Effect of the Functionalization of Porous Silicon/WO3 Nanorods with Pd Nanoparticles and Their Enhanced NO2-Sensing Performance at Room Temperature
Abstract
:1. Introduction
2. Materials and Methods
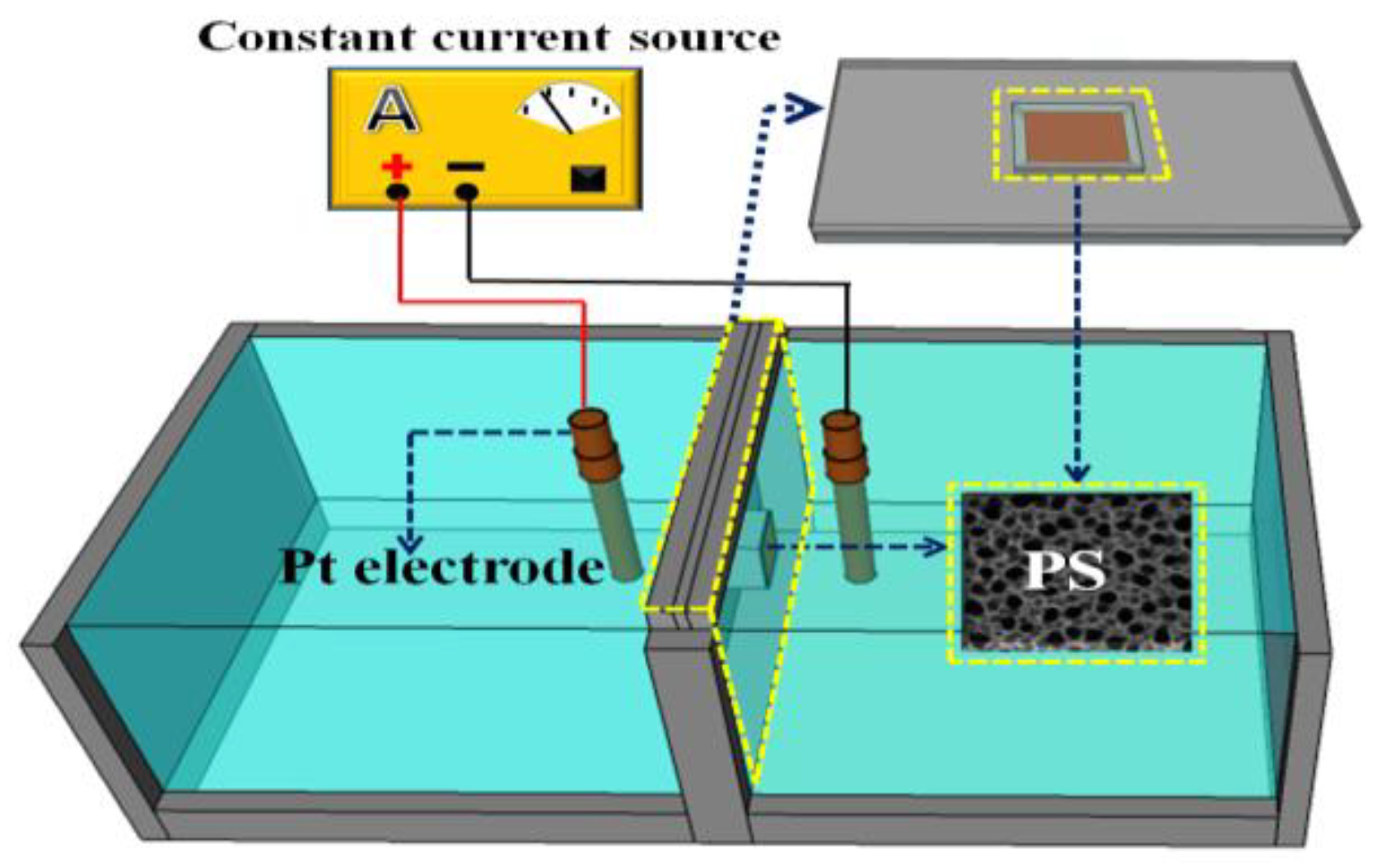
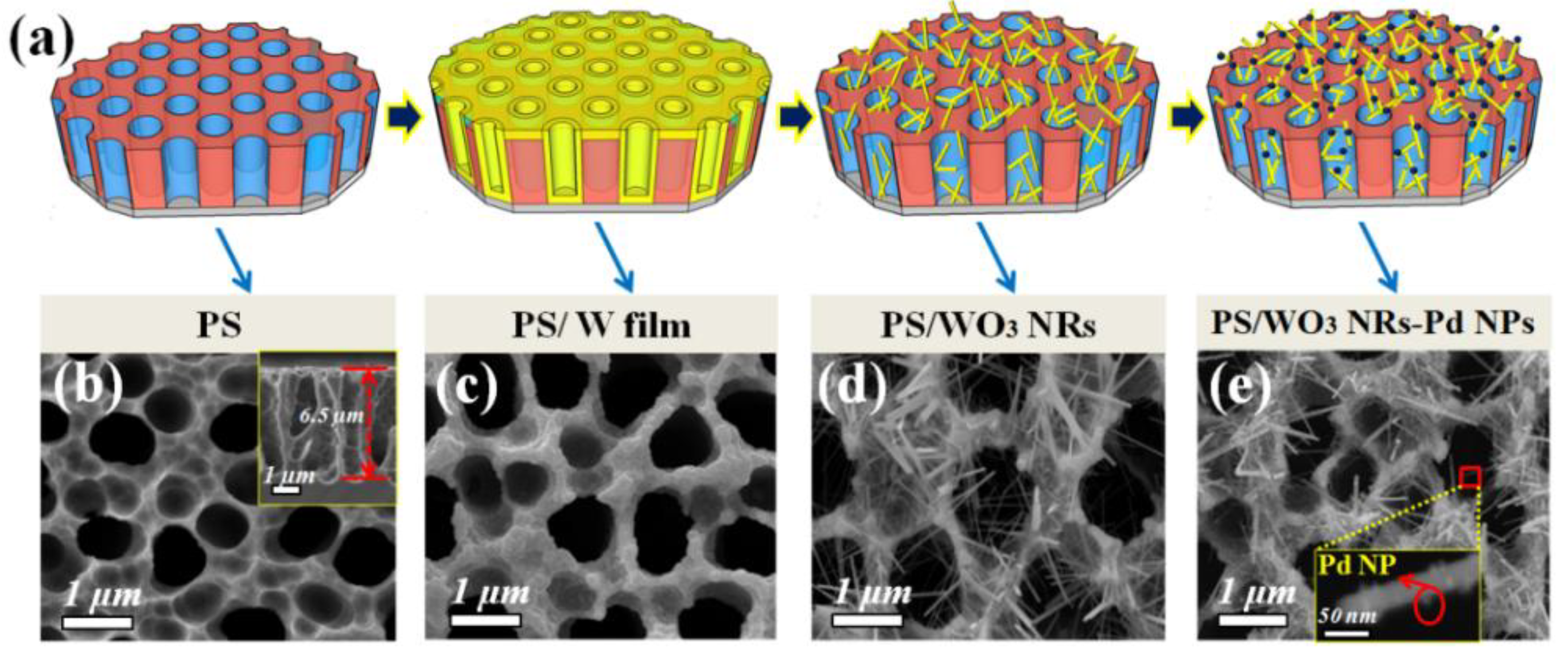
2.1. Synthesis and Characterization of PS/WO3 NRs–Pd NPs
2.2. Gas-Sensing Characterizations
3. Results and Discussion
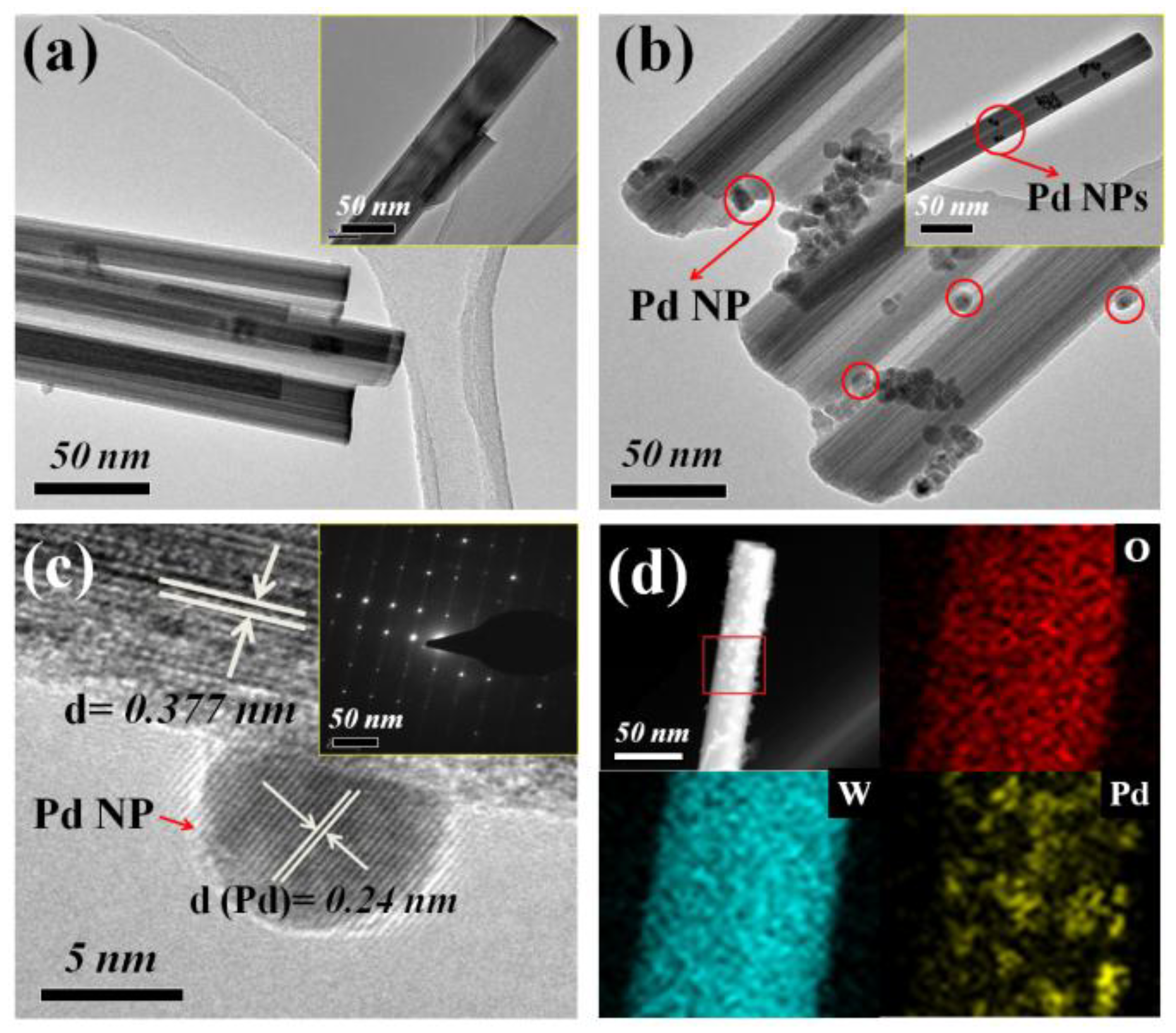
3.1. Materials Characterization
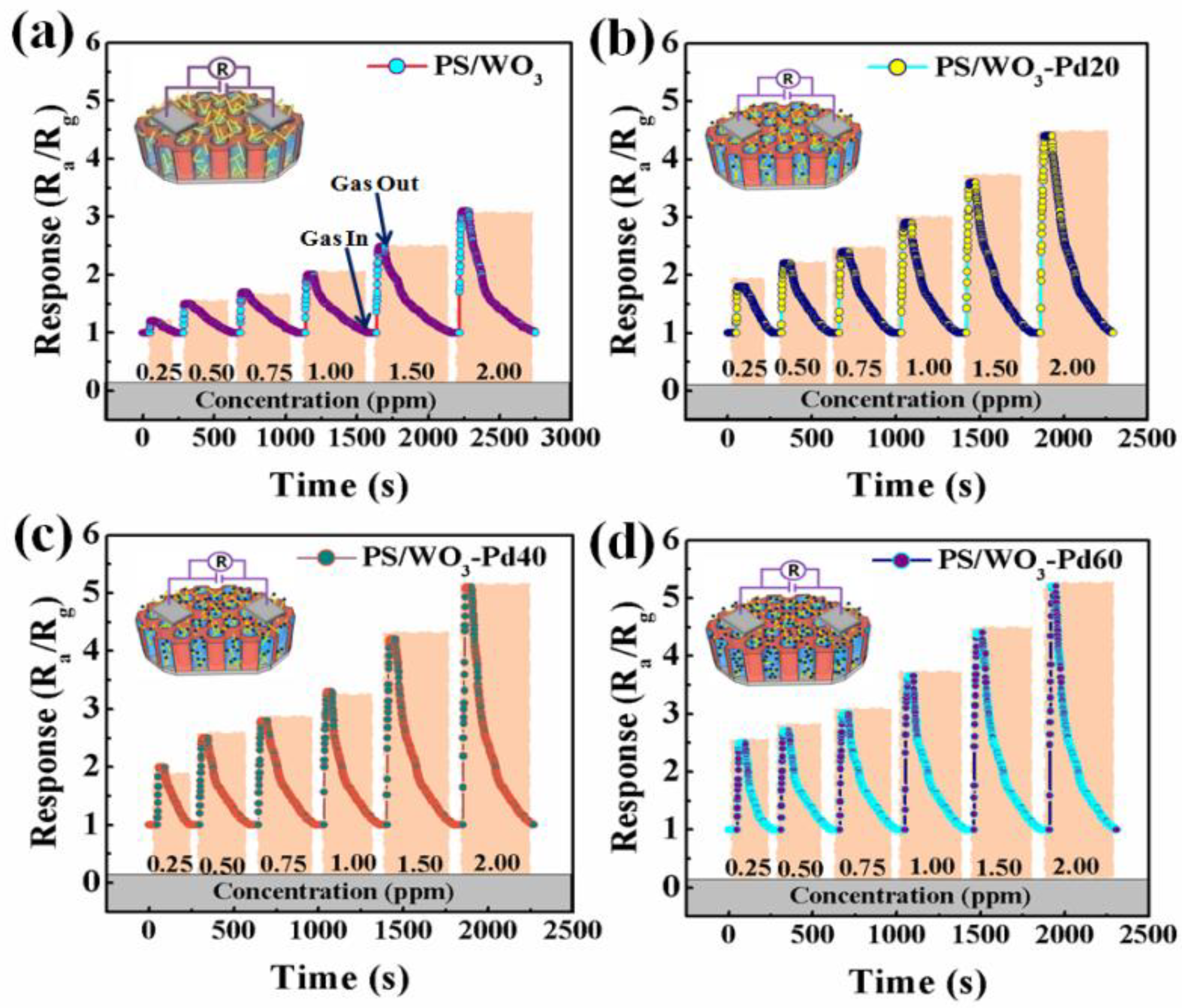
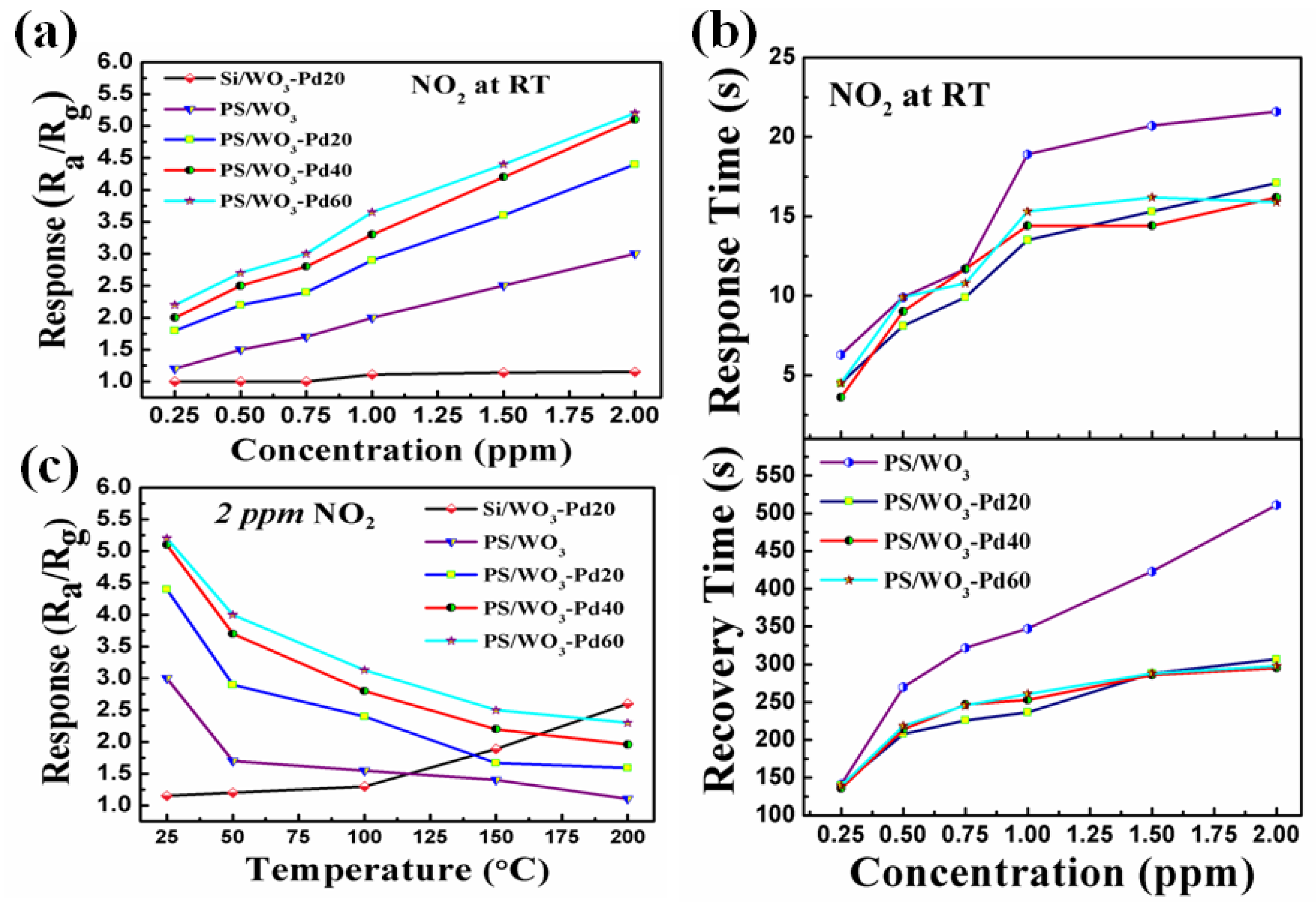
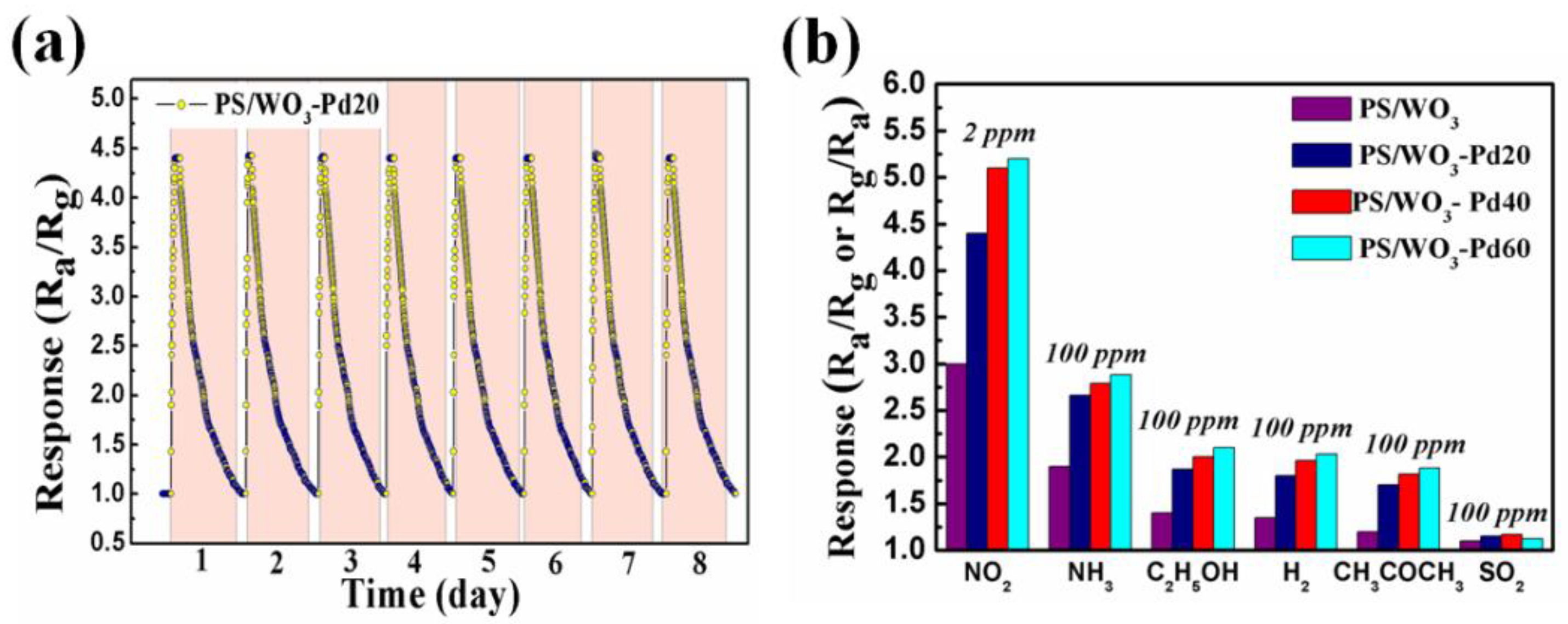
3.2. NO2-Sensing Performance
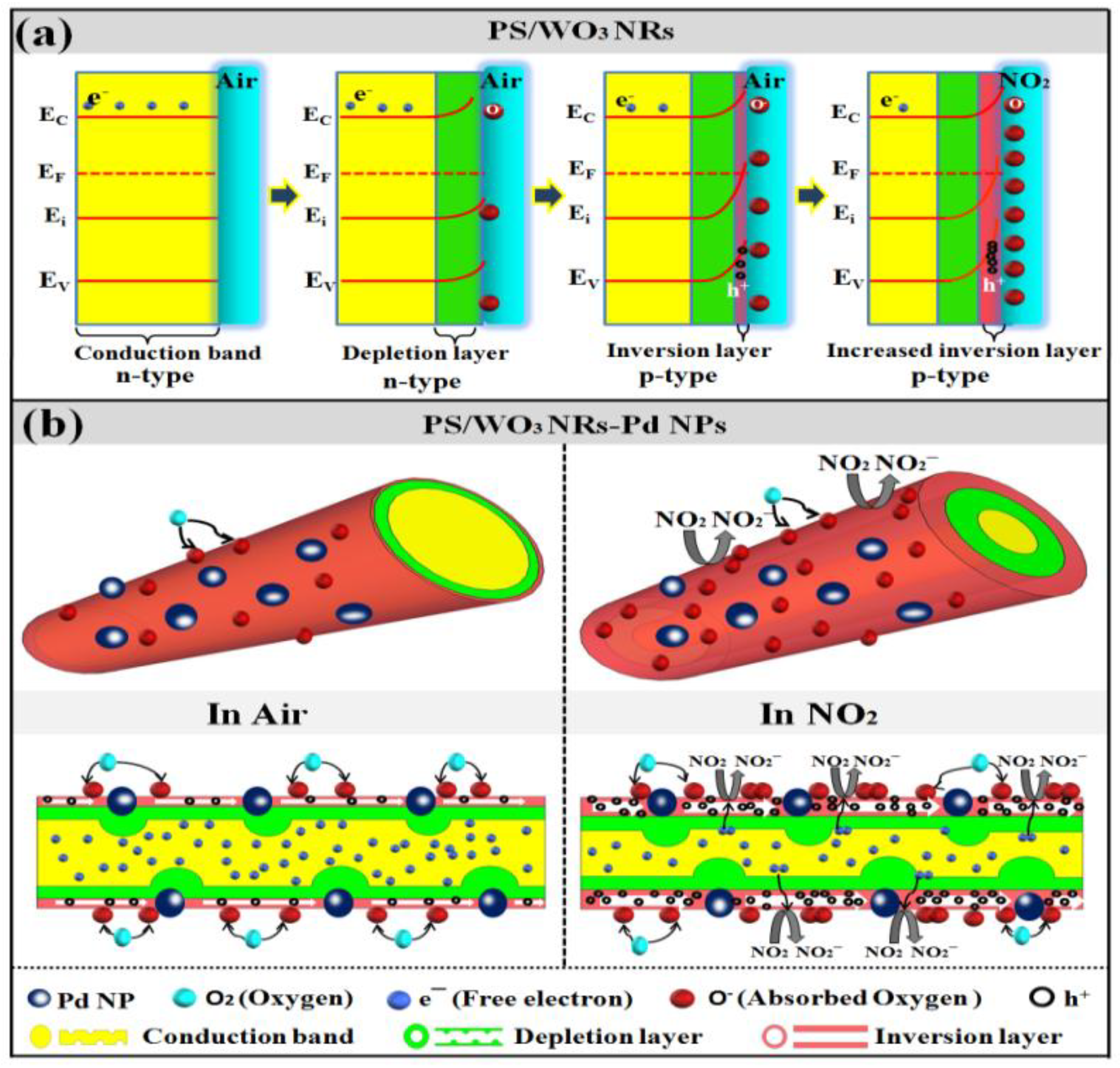
3.3. NO2-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Qin, Y.; Li, X.; Wang, F.; Hu, M. Solvothermally synthesized tungsten oxide nanowires/nanorods for NO2 gas sensor applications. J. Alloy. Compd. 2011, 509, 8401–8406. [Google Scholar] [CrossRef]
- Lu, G.; Xu, J.; Sun, J.; Yu, Y.; Zhang, Y.; Liu, F. UV-enhanced room temperature NO2 sensor using ZnO nanorods modified with SnO2 nanoparticles. Sens. Actuators B 2012, 162, 82–88. [Google Scholar] [CrossRef]
- Penza, M.; Martucci, C.; Cassano, G. NOx gas sensing characteristics of WO3 thin films activated by noble metals (Pd, Pt, Au) layers. Sens. Actuators B 1998, 50, 52–59. [Google Scholar] [CrossRef]
