Combined Effects of Set Retarders and Polymer Powder on the Properties of Calcium Sulfoaluminate Blended Cement Systems
Abstract
:1. Introduction
2. Experimental Program
2.1. Materials
2.2. Mix Proportions
2.3. Sample Preparation
2.4. Test Methods
2.4.1. Compression Test
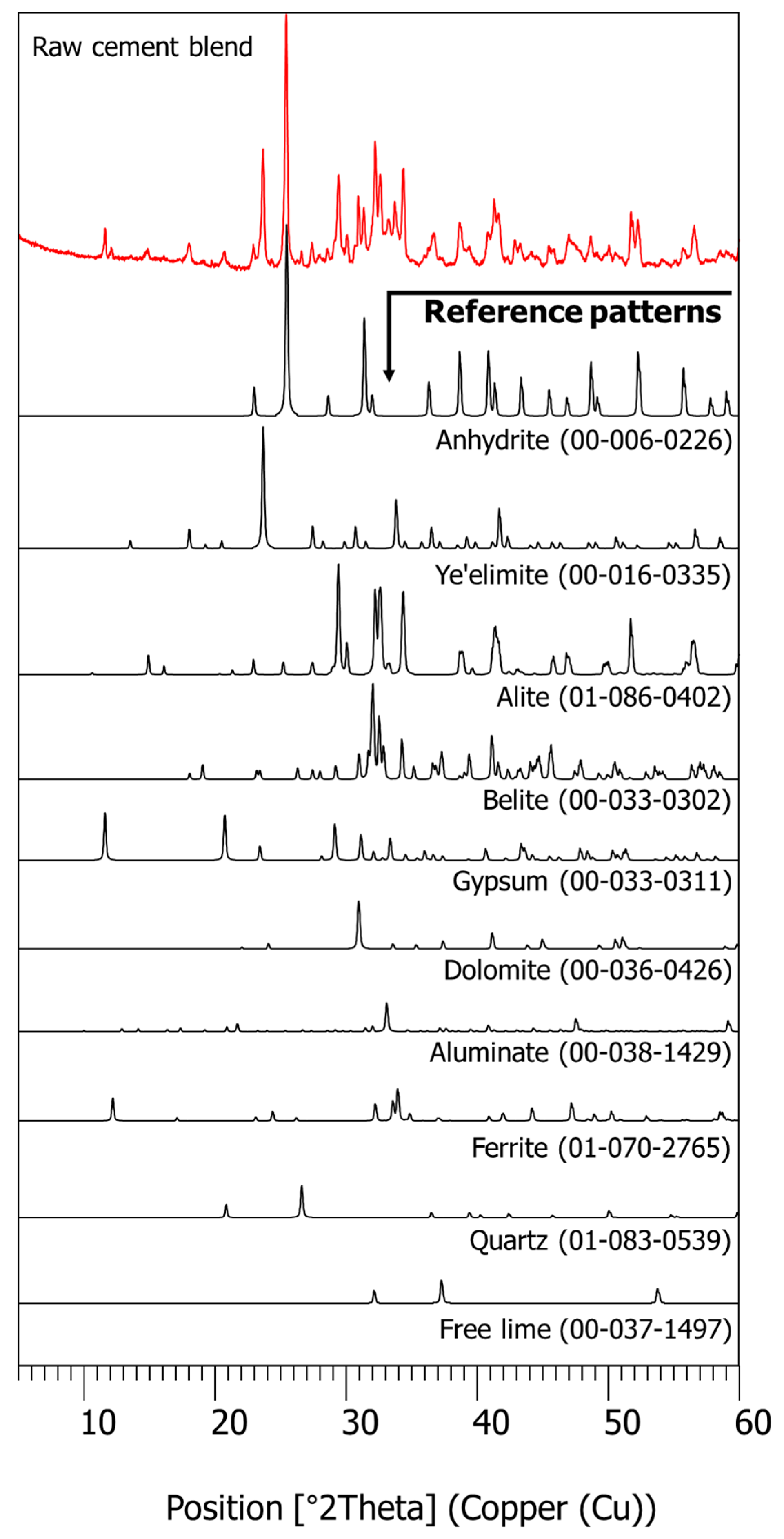
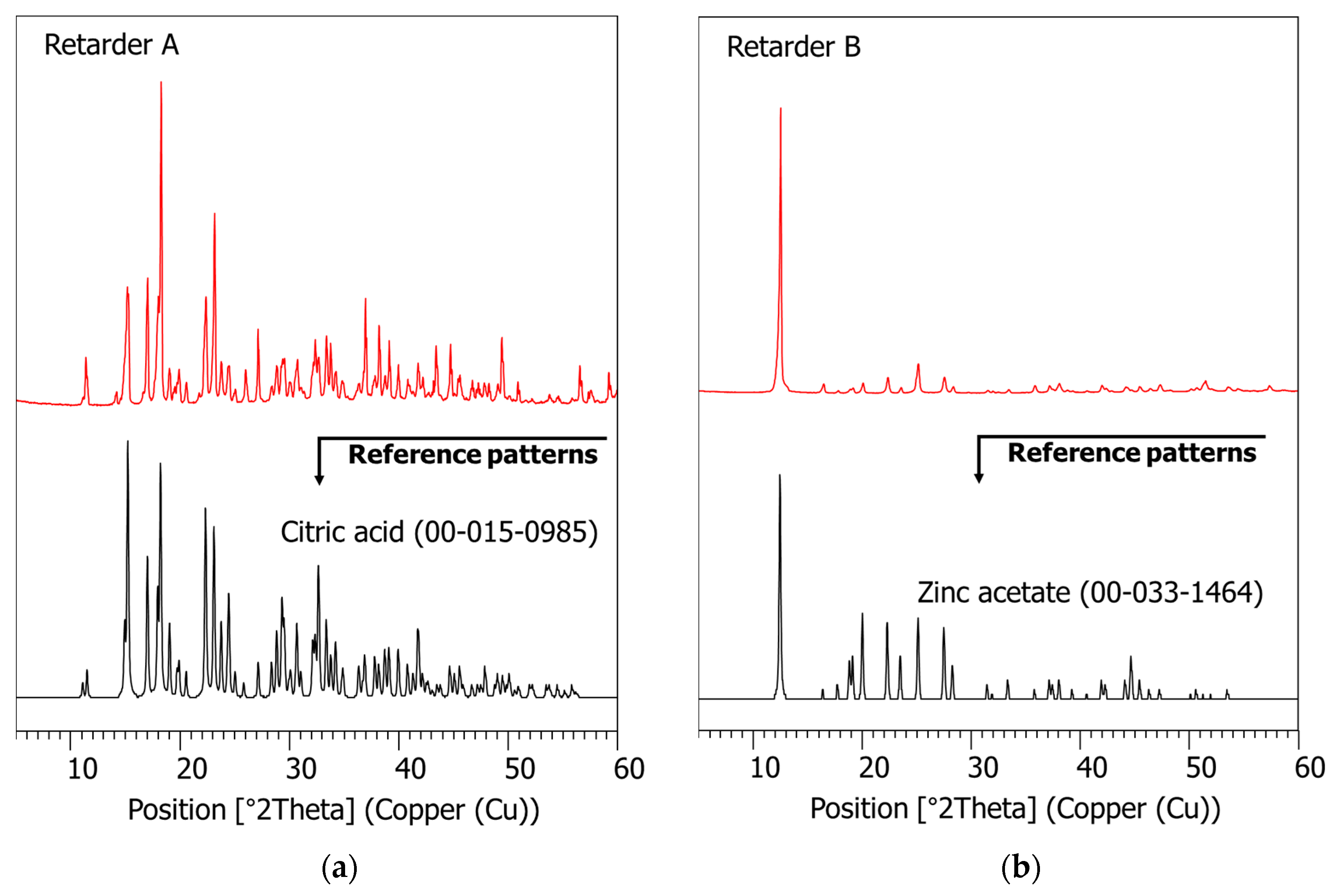
