Manufacturing Technology of Ceramic Pebbles for Breeding Blanket
Abstract
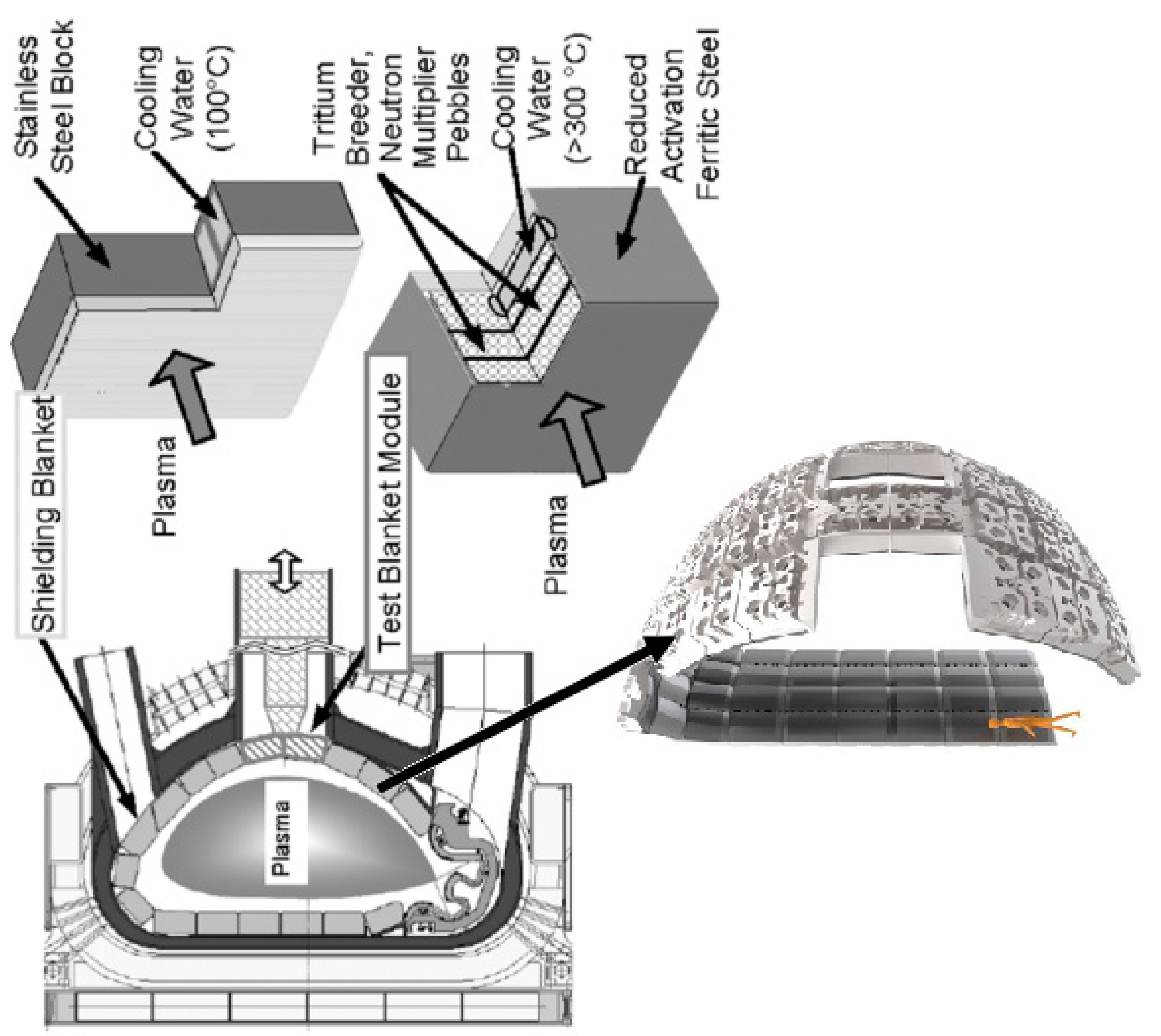
:1. Introduction
2. Materials and Methods
2.1. Drip Casting
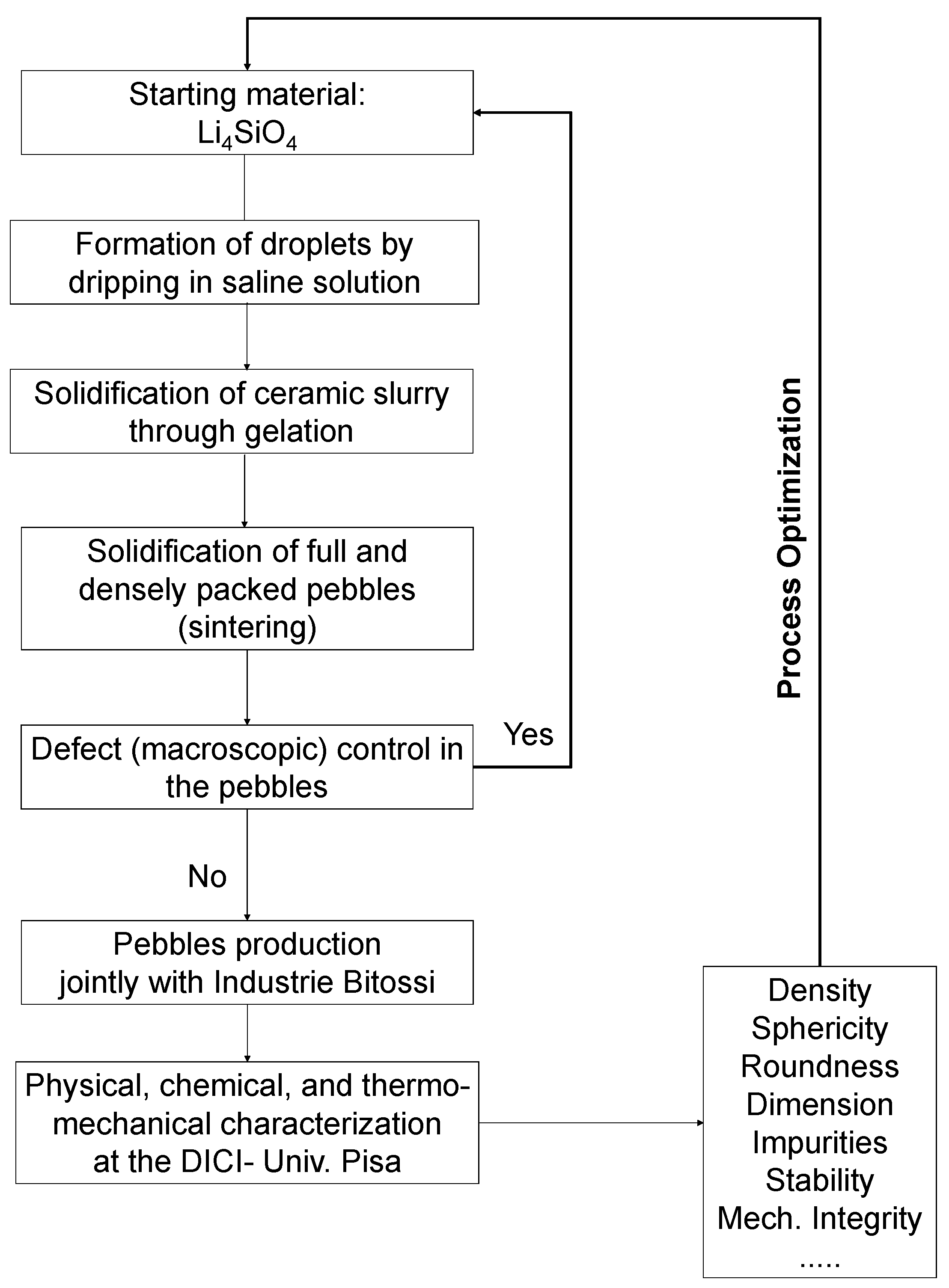
2.1.1. Li4SiO4 Pebbles Fabrication Process
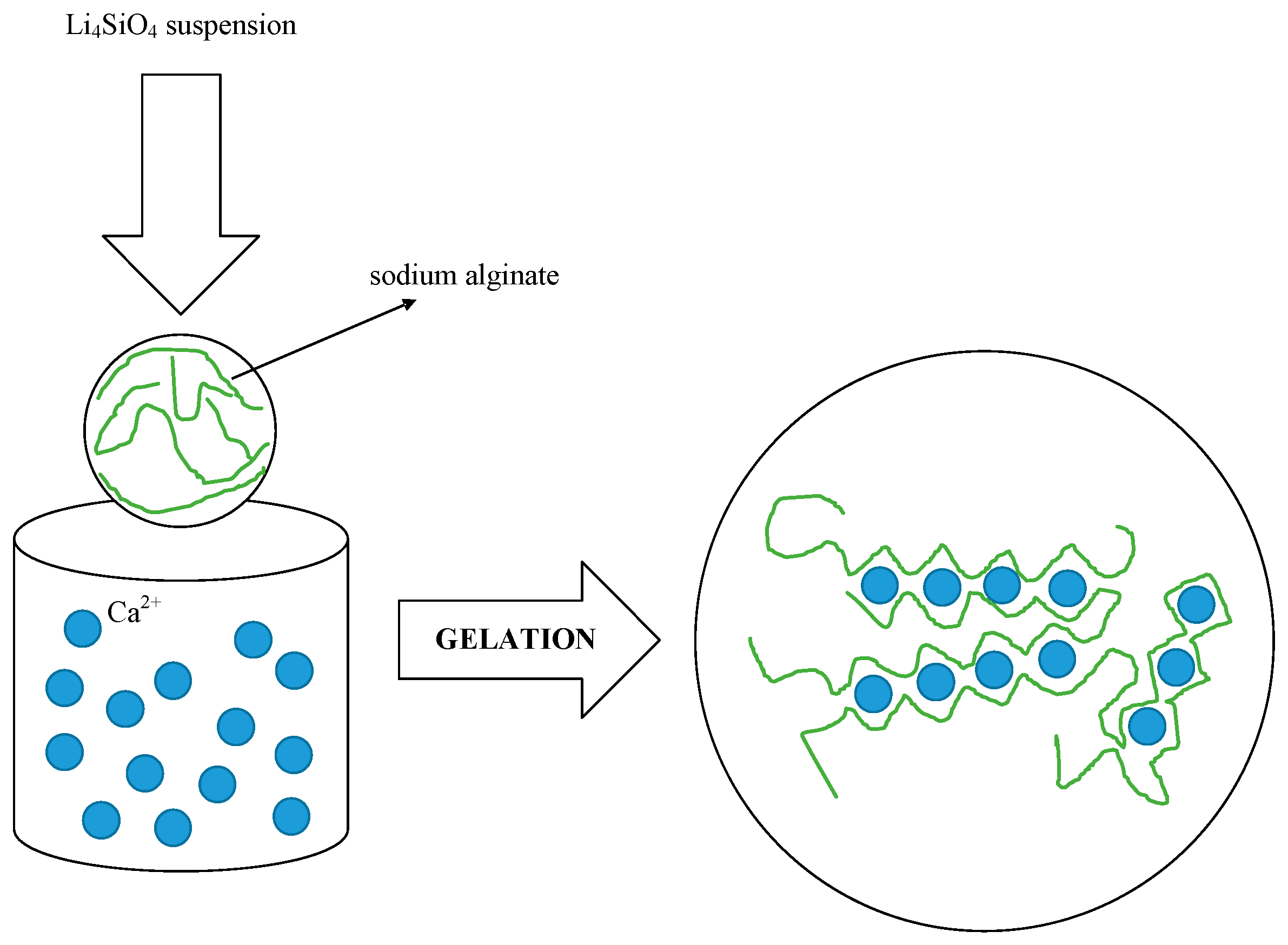
2.1.2. Slurry and CaCl2 Solution Preparation
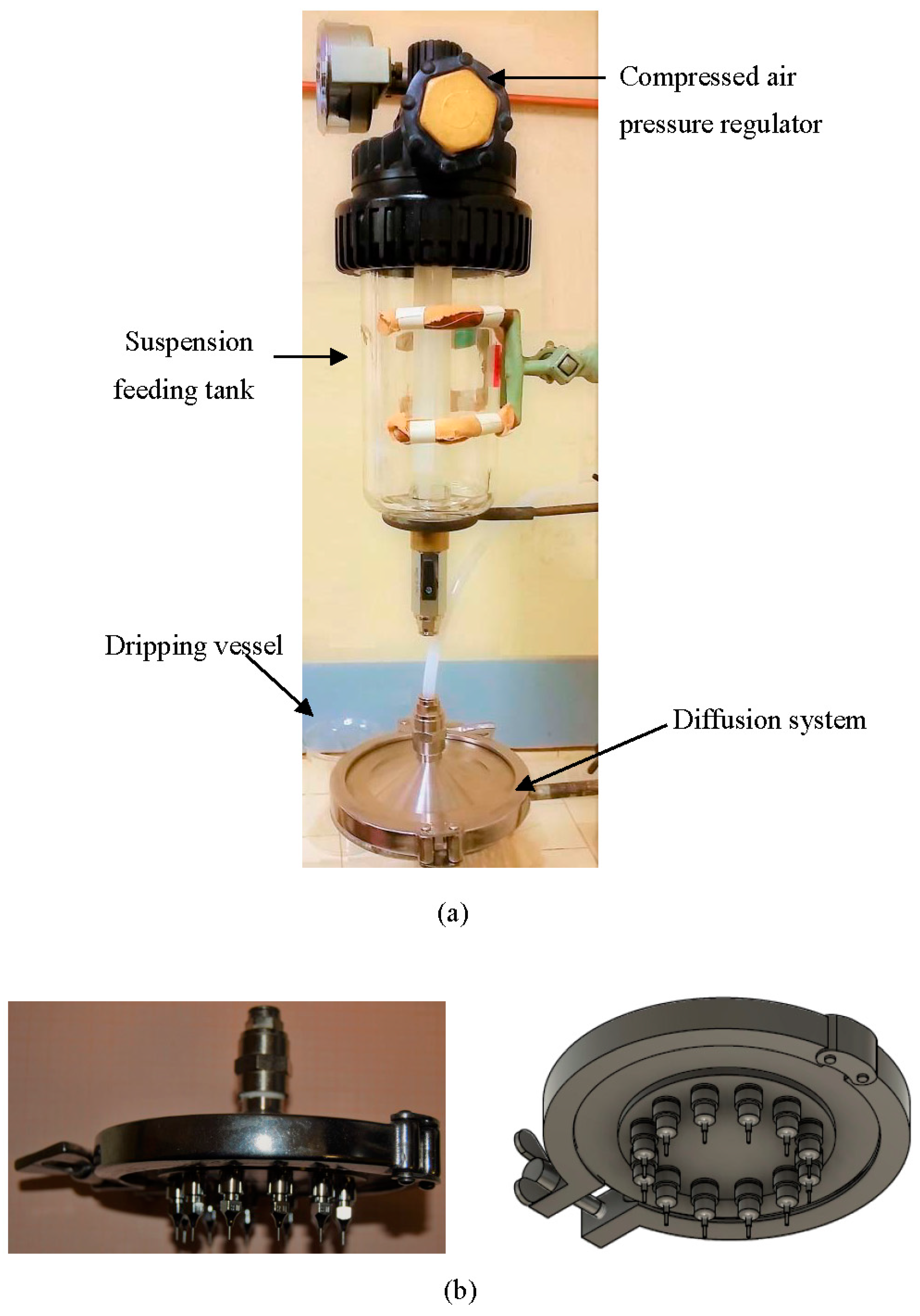
2.2. Experimental Apparatus
3. Experimental
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Konishi, S.; Enoeda, M.; Nakamichi, M.; Hoshino, T.; Ying, A.; Sharafat, S.; Smolentsev, S. Functional materials for breeding blankets—Status and developments. Nucl. Fusion 2017, 57, 092014. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Kawamura, H.; Takayama, T.; Kato, S. Control of particle size and density of Li2TiO3 pebbles fabricated by indirect wet processes. J. Nucl. Mater. 2005, 345, 239–244. [Google Scholar] [CrossRef]
