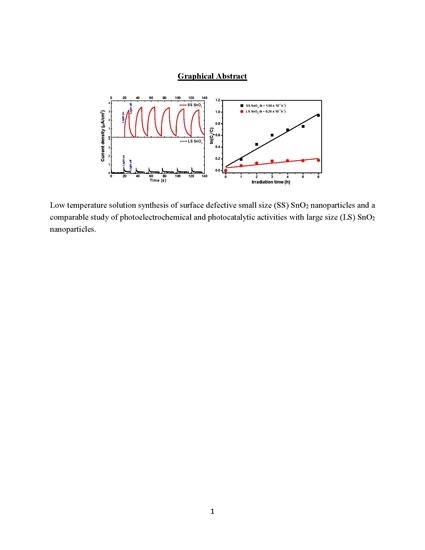
Influence of Surface Defects and Size on Photochemical Properties of SnO2 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Small Size (SS) and Large Size (LS) SnO2 NPs
2.2. Characterization of SnO2 NPs
2.3. Photoelectrochemical and Photocatalytic Activities
3. Results and Discussion
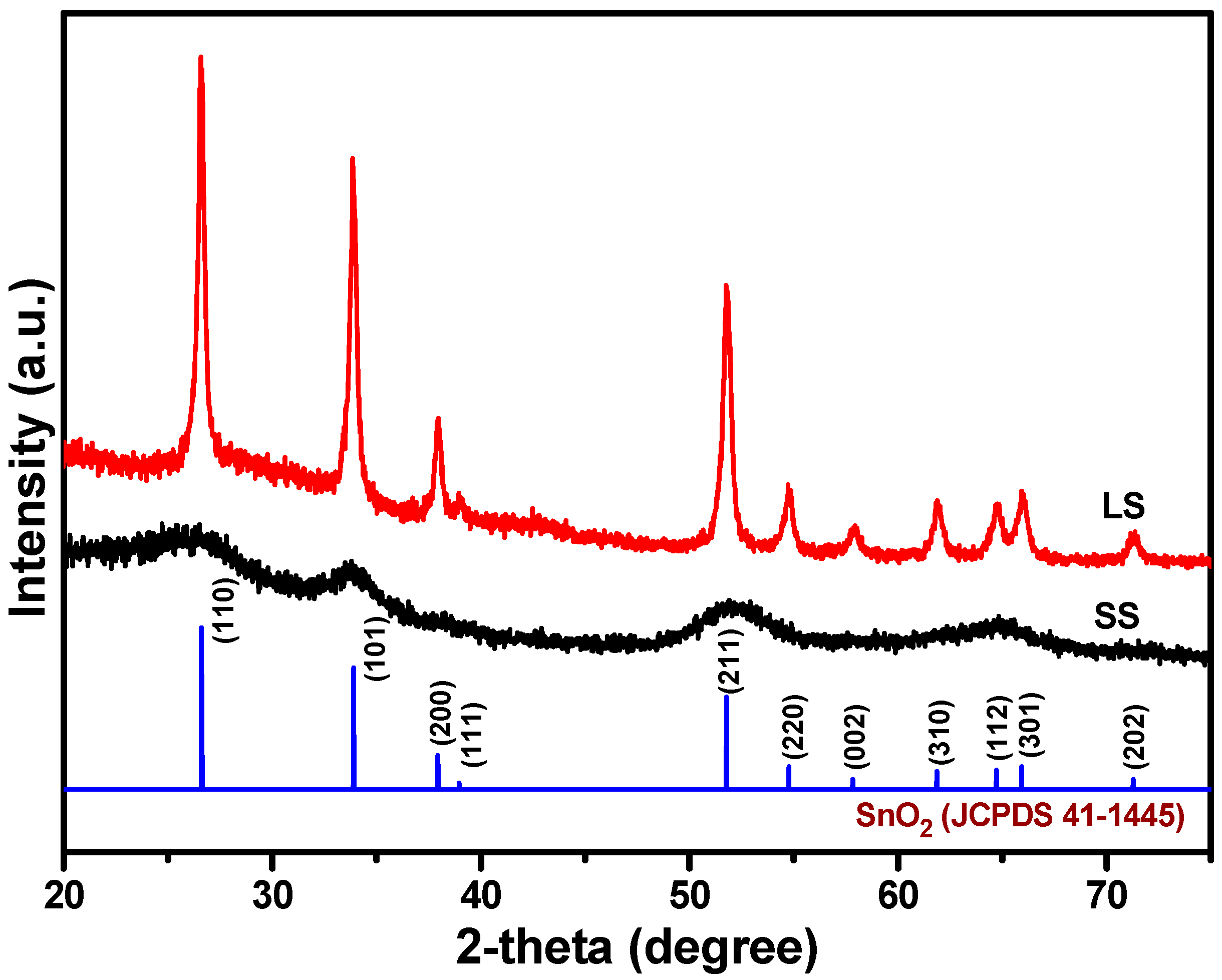
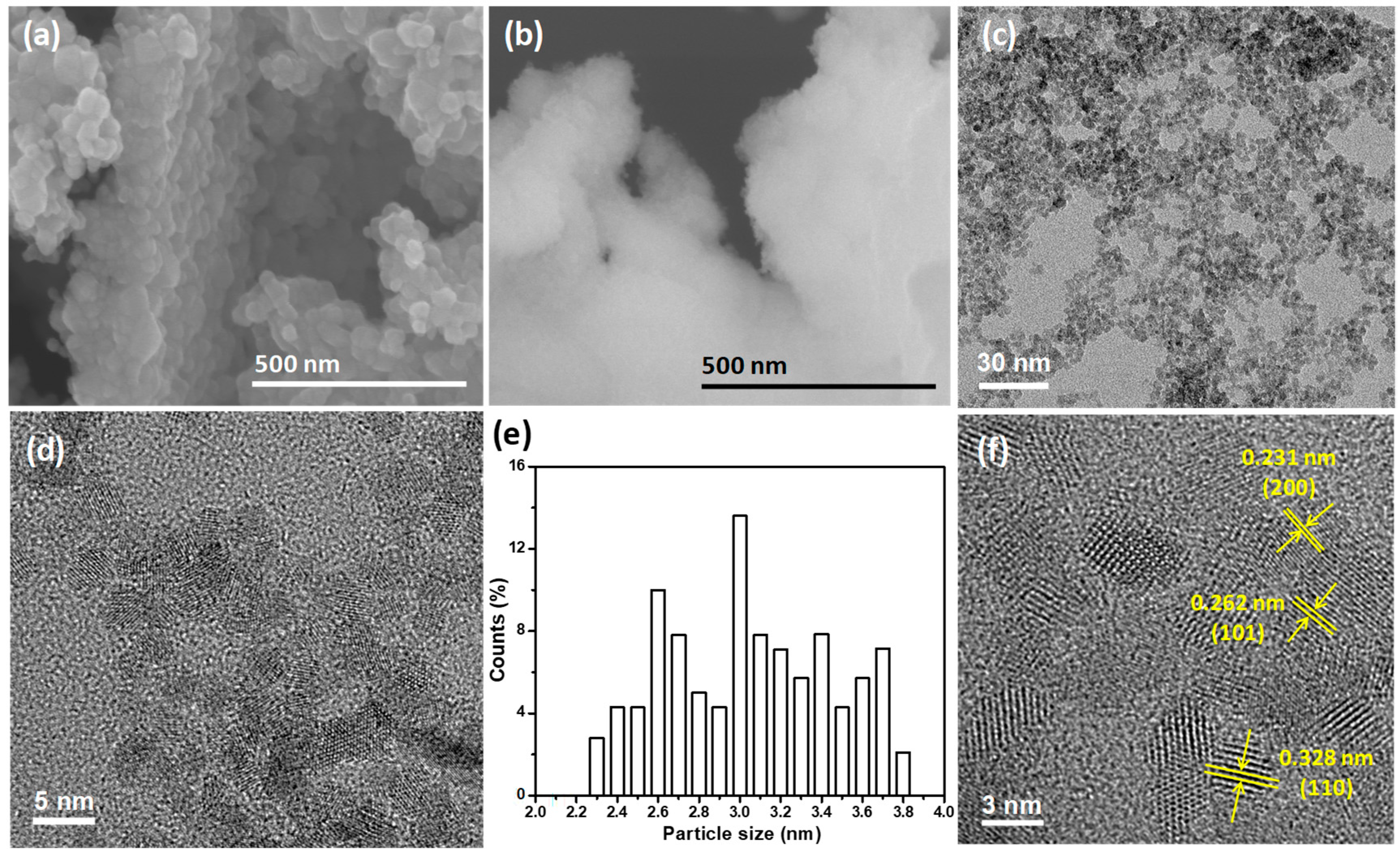
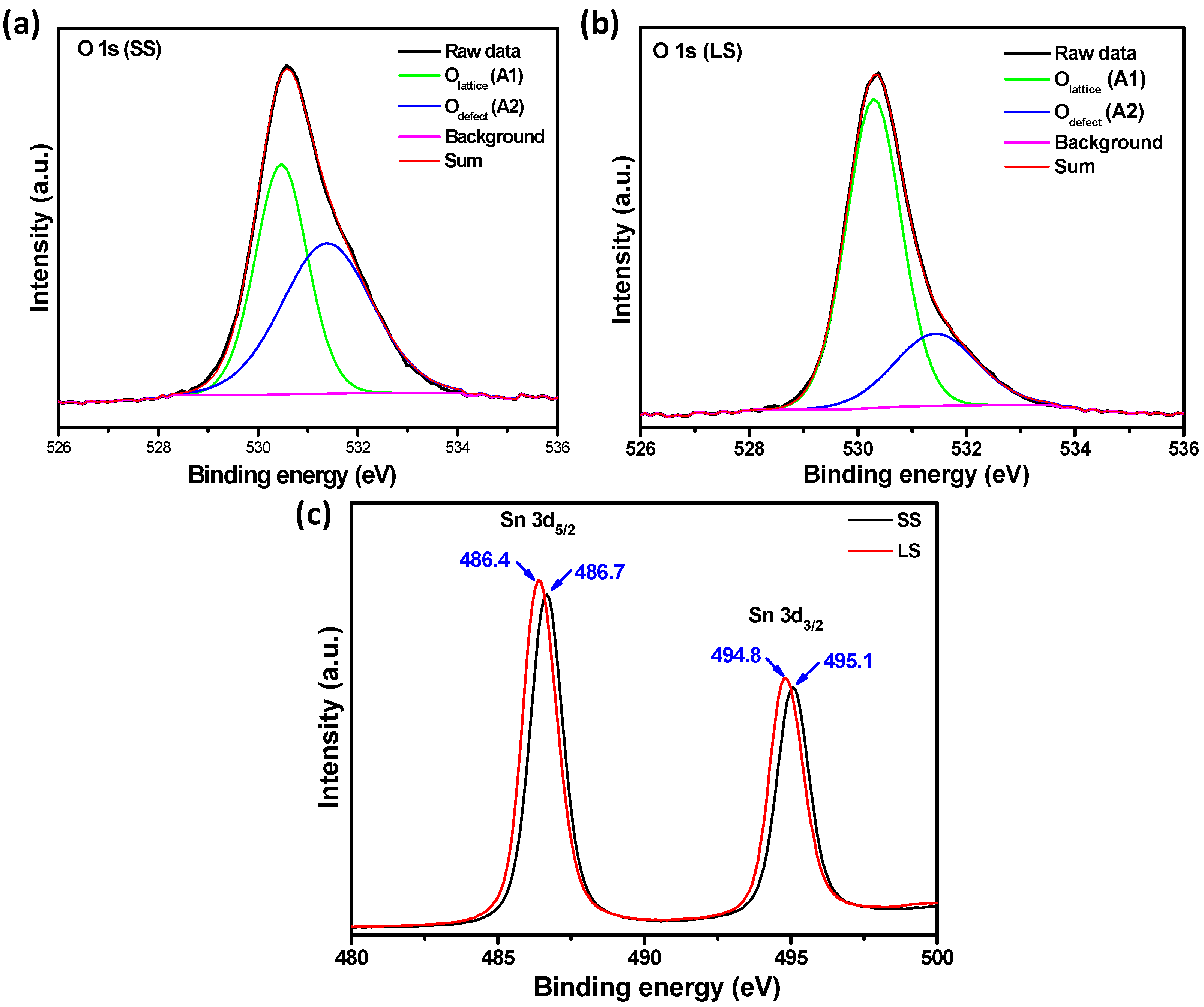
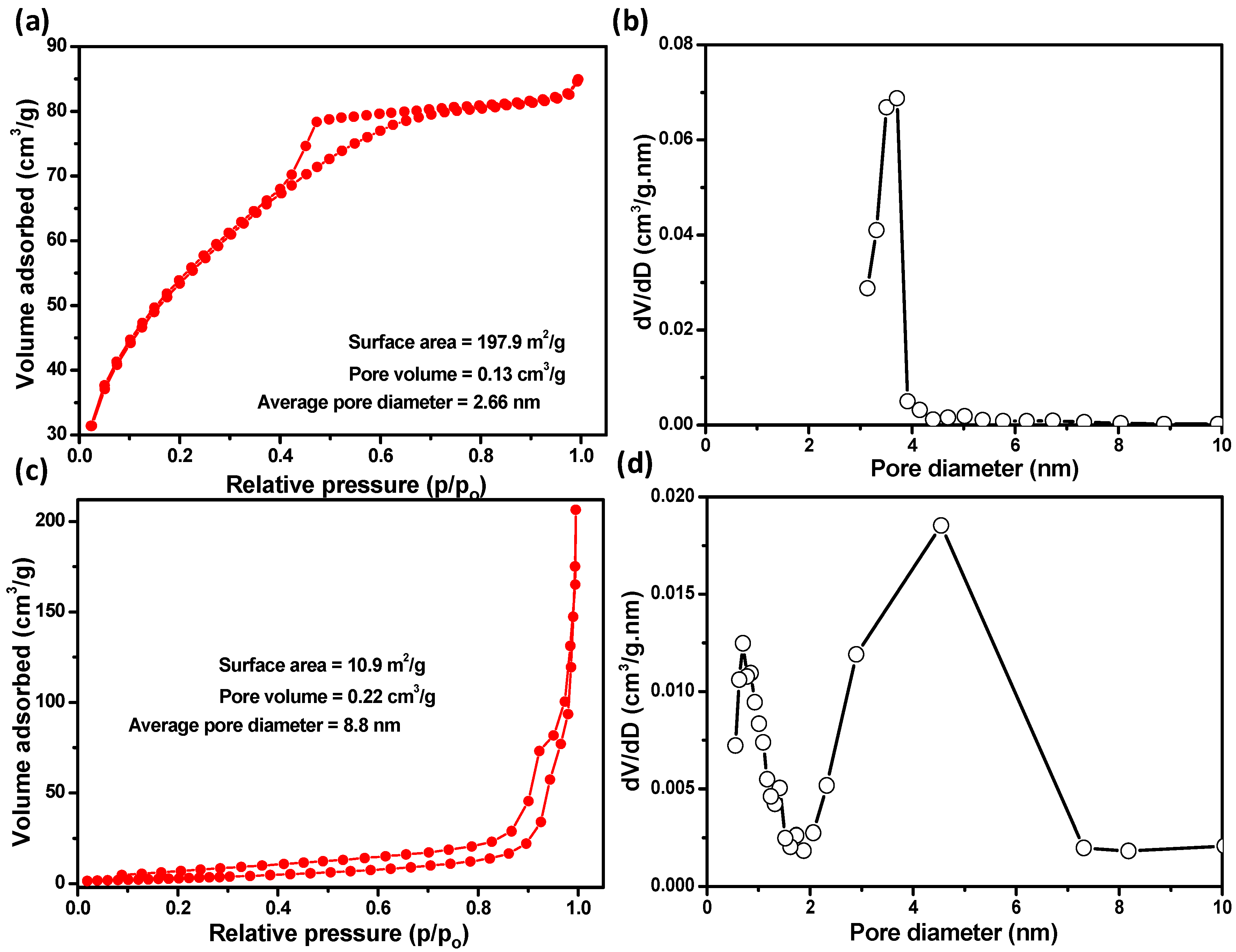
3.1. Material Properties
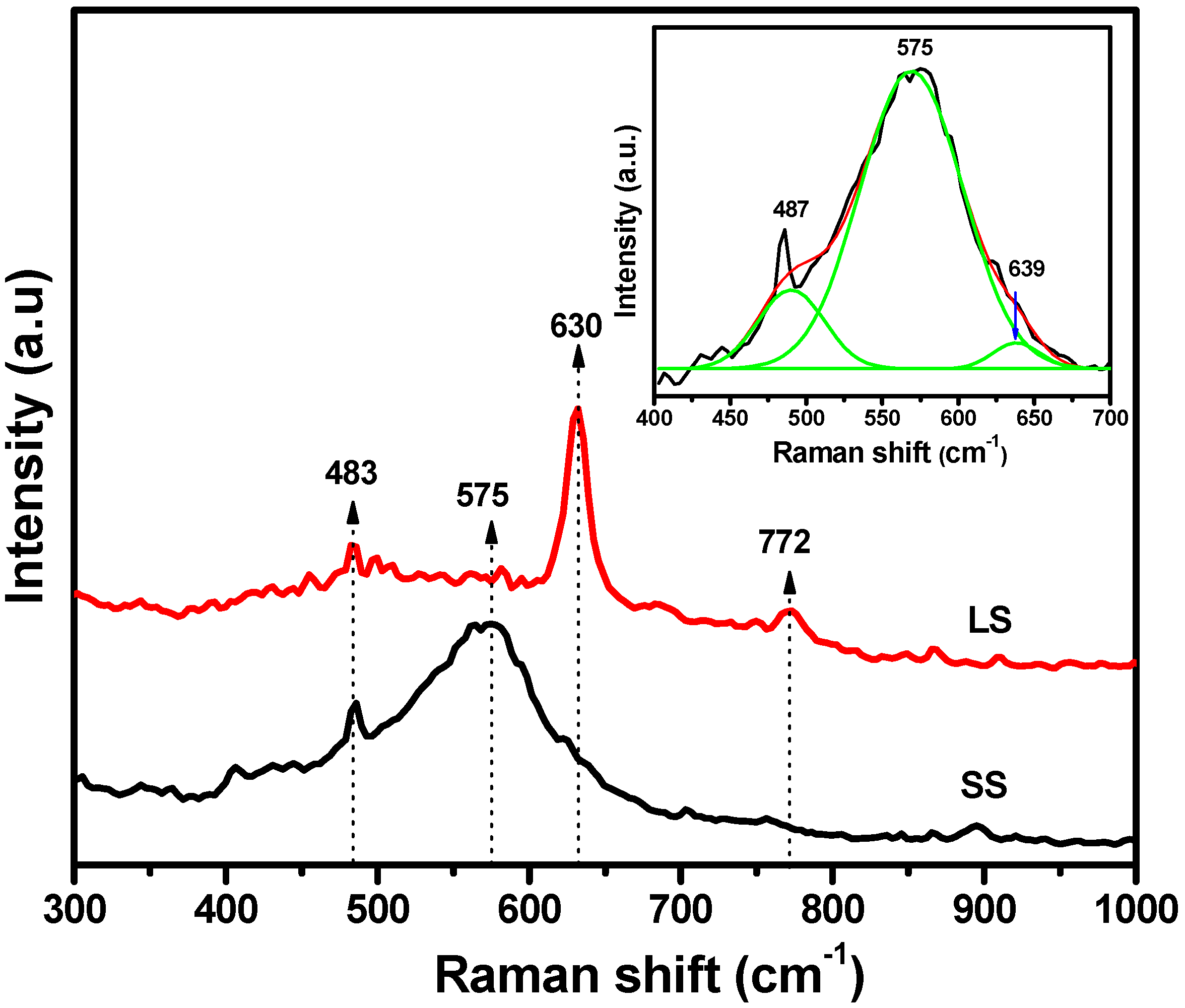
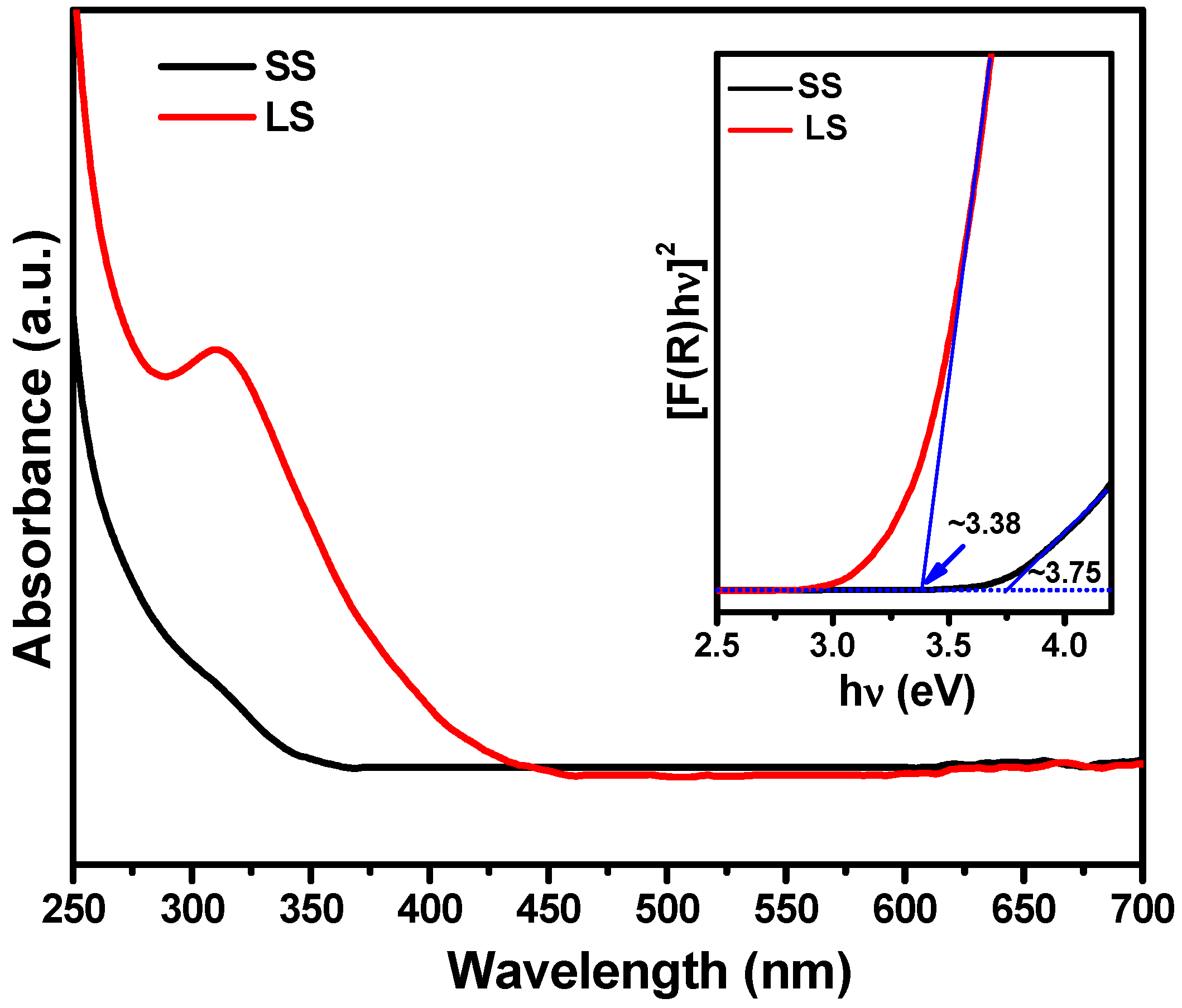
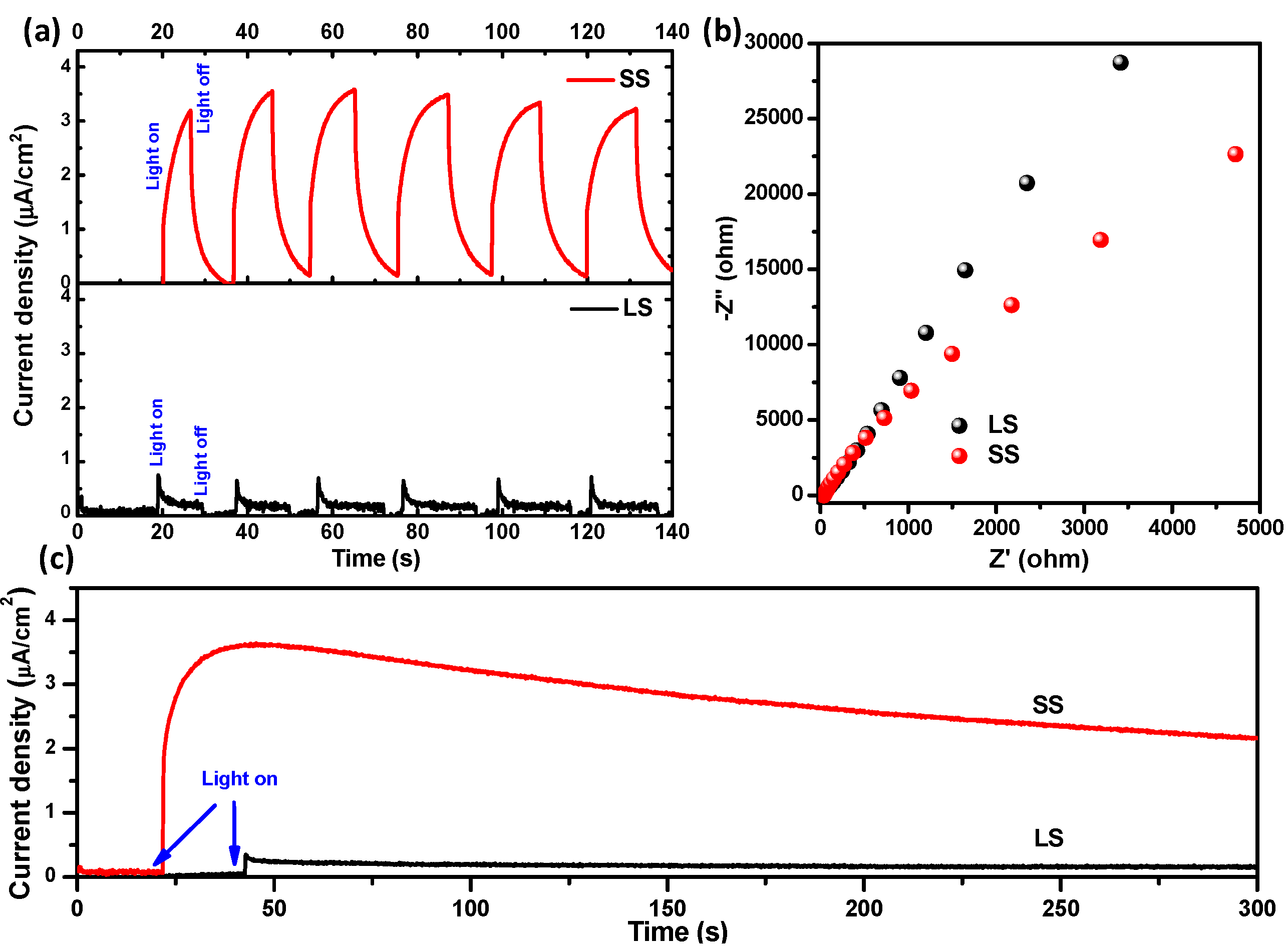
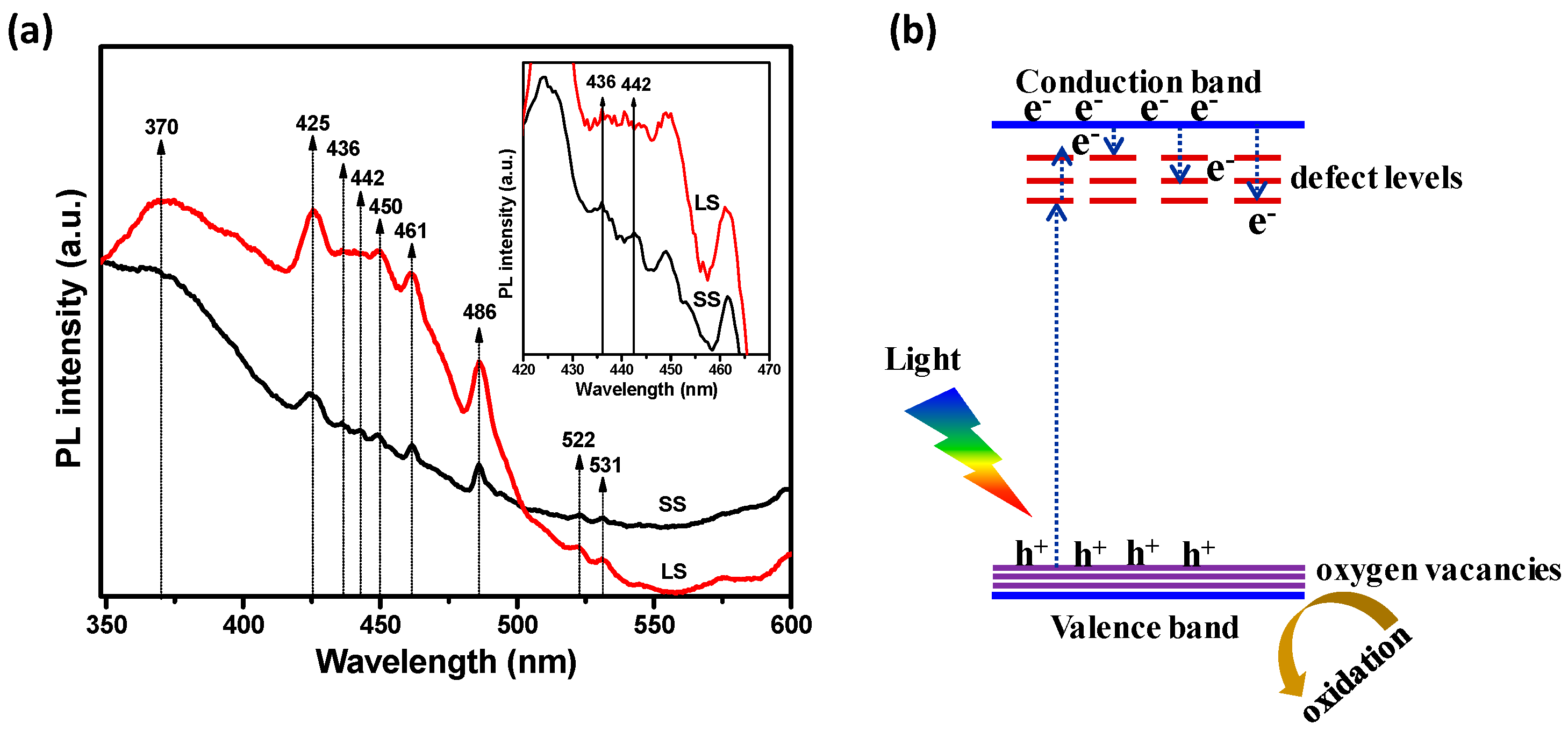
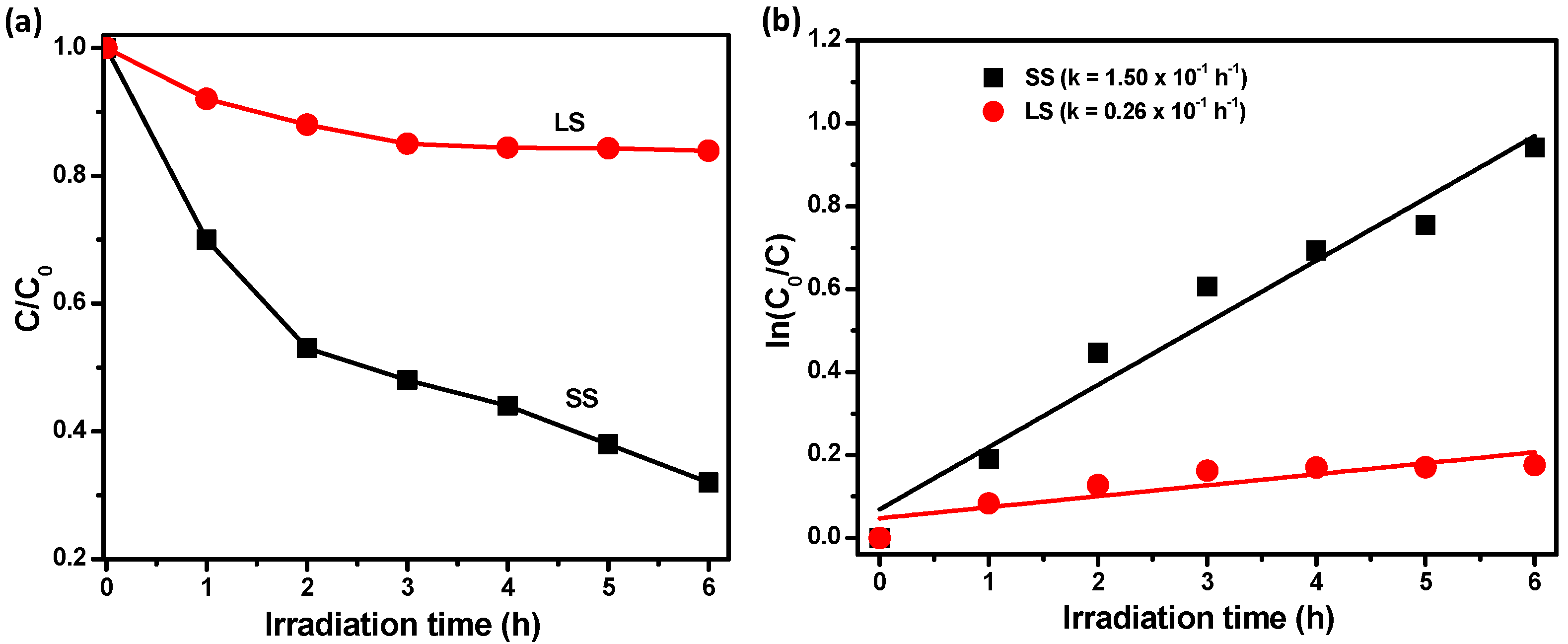
3.2. Optical and Photochemical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Khan, H.; Biswas, I.; Jana, S. Polyaniline hybridized surface defective ZnO nanorods with long-term stable photoelectrochemical activity. Appl. Surf. Sci. 2016, 383, 165–176. [Google Scholar] [CrossRef]
