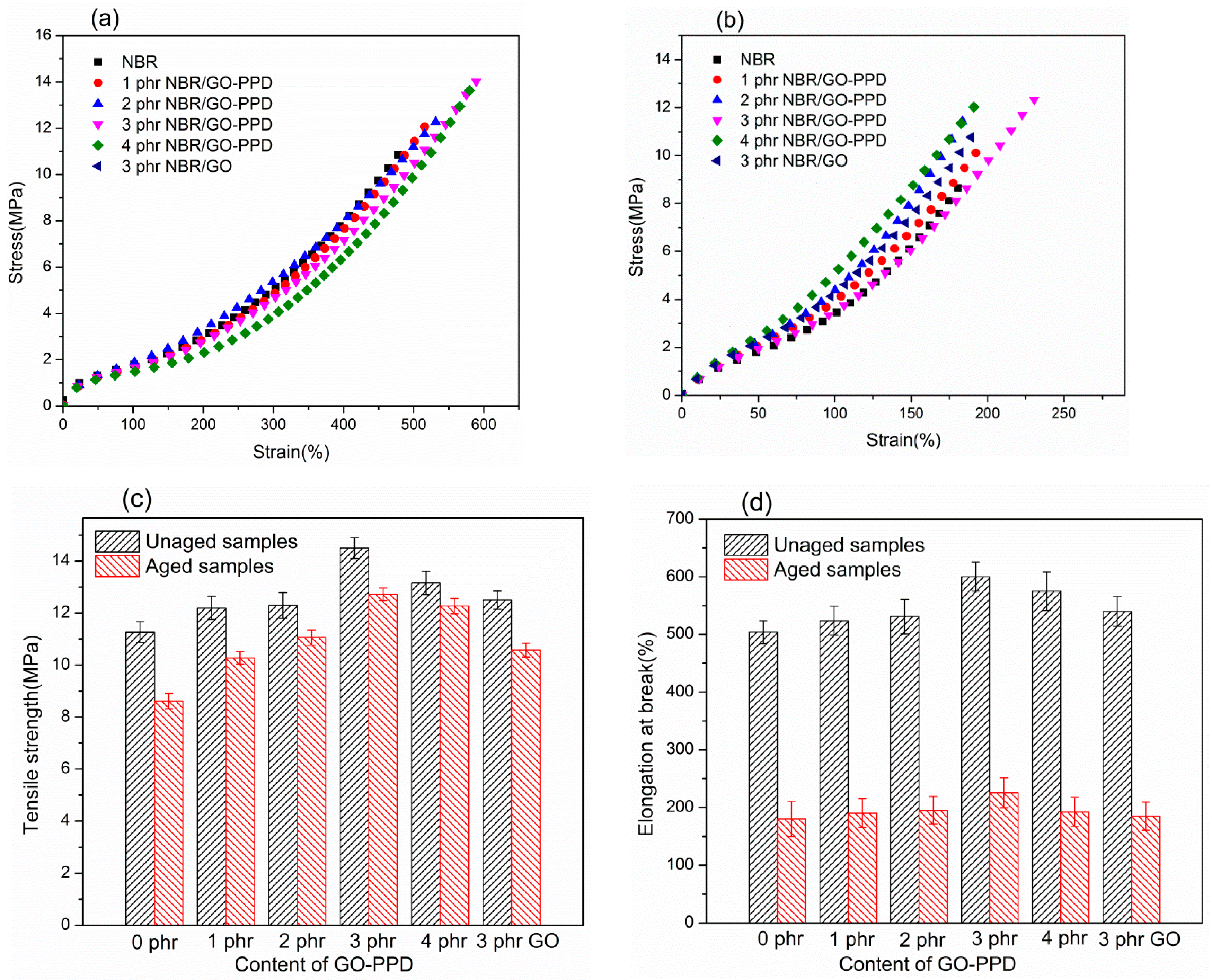
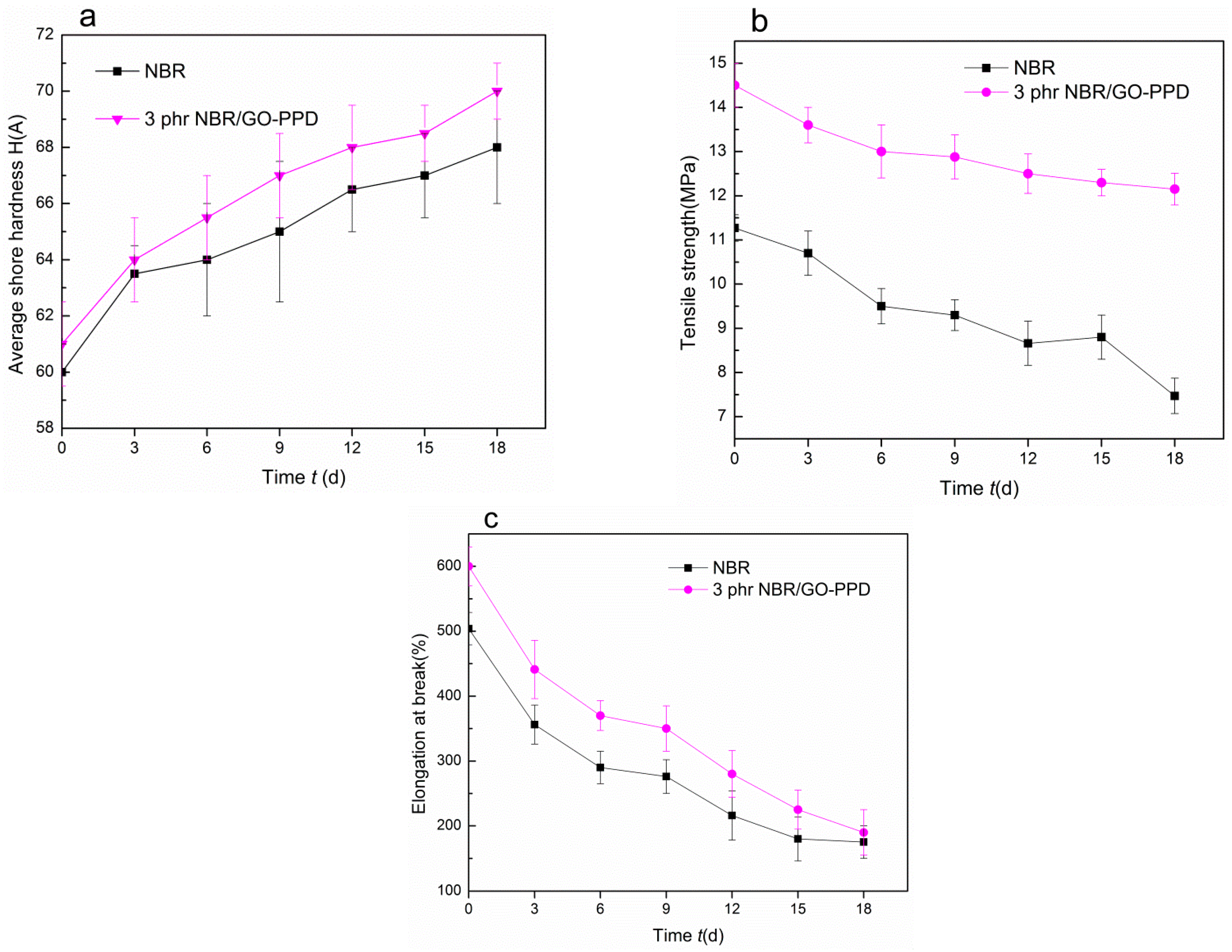


| Material | phr/NBR | phr/NBR/GO-PPD | phr/NBR/GO |
|---|---|---|---|
| NBR | 100 | 100 | 100 |
| graphene oxide–p-phenylenediamine (GO–PPD) | 0 | 1/2/3/4 | 0 |
| GO | 0 | 0 | 3 |
| zinc oxide | 5 | 5 | 5 |
| stearic acid | 1 | 1 | 1 |
| carbon black 234 | 20 | 20 | 20 |
| sulfur | 1.5 | 1.5 | 1.5 |
| accelerator 2,2′-dithiobis (benzothiazole) | 1.5 | 1.5 | 1.5 |
| dioctyl phthalate (DOP) | 1.5 | 1.5 | 1.5 |
| Content of Filler | GO-PPD | GO-PPD | GO-PPD | GO-PPD | GO-PPD | GO |
|---|---|---|---|---|---|---|
| 0 phr | 1 phr | 2 phr | 3 phr | 4 phr | 3 phr | |
| Before aging | 11.27 | 12.2 | 12.3 | 14.5 | 13.16 | 12.5 |
| After aging for 15 days | 8.8 | 10.5 | 11.3 | 13 | 12.54 | 10.8 |
| Retention ratio | 78.1% | 86.1% | 91.9% | 89.7% | 95.3% | 86.4% |