- Guillen, M.G.; Gamez, F.; Suarez, B.; Queiros, C.; Silva, A.M.G.; Barranco, A.; Sanchez-Valencia, J.R.; Pedrosa, J.M.; Lopes-Costa, T. Preparation and Optimization of Fluorescent Thin Films of Rosamine-SiO2/TiO2 Composites for NO2 Sensing. Materials 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Park, S. Acetone gas detection using TiO2 nanoparticles functionalized In2O3 nanowires for diagnosis of diabetes. J. Alloy. Compd. 2017, 696, 655–662. [Google Scholar] [CrossRef]
- Cao, J.; Qin, C.; Wang, Y.; Zhang, H.; Sun, G.; Zhang, Z. Solid-State Method Synthesis of SnO2-Decorated g-C3N4 Nanocomposites with Enhanced Gas-Sensing Property to Ethanol. Materials 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pozos, H.; Arredondo, E.J.L.; Alvarez, A.M.; Biswal, R.; Kudriavtsev, Y.; Perez, J.V.; Casallas-Moreno, Y.L.; Amador, M.D.O. Cu-Doped ZnO Thin Films Deposited by a Sol-Gel Process Using Two Copper Precursors: Gas-Sensing Performance in a Propane Atmosphere. Materials 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Katoch, A.; Kim, J.H.; Kim, S.S. A novel approach to improving oxidizing-gas sensing ability of p-CuO nanowires using biased radial modulation of a hole-accumulation layer. J. Mater. Chem. C 2014, 2, 8911–8917. [Google Scholar] [CrossRef]
- Lavanya, N.; Anithaa, A.C.; Sekar, C.; Asokan, K.; Bonavita, A.; Donato, N.; Leonardi, S.G.; Neri, G. Effect of gamma irradiation on structural, electrical and gas sensing properties of tungsten oxide nanoparticles. J. Alloy. Compd. 2017, 693, 366–372. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Akiyama, M.; Tamaki, J.; Miura, N.; Yamazoe, N. Tungsten oxide-based semiconductor sensor highly sensitive to NO and NO2. Chem. Lett. 1991, 20, 1611–1614. [Google Scholar] [CrossRef]
