Coextrusion-Based 3D Plotting of Ceramic Pastes for Porous Calcium Phosphate Scaffolds Comprised of Hollow Filaments
Abstract
:1. Introduction
2. Materials and Methods
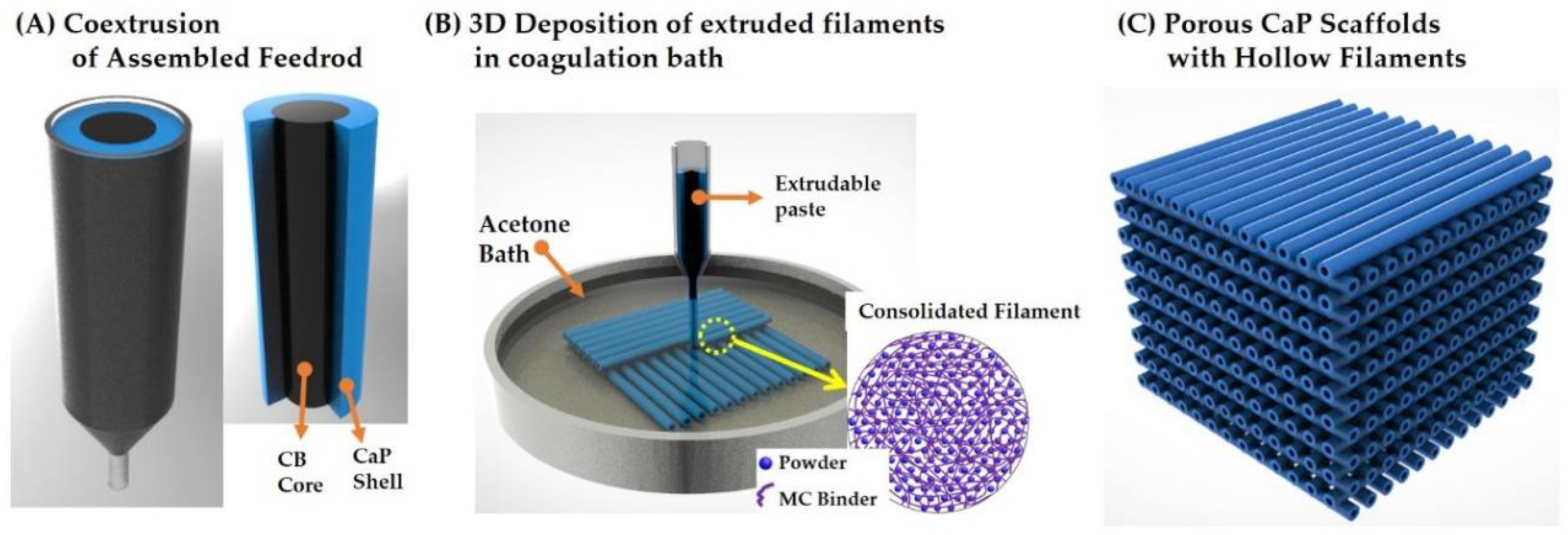
2.1. Principle of CoEx-3DP Technique
2.2. Starting Materials
2.3. CaP and CB Pastes Preparation
2.4. Initial Feedrod Preparation
2.5. Porous Green Scaffolds Production
2.6. Porous Structure, Microstructure, and Crystalline Structure Characterization
2.7. Compressive Strength Tests
2.8. In Vitro Biocompatibility Tests
2.9. Polymer-Infiltrated Scaffold Production
2.10. Statistical Analysis
3. Results and Discussion
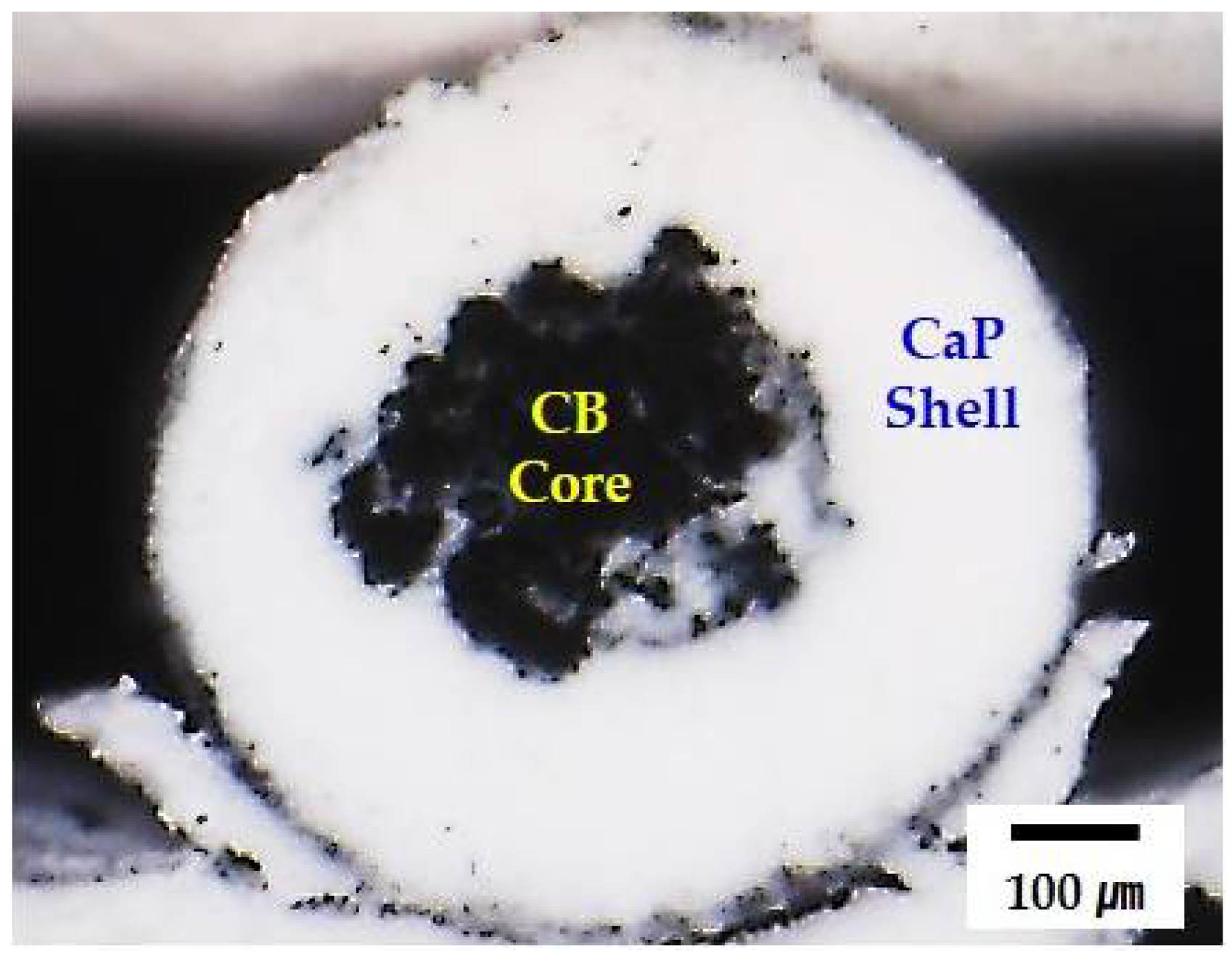
3.1. Designing of Feedrod with Core/Shell Structure for Coextrusion
3.2. Geometry and Microstructure of Green Filaments
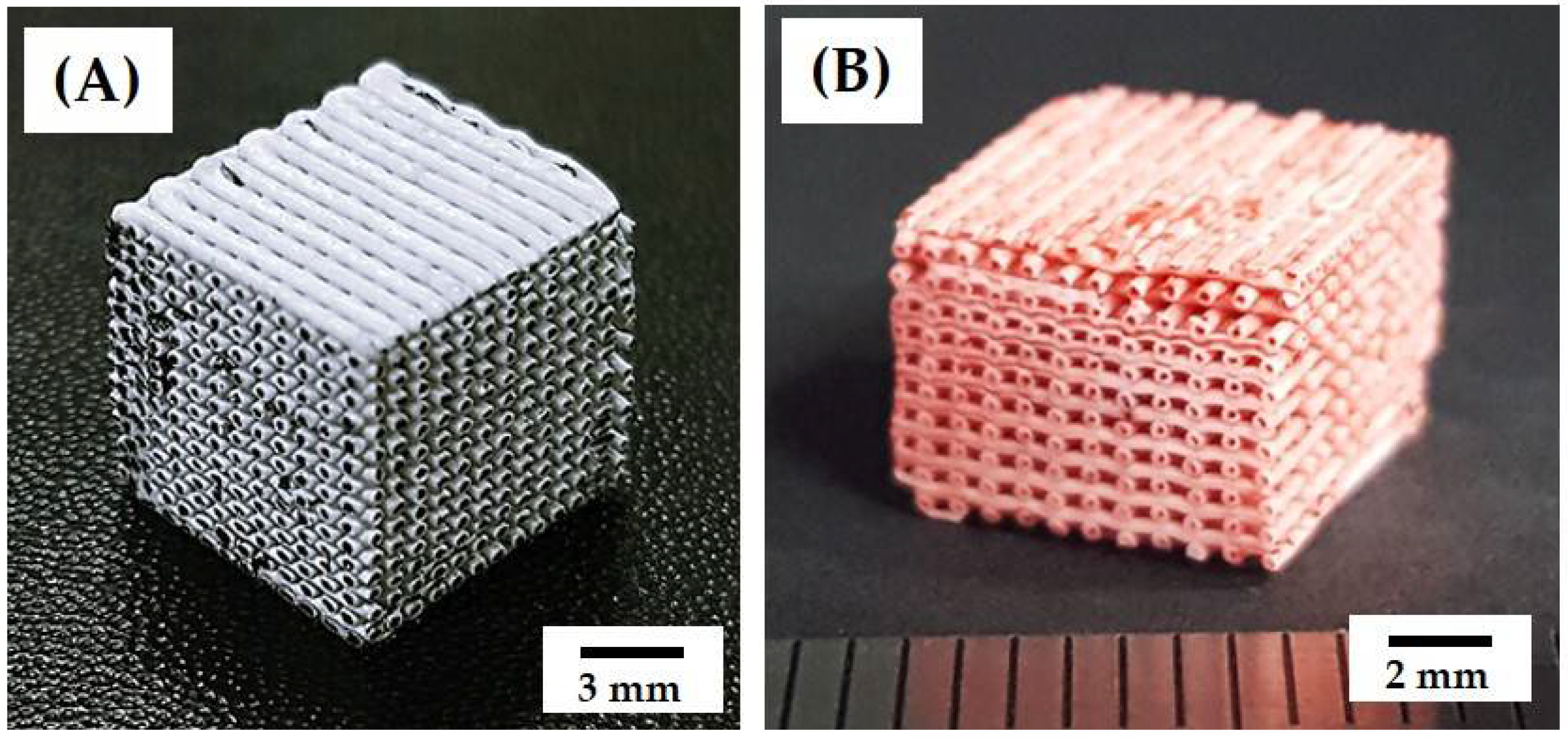
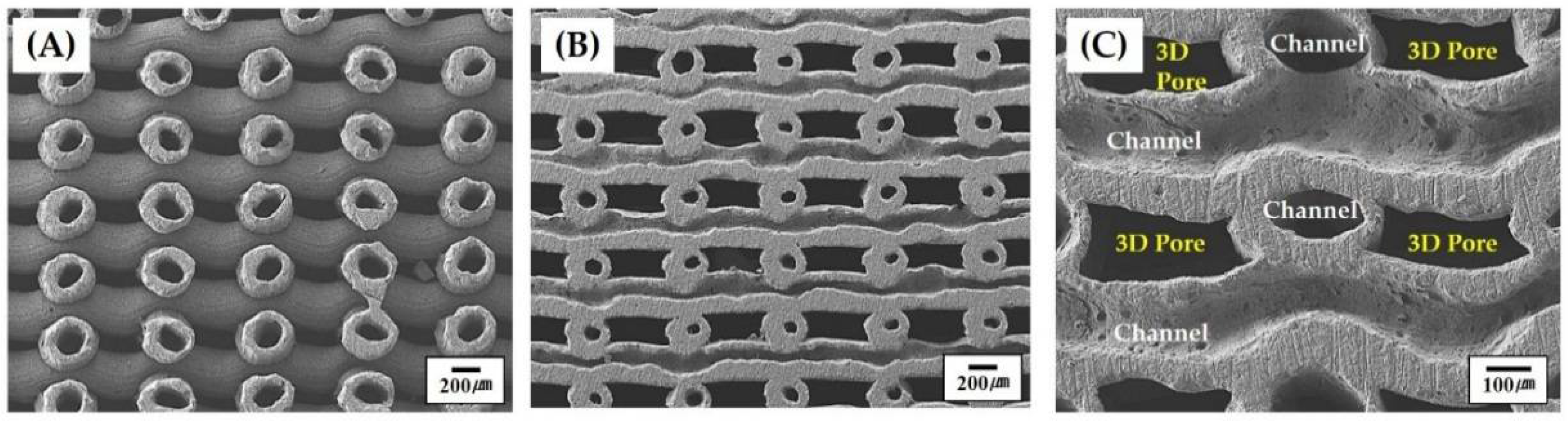
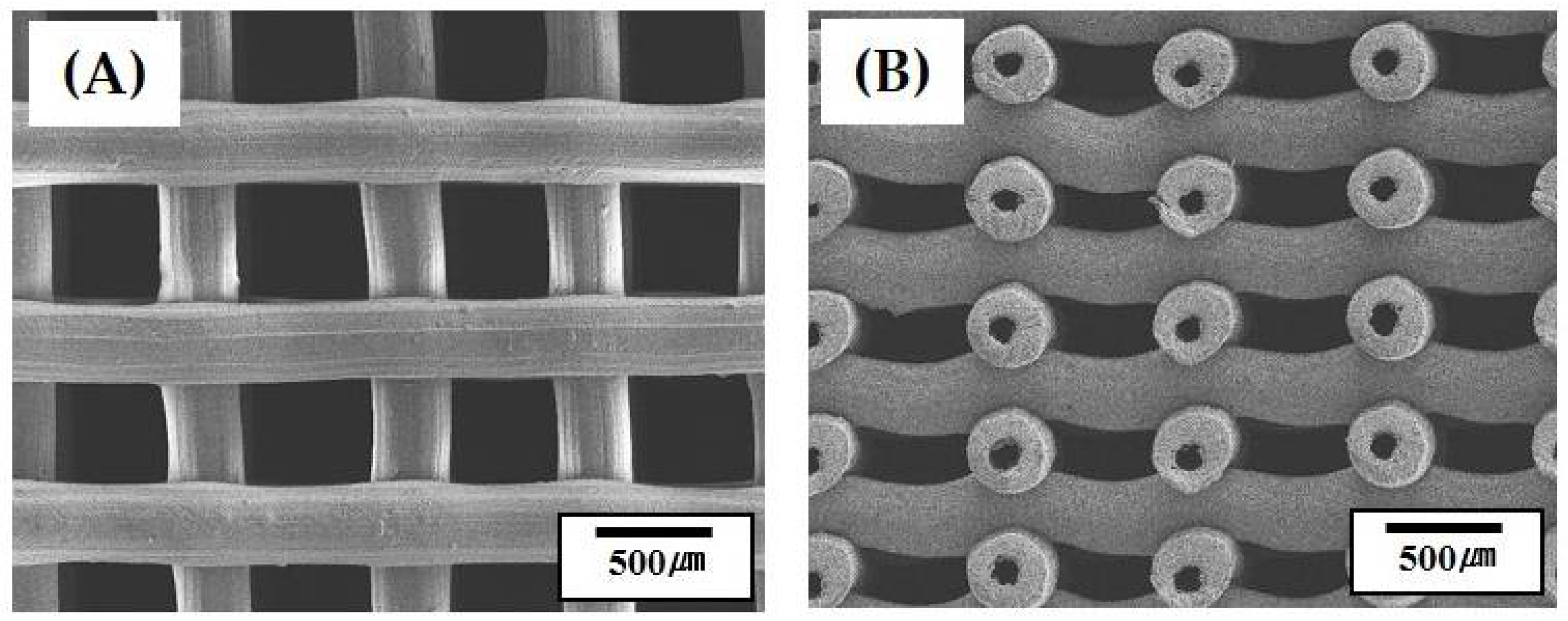
3.3. 3D Porous Structure of Green Scaffolds
3.4. 3D Porous Structure of Porous Scaffolds with Hollow Filaments
3.5. Overall Porosity and Fraction of 3D Pores
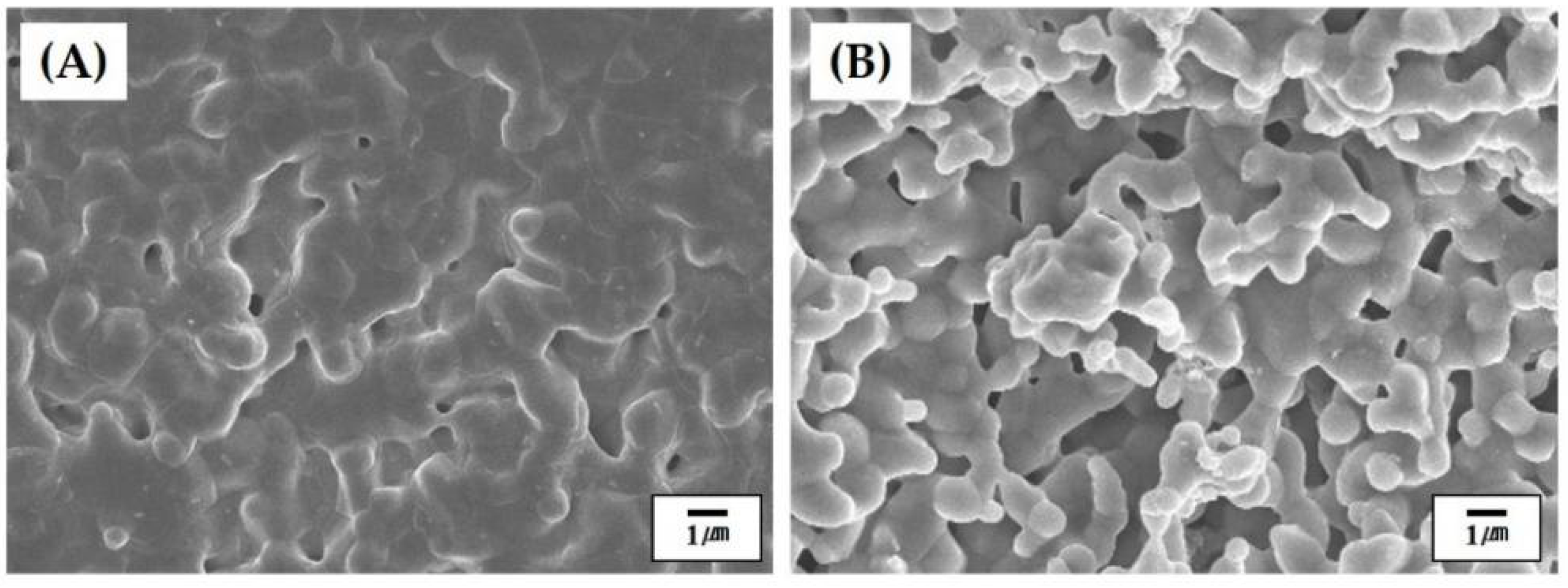
3.6. Microstructure of Hollow CaP Filaments
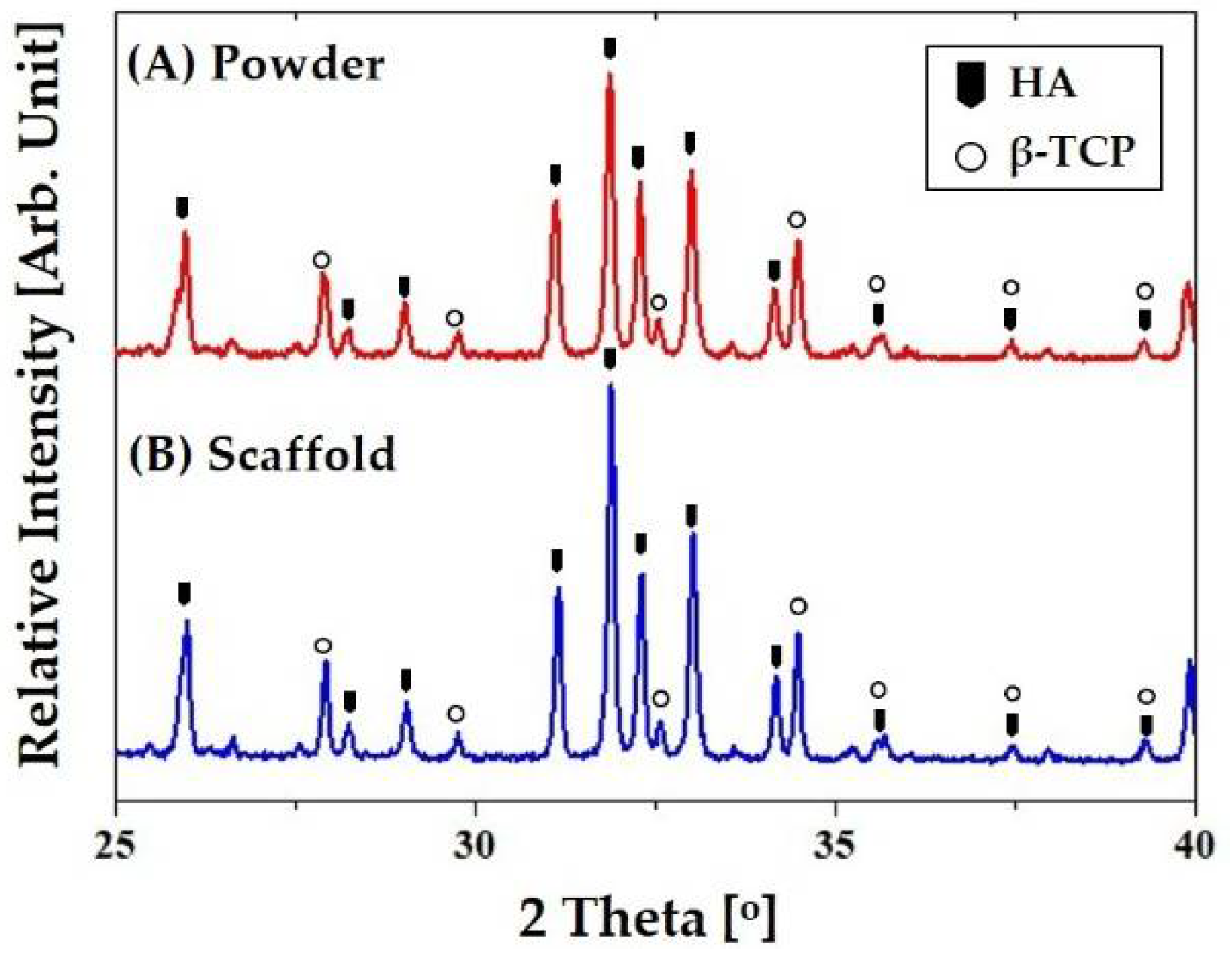
3.7. Crystalline Phases of CaP Scaffold
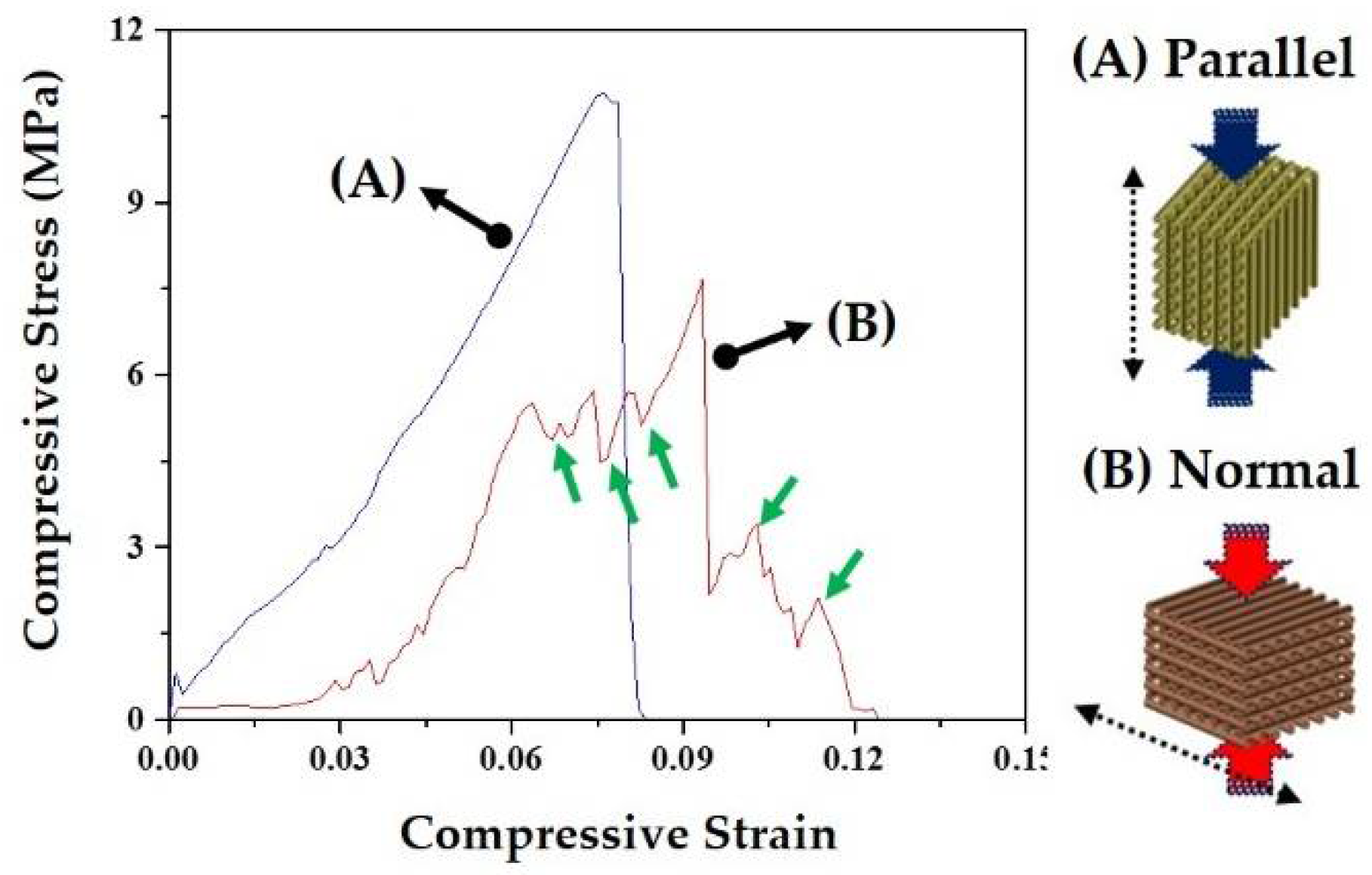
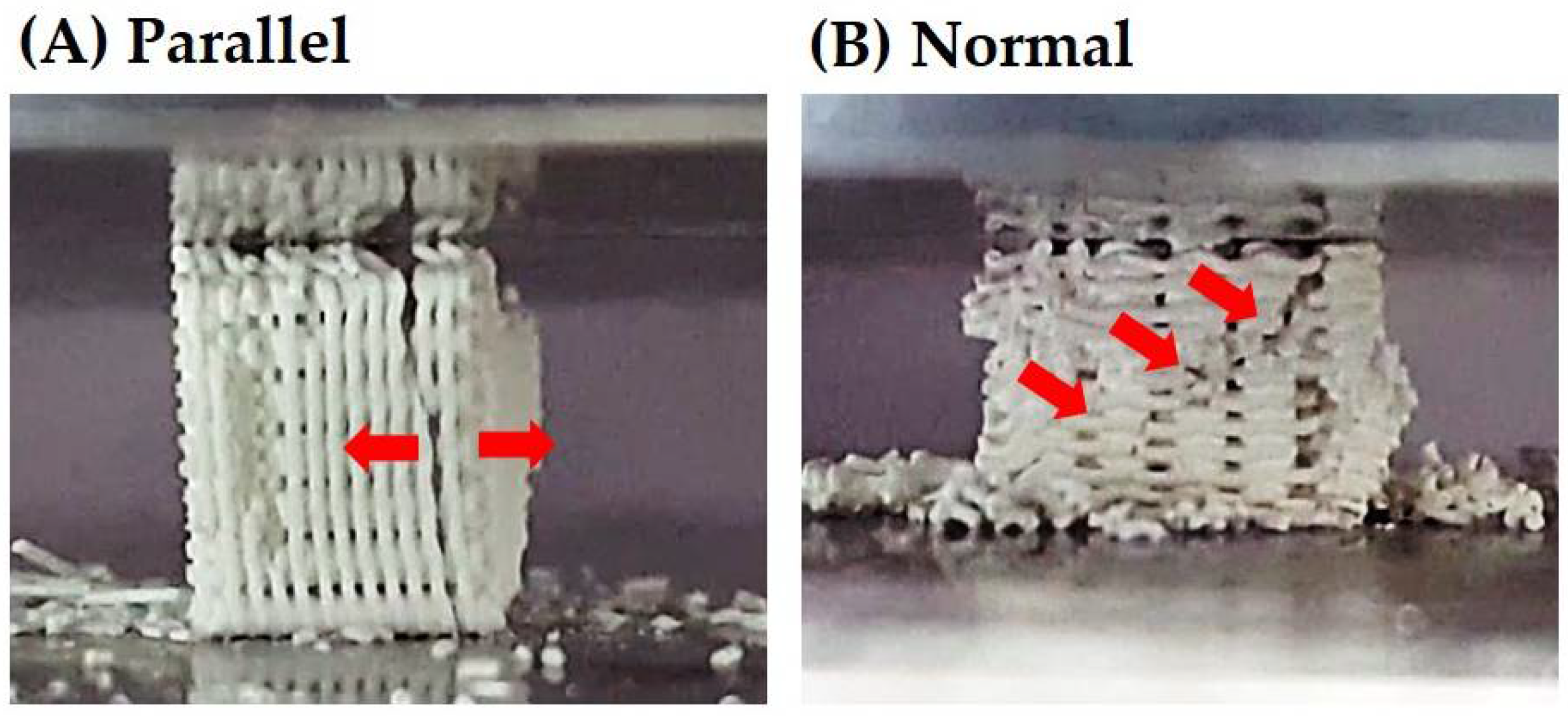
3.8. Mechanical Properties of CaP Scaffold
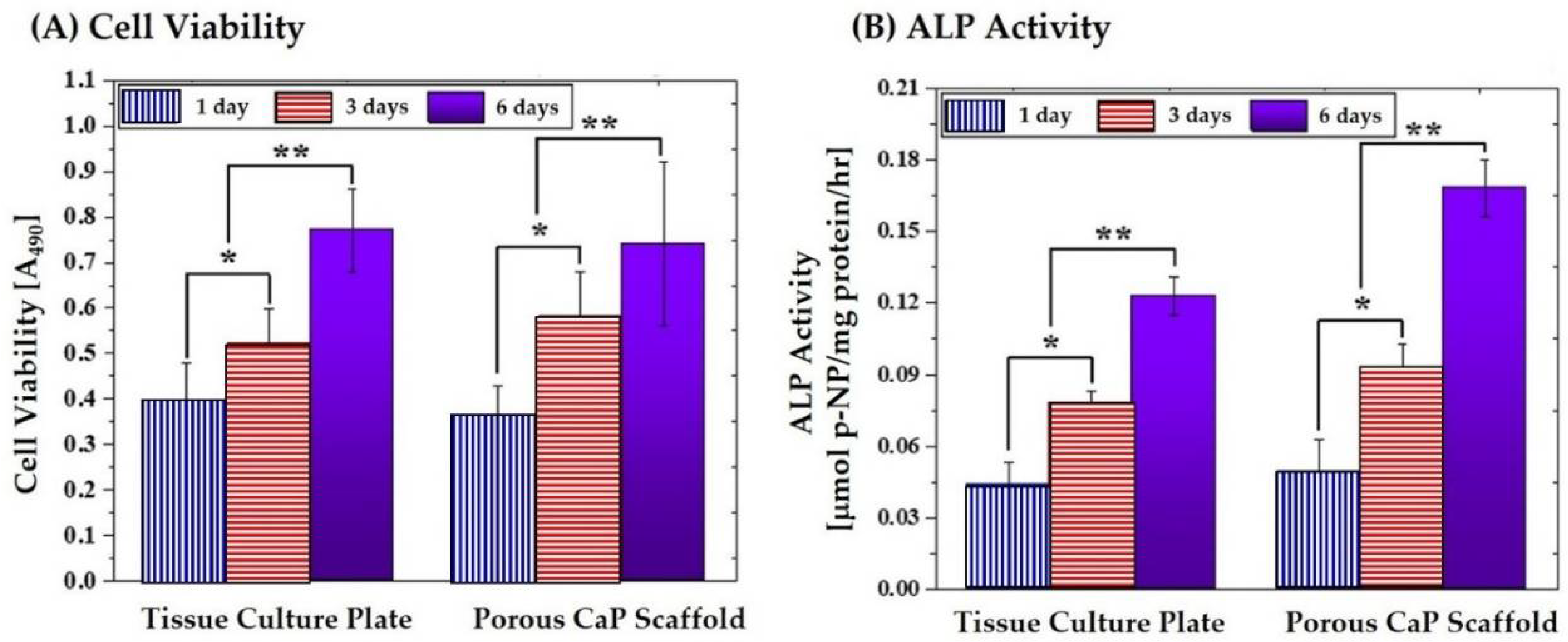
3.9. In Vitro Biocompatibility of Porous Scaffold
