Redox Activity of Sodium Vanadium Oxides towards Oxidation in Na Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
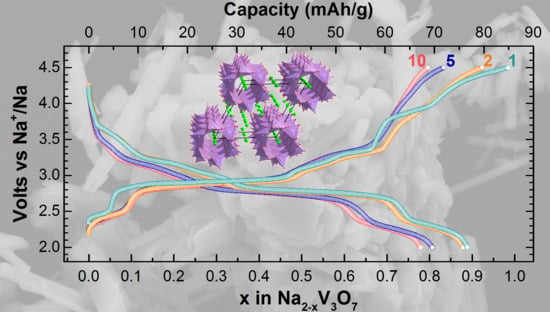
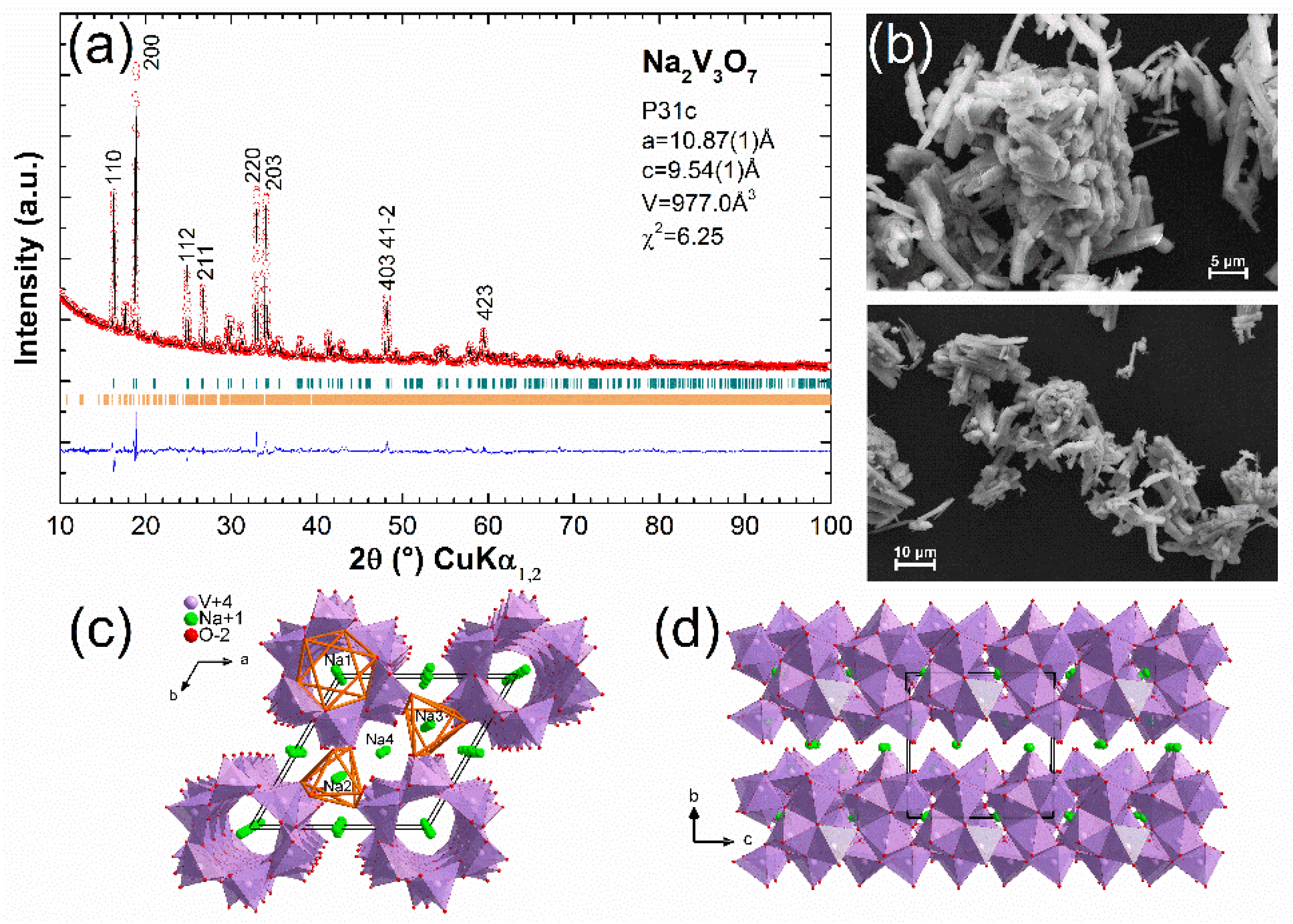
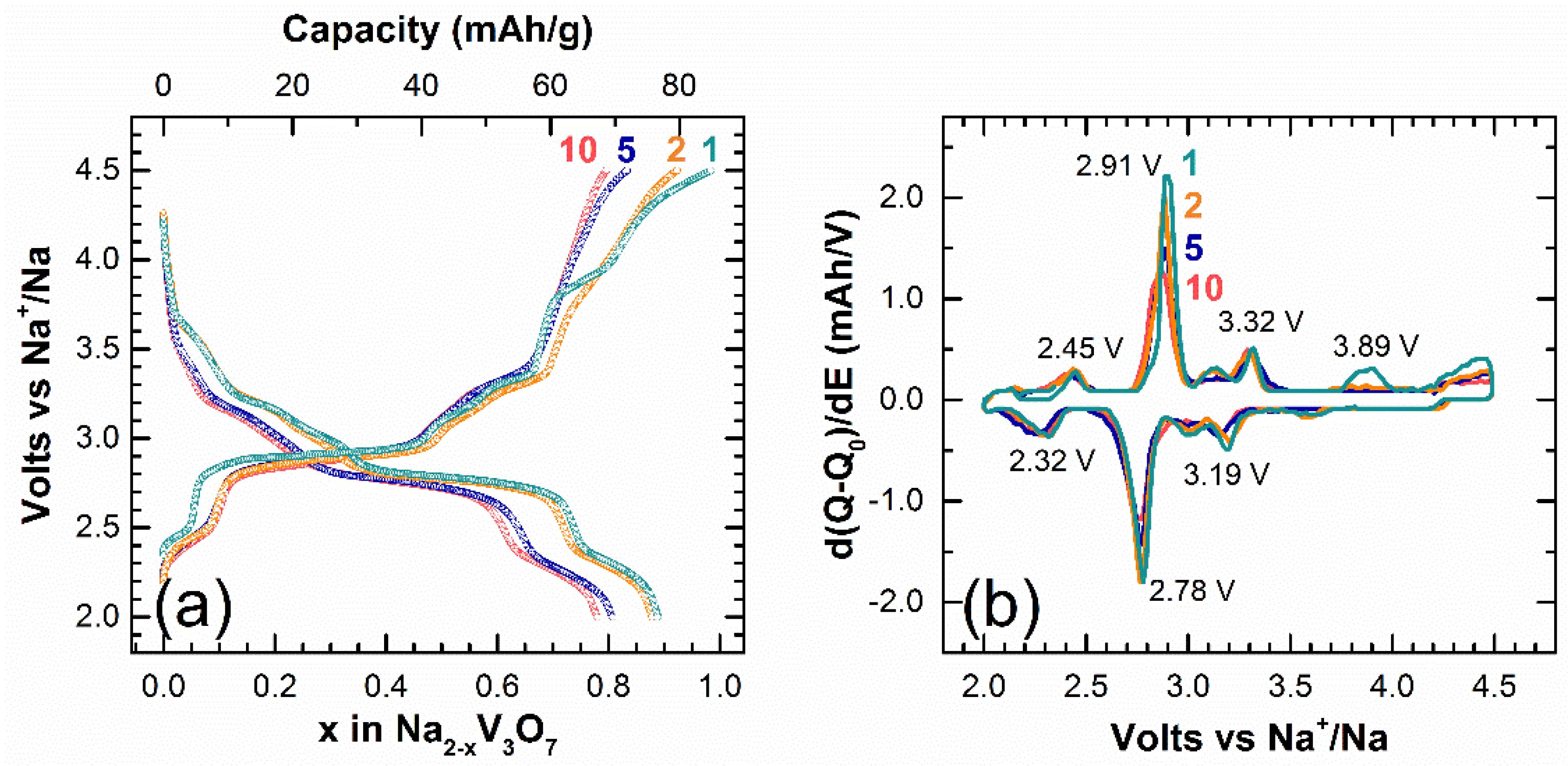
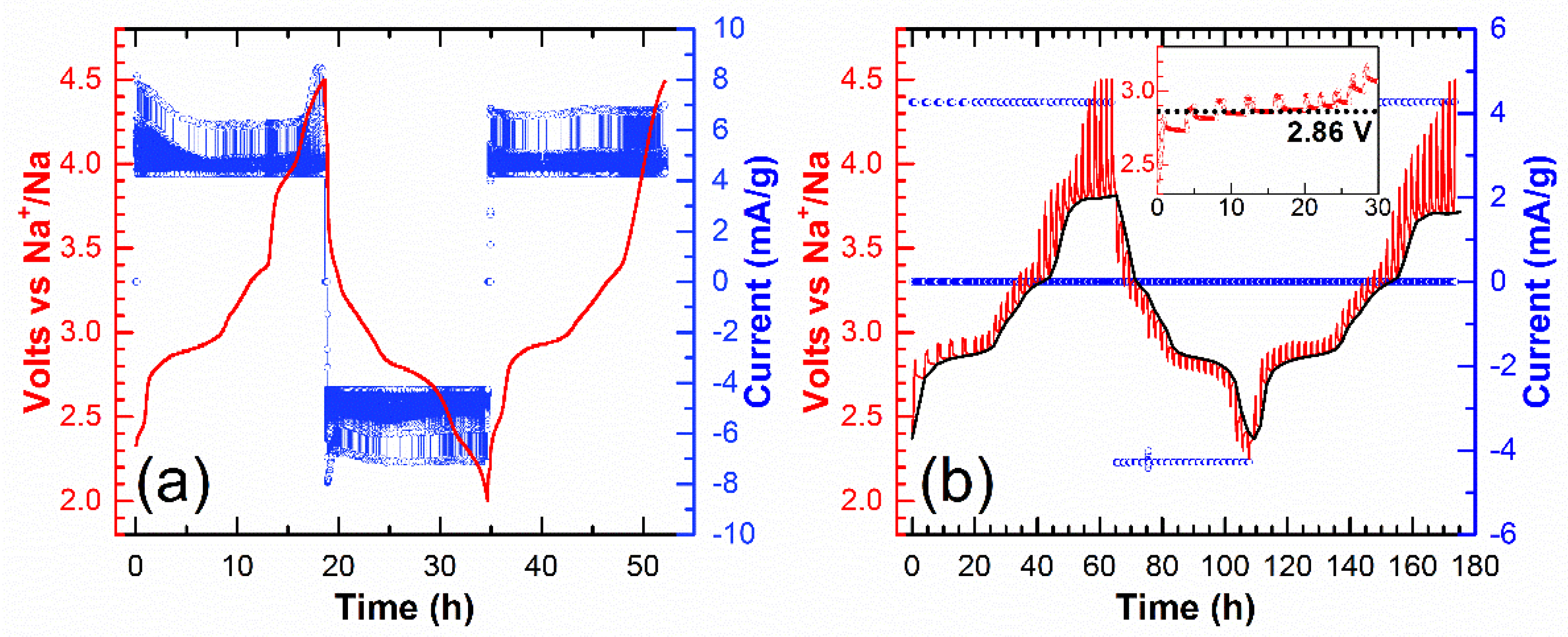
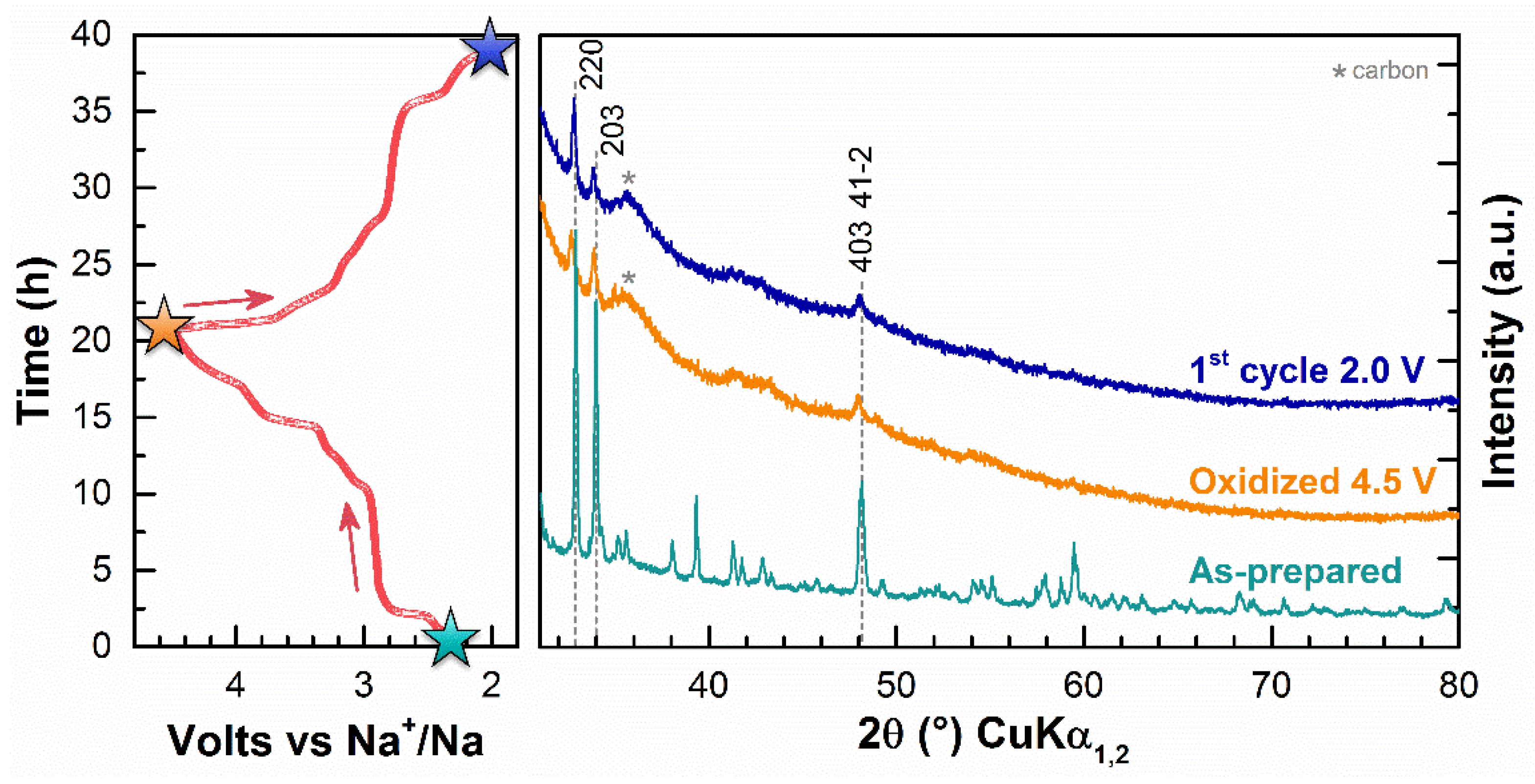
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindley, D. Smart grids: The energy storage problem. Nature 2010, 463, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- West, K.; Zachau-Christiansen, B.; Jacobsen, T.; Skaarup, S. Sodium insertion in vanadium oxides. Solid State Ion. 1988, 28, 1128–1131. [Google Scholar] [CrossRef]
- Tepavcevic, S.; Xiong, H.; Stamenkovic, V.R.; Zuo, X.; Balasubramanian, M.; Prakapenka, V.B.; Johnson, C.S.; Rajh, T. Nanostructured Bilayered Vanadium Oxide Electrodes for Rechargeable Sodium-Ion Batteries. ACS Nano 2012, 6, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Su, D.W.; Dou, S.X.; Wang, G.X. Hierarchical orthorhombic V2O5 hollow nanospheres as high performance cathode materials for sodium-ion batteries. J. Mater. Chem. A 2014, 2, 11185–11194. [Google Scholar] [CrossRef]