- Prades, J.D.; Díaz, R.J.; Ramirez, F.H.; Barth, S.; Cirera, A.; Rodríguez, A.R.; Mathur, S.; Morante, J.R. Equivalence between thermal and room temperature UV light-modulated responses of gas sensors based on individual SnO2 nanowires. Sens. Actuators B 2009, 140, 337–341. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.-W.; Sun, G.-J.; Kim, S.S. Pt Nanoparticle-Decorated ZnO Nanowire Sensors for Detecting Benzene at Room Temperature. J. Nanosci. Nanotechnol. 2013, 13, 7097–7099. [Google Scholar] [CrossRef] [PubMed]
- Minh Vuong, N.; Kim, D.; Kim, H. Porous Au-embedded WO3 Nanowire Structure for Efficient Detection of CH4 and H2S. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Q.; Cui, Z.D.; Yang, X.J.; Zhu, S.L.; Li, Z.Y.; Liang, Y.Q. Pd-loaded In2O3 nanowire-like network synthesized using carbon nanotube templates for enhancing NO2 sensing performance. RSC Adv. 2015, 5, 30038–30045. [Google Scholar] [CrossRef]
- An, S.; Park, S.; Ko, H.; Lee, C. Enhanced NO2 gas sensing properties of WO3nanorods encapsulated with ZnO. Appl. Phys. A 2012, 108, 53–58. [Google Scholar] [CrossRef]
- Kanungo, J.; Basu, S.; Sarkar, C.K. Fabrication and Characterization of ZnO/p-Si and TiO2/p-Si Heterojunctions for Hydrogen Detection-Influence of Pd Functionalization. IEEE Sens. J. 2015, 15, 6954–6961. [Google Scholar] [CrossRef]
- Ibrahim, I.M.; Sharhan, S.I.; Ibrahim, F.T. Sensing behavior of CuO-doped ZnO/PS nanoparticles. Mater. Lett. 2015, 157, 57–62. [Google Scholar] [CrossRef]