3.10. Potential as Framework for Porous Ceramic/Polymer Composite Scaffolds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dorozhkin, S.V. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomater. 2012, 8, 963–977. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.; McCarthy, H.O.; Montufar, E.B.; Ginebra, M.P.; Wilson, D.I.; Lennon, A.; Dunne, N. Critical review: Injectability of calcium phosphate pastes and cements. Acta Biomater. 2017, 50, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wilcock, C.J.; Stafford, G.P.; Miller, C.A.; Ryabenkova, Y.; Fatima, M.; Gentile, P.; Möbus, G.; Hatton, P.V. Preparation and antibacterial properties of silver-doped nanoscale hydroxyapatite pastes for bone repair and augmentation. J. Biomed. Nanotechnol. 2017, 13, 1168–1176. [Google Scholar] [CrossRef]
- Lew, K.S.; Othman, R.; Ishikawa, K.; Yeoh, F.Y. Macroporous bioceramics: A remarkable material for bone regeneration. J. Biomater. Appl. 2012, 27, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.A. Bioceramic Bone graft substitutes: Influence of porosity and chemistry. Int. J. Appl. Ceram. Technol. 2005, 2, 184–199. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Butscher, A.; Bohner, M.; Hofmann, S.; Gauckler, L.; Muller, R. Structural and material approaches to bone tissue engineering in powder-based tree-dimensional printing. Acta Biomater. 2011, 7, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Travitzky, N.; Bonet, A.; Dermeik, B.; Fey, T.; Filbert-Demut, I.; Schlier, L.; Schlordt, T.; Greil, P. Additive manufacturing of ceramic-based materials. Adv. Eng. Mater. 2014, 16, 729–754. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Li, S.J.; Wang, H.L.; Hou, W.T.; Hao, Y.L.; Yang, R.; Sercombe, T.B.; Zhang, L.C. Microstructure, defects and mechanical behavior of beta-type titanium porous structures manufactured by electron beam melting and selective laser melting. Acta Mater. 2016, 113, 56–67. [Google Scholar] [CrossRef]
- Zhang, L.C.; Attar, H. Selective laser melting of titanium alloys and titanium matrix composites for biomedical applications: A review. Adv. Eng. Mater. 2016, 18, 463–475. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, H.L.; Li, S.J.; Wang, S.G.; Wang, W.J.; Hou, W.T.; Hao, Y.L.; Yang, R.; Zhang, L.C. Compressive and fatigue behavior of beta-type titanium porous structures fabricated by electron beam melting. Acta Mater. 2017, 126, 58–66. [Google Scholar] [CrossRef]
- Qiang, F.; Eduardo, S.; Tomsia, A.P. Bioinspired strong and highly porous glass scaffolds. Adv. Funct. Mater. 2011, 21, 1058–1063. [Google Scholar]
- Huang, T.S.; Rahaman, M.N.; Doiphode, N.D.; Leu, M.C.; Bal, B.S.; Day, D.E.; Liu, X. Porous and strong bioactive glass (13–93) scaffolds fabricated by freeze extrusion technique. Mater. Sci. Eng. C 2011, 31, 1482–1489. [Google Scholar] [CrossRef]
- Chu, T.M.; Orton, D.G.; Hollister, S.J.; Feinberg, S.E.; Halloran, J.W. Mechanical and in vivo performance of hydroxyapatite implants with controlled architectures. Biomaterials 2002, 23, 1283–1293. [Google Scholar] [CrossRef]