- Li, H.-Y.; Yang, C.-H.; Tseng, C.-M.; Lee, S.-W.; Yang, C.-C.; Wu, T.-Y.; Chang, J.-K. Electrochemically grown nanocrystalline V2O5 as high-performance cathode for sodium-ion batteries. J. Power Sources 2015, 285, 418–424. [Google Scholar] [CrossRef]
- Muller-Bouvet, D.; Baddour-Hadjean, R.; Tanabe, M.; Huynh, L.T.N.; Le, M.L.P.; Pereira-Ramos, J.P. Electrochemically formed α’-NaV2O5: A new sodium intercalation compound. Electrochim. Acta 2015, 176, 586–593. [Google Scholar] [CrossRef]
- Safrany Renard, M.; Emery, N.; Baddour-Hadjean, R.; Pereira-Ramos, J.-P. γ’-V2O5: A new high voltage cathode material for sodium-ion battery. Electrochim. Acta 2017, 252, 4–11. [Google Scholar] [CrossRef]
- Safrany Renard, M.; Emery, N.; Roginskii, E.M.; Baddour-Hadjean, R.; Pereira-Ramos, J.-P. Crystal structure determination of a new sodium vanadium bronze electrochemically formed. J. Solid State Chem. 2017, 254, 62–68. [Google Scholar] [CrossRef]