- Saboor, F.H.; Ueda, T.; Kamada, K.; Hyodo, T.; Mortazavi, Y.; Khodadadi, A.A.; Shimizu, Y. Enhanced NO2 gas sensing performance of bare and Pd-loaded SnO2 thick film sensors under UV-light irradiation at room temperature. Sens. Actuators B 2016, 223, 429–439. [Google Scholar] [CrossRef]
- Samerjai, T.; Liewhiran, C.; Wisitsoraat, A.; Tuantranont, A.; Khanta, C.; Phanichphant, S. Highly selective hydrogen sensing of Pt-loaded WO3 synthesized by hydrothermal/impregnation methods. Int. J. Hydrog. Energy 2014, 39, 6120–6128. [Google Scholar] [CrossRef]
- Zhao, P.X.; Tang, Y.; Mao, J.; Chen, Y.X.; Song, H.; Wang, J.W.; Song, Y.; Liang, Y.Q.; Zhang, X.M. One-Dimensional MoS2-Decorated TiO2 nanotube gas sensors for efficient alcohol sensing. J. Alloy. Compd. 2016, 674, 252–258. [Google Scholar] [CrossRef]
- Şahin, Y.; Öztürk, S.; Kılınç, N.; Kösemen, A.; Erkovan, M.; Öztürk, Z.Z. Electrical conduction and NO2 gas sensing properties of ZnOnanorods. Appl. Surf. Sci. 2014, 303, 90–96. [Google Scholar] [CrossRef]
- Kim, S.S.; Choi, S.-W.; Na, H.G.; Kwak, D.S.; Kwon, Y.J.; Kim, H.W. ZnO-SnO2 branch-stem nanowires based on a two-step process: Synthesis and sensing capability. Curr. Appl. Phys. 2013, 13, 526–532. [Google Scholar] [CrossRef]
- Uhlir, A. Electrolytic shaping of germanium and silicon. Bell Syst. Tech. J. 1956, 35, 333–347. [Google Scholar] [CrossRef]