- Mattas, R.F.; Billone, M.C. Materials for breeding blankets. J. Nucl. Mater. 1996, 233–237, 72–81. [Google Scholar] [CrossRef]
- Raffray, A.R.; Akiba, M.; Chuyanov, V.; Giancarli, L.; Malang, S. Breeding blanket concepts for fusion and materials requirements. J. Nucl. Mater. 2002, 307–311, 21–30. [Google Scholar] [CrossRef]
- Lo Frano, R.; Aquaro, D.; Scaletti, L. Thermo-mechanical characterization of ceramic pebbles for breeding blanket. Fusion Eng. Des. 2016, 109–111, 383–388. [Google Scholar] [CrossRef]
- Zaccari, N.; Aquaro, D. Mechanical characterization of Li2TiO3 and Li4SiO4 pebble beds: Experimental determination of the material properties and of the pebble bed effective values. Fusion Eng. Des. 2007, 82, 2375–2382. [Google Scholar] [CrossRef]
- An, Z.; Ying, A.; Abdou, M. Numerical characterization of thermo-mechanical performance of breeder pebble beds. J. Nucl. Mater. 2007, 367–370, 1393–1397. [Google Scholar] [CrossRef]
- Piazza, G.; Enoeda, M.; Ying, A. Measurements of effective thermal conductivity of ceramic breeder pebble beds. Fusion Eng. Des. 2001, 58–59, 661–666. [Google Scholar] [CrossRef]
- Aquaro, D.; Frano, R.L. Preliminary experimental evaluation of thermal conductivity of ceramic pebble beds. J. Phys. Conf. Ser. 2014, 501, 012031. [Google Scholar] [CrossRef]
- Tanigawa, H.; Hatano, T.; Enoeda, M.; Akiba, M. Effective thermal conductivity of a compressed Li2TiO3 pebble bed. Fusion Eng. Des. 2005, 75–79, 801–805. [Google Scholar] [CrossRef]
- Lo Frano, R.; Aquaro, D.; Pupeschi, S.; Moscardini, M. Thermo-mechanical test rig for experimental evaluation of thermal conductivity of ceramic pebble beds. Fusion Eng. Des. 2014, 89, 1309–1313. [Google Scholar] [CrossRef]