- Adamczyk, E.; Anger, E.; Freire, M.; Pralong, V. A chemical redox reaction to generate rock salt-type materials: The case of Na3V2O5. Dalton Trans. 2018, 47, 3112–3118. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, G.; Pralong, V.; Lebedev, O.I.; Caignaert, V.; Bazin, P.; Raveau, B. Amorphous sodium vanadate Na1.5+yVO3, a promising matrix for reversible sodium intercalation. Electrochem. Commun. 2014, 40, 100–102. [Google Scholar] [CrossRef]
- Didier, C.; Guignard, M.; Denage, C.; Szajwaj, O.; Ito, S.; Saadoune, I.; Darriet, J.; Delmas, C. Electrochemical Na-Deintercalation from NaVO2. Electrochem. Solid State Lett. 2011, 14, A75. [Google Scholar] [CrossRef]
- Hamani, D.; Ati, M.; Tarascon, J.-M.; Rozier, P. NaxVO2 as possible electrode for Na-ion batteries. Electrochem. Commun. 2011, 13, 938–941. [Google Scholar] [CrossRef]
- Guignard, M.; Didier, C.; Darriet, J.; Bordet, P.; Elkaïm, E.; Delmas, C. P2-NaxVO2 system as electrodes for batteries and electron-correlated materials. Nat. Mater. 2013, 12, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Millet, P.; Henry, J.Y.; Mila, F.; Galy, J. Vanadium(IV)–Oxide Nanotubes: Crystal Structure of the Low-Dimensional Quantum Magnet Na2V3O7. J. Solid State Chem. 1999, 147, 676–678. [Google Scholar] [CrossRef]