- Abarrategi, A.; Moreno-Vicente, C.; Martínez-Vázquez, F.J.; Civantos, A.; Ramos, V.; Vicente Sanz-Casado, J.; Martínez-Corria, R.; Perera, F.H.; Mulero, F.; Miranda, P.; López-Lacomba, J.L. Biological properties of solid free form designed ceramic scaffolds with BMP-2: In vitro and in vivo evaluation. PLoS ONE 2012, 7, e34117. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Rahaman, M.N.; Hilmas, G.E.; Bal, B.S. Mechanical properties of bioactive glass (13–93) scaffolds fabricated by robotic deposition for structural bone repair. Acta. Biomater. 2013, 9, 7025–7034. [Google Scholar] [CrossRef] [PubMed]
- Michna, S.; Willie, W.; Lewis, J.A. Concentrated hydroxyapatite inks for direct-write assembly of 3-D periodic scaffolds. Biomaterials 2005, 26, 5632–5639. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Saiz, E.; Tomsia, A.P. Direct ink writing of highly porous and strong glass scaffolds for load-bearing bone defects repair and regeneration. Acta. Biomater. 2011, 7, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Schlordt, T.; Schwanke, S.; Keppner, F.; Fey, T.; Travitzky, N.; Greil, P. Robocasting of alumina hollow filament lattice structures. J. Eur. Ceram. Soc. 2013, 33, 3243–3248. [Google Scholar] [CrossRef]
- Martínez-Vázquez, F.; Pajares, A.; Guiberteau, F.; Miranda, P. Effect of polymer infiltration on the flexural behavior of β-tricalcium phosphate robocast scaffolds. Materials 2014, 7, 4001–4018. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, F.C.G.; Evans, J.R.G. Sintered hydroxyapatite latticework for bone substitute. J. Am. Ceram. Soc. 2003, 86, 517–519. [Google Scholar] [CrossRef]
- Grida, I.; Evans, J.R.G. Extrusion freeforming of ceramics through fine nozzles. J. Eur. Ceram. Soc. 2003, 23, 629–635. [Google Scholar] [CrossRef]