- Enoeda, M.; Kosaku, Y.; Hatano, T.; Kuroda, T.; Miki, N.; Honma, T.; Akiba, M.; Konishi, S.; Nakamura, H.; Kawamura, Y.; et al. Design and technology development of solid breeder blanket cooled by supercritical water in Japan. Nucl. Fusion 2003, 43, 1837–1844. [Google Scholar] [CrossRef]
- Reimann, J.; Boccaccini, L.; Enoeda, M.; Ying, A. Thermomechanics of solid breeder and be pebble bed materials. Fusion Eng. Des. 2002, 61–62, 319–331. [Google Scholar] [CrossRef]
- Del Serra, D.; Lo Frano, R.; Puccini, M.; Aquaro, D.; Vitolo, S.; Stefanelli, E.; Forgione, N.; De Santis, S.; Marco, A.; Ciolini, R.; et al. Manufacturing Challenges Of Fusion Pebbles Bed Material 1. In Proceedings of the 26th International Conference Nuclear Energy for New Europe, Bled, Slovenia, 11–14 September 2017; pp. 1–9. [Google Scholar]
- Knitter, R.; Chaudhuri, P.; Feng, Y.J.; Hoshino, T.; Yu, I.-K. Recent developments of solid breeder fabrication. J. Nucl. Mater. 2013, 442, S420–S424. [Google Scholar] [CrossRef]
- Feng, Y.J.; Feng, K.M.; Cao, Q.X.; Hu, J.; Tang, H. Fabrication and characterization of Li4SiO4pebbles by melt spraying method. Fusion Eng. Des. 2012, 87, 753–756. [Google Scholar] [CrossRef]
- Knitter, R.; Kolb, M.H.H.; Kaufmann, U.; Goraieb, A.A. Fabrication of modified lithium orthosilicate pebbles by addition of titania. J. Nucl. Mater. 2013, 442, S433–S436. [Google Scholar] [CrossRef]
- Gao, X.; Chen, X.; Gu, M.; Xiao, C.; Peng, S. Fabrication and characterization of Li4SiO4ceramic pebbles by wet method. J. Nucl. Mater. 2012, 424, 210–215. [Google Scholar] [CrossRef]
- Wu, X.; Wen, Z.; Xu, X.; Liu, Y. Fabrication of Li4SiO4 pebbles by a sol–gel technique. Fusion Eng. Des. 2010, 85, 222–226. [Google Scholar] [CrossRef]