- Rahman, M.A.; Bazargan, S.; Srivastava, S.; Wang, X.; Ellah, M.A.; Thomas, J.P.; Heinig, N.F.; Pradhan, D.; Leung, K.T. Defect-rich Decorated TiO2 Nanowires for super-efficient photoelectrochemical water splitting driven by visible light. Energy Environ. Sci. 2015, 8, 3363–3373. [Google Scholar] [CrossRef]
- Anuchai, S.; Phanichphant, S.; Tantraviwat, D.; Pluengphon, P.; Bovornratanaraks, T.; Inceesungvorn, B. Low temperature preparation of oxygen-deficient tin dioxide nanocrystals and a role of oxygen vacancy in photocatalytic activity improvement. J. Colloid Interface Sci. 2018, 512, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Kundu, S.; Patra, A. Surface defect-related luminescence properties of SnO2 nanorods and nanoparticles. J. Phys. Chem. C 2011, 115, 118–124. [Google Scholar] [CrossRef]
- Kar, A.; Sain, S.; Kundu, S.; Bhattacharyya, A.; Pradhan, S.K.; Patra, A. Influence of size and shape on the photocatalytic properties of SnO2 nanocrystals. ChemPhysChem 2015, 16, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Yin, S. Oxygen vacancies confined in SnO2 nanoparticles for desirable electronic structure and enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2017, 420, 399–406. [Google Scholar] [CrossRef]
- Sadhu, S.; Patra, A. A brief overview of some physical studies on the relaxation dynamics and förster resonance energy transfer of semiconductor quantum dots. ChemPhysChem 2013, 14, 2641–2653. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ren, Y.; Yang, J.; Wang, L.; Jiang, W.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y.; Zhao, D.; Luo, W. Hierarchical ordered macro/mesoporous titania with a highly interconnected porous structure for efficient photocatalysis. J. Mater. Chem. A 2016, 4, 16446–16453. [Google Scholar] [CrossRef]
- Dong, W.; Sun, Y.; Hua, W.; Yao, Y.; Zhuang, G.; Lv, X.; Ma, Q.; Zhao, D. Preparation of secondary mesopores in mesoporous anatase–silica nanocomposites with unprecedented-high photocatalytic degradation performances. Adv. Funct. Mater. 2016, 26, 964–976. [Google Scholar] [CrossRef]