2.4.2. XRD and XRF
2.4.3. EA
2.4.4. MIP
2.4.5. SEM/EDS
3. Results and Discussion
3.1. Characterization of Raw Materials
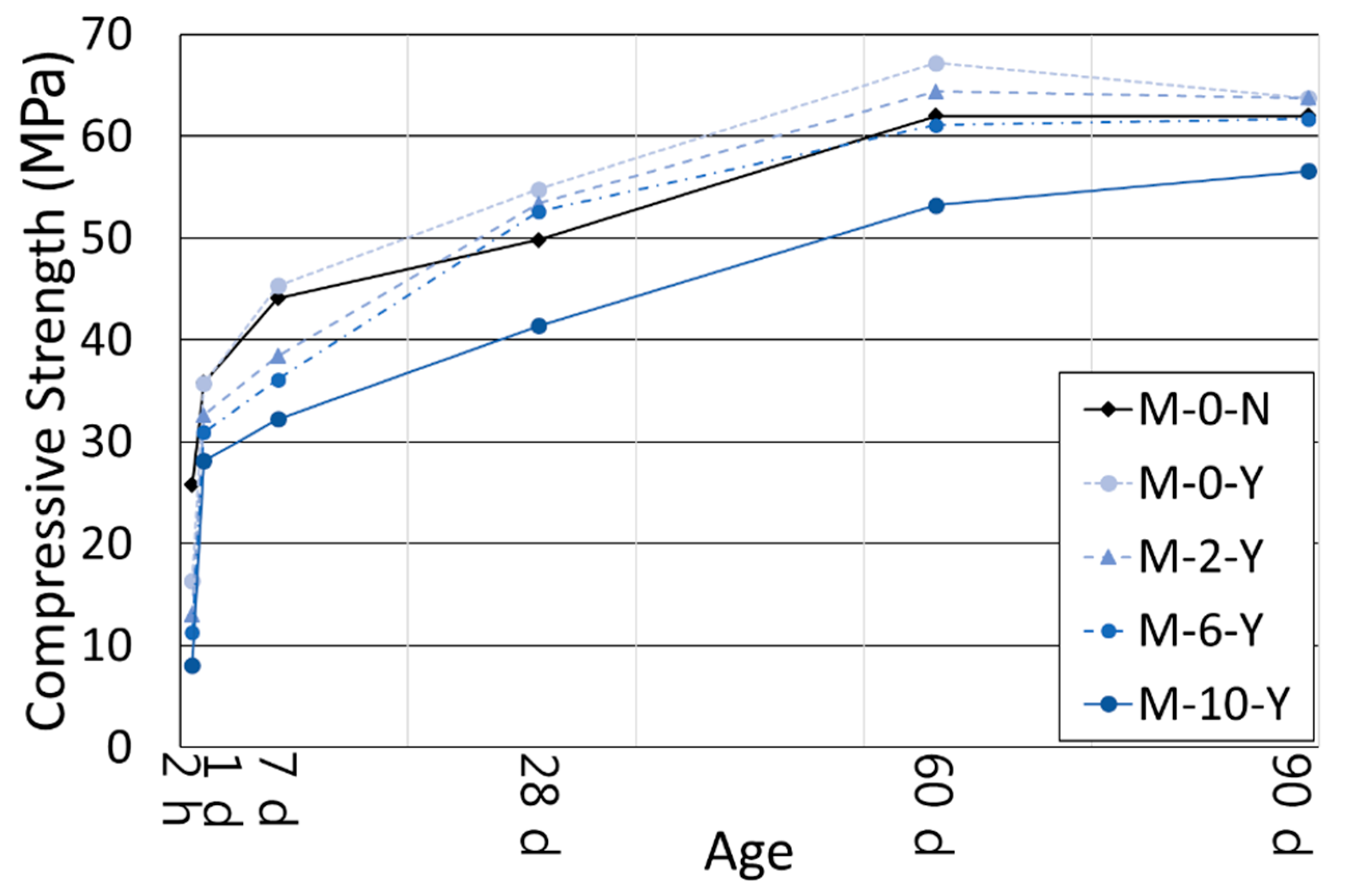
3.2. Compressive Strength
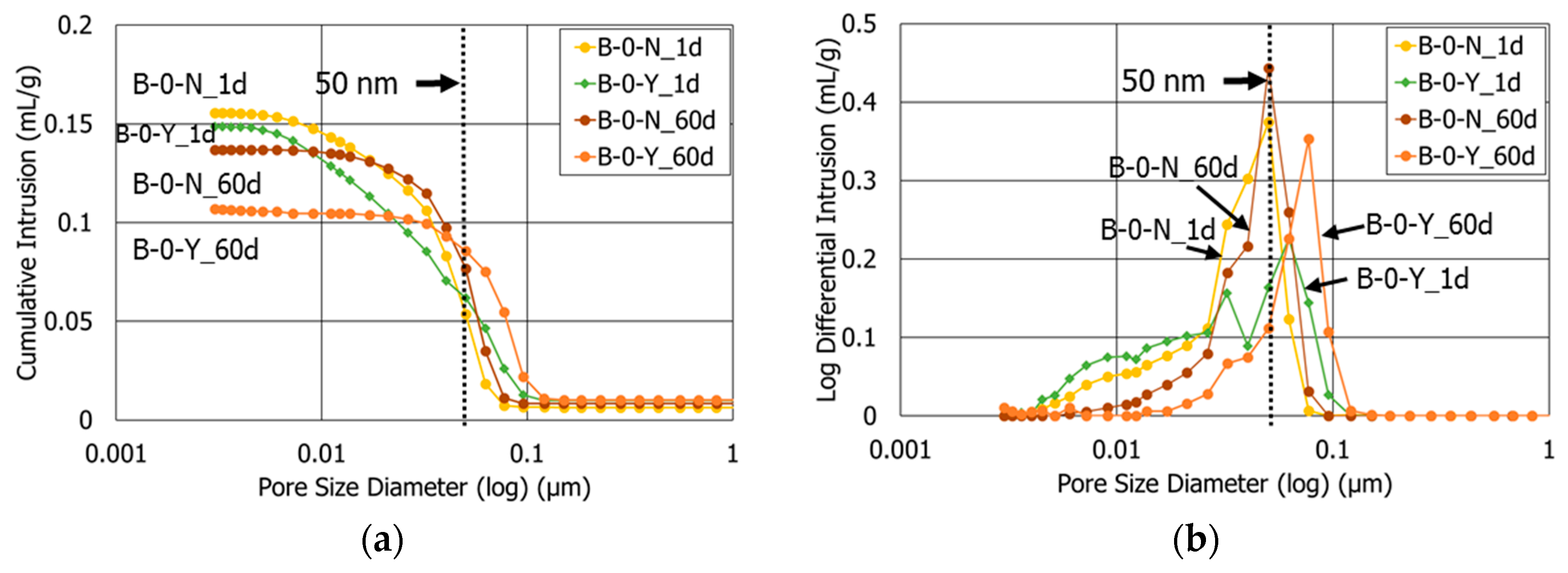
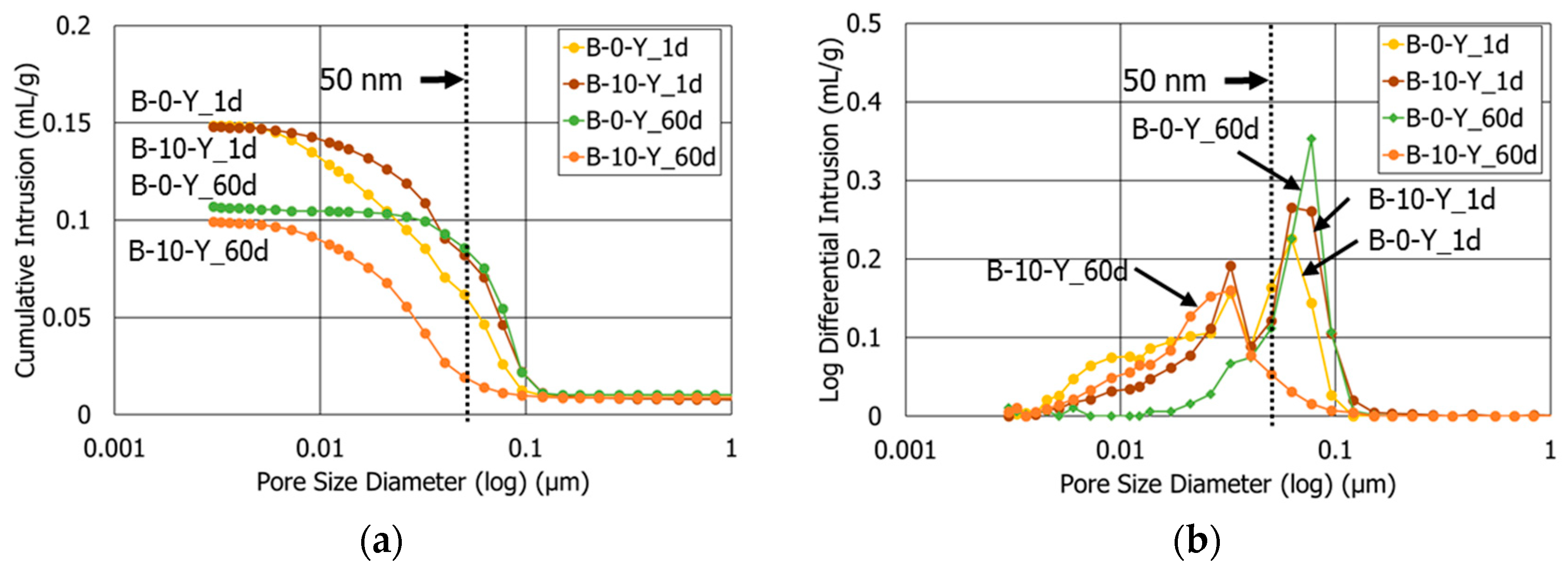
3.3. Porosity
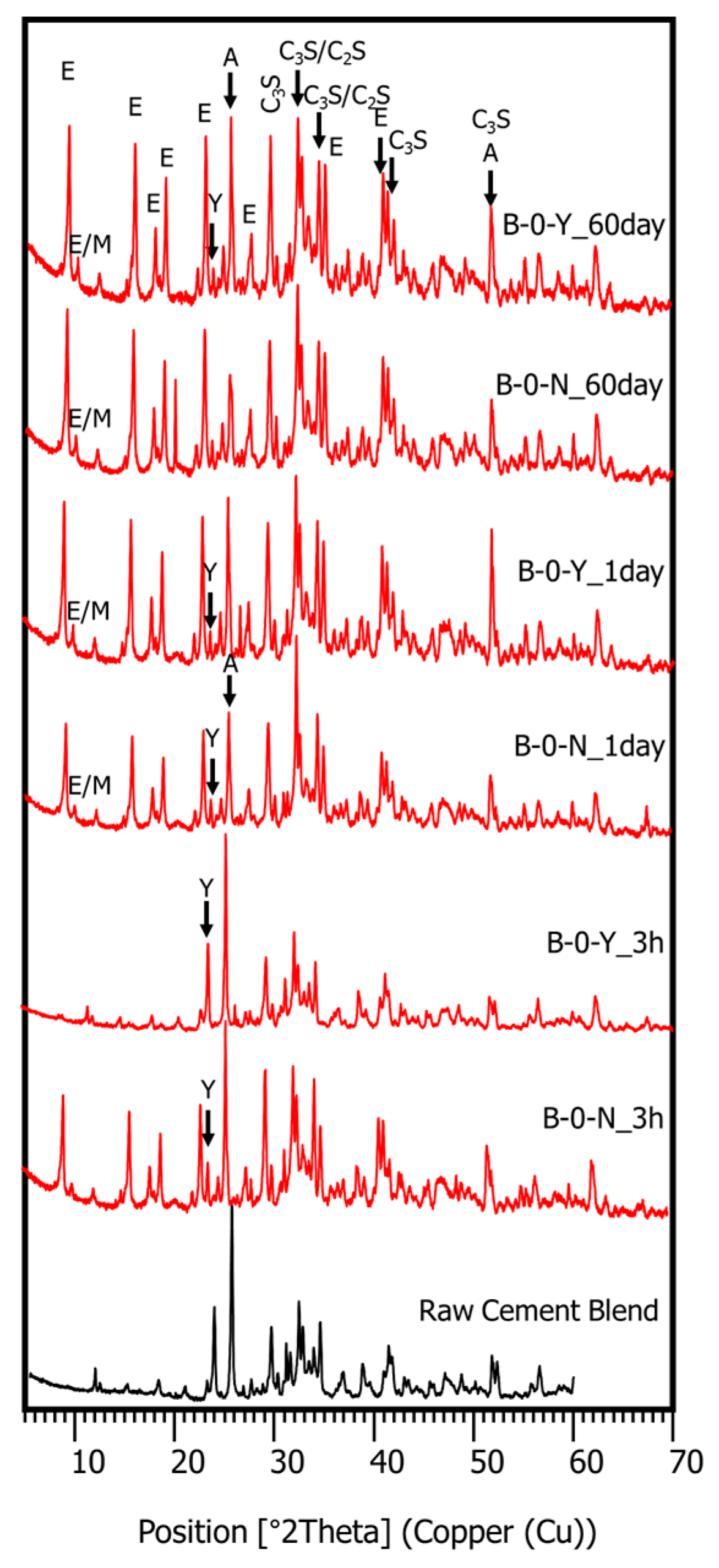
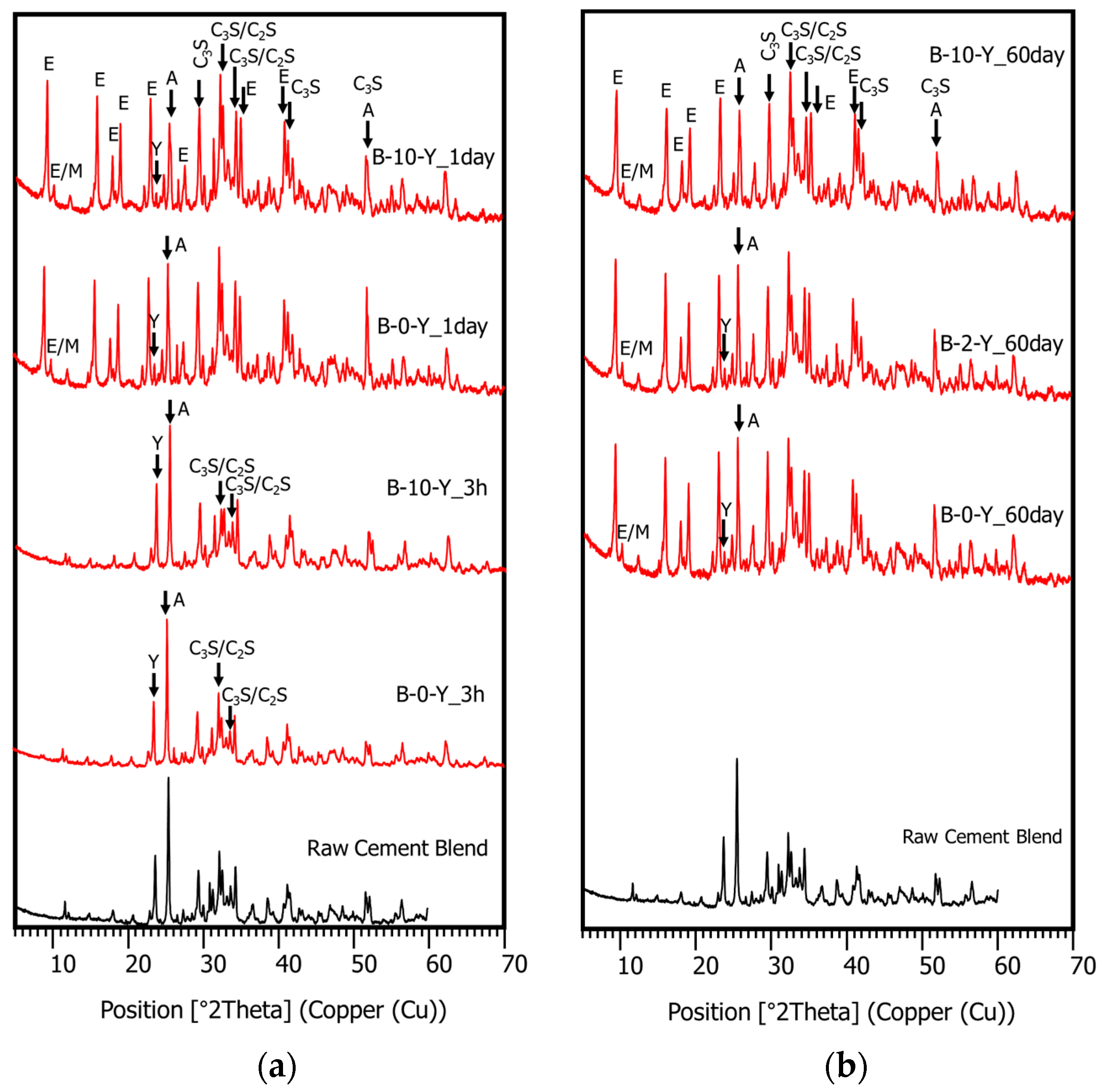