- Gavilano, J.L.; Rau, D.; Mushkolaj, S.; Ott, H.R.; Millet, P.; Mila, F. Low-Dimensional Spin S=1/2 System at the Quantum Critical Limit: Na2V3O7. Phys. Rev. Lett. 2003, 90, 167202. [Google Scholar] [CrossRef] [PubMed]
- Gavilano, J.L.; Felder, E.; Rau, D.; Ott, H.R.; Millet, P.; Mila, F.; Cichorek, T.; Mota, A.C. Unusual magnetic properties of the low-dimensional quantum magnet Na2V3O7. Phys. Rev. B 2005, 72, 064431. [Google Scholar] [CrossRef]
- Gavilano, J.L.; Felder, E.; Rau, D.; Ott, H.R.; Millet, P.; Mila, F.; Cichorek, T.; Mota, A.C. Na2V3O7: An unusual low-dimensional quantum magnet. Phys. B Condens. Matter 2006, 378–380, 123–124. [Google Scholar] [CrossRef]
- Choi, J.; Musfeldt, J.L.; Wang, Y.J.; Koo, H.-J.; Whangbo, M.-H.; Galy, J.; Millet, P. Optical Investigation of Na2V3O7 Nanotubes. Chem. Mater. 2002, 14, 924–930. [Google Scholar] [CrossRef]
- Delacourt, C.; Ati, M.; Tarascon, J.M. Measurement of Lithium Diffusion Coefficient in LiyFeSO4F. J. Electrochem. Soc. 2011, 158, A741. [Google Scholar] [CrossRef]
- Adamczyk, E.; Pralong, V. Na2Mn3O7: A Suitable Electrode Material for Na-Ion Batteries? Chem. Mater. 2017, 29, 4645–4648. [Google Scholar] [CrossRef]