| Content of Filler | GO-PPD | GO-PPD | GO-PPD | GO-PPD | GO-PPD | GO |
|---|---|---|---|---|---|---|
| 0 phr | 1 phr | 2 phr | 3 phr | 4 phr | 3 phr | |
| Before aging | 504 | 524 | 531 | 600 | 575 | 540 |
| After aging for 15 days | 180 | 190 | 191 | 225 | 192 | 186 |
| The Contents of Filler | 0 phr | 1 phr | 2 phr | 3 phr | 4 phr |
|---|---|---|---|---|---|
| G′(MPa) of 10 °C | 7.18 | 16.54 | 9.3 | 14.02 | 10.11 |
| Area (tan δ) | 15.4 | 13.5 | 14.6 | 13.1 | 14.3 |
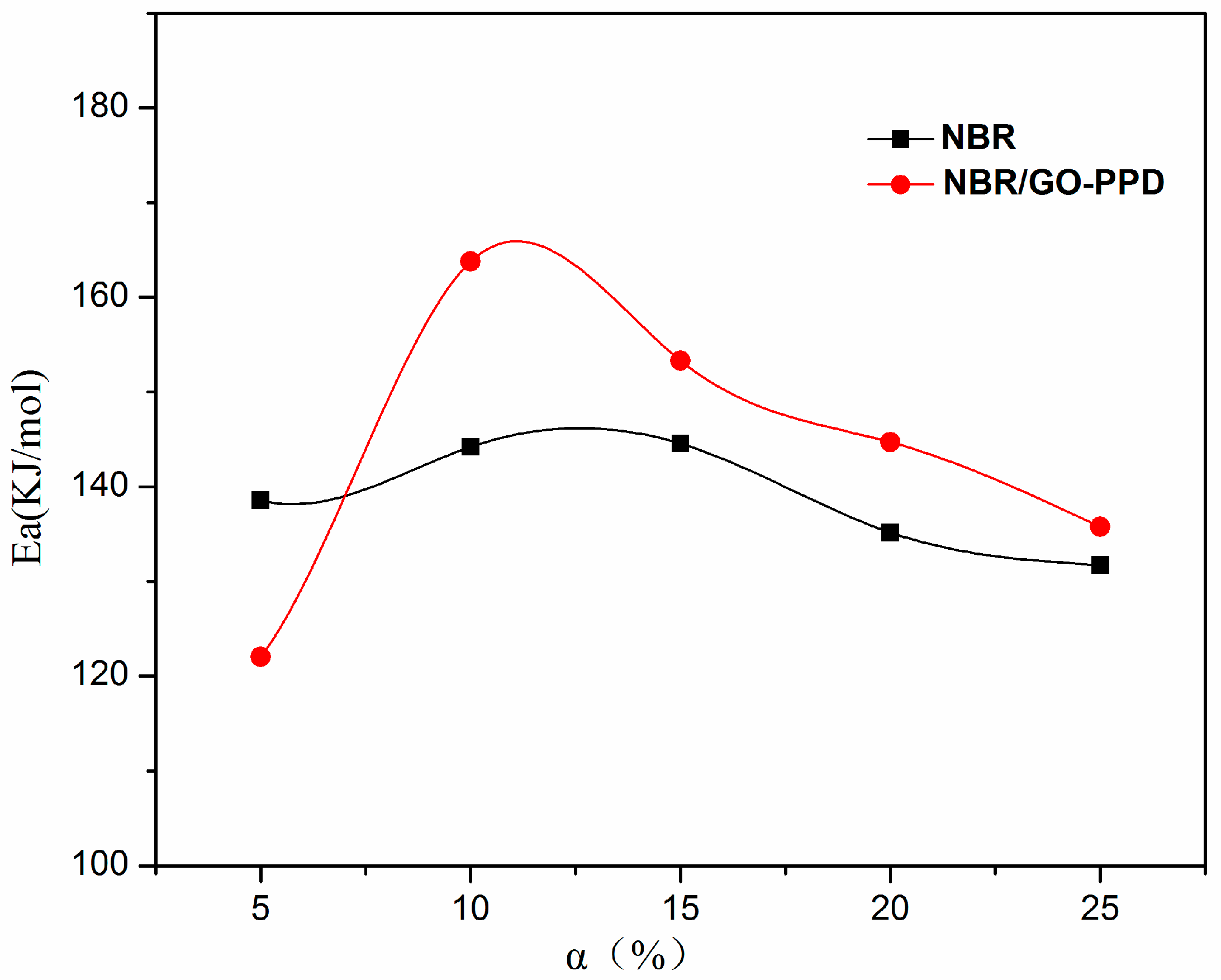
| The Mass Loss (%) | 5 | 10 | 15 | 20 | 25 |
|---|---|---|---|---|---|
| The Ea value of NBR (KJ/mol) | 138 | 145 | 143 | 135 | 131 |
| The Ea value of NBR/GO–PPD (KJ/mol) | 120 | 163 | 153 | 144 | 136 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, R.; Zhang, Z.; Zhao, H.; He, X.; Wang, X.; Zhang, R. Improving Thermo-Oxidative Stability of Nitrile Rubber Composites by Functional Graphene Oxide. Materials 2018, 11, 921. https://doi.org/10.3390/ma11060921
Zhong R, Zhang Z, Zhao H, He X, Wang X, Zhang R. Improving Thermo-Oxidative Stability of Nitrile Rubber Composites by Functional Graphene Oxide. Materials. 2018; 11(6):921. https://doi.org/10.3390/ma11060921
Chicago/Turabian StyleZhong, Rui, Zhao Zhang, Hongguo Zhao, Xianru He, Xin Wang, and Rui Zhang. 2018. "Improving Thermo-Oxidative Stability of Nitrile Rubber Composites by Functional Graphene Oxide" Materials 11, no. 6: 921. https://doi.org/10.3390/ma11060921
APA StyleZhong, R., Zhang, Z., Zhao, H., He, X., Wang, X., & Zhang, R. (2018). Improving Thermo-Oxidative Stability of Nitrile Rubber Composites by Functional Graphene Oxide. Materials, 11(6), 921. https://doi.org/10.3390/ma11060921