3.4. Hydration Phase Evolution
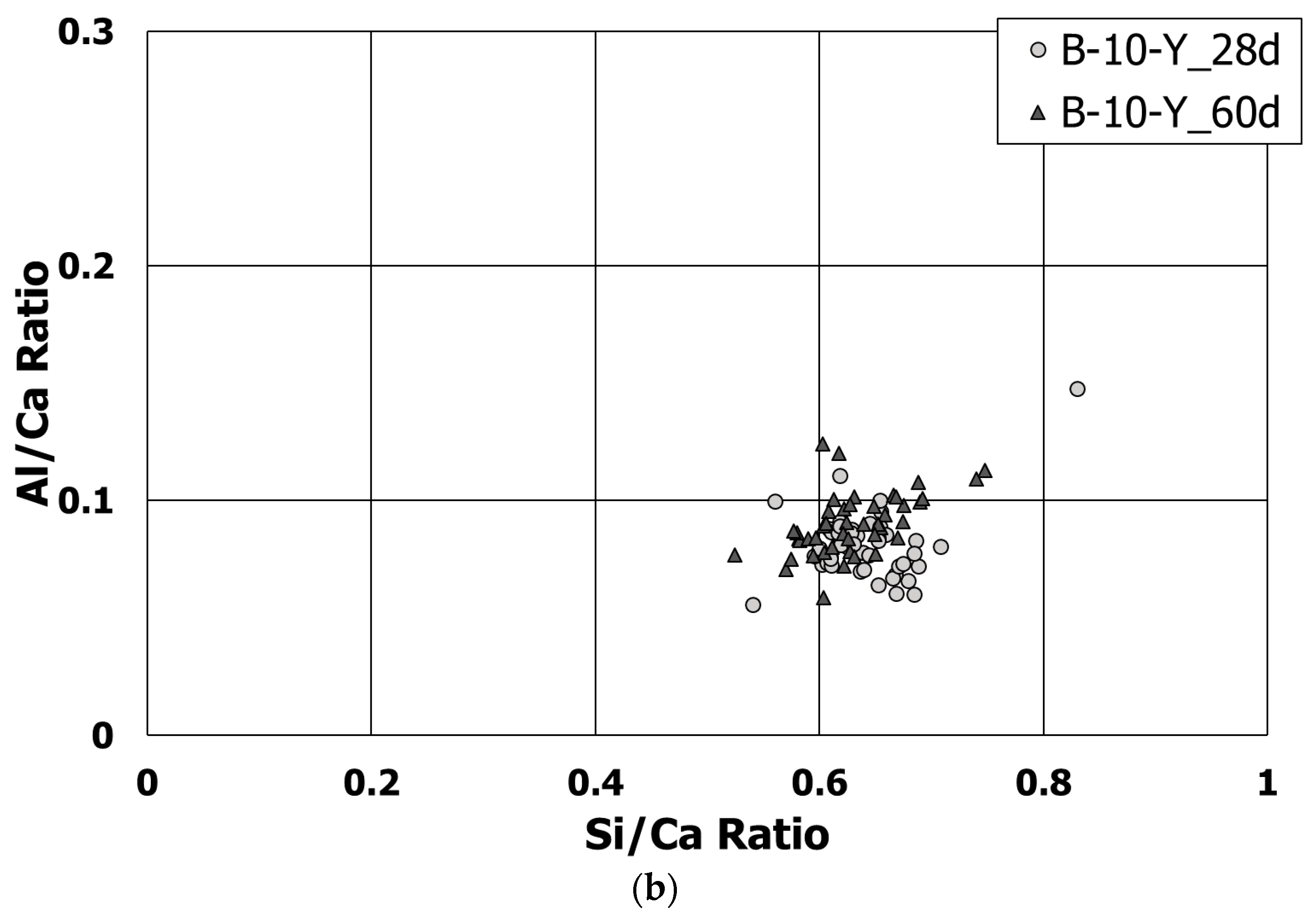
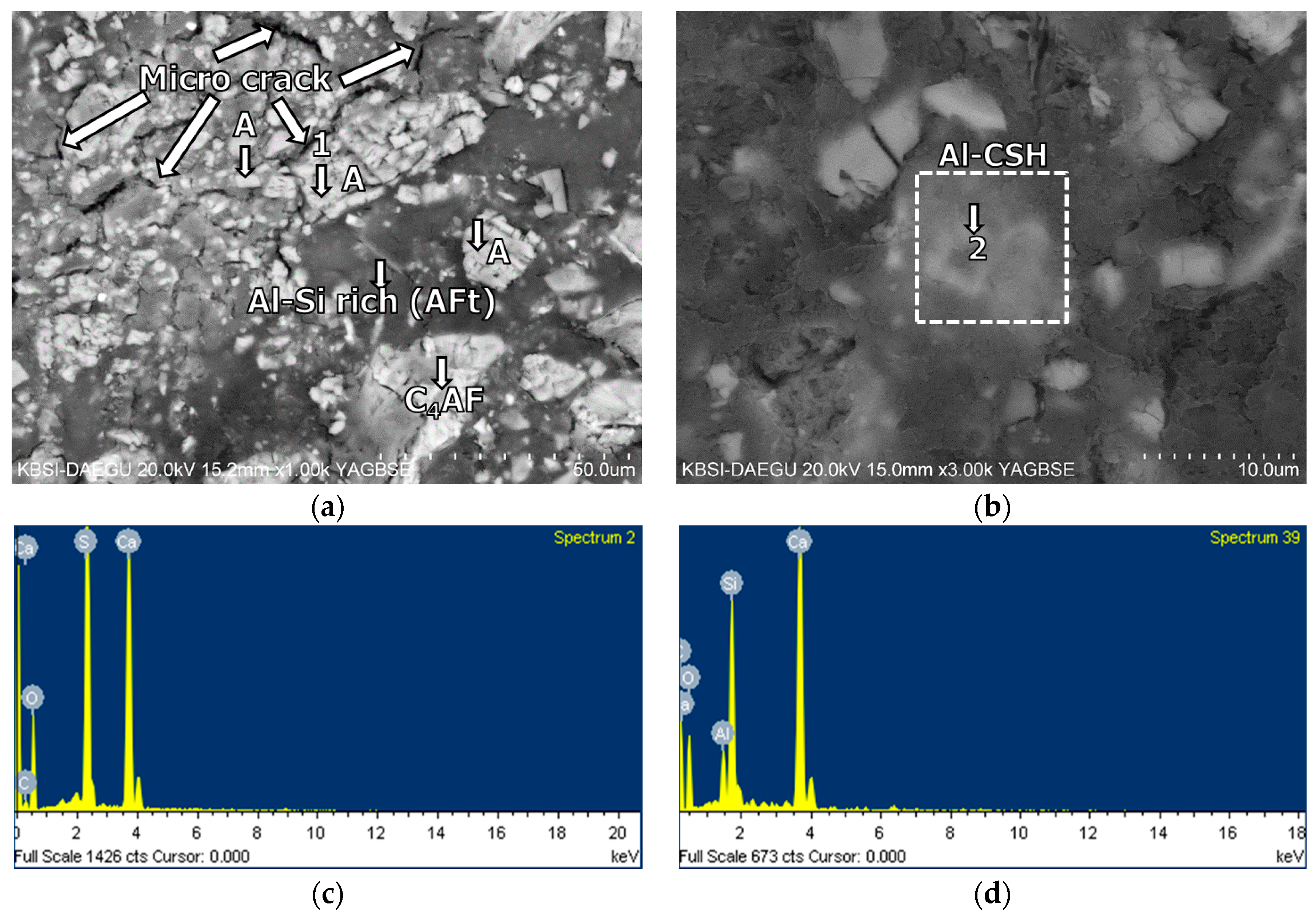
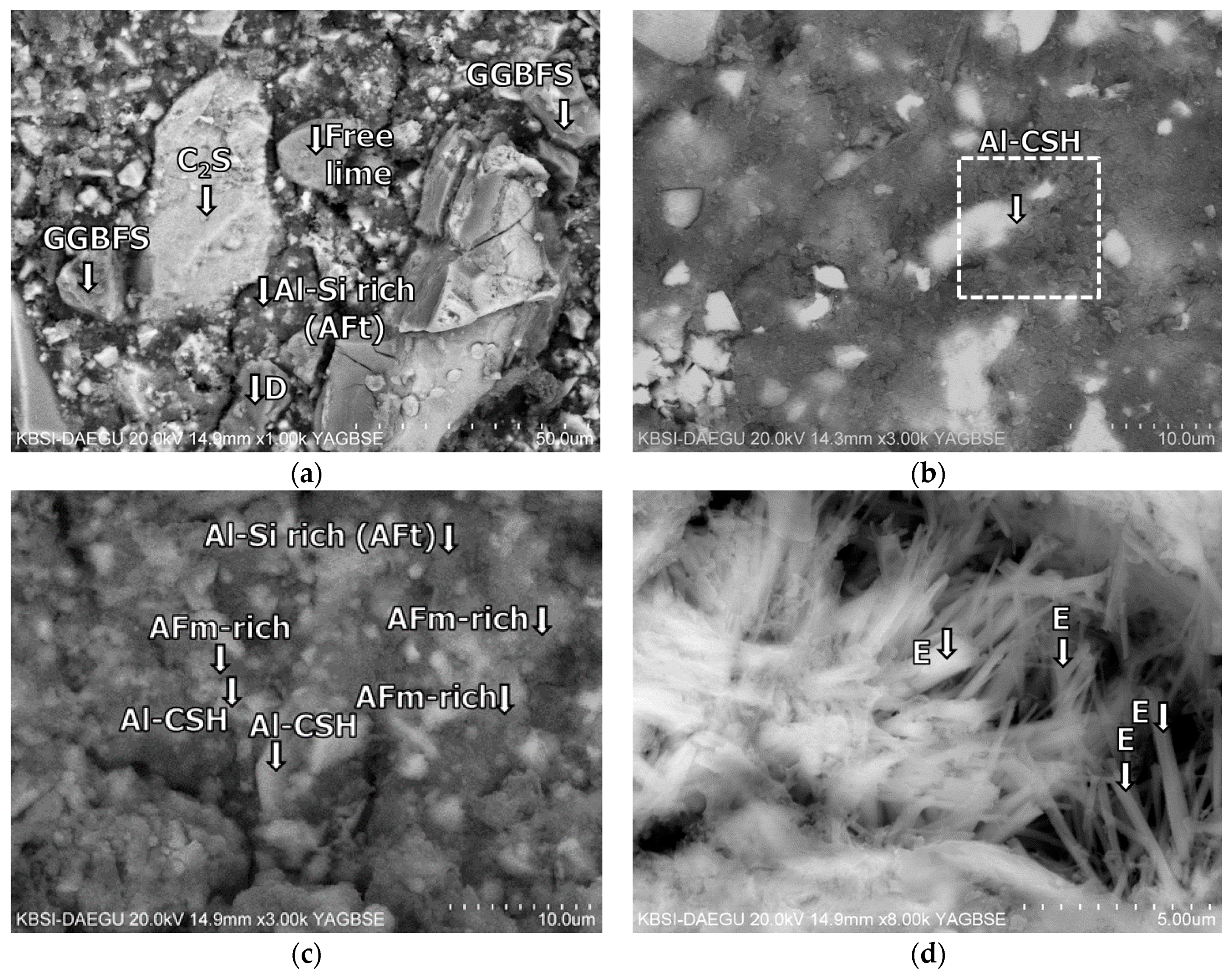
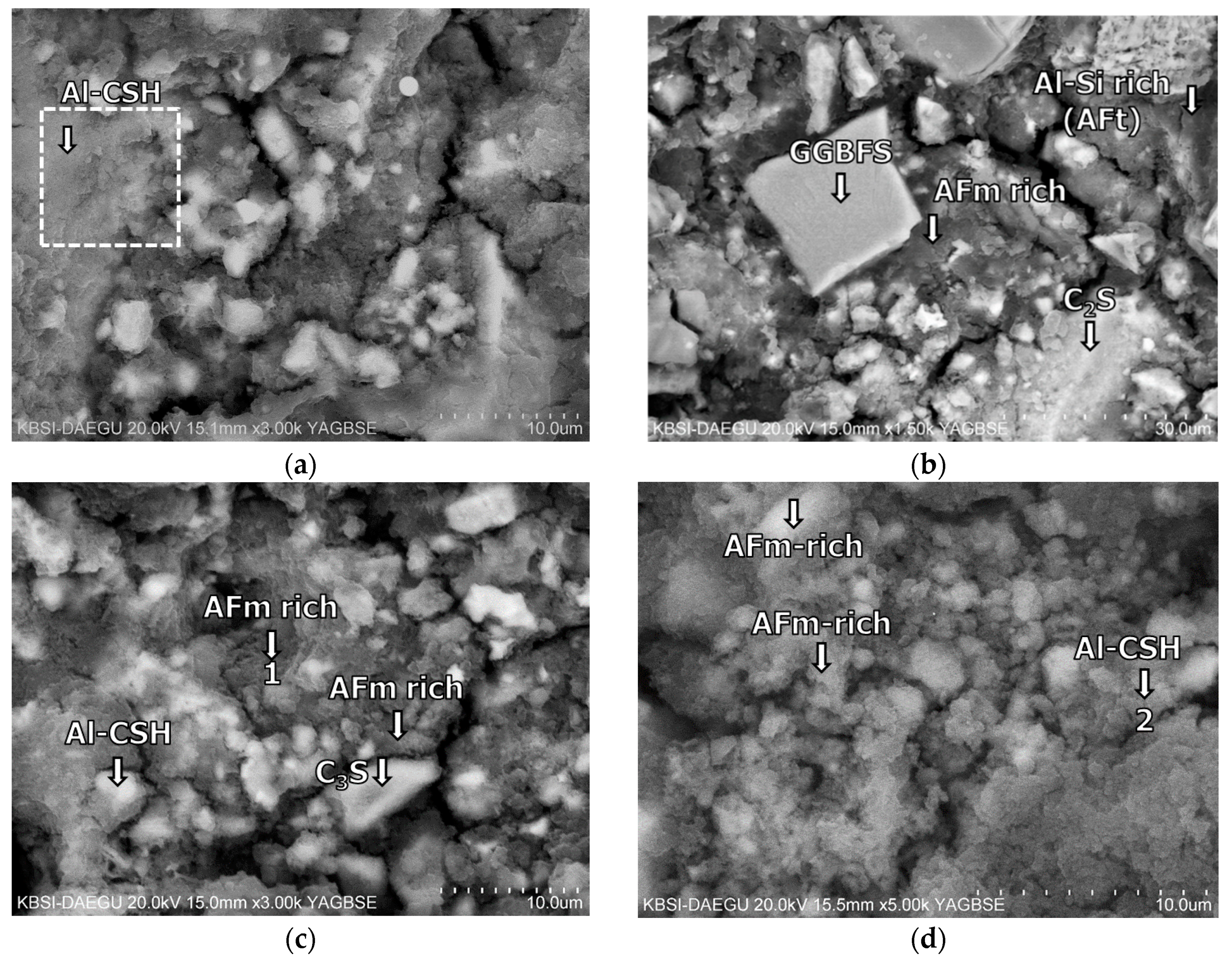
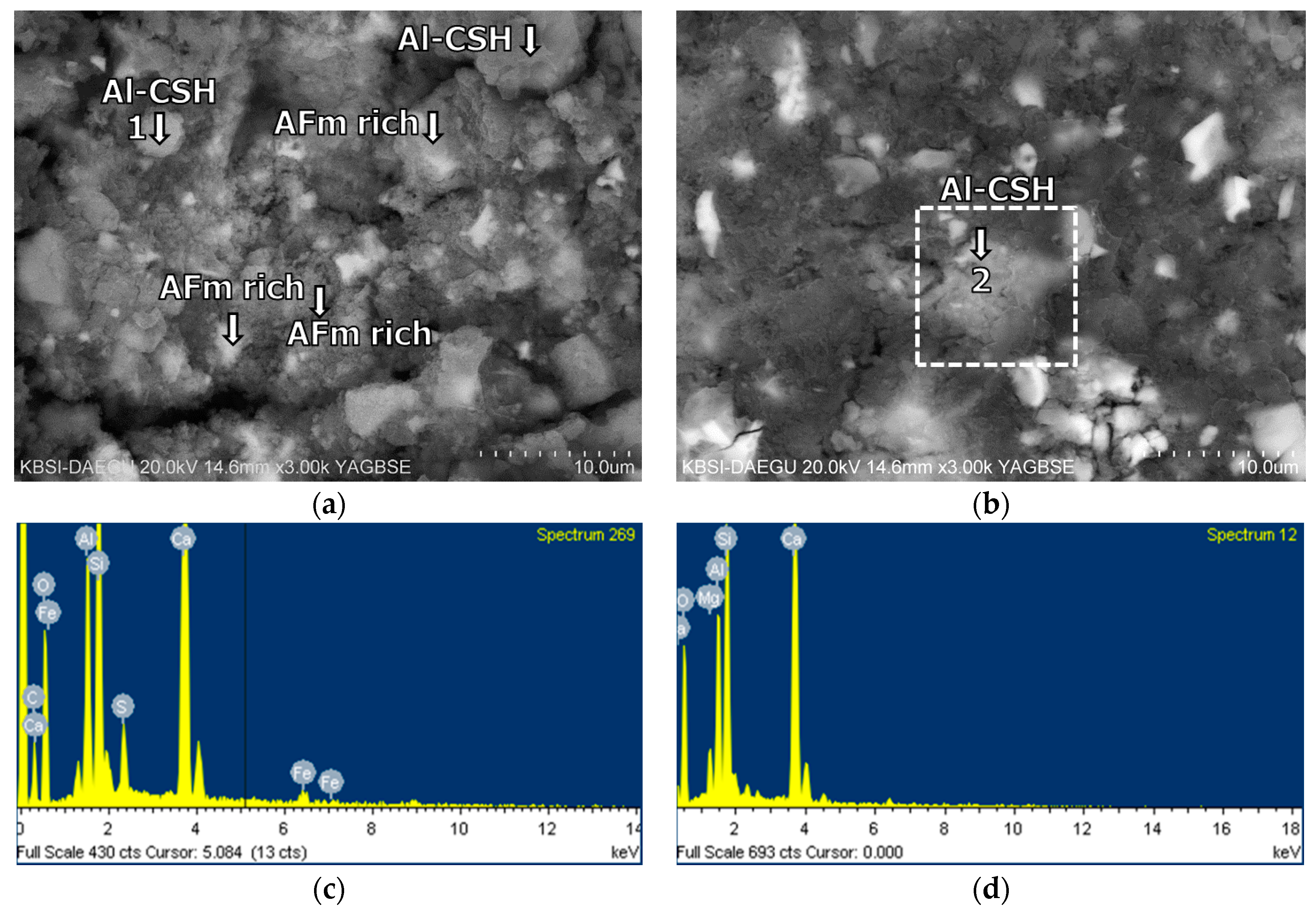
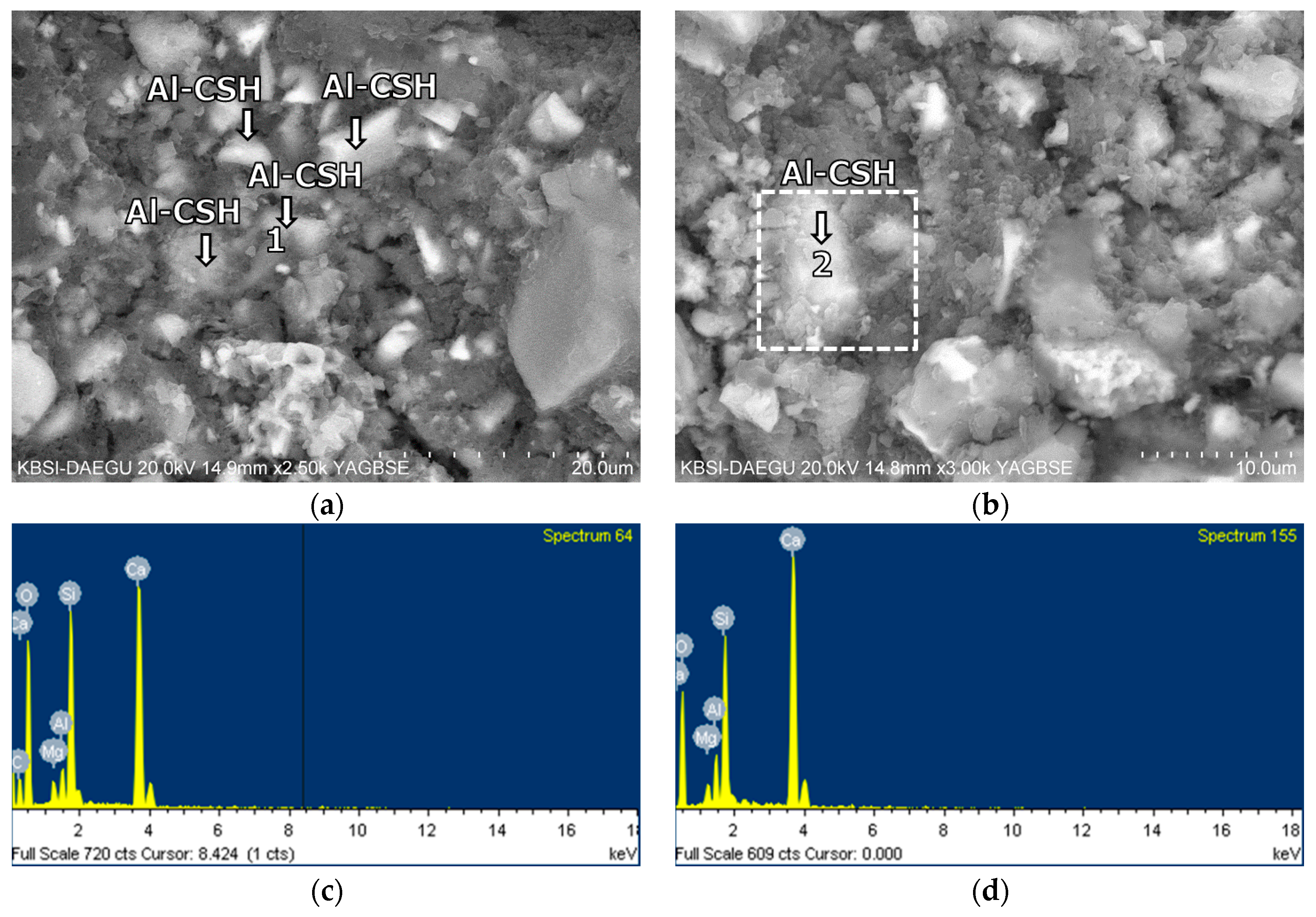
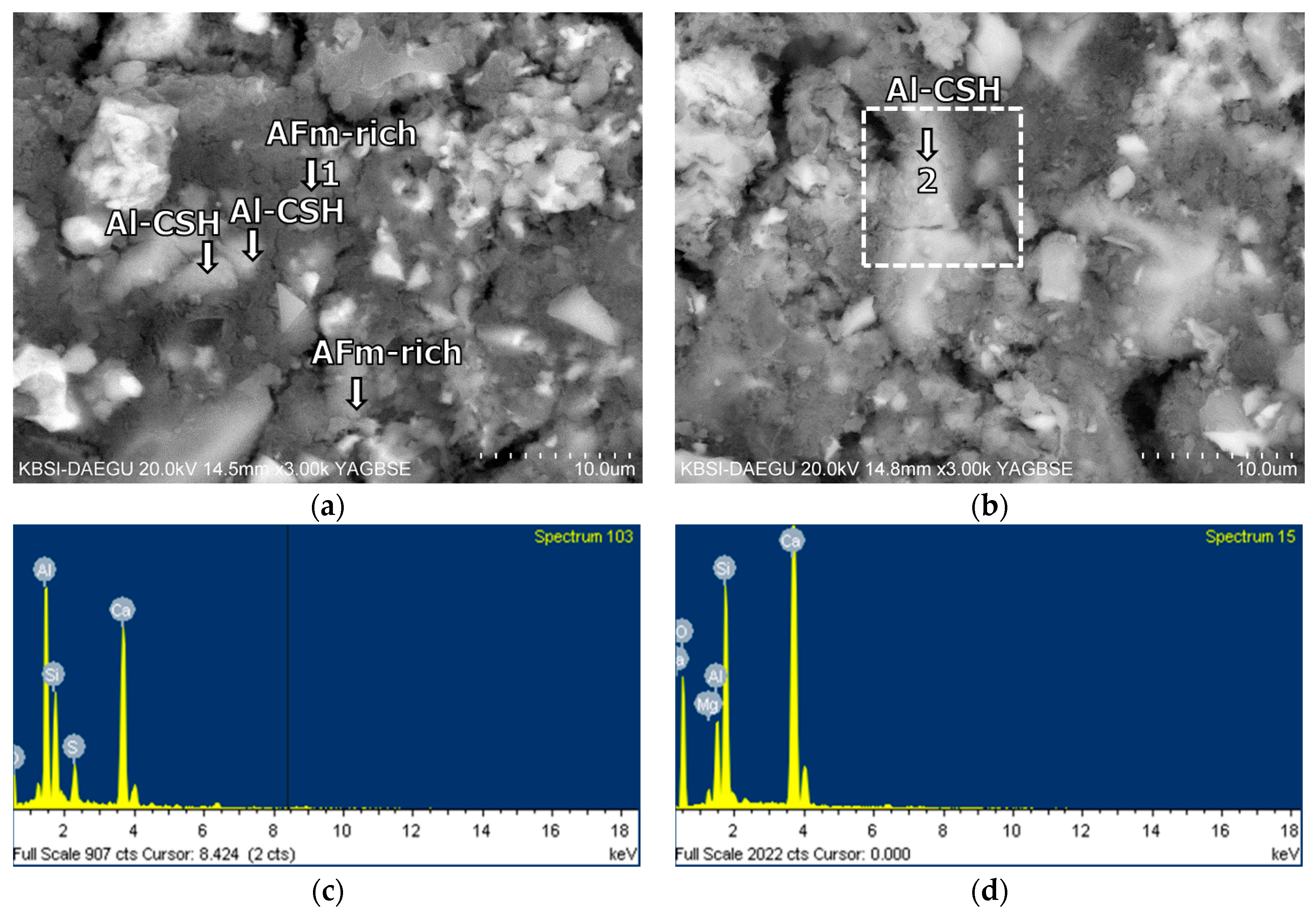
3.5. Morphological Transition
4. Conclusions
- The XRD and SEM results show that the growth of the hydrated phases (for example, ettringite, monosulfate, C-S-Hs) was substantially restrained with the retarders at the very early age (that is, 2–3 h). This likely occurred because the retarders formed hydrophobic barrier layers surrounding anhydrous mineral phases (for example, anhydrite, ye’elimite, C3S, C2S, C3A) in the finely dispersed cement grains. Moreover, the delaying effect of the retarders cumulatively added to the delaying effect of the polymer.
- The use of the retarders increased the ultimate strength of the cement blend systems at the long-term age. Even with a polymer ratio up to 6%, the mortars with the retarders showed higher compressive strengths than the mortar without both retarders and polymers after 28 days of curing. This was likely to happen because the retarders created a finer and denser hydrated cementitious matrix as observed in the MIP results, by increasing the dispersibility of cement grains, their specific surface areas, and the accessibility of water to them.