- Yang, S.; Yang, H.; Chi, X.; Evans, J.R.G.; Thompson, I.; Cook, R.J.; Robinson, P. Rapid prototyping of ceramic lattices for hard tissue scaffolds. Mater. Des. 2008, 29, 1802–1809. [Google Scholar] [CrossRef]
- Doiphode, N.D.; Huang, T.S.; Leu, M.C.; Rahaman, M.N.; Day, D.E. Freeze extrusion fabrication of 13-93 bioactive glass scaffolds for bone repair. J. Mater. Sci. Mater. Med. 2011, 22, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Jo, I.H.; Ahn, M.K.; Moon, Y.W.; Koh, Y.H.; Kim, H.E. Novel rapid direct deposition of ceramic paste for porous biphasic calcium phosphate (BCP) scaffolds with tightly controlled 3-d macrochannels. Ceram. Int. 2014, 40, 11079–11084. [Google Scholar] [CrossRef]
- Moon, Y.W.; Shin, K.H.; Koh, Y.H.; Jung, H.D.; Kim, H.E. Three-dimensional ceramic/camphene-based co-extrusion for unidirectionally macrochanneled alumina ceramics with controlled porous walls. J. Am. Ceram. Soc. 2014, 97, 32–34. [Google Scholar] [CrossRef]
- Moon, Y.W.; Shin, K.H.; Koh, Y.H.; Jung, H.D.; Kim, H.E. Macroporous alumina scaffolds consisting of highly microporous hollow filaments using three-dimensional ceramic/camphene-based co-extrusion. J. Eur. Ceram. Soc. 2015, 35, 4623–4627. [Google Scholar] [CrossRef]
- Bian, P.W.; Li, D.; Lian, Q.; Zhang, W.; Zhu, L.; Li, X.; Jin, Z. Design and fabrication of a novel porous implant with pre-set channels based on ceramic stereolithography for vascular implantation. Biofabrication 2011, 3, 034103. [Google Scholar] [CrossRef] [PubMed]