- Levitsky, I.A. Porous Silicon Structures as Optical Gas Sensors. Sensors 2015, 15, 19968–19991. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Kwon, E.J.; Kang, J.Y.; Skalak, M.; Anglin, E.J.; Mann, A.P.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Porous silicon-graphene oxide core-shell nanoparticles for targeted delivery of siRNA to the injured brain. Nanoscale Horiz. 2016, 1, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Dutt, A.; de la Mora, B.; Mon-Pérez, E.; Villagrán-Muniz, M.; García-Sánchez, M.F.; Santana, G. Effect of ammonia plasma treatment on the luminescence and stability of porous silicon. Mater. Lett. 2018, 216, 277–280. [Google Scholar] [CrossRef]
- Li, X.; Gu, M.; Hu, S.; Kennard, R.; Yan, P.; Chen, X.; Wang, C.; Sailor, M.J.; Zhang, J.-G.; Liu, J. Mesoporous silicon sponge as an anti-pulverization structure for high-performance lithium-ion battery anodes. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Dutt, A.; de la Mora, B.; Mon-Perez, E.; Villagran-Muniz, M.; Garcia-Sanchez, M.F.; Santana, G.; Lukianov, A.; Ihara, M. Free-standing epitaxial silicon thin films for solar cells grown on double porous layers of silicon and electrochemically oxidized porous silicon dioxide. Thin Solid Films 2018, 648, 1–7. [Google Scholar]
- Sainato, M.; Strambini, L.M.; Rella, S.; Mazzotta, E.; Barillaro, G. Sub-Parts Per Million NO2Chemi-Transistor Sensors Based on Composite Porous Silicon/Gold Nanostructures Prepared by Metal-Assisted Etching. ACS Appl. Mater. Interfaces 2015, 7, 7136–7145. [Google Scholar] [CrossRef] [PubMed]
- Tebizi-Tighilt, F.-Z.; Zane, F.; Belhaneche-Bensemra, N.; Belhousse, S.; Sam, S.; Gabouze, N.-E. Electrochemical gas sensors based on polypyrrole-porous silicon. Appl. Surf. Sci. 2013, 269, 180–183. [Google Scholar] [CrossRef]