- Despite variations in the polymer ratio, the compressive strengths of all the mortars with retarders tended to converge at the age of 90 days. This reflects the formation of a more refined pore structure with a higher polymer ratio that compensated the inherited weak strength of the polymer powder itself, as well as the formation of a monolithic co-matrix between the cement hydrates and polymer phases. The authors will examine the combined effects of the set retarders and polymer powder on the ITZs in the near future.
- At the age after 60 days, the sample with retarders and 10% polymer exhibited both the smallest porosity and average pore diameter among all the HCP samples. This highlights that the combined use of retarders and polymer had a synergetic effect to refine the pore structures of the cement blend systems.
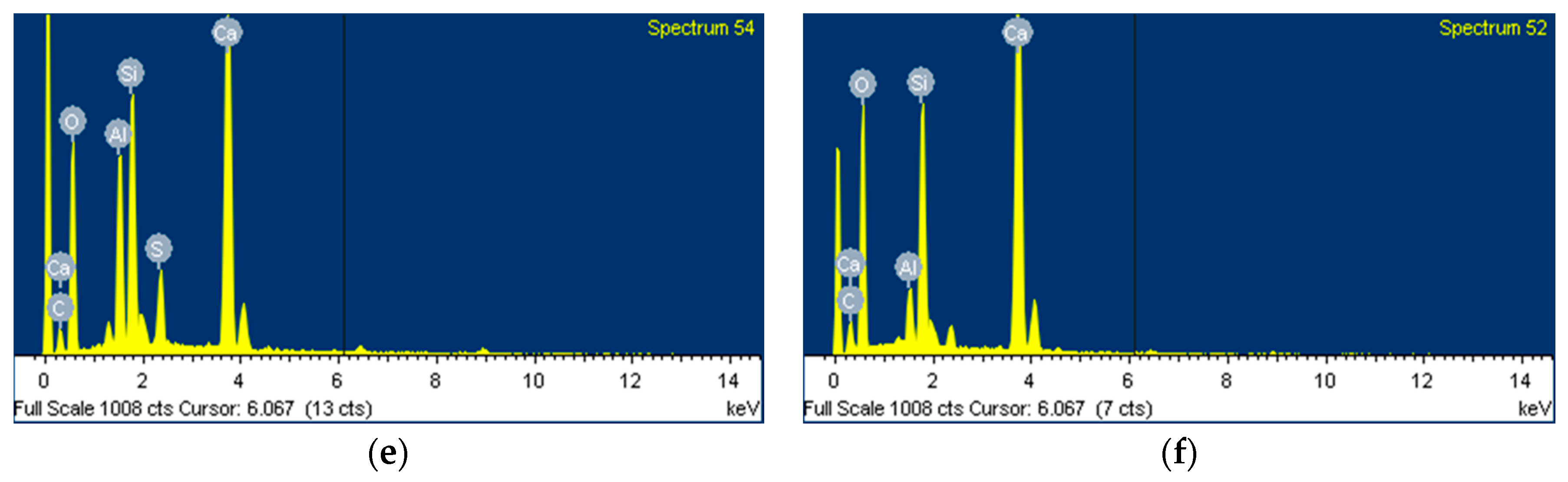
- According to the EDS spot analyses, the paste with the retarders at 60 days of curing had a slightly more Al uptake on average than the paste with no retarders. This supports the compression test results that the mortar with the retarders showed higher strengths than the mortar without retarders after 28 days of curing.
Author Contributions
Funding
Conflicts of Interest
References
- Su, M.; Deng, J.; Wu, Z.; Liu, X. Research on the chemical composition and microstructures of sulpho-aluminate cement clinker. In Proceedings of the 9th International Congress on the Chemistry of Cements, New Delhi, India, 23–28 November 1992; pp. 94–100. [Google Scholar]
- Ali, M.M.; Gopal, S.; Handoo, S.K. Studies on the formation kinetics of calcium sulphoaluminate. Cem. Concr. Res. 1994, 24, 715–720. [Google Scholar] [CrossRef]
- Beretka, J.; de Vito, B.; Santoro, L.; Sherman, N.; Valenti, G.L. Hydraulic behaviour of calcium sulphoaluminate-based cements derived from industrial process wastes. Cem. Concr. Res. 1993, 23, 1205–1214. [Google Scholar] [CrossRef]
- Sahu, S.; Majling, J. Preparation of sulphoaluminate belite cement from fly ash. Cem. Concr. Res. 1994, 24, 1065–1072. [Google Scholar] [CrossRef]
- Gartner, E.M. Industrially interesting approaches to “low-CO2” cements. Cem. Concr. Res. 2004, 34, 1489–1498. [Google Scholar] [CrossRef]
- Sahu, S.; Majling, J. Phase compatibility in the system CaO–SiO2–Al2O3–Fe2O3–SO3 referred to sulphoaluminate belite cement clinker. Cem. Concr. Res. 1993, 23, 1331–1339. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C.G. Incorporation of coal combustion residuals into calcium sulfoaluminate–belite cement clinkers. Cem. Concr. Res. 2012, 34, 893–902. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F.P. Hydration of calcium sulfoaluminate cement at less than 24 h. Adv. Cem. Res. 2002, 14, 141–155. [Google Scholar] [CrossRef]
- Lee, I.W.; Pyo, S.; Jung, Y.H. Development of quick-hardening infilling materials for composite railroad tracks to strengthen existing ballasted track. Compos. Part B Eng. 2016, 92, 37–45. [Google Scholar] [CrossRef]
- Jang, S.Y.; Lee, I.W.; Kang, Y.S. Track system technology for future advanced railway construction. J. Korean Soc. Civ. Eng. 2013, 61, 41–51. (In Korean) [Google Scholar]
- Gwon, S.; Jang, S.Y.; Shin, M. Age-dependent hydration characteristics of calcium sulfoaluminate and Portland cement blend containing redispersible polymer powder. Constr. Build. Mater 2018. under review. [Google Scholar]
- Czarnecki, L. Polymer concretes. Cem. Wapno Beton 2010, 15, 63–85. [Google Scholar]
- Czarnecki, L.; Łukowski, P. Polymer-cement concretes. Cem. Wapno Beton 2010, 5, 243–258. [Google Scholar]
- Zingg, A. Cement-Superplasticizer Interaction: Link between Macroscopic Phenomena and Microstructural Data of the Early Cement Hydration. Ph.D. Thesis, Swiss Federal Institute of Technology, Zurich, Switzerland, 2008. [Google Scholar]
- Ortega, J.M.; Sánchez, I.; Climent, M.A. Impedance spectroscopy study of the effect of environmental conditions in the microstructure development of OPC and slag cement mortars. Arch. Civ. Mech. Eng. 2015, 15, 569–583. [Google Scholar] [CrossRef]
- Joshaghani, A.; Balapour, M.; Ramezanianpour, A.A. Effect of controlled environmental conditions on mechanical, microstructural and durability properties of cement mortar. Constr. Build. Mater. 2018, 164, 134–149. [Google Scholar] [CrossRef]
- Ortega, J.M.; Esteban, M.D.; Rodríguez, R.R.; Pastor, J.L.; Ibanco, F.J.; Sánchez, I.; Climent, M.Á. Long-Term Behaviour of fly ash and slag cement grouts for micropiles exposed to a sulphate aggressive medium. Materials 2017, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Ramezanianpour, A.A.; Malhotra, V.M. Effect of curing on the compressive strength, resistance to chloride-ion penetration and porosity of concretes incorporating slag, fly ash or silica fume. Cem. Concr. Compos. 1995, 17, 125–133. [Google Scholar] [CrossRef]
- Standard Specification for Chemical Admixtures for Concrete; ASTM C494/C494M-04; ASTM International: West Conshohocken, PA, USA, 1999.
- Mailvaganam, N.P.; Rixom, M.R. Chemical Admixtures for Concrete; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Cement Admixtures Association. “Admixture Technical Sheet—ATS3—Set Retarding”. 2012. Available online: http://www.admixtures.org.uk/publications/admixture-technical-sheets (accessed on 16 May 2018).
- Young, J.F. A review of the mechanisms of set-retardation in Portland cement pastes containing organic admixtures. Cem. Concr. Res. 1972, 2, 415–433. [Google Scholar] [CrossRef]
- Singh, N.B.; Singh, A.K.; Singh, S.P. Effect of citric acid on the hydration of Portland cement. Cem. Concr. Res. 1986, 16, 911–920. [Google Scholar] [CrossRef]
- Richard, L.A.; Keenum John, C., Jr. Cement Set Retarding Composition. U.S. Patent No 3,429,724, 25 February 1969. [Google Scholar]
- Papageorgiou, A.; Tzouvalas, G.; Tsimas, S. Use of inorganic setting retarders in cement industry. Cem. Concr. Compos. 2005, 27, 183–189. [Google Scholar] [CrossRef]
- Blanc, A.; Fontaine, C. Setting-Retarder and its Application to Concrete, Mortars and/or Grouts. U.S. Patent No. 5,976,242, 2 November 1999. [Google Scholar]
- Standard Specification for Portland Cement; ASTM C150/C150M-18; ASTM International: West Conshohocken, PA, USA, 2018.
