Impacts of Different Functional Groups on the Kinetic Rates of α-Amine Ketoximesilanes Hydrolysis in the Preparation of Room Temperature Vulcanized Silicone Rubber
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Instruments
2.3. Synthesis of α-Amine Ketoximesilanes
2.4. α-Amine Ketoximesilanes Hydrolysis
2.5. Preparation of RTV Silicone Rubber
3. Results and Discussion
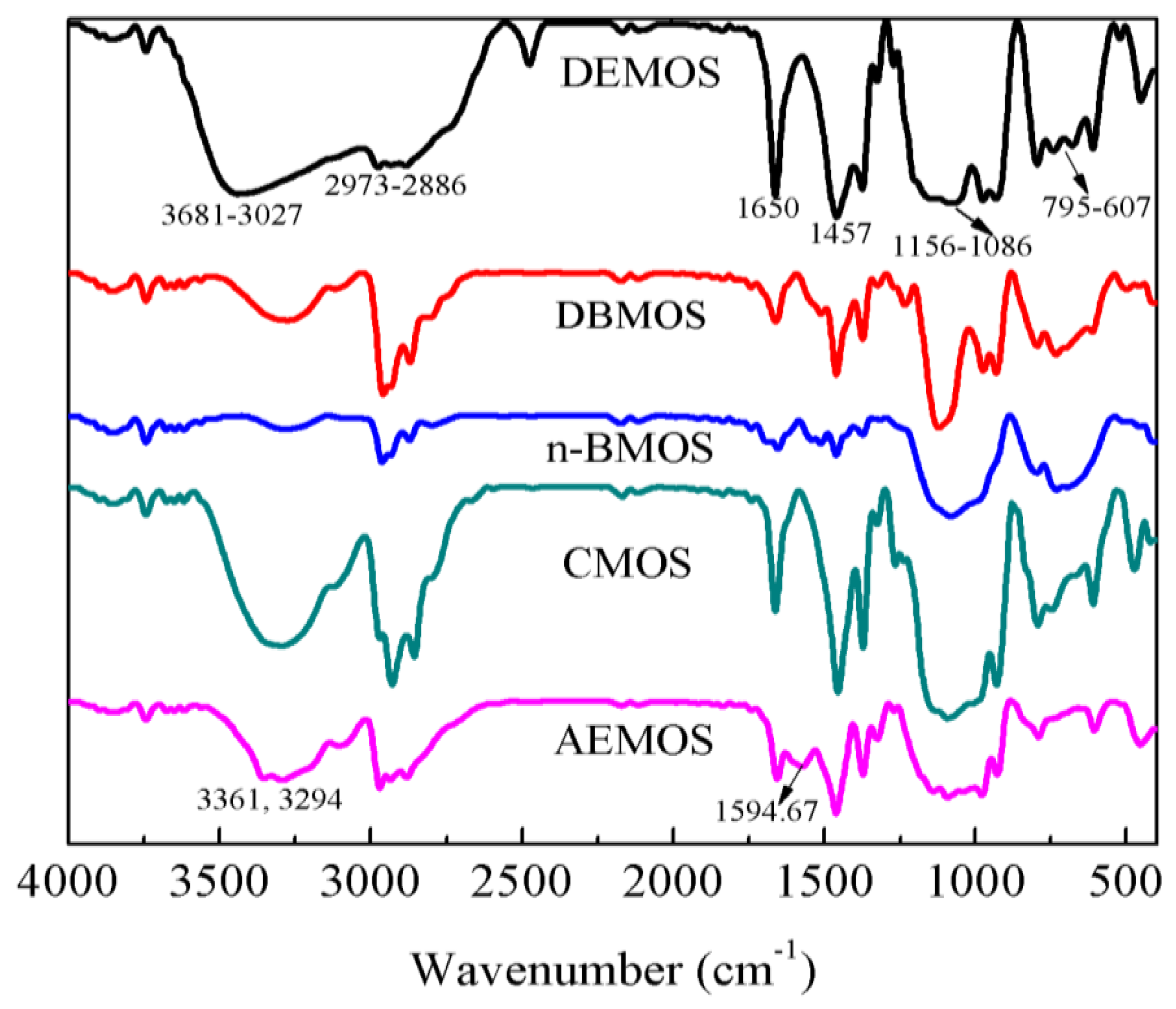
3.1. Synthesis and Characterization
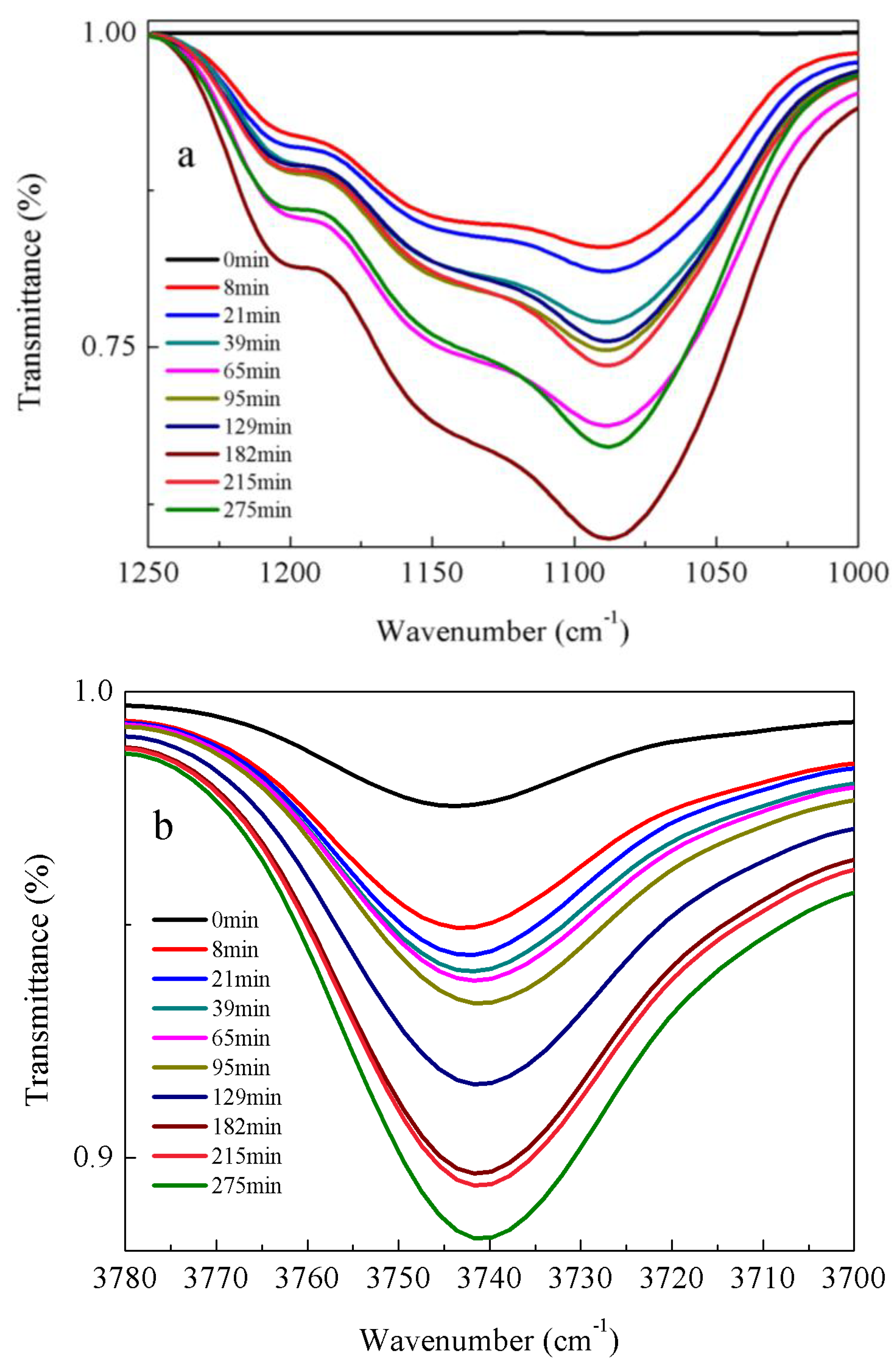
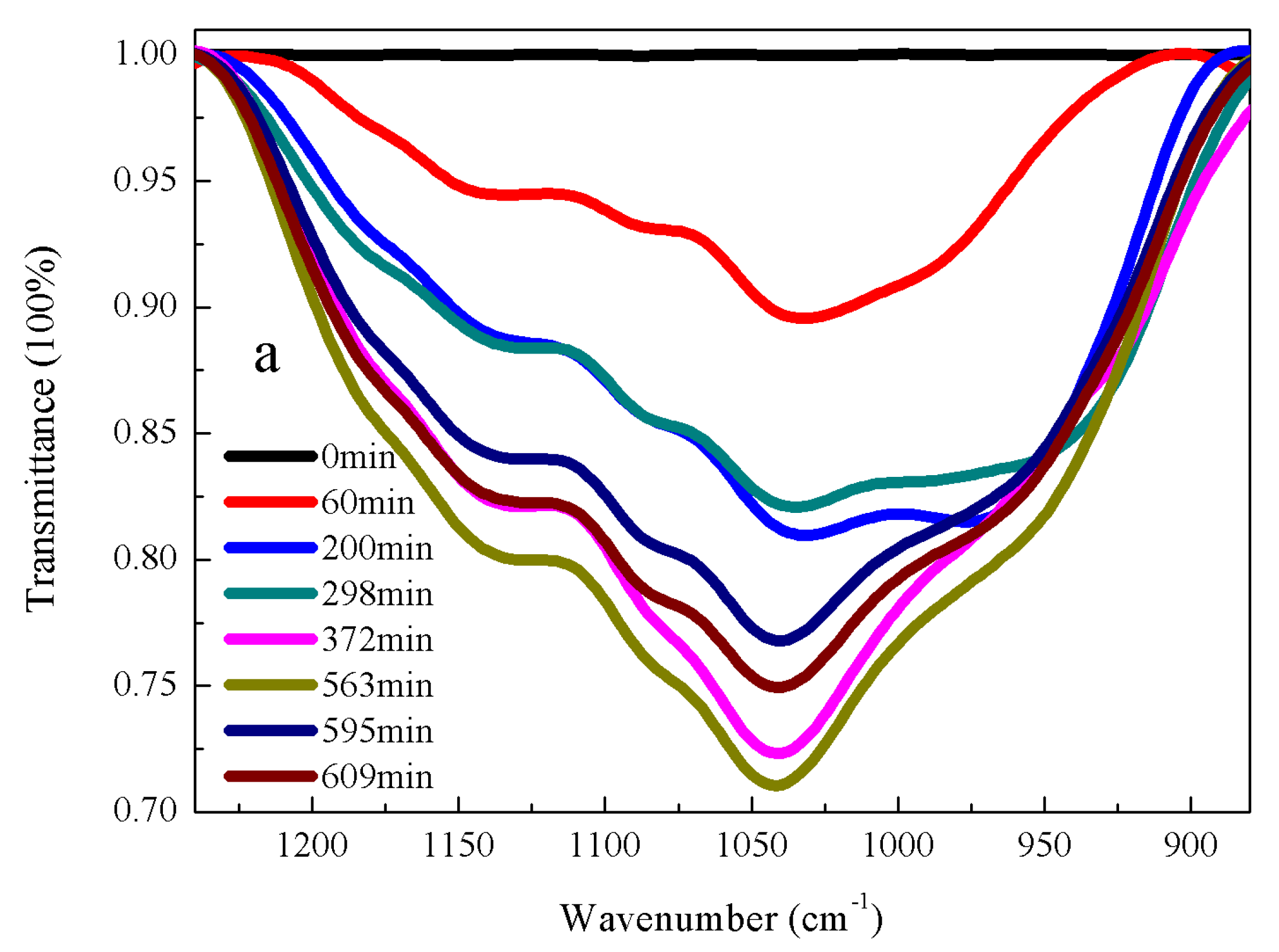
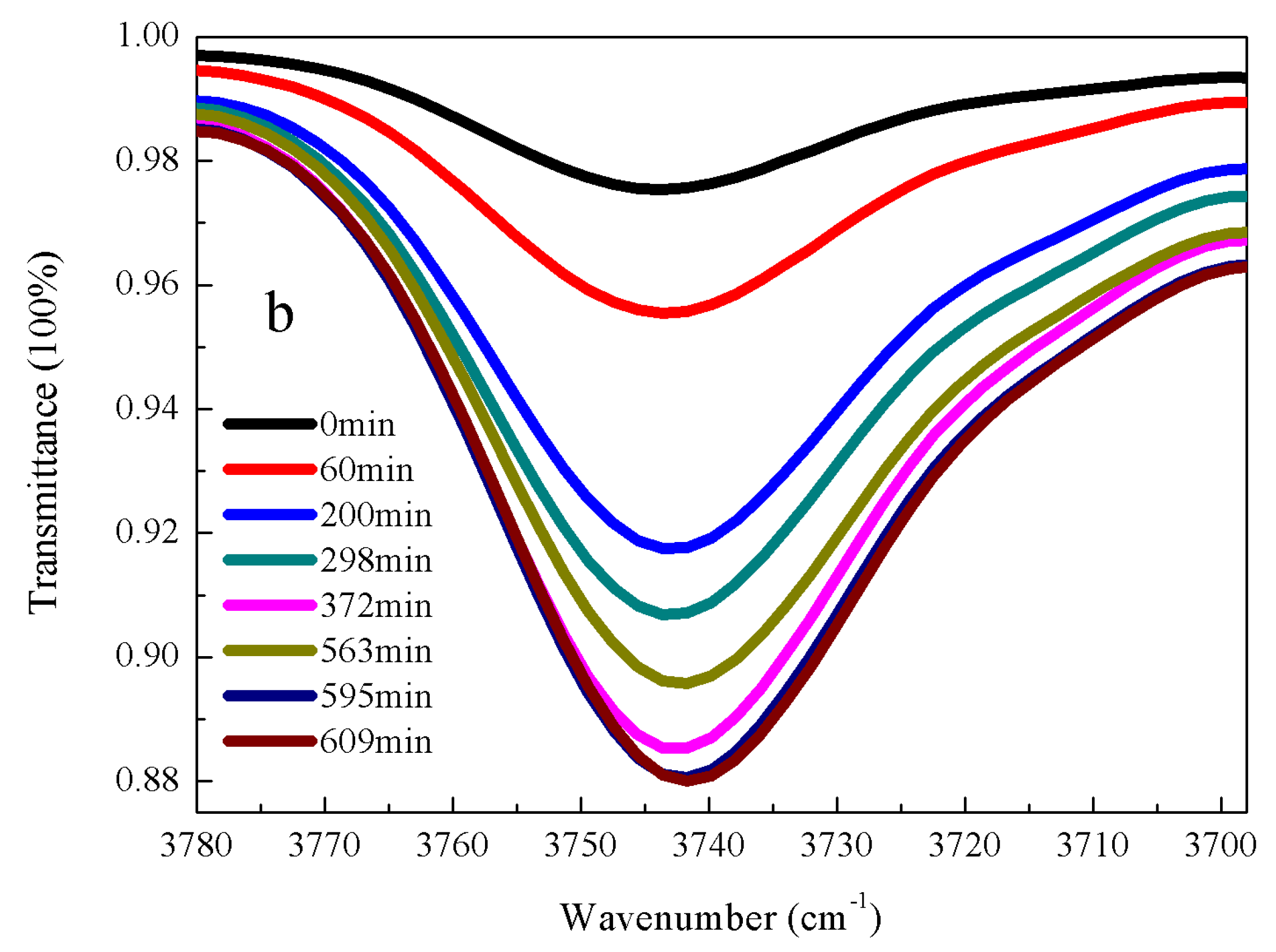
3.2. Kinetic Rate of α-Amine Ketoximesilanes Hydrolysis
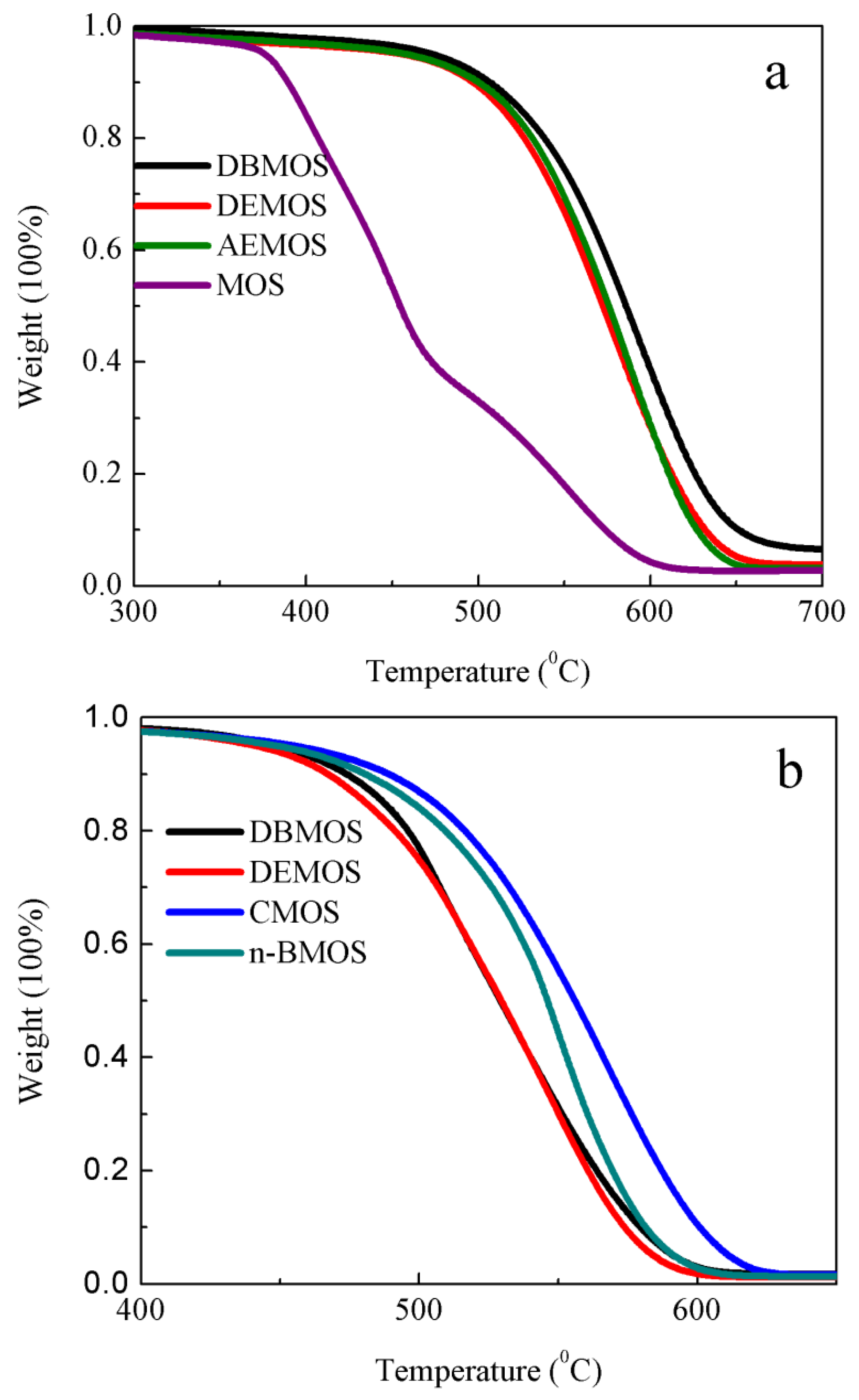
3.3. Thermal Stability of RTV Silicone Rubber
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pelster, S.A.; Schrader, W.; Schüth, F. Monitoring temporal evolution of silicate species during hydrolysis and condensation of silicates using mass spectrometry. J. Am. Chem. Soc. 2006, 128, 4310–4317. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Feng, J.; Zhang, W.; Qu, J.E. Experimental and computational study on hydrolysis and condensation kinetics of γ-glycidoxypropyltrimethoxysilane (γ-GPS). Appl. Surf. Sci. 2010, 257, 990–996. [Google Scholar] [CrossRef]
- Diao, S.; Dong, F.; Meng, J.; Ma, P.; Zhao, Y.; Feng, S. Preparation and properties of heat-curable silicone rubber through chloropropyl/amine crosslinking reactions. Mater. Chem. Phys. 2015, 153, 161–167. [Google Scholar] [CrossRef]
- Wang, Z.; Nelson, K.J.; Hillborg, H.; Zhao, S.; Schadler, L.S. Graphene oxide filled nanocomposite with novel electrical and dielectric properties. Adv. Mater. 2012, 24, 3134–3137. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Don, T.M.; Lee, H.S.J.; Sha, Y.O. Studies on the aminolysis of RTV silicone rubber and modifications of degradation products. Polym. Degrad. Stab. 2004, 85, 769–777. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Olsson, R.T.; Hedenqvist, M.S. The role of interfaces in polyethylene/metal-oxide nanocomposites for ultrahigh-voltage insulating materials. Adv. Mater. 2018, 30, 1703624. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Kim, J.; Nguyen, T.D.; Lisko, B.; Purohit, P.K.; McAlpine, M.C. Enhanced piezoelectricity and stretchability in energy harvesting devices fabricated from buckled PZT ribbons. Nano Lett. 2011, 11, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Liles, D.T.; Lin, F. Silicone elastomeric particles in skin care applications. ACS. Ser. Polym. Deliv. Ther. 2013, 1053, 207–219. [Google Scholar]
- Almarzouqi, M.H.; Marzouk, S.A.M.; EI-Naas, M.; Abdullatif, N. Removal from CO2−CH4 gas mixture using different solvents and hollow fiber membranes. Ind. Eng. Chem. Res. 2009, 48, 3600–3605. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, S. Hydrogen-containing silicone resin as the crosslinking agent of silicone rubber. J. Appl. Polym. Sci. 2003, 49, 139–142. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, L.; Pan, H.; Fang, S. Influence principle of mechanical properties of additional RTV silicone rubber. Silicone Mater. 2011, 5, 314–317. [Google Scholar]
- Zhao, S.; Feng, S. Vinyl-containing silicone resin as the crosslinking aging of heat-curable silicone rubber. J. Appl. Polym. Sci. 2002, 83, 3123–3127. [Google Scholar] [CrossRef]
- Dong, F.; Diao, S.; Ma, D.; Zhang, S.; Feng, S. Preparation and characterization of 3-chloropropyl polysiloxane-based heat-curable silicone rubber using polyamidoamine dendrimers as cross-linkers. React. Funct. Polym. 2015, 96, 14–20. [Google Scholar] [CrossRef]
- Torry, S.A.; Campbell, A.; Cunliffe, A.V.; Tod, D.A. Kinetic analysis of organosilane hydrolysis and condensation. Int. J. Adhes. Adhes. 2006, 26, 40–49. [Google Scholar] [CrossRef]
- Dubitsky, Y.; Zaopo, A.; Zannoni, G.; Zetta, L. 1H NMR study of the hydrolysis of vinyltrialkoxysilanes. Mater. Chem. Phys. 2000, 64, 45–53. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, G.; Nie, L.; Dinse, A.; Buda, C.; Shabaker, J.; Resasco, D.E. Different product distributions and mechanistic aspects of the hydrodeoxygenation of m-cresol over platinum and ruthenium catalysts. ACS Catal. 2015, 5, 6271–6283. [Google Scholar] [CrossRef]
- Chen, D.; Yi, S.; Wu, W.; Zhong, Y.; Liao, J.; Huang, C.; Shi, W. Synthesis and characterization of novel room temperature vulcanized (RTV) silicone rubbers using vinyl-POSS derivatives as cross linking agents. Polymer 2010, 51, 3867–3878. [Google Scholar] [CrossRef]
- Chen, D.; Yi, S.; Fang, P.; Zhong, Y.; Huang, C.; Wu, X. Synthesis and characterization of novel room temperature vulcanized (RTV) silicone rubbers using octa[(trimethoxysilyl)ethyl]-POSS as cross-linker. React. Funct. Polym. 2011, 71, 502–511. [Google Scholar] [CrossRef]