- De Boisse, B.M.; Nishimura, S.; Watanabe, E.; Lander, L.; Tsuchimoto, A.; Kikkawa, J.; Kobayashi, E.; Asakura, D.; Okubo, M.; Yamada, A. Highly Reversible Oxygen-Redox Chemistry at 4.1 V in Na4/7−x[□1/7Mn6/7]O2 (□: Mn Vacancy). Adv. Energy Mater. 2018. [Google Scholar] [CrossRef]
- Johnson, C.S.; Kim, J.-S.; Lefief, C.; Li, N.; Vaughey, J.T.; Thackeray, M.M. The significance of the Li2MnO3 component in ‘composite’ xLi2MnO3·(1−x)LiMn0.5Ni0.5O2 electrodes. Electrochem. Commun. 2004, 6, 1085–1091. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Johnson, C.S.; Vaughey, J.T.; Li, N.; Hackney, S.A. Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J. Mater. Chem. 2005, 15, 2257–2267. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.-H.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112–3125. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Hara, R.; Kubota, K.; Paulsen, J.; Kumakura, S.; Komaba, S. A new electrode material for rechargeable sodium batteries: P2-type Na2/3[Mg0.28Mn0.72]O2 with anomalously high reversible capacity. J. Mater. Chem. A 2014, 2, 16851–16855. [Google Scholar] [CrossRef]