- Sugama, T.; Brothers, L. Citric Acid as a Set Retarder for Calcium Aluminate Phosphate Cements; (No. BNL–73600-2005-IR); Brookhaven National Laboratory: Upton, NY, USA, 2005. [Google Scholar]
- Peysson, S. Contribution à L’étude de la Stabilization de Déchets par du Ciment Sulfoalumineux. Ph.D. Thesis, INSA de Lyon, Villeurbanne, France, 2005. [Google Scholar]
- Wilding, C.R.; Walter, A.; Double, D.D. A classification of inorganic and organic admixtures by conduction calorimetry. Cem. Concr. Res. 1984, 14, 185–194. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Lowery, M.S. Conduction calorimetric investigation of the effect of retarders on the hydration of Portland cement. Thermochim. Acta 1992, 195, 373–387. [Google Scholar] [CrossRef]
- Pelletier, L.; Winnefeld, F.; Lothenbach, B. The ternary system Portland cement–calcium sulphoaluminate clinker–anhydrite: Hydration mechanism and mortar properties. Cem. Concr. Compos. 2010, 32, 497–507. [Google Scholar] [CrossRef]
- Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens); ASTM C109/C109M-16a; ASTM International: West Conshohocken, PA, USA, 2016.
- Zhang, J.; Scherer, G.W. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar] [CrossRef]
- Aligizaki, K.K. Pore Structure of Cement-Based Materials: Testing, Interpretation and Requirements; Taylor & Francis: Boca Raton, FL, USA, 2006. [Google Scholar]
- Taylor, H.F. Cement Chemistry; Thomas Telford: London, UK, 1997. [Google Scholar]
- Diao, J.J. (Ed.) Production and Application of Chinese CSAs; Chinese Buidling Material Industrial Press: Chongqing, China, 2006. (In Chinese) [Google Scholar]
- Alaoui, A.; Nguyen, V.H.; Divet, L.; Feraille, A.; le Roy, R. Experimental studies of hydration mechanism of sulfoaluminate clinker. In Proceedings of the 12th International Congress on the Chemistry of Cement, Montreal, QC, Canada, 8–13 July 2007. [Google Scholar]
- Kurdowski, W. Cement and Concrete Chemistry; Springer Science & Business: Berlin, Germany, 2014. [Google Scholar]
- Khan, B. Effect of a retarding admixture on the setting time of cement pastes in hot weather. Eng. Sci. 2004, 15, 63–79. [Google Scholar]
- Banfill, P.F.G.; Saunders, D.C. The relationship between the sorption of organic compounds on cement and the retardation of hydration. Cem. Concr. Res. 1986, 16, 399–410. [Google Scholar] [CrossRef]
- Ohama, Y. Handbook of Polymer-Modified Concrete and Mortars: Properties and Process Technology; William Andrew: Norwich, NY, USA, 1995. [Google Scholar]
- Beeldens, A.; van Gemert, D. Paste-aggregate adhesion in polymer-cement porous concrete. In Proceedings of the 2nd International RILEM Symposium on Adhesion between Polymers and Concrete—ISAP; Puterman, M., Ed.; RILEM Publications: Bagneux, France, 1999; Volume 99, pp. 93–102. [Google Scholar]
- Short, N.R.; Salbin, M.K. The Influence of Curing Conditions on the Surface Porosity of Polymer-Modified Cements; Special Publication: Tulsa, OK, USA, 1997; Volume 170, pp. 1009–1028. [Google Scholar]
- Richardson, I.G. The nature of C-S-H in hardened cements. Cem. Concr. Res. 1999, 29, 1131–1147. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]



| Mixture Label | Mortar (Unit: kg/m3) | |||||||||
| Water | Cement | Sand | Polymer | Retarder A | Retarder B | |||||
| M-0-N | 339 | 892 | 892 | 0 | 0 | 0 | ||||
| M-0-Y | 339 | 892 | 892 | 0 | 1.43 | 1.07 | ||||
| M-2-Y | 339 | 892 | 892 | 17.9 | 1.43 | 1.07 | ||||
| M-6-Y | 339 | 892 | 892 | 53.6 | 1.43 | 1.07 | ||||
| M-10-Y | 339 | 892 | 892 | 89.3 | 1.43 | 1.07 | ||||
| Mixture Label | Cement Paste (Unit: kg/m3) | |||||||||
| Water | Cement | Polymer | Retarder A | Retarder B | ||||||
| B-0-N | 512 | 1346 | 0 | 0 | 0 | |||||
| B-0-Y | 512 | 1346 | 0 | 2.15 | 1.62 | |||||
| B-2-Y | 512 | 1346 | 26.9 | 2.15 | 1.62 | |||||
| B-6-Y | 512 | 1346 | 80.8 | 2.15 | 1.62 | |||||
| B-10-Y | 512 | 1346 | 134.6 | 2.15 | 1.62 | |||||
| Oxide in Cement Blend (wt.%) | ||||||
|---|---|---|---|---|---|---|
| CaO | SO3 | SiO2 | Al2O3 | MgO | Fe2O3 | Others |
| 54.6 | 14.6 | 12.4 | 11.8 | 2.3 | 2.3 | 2.0 |
| Oxide Composition (wt.%) | CaO | SO3 | SiO2 | Others |
| 24.9 | 45.9 | 26.2 | 3.0 | |
| Element Composition (wt.%) | Carbon | Hydrogen | Sulfur | Nitrogen |
| 71.1 | 7.7 | 5.3 | 0.7 |
| Age | 2 h | 1 Day | 7 Days | 28 Days | 60 Days | 90 Days |
|---|---|---|---|---|---|---|
| Mixture Label | Compressive Strength (MPa) | |||||
| Average (std. dev.) | Average (std. dev.) | Average (std. dev.) | Average (std. dev.) | Average (std. dev.) | Average (std. dev.) | |
| M-0-N | 25.8 (0.14) | 35.8 (2.65) | 44.1 (1.72) | 49.8 (4.21) | 62.0 (3.84) | 62.0 (4.17) |
| M-0-Y | 16.3 (0.60) | 35.7 (1.05) | 45.3 (2.20) | 54.8 (5.91) | 67.2 (4.34) | 63.7 (0.75) |
| M-2-Y | 13.0 (0.07) | 32.6 (1.38) | 38.4 (0.90) | 53.4 (2.40) | 64.4 (4.92) | 63.8 (5.65) |
| M-6-Y | 11.3 (0.35) | 30.9 (1.40) | 36.1 (1.54) | 52.6 (2.14) | 61.1 (5.39) | 61.7 (1.94) |
| M-10-Y | 8.0 (0.08) | 28.1 (1.00) | 32.2 (0.15) | 41.4 (1.64) | 53.2 (2.18) | 56.6 (4.26) |
| Mixture Label | Age (day) | Total Porosity (%) | Average Pore Diameter (nm) |
|---|---|---|---|
| B-0-N | 1 | 27.5 | 27.9 |
| 60 | 23.8 | 42.8 | |
| B-0-Y | 1 | 25.5 | 23.6 |
| 60 | 20.0 | 52.4 | |
| B-10-Y | 1 | 24.5 | 34.5 |
| 28 | 20.1 | 21.0 | |
| 60 | 18.1 | 21.4 |
| Mixture Label | Curing Age | Average Ca/Si Ratio | Average Al/Ca Ratio |
|---|---|---|---|
| B-0-N | 2 h | 1.68 | 0.072 |
| 1 day | 1.47 | 0.103 | |
| 60 days | 1.30 | 0.242 | |
| B-0-Y | 60 days | 1.26 | 0.254 |
| B-10-Y | 28 days | 1.57 | 0.082 |
| 60 days | 1.59 | 0.091 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwon, S.; Jang, S.Y.; Shin, M. Combined Effects of Set Retarders and Polymer Powder on the Properties of Calcium Sulfoaluminate Blended Cement Systems. Materials 2018, 11, 825. https://doi.org/10.3390/ma11050825
Gwon S, Jang SY, Shin M. Combined Effects of Set Retarders and Polymer Powder on the Properties of Calcium Sulfoaluminate Blended Cement Systems. Materials. 2018; 11(5):825. https://doi.org/10.3390/ma11050825
Chicago/Turabian StyleGwon, Seongwoo, Seung Yup Jang, and Myoungsu Shin. 2018. "Combined Effects of Set Retarders and Polymer Powder on the Properties of Calcium Sulfoaluminate Blended Cement Systems" Materials 11, no. 5: 825. https://doi.org/10.3390/ma11050825
APA StyleGwon, S., Jang, S. Y., & Shin, M. (2018). Combined Effects of Set Retarders and Polymer Powder on the Properties of Calcium Sulfoaluminate Blended Cement Systems. Materials, 11(5), 825. https://doi.org/10.3390/ma11050825