- Karacali, T.; Hasar, U.C.; Ozbek, I.Y.; Oral, E.A.; Efeoglu, H. Novel Design of Porous Silicon Based Sensor for Reliable and Feasible Chemical Gas Vapor Detection. J. Lightwave Technol. 2013, 31, 295–305. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, M.; Wang, D.; Zhang, W.; Qin, Y. Room temperature NO2-sensing properties of porous silicon/tungsten oxide nanorods composite. J. Alloy. Compd. 2015, 640, 517–524. [Google Scholar] [CrossRef]
- Li, M.; Hu, M.; Yan, W.; Ma, S.; Zeng, P.; Qin, Y. NO2 sensing performance of p-type intermediate size porous silicon by a galvanostatic electrochemical etching method. Electrochim. Acta 2013, 113, 354–360. [Google Scholar] [CrossRef]
- Lim, B.; Jiang, M.; Camargo, P.H.C.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wei, G.; Ispas, A.; Hickey, S.G.; Eychmüller, A. Synthesis of palladium nanoparticles and their applications for surface-enhanced Raman scattering and electrocatalysis. J. Phys. Chem. C 2010, 114, 21976–21981. [Google Scholar] [CrossRef]
- Yan, D.L.; Hu, M.; Li, S.Y.; Liang, J.R.; Wu, Y.Q.; Ma, S.Y. Electrochemical deposition of ZnO nanostructures onto porous silicon and their enhanced gas sensing to NO2 at room temperature. Electrochim. Acta 2014, 115, 297–305. [Google Scholar] [CrossRef]
- Halimaoui, A.; Canham, L. Properties of Porous Silicon; INSPEC: London, UK, 1997; Chapter 1; p. 12. [Google Scholar]