- Tesavibul, P.; Felzmann, R.; Gruber, S.; Liska, R.; Thompson, I.; Boccaccini, A.R.; Stampfl, J. Processing of 45S5 Bioglass (R) by lithography-based additive manufacturing. Mater. Lett. 2012, 74, 81–84. [Google Scholar] [CrossRef]
- Leukers, B.; Gulkan, H.; Irsen, S.H.; Milz, S.; Tille, C.; Schieker, M.; Seitz, H. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J. Mater. Sci. Mater. Med. 2005, 16, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Moseke, C.; Ewald, A.; Gbureck, U.; Groll, J.; Pires, I.; Teßmar, J.; Vorndran, E. Direct 3D powder printing of biphasic calcium phosphate scaffolds for substitution of complex bone defects. Biofabrication 2014, 6, 015006. [Google Scholar] [CrossRef] [PubMed]
- Gomes de Sousa, F.C.; Evans, J.E.G. Tubular hydroxyapatite scaffolds. Adv. Appl. Ceram. 2015, 104, 30–34. [Google Scholar] [CrossRef]
- Koh, Y.H.; Kim, H.W.; Kim, H.E.; Halloran, J.W. Fabrication of macrochannelled-hydroxyapatite bioceramic by coextrusion process. J. Am. Ceram. Soc. 2002, 85, 2578–2580. [Google Scholar] [CrossRef]
- Koh, Y.H.; Kim, H.W.; Kim, H.E.; Halloran, J.W. Fabrication and compressive strength of macrochannelled tetragonal zirconia polycrystals with calcium phosphate coating layer. J. Mater. Res. 2003, 18, 2009–2012. [Google Scholar] [CrossRef]
- Prins, H.J.; Braat, A.K.; Gawlitta, D.; Dhert, W.J.; Egan, D.A.; Tijssen-Slump, E.; Yuan, H.; Coffer, P.J.; Rozemuller, H.; Martens, A.C. In vitro induction of alkaline phosphatase levels predicts in vivo bone forming capacity of human bone marrow stromal cells. Stem Cell Res. 2014, 12, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Tapan, K.; Dash, V.; Konkimalla, B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, M.; Czernuszka, J.T. The need for hierarchical scaffolds in bone tissue engineering. Hard Tissue 2013, 2, 37. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, X.P.; Zhang, L.C.; Sercombe, T.B. Processing and properties of topologically optimised biomedical Ti-24Nb-4Zr-8Sn scaffolds manufactured by selective laser melting. Mater. Sci. Eng. A. 2015, 642, 268–278. [Google Scholar] [CrossRef]
- Attar, H.; Löber, L.; Funk, A.; Calin, M.; Zhang, L.C.; Prashanth, K.G.; Scudino, S.; Zhang, Y.S.; Eckert, J. Mechanical behavior of porous commercially pure Ti and Ti–TiB composite materials manufactured by selective laser melting. Mater. Sci. Eng. A. 2015, 625, 350–356. [Google Scholar] [CrossRef]
- Jun, I.K.; Koh, Y.H.; Kim, H.E. Fabrication of ultrahigh porosity ceramics with biaxial pore channels. Mater. Lett. 2006, 60, 878–882. [Google Scholar] [CrossRef]
- Gibson, L.J. Biomechanics of cellular solids. J. Biomech. 2005, 38, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Meza, L.R.; Das, S.; Greer, J.R. Strong, lightweight, and recoverable three-dimensional ceramic nanolattices. Science 2014, 345, 1322–1326. [Google Scholar] [CrossRef] [PubMed]



| Components | CaP Paste | CB Paste |
|---|---|---|
| Powder Content | 13.9 g (30 vol % in CaP Paste) | 2.52 g (10 vol % in CB Paste) |
| Aqueous Solution | 10 g (5 wt % MC solution) | 10 g (5 wt % MC solution) |
| Dispersant (KD 6) | 0.20 g (1.5 wt % in relation to CaP powder) | 0.07 g (2.5 wt % in relation to CB powder) |