- Amgoune, A.; Krumova, M.; Mecking, S. Nanoparticle-supported molecular polymerization catalysts. Macromolecules 2008, 22, 8388–8396. [Google Scholar] [CrossRef][Green Version]
- Chojnowski, J.; Kurjata, J.; Fortuniak, W.; Rubinsztajn, W.; Trzebicka, B. Hydride transfer ring-opening polymerization of a cyclic oligomethylhydrosiloxane. Route to a polymer of closed multicyclic structure. Macromolecules 2012, 45, 2654–2661. [Google Scholar] [CrossRef]
- Berg, D.T.; Joffre, E.J. Elastomers from Silicone Emulsions Having Self-Catalytic Crosslinkers. US Patent 5994459, 30 September 1997. [Google Scholar]
- Zhao, Q.; Liu, Q.; Xu, H.; Bei, Y. Preparation and characterization of room temperature vulcanized silicone rubber using a-amine ketoximesilanes as auto-catalyzed cross-linkers. RSC Adv. 2016, 6, 38447–38453. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, Z.; Wang, X. Kinetic study of methyltriethoxysilane (MTES) hydrolysis by FTIR spectroscopy under different temperatures and solvents. Vib. Spectrosc. 2008, 46, 1–7. [Google Scholar] [CrossRef]
- Liu, D.; Pourrahimi, A.M.; Pallon, K.H.; Andersson, R.L.; Hedenquvist, M.S.; Gedde, U.W.; Olsson, R.T. Morphology and properties of silica-based coatings with different functionalities for Fe3O4, ZnO and Al2O3 nanoparticles. RSC Adv. 2015, 5, 48094–48103. [Google Scholar] [CrossRef]
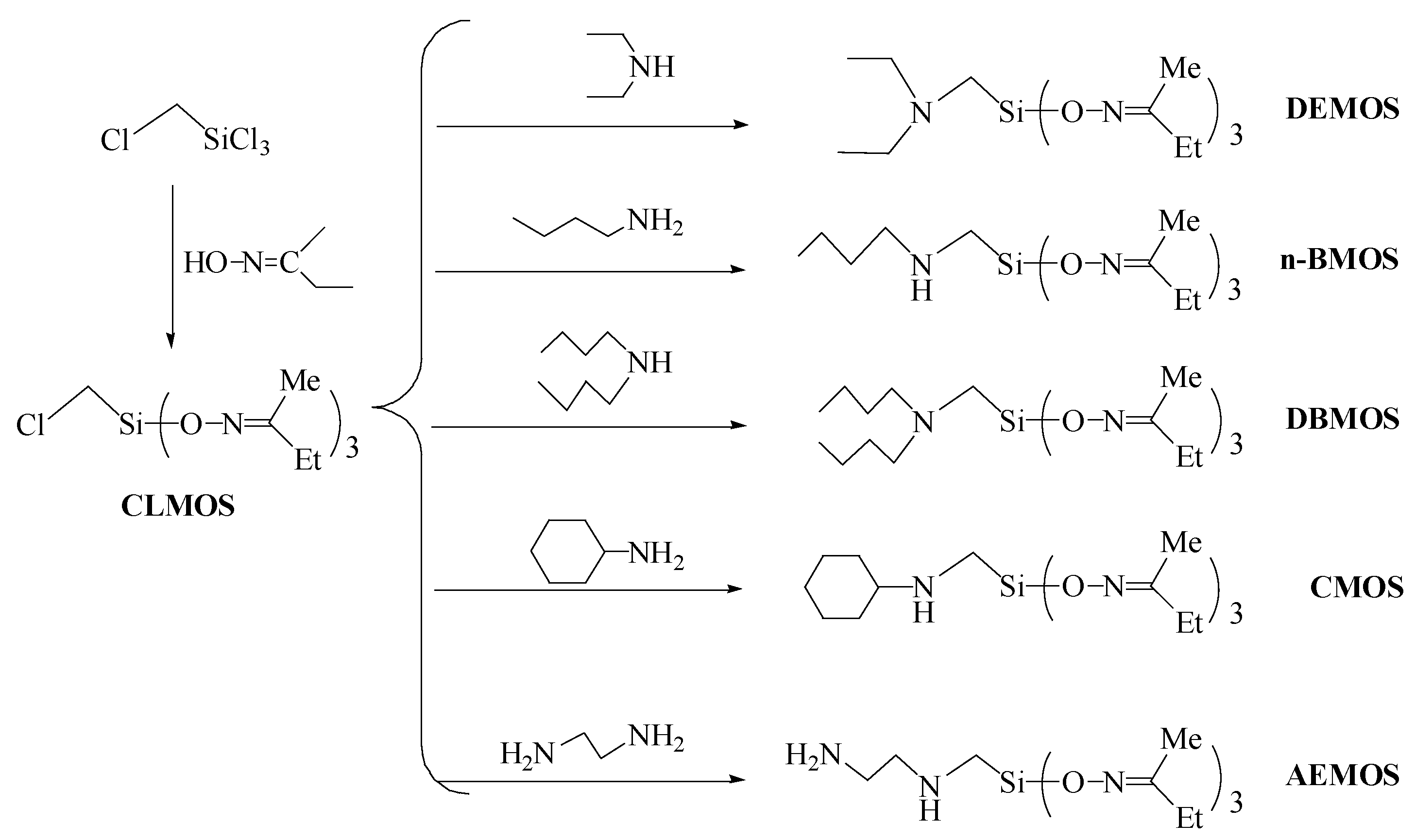
| Compounds | Rate Constant (×10−4 s−1) | Dependence |
|---|---|---|
| DEMOS | 12.2 | R2 = 0.611 |
| n-BMOS | 9.1 | R2 = 0.829 |
| DBMOS | 8.6 | R2 = 0.613 |
| CMOS | 8.2 | R2 = 0.549 |
| AEMOS | 7.6 | R2 = 0.795 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Liu, Z.; Liu, Q.; Bei, Y.; Zhu, Q. Impacts of Different Functional Groups on the Kinetic Rates of α-Amine Ketoximesilanes Hydrolysis in the Preparation of Room Temperature Vulcanized Silicone Rubber. Materials 2018, 11, 790. https://doi.org/10.3390/ma11050790
Xu H, Liu Z, Liu Q, Bei Y, Zhu Q. Impacts of Different Functional Groups on the Kinetic Rates of α-Amine Ketoximesilanes Hydrolysis in the Preparation of Room Temperature Vulcanized Silicone Rubber. Materials. 2018; 11(5):790. https://doi.org/10.3390/ma11050790
Chicago/Turabian StyleXu, Huihui, Zihou Liu, Qingyang Liu, Yiling Bei, and Qingzeng Zhu. 2018. "Impacts of Different Functional Groups on the Kinetic Rates of α-Amine Ketoximesilanes Hydrolysis in the Preparation of Room Temperature Vulcanized Silicone Rubber" Materials 11, no. 5: 790. https://doi.org/10.3390/ma11050790
APA StyleXu, H., Liu, Z., Liu, Q., Bei, Y., & Zhu, Q. (2018). Impacts of Different Functional Groups on the Kinetic Rates of α-Amine Ketoximesilanes Hydrolysis in the Preparation of Room Temperature Vulcanized Silicone Rubber. Materials, 11(5), 790. https://doi.org/10.3390/ma11050790