- Trung, D.D.; Hoa, N.D.; Tong, P.V.; Duy, N.V.; Dao, T.D.; Chung, H.V.; Nagao, T.; Hieu, N.V. Effective decoration of Pd nanoparticles on the surface of SnO2 nanowires for enhancement of CO gas-sensing performance. J. Hazard. Mater. 2014, 265, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Tong, P.V.; Hoa, N.D.; Duy, N.V.; Le, D.T.T.; Hieu, N.V. Enhancement of gas-sensing characteristics of hydrothermally synthesized WO3 nanorods by surface decoration with Pd nanoparticles. Sens. Actuators B 2016, 223, 453–460. [Google Scholar] [CrossRef]
- Chávez, F.; Pérez-Sánchez, G.F.; Goiz, O.; Zaca-Morán, P.; Pena-Sierra, R.; Morales-Acevedo, A.; Felipe, C.; Soledad-Priego, M. Sensing performance of palladium-functionalized WO3 nanowires by a drop-casting method. Appl. Surf. Sci. 2013, 275, 28–35. [Google Scholar] [CrossRef]
- Yuan, L.; Hu, M.; Wei, Y.L.; Ma, W.F. Enhanced NO2 sensing characteristics of Au modified porous silicon/thorn-sphere-like tungsten oxide composites. Appl. Surf. Sci. 2016, 389, 824–834. [Google Scholar] [CrossRef]
- Wisitsoorat, A.; Ahmad, M.Z.; Yaacob, M.H.; Horpratum, M.; Phakaratkul, D.; Lomas, T.; Tuantranont, A.; Wlodarski, W. Optical H2 sensing properties of vertically aligned Pd/WO3 nanorods thin films deposited via glancing angle RF magnetron sputtering. Sens. Actuators B 2013, 182, 795–801. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Yamazaki, T.; Kikuta, T. NO2 response enhancement and anomalous behavior of n-type SnO2 nanowires functionalized by Pd nanodots. Sens. Actuators B 2012, 166, 671–677. [Google Scholar] [CrossRef]

| Nanomaterials | NO2 Concentration (ppm) | Response | Working Temperature (°C) | References |