| Scaffolds | Green Scaffold | Porous CaP Scaffold |
|---|---|---|
| Dimensions [mm] | ~9.48 × 9.37 × 9.55 | ~6.52 × 6.43 × 6.58 |
| Scaffolds | Designed Scaffold | Green Scaffold | Sintered Scaffold |
|---|---|---|---|
| Dimensions of 3D Pores (μm) | ~500 × 440 | ~457 × 326 | ~360 × 230 |
| Diameter of Channels (μm) | ~500 | ~344 | ~187 |
| Loading Direction | Parallel | Normal |
|---|---|---|
| Compressive Strength (MPa) | 12.3 ± 2.2 | 7.1 ± 1.9 |
| Overall Porosity (vol %) | 82 ± 2.2 | 73 ± 1.5 |
| Compressive Strength (Mpa) | 4.8 ± 1.1 | 7.1 ± 1.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, I.-H.; Koh, Y.-H.; Kim, H.-E. Coextrusion-Based 3D Plotting of Ceramic Pastes for Porous Calcium Phosphate Scaffolds Comprised of Hollow Filaments. Materials 2018, 11, 911. https://doi.org/10.3390/ma11060911
Jo I-H, Koh Y-H, Kim H-E. Coextrusion-Based 3D Plotting of Ceramic Pastes for Porous Calcium Phosphate Scaffolds Comprised of Hollow Filaments. Materials. 2018; 11(6):911. https://doi.org/10.3390/ma11060911
Chicago/Turabian StyleJo, In-Hwan, Young-Hag Koh, and Hyoun-Ee Kim. 2018. "Coextrusion-Based 3D Plotting of Ceramic Pastes for Porous Calcium Phosphate Scaffolds Comprised of Hollow Filaments" Materials 11, no. 6: 911. https://doi.org/10.3390/ma11060911
APA StyleJo, I.-H., Koh, Y.-H., & Kim, H.-E. (2018). Coextrusion-Based 3D Plotting of Ceramic Pastes for Porous Calcium Phosphate Scaffolds Comprised of Hollow Filaments. Materials, 11(6), 911. https://doi.org/10.3390/ma11060911