- Niederberger, M. Nonaqueous sol–gel routes to metal oxide nanoparticles. Acc. Chem. Res. 2007, 40, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Pramata, A.D.; Suematsu, S.; Quitain, A.T.; Sasaki, M.; Kida, T. Synthesis of highly luminescent sno2 nanocrystals: analysis of their defect-related photoluminescence using polyoxometalates as quenchers. Adv. Funct. Mater. 2018, 28, 1704620. [Google Scholar] [CrossRef]
- Wang, H.; Dou, K.; Teoh, W.Y.; Zhan, Y.; Hung, T.F.; Zhang, F.; Xu, J.; Zhang, R.; Rogach, A.L. Engineering of facets, band structure, and gas sensing properties of hierarchical Sn2+ doped sno2 nanostructures. Adv. Funct. Mater. 2013, 23, 4847–4853. [Google Scholar]
- Kumar, V.; Swart, H.C.; Gohain, M.; Bezuidenhoudt, B.C.B.; van Vuuren, A.J.; Lee, M.; Ntwaeaborwa, O.M. The role of neutral and ionized oxygen defects in the emission of tin oxide nanocrystals for near white light application. Nanotechnology 2015, 26, 295703. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Pal, M.; Sarkar, S.; Jana, S. Hierarchically Structured Macro with Nested Mesoporous Zinc Indium Oxide Conducting Film. ACS Appl. Mater. Interfaces 2017, 9, 4420–4424. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.; Murmua, P.P.; Manikandan, E.; Lee, S.Y. Investigation of structural and photoluminescence properties of gas and metal ions doped zinc oxide single crystals. J. Alloys Compd. 2014, 616, 614–617. [Google Scholar] [CrossRef]
- Kennedy, J.; Murmua, P.P.; Leveneura, J.; Markwitz, A.; Futter, J. Controlling preferred orientation and electrical conductivity of zincoxide thin films by post growth annealing treatment. Appl. Surf. Sci. 2016, 367, 52–58. [Google Scholar] [CrossRef]
- Pal, M.; Bera, S.; Jana, S. Sol–gel based simonkolleite nanopetals with SnO2 nanoparticles in graphite-like amorphous carbon as an efficient and reusable photocatalyst. RSC Adv. 2015, 5, 75062–75074. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, J.; Chen, F.; Tian, B. An economic method to prepare vacuum activated photocatalysts with high photo-activities and photosensitivities. Chem. Commun. 2011, 47, 4947–4949. [Google Scholar] [CrossRef] [PubMed]
- Sangwichien, C.; Aranovich, G.L.; Donohue, M.D. Density Functional theory predictions of adsorption isotherms with hysteresis loops. Colloids Surf. A 2002, 206, 313–320. [Google Scholar] [CrossRef]
- Li, P.; Lan, Y.; Zhang, Q.; Zhao, Z.; Pullerits, T.; Zheng, K.; Zhou, Y. Iodinated SnO2 Quantum Dots: A Facile and Efficient Approach to Increase Solar absorption for visible-light photocatalysis. J. Phys. Chem. C 2016, 120, 9253–9262. [Google Scholar] [CrossRef]
- Lei, F.; Sun, Y.; Liu, K.; Gao, S.; Liang, L.; Pan, B.; Xie, Y. Oxygen vacancies confined in ultrathin indium oxide porous sheets for promoted visible-light water splitting. J. Am. Chem. Soc. 2014, 136, 6826–6829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, C.; Wu, Z.; Han, W.; Wang, Y.; Fua, W.; Li, X.; Xie, E. Toward efficient