|---|---|---|---|---|
| PS/WO3–Pd60 | 2 | 5.2 | RT | present work |
| WO3 film–Pd | 10 | 0.42 | 200 | [3] |
| Rosemine–SiO2/TiO2 composite | 50 | 84% | RT | [4] |
| TiO2 NPs attached CuO NWs | 10 | 5 | 300 | [8] |
| Pd-loaded In2O3 nanowires | 5 | 4.05 | 110 | [15] |
| WO3 encapsulated with ZnO | 5 | 2.8 | 300 | [16] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiang, X.; Hu, M.; Zhao, B.; Qin, Y.; Yang, R.; Zhou, L.; Qin, Y. Effect of the Functionalization of Porous Silicon/WO3 Nanorods with Pd Nanoparticles and Their Enhanced NO2-Sensing Performance at Room Temperature. Materials 2018, 11, 764. https://doi.org/10.3390/ma11050764
Qiang X, Hu M, Zhao B, Qin Y, Yang R, Zhou L, Qin Y. Effect of the Functionalization of Porous Silicon/WO3 Nanorods with Pd Nanoparticles and Their Enhanced NO2-Sensing Performance at Room Temperature. Materials. 2018; 11(5):764. https://doi.org/10.3390/ma11050764
Chicago/Turabian StyleQiang, Xiaoyong, Ming Hu, Boshuo Zhao, Yue Qin, Ran Yang, Liwei Zhou, and Yuxiang Qin. 2018. "Effect of the Functionalization of Porous Silicon/WO3 Nanorods with Pd Nanoparticles and Their Enhanced NO2-Sensing Performance at Room Temperature" Materials 11, no. 5: 764. https://doi.org/10.3390/ma11050764
APA StyleQiang, X., Hu, M., Zhao, B., Qin, Y., Yang, R., Zhou, L., & Qin, Y. (2018). Effect of the Functionalization of Porous Silicon/WO3 Nanorods with Pd Nanoparticles and Their Enhanced NO2-Sensing Performance at Room Temperature. Materials, 11(5), 764. https://doi.org/10.3390/ma11050764