- Maitra, U.; House, R.A.; Somerville, J.W.; Tapia-Ruiz, N.; Lozano, J.G.; Guerrini, N.; Hao, R.; Luo, K.; Jin, L.; Pérez-Osorio, M.A.; et al. Oxygen redox chemistry without excess alkali-metal ions in Na2/3[Mg0.28Mn0.72]O2. Nat. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, E.; Gnanavel, M.; Pralong, V. Redox Activity of Sodium Vanadium Oxides towards Oxidation in Na Ion Batteries. Materials 2018, 11, 1021. https://doi.org/10.3390/ma11061021
Adamczyk E, Gnanavel M, Pralong V. Redox Activity of Sodium Vanadium Oxides towards Oxidation in Na Ion Batteries. Materials. 2018; 11(6):1021. https://doi.org/10.3390/ma11061021
Chicago/Turabian StyleAdamczyk, Evan, Muthaiyan Gnanavel, and Valerie Pralong. 2018. "Redox Activity of Sodium Vanadium Oxides towards Oxidation in Na Ion Batteries" Materials 11, no. 6: 1021. https://doi.org/10.3390/ma11061021
APA StyleAdamczyk, E., Gnanavel, M., & Pralong, V. (2018). Redox Activity of Sodium Vanadium Oxides towards Oxidation in Na Ion Batteries. Materials, 11(6), 1021. https://doi.org/10.3390/ma11061021