photoelectrochemical water-splitting by using screw-like SnO2 nostructures as photoanode after being decorated with CdS quantum dots. Nano Energy 2016, 19, 318–327. [Google Scholar] [CrossRef]
- Liu, J.; Shahid, M.; Ko, Y.-S.; Kim, E.; Ahn, T.K.; Park, J.H.; Kwon, Y.-U. Investigation of porosity and heterojunction effects of a mesoporous hematite electrode on photoelectrochemical water splitting. Phys. Chem. Chem. Phys. 2013, 15, 9775–9782. [Google Scholar] [CrossRef] [PubMed]
- Yanga, L.; Huang, J.; Shi, L.; Cao, L.; Zhou, W.; Chang, K.; Meng, X.; Liu, G.; Jie, Y.; Ye, J. Efficient hydrogen evolution over Sb doped SnO2 photocatalyst sensitized by Eosin Y under visible light irradiation. Nano Energy 2017, 36, 331–340. [Google Scholar] [CrossRef]
- Kasinathan, K.; Kennedy, J.; Elayaperumal, M.; Henini, M.; Malik, M. Photodegradation of organic pollutants RhB dye using UV simulated sunlight on ceria based TiO2 nanomaterials for antibacterial applications. Sci. Rep. 2016, 6, 38064. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Shi, J.; Wang, Z.; Li, C. Photoelectrocatalytic water splitting: significance of cocatalysts, electrolyte, and interfaces. ACS Catal. 2017, 7, 675–688. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilka, M.; Bera, S.; Kwon, S.-H. Influence of Surface Defects and Size on Photochemical Properties of SnO2 Nanoparticles. Materials 2018, 11, 904. https://doi.org/10.3390/ma11060904
Ilka M, Bera S, Kwon S-H. Influence of Surface Defects and Size on Photochemical Properties of SnO2 Nanoparticles. Materials. 2018; 11(6):904. https://doi.org/10.3390/ma11060904
Chicago/Turabian StyleIlka, Mahdi, Susanta Bera, and Se-Hun Kwon. 2018. "Influence of Surface Defects and Size on Photochemical Properties of SnO2 Nanoparticles" Materials 11, no. 6: 904. https://doi.org/10.3390/ma11060904
APA StyleIlka, M., Bera, S., & Kwon, S.-H. (2018). Influence of Surface Defects and Size on Photochemical Properties of SnO2 Nanoparticles. Materials, 11(6), 904. https://doi.org/10.3390/ma11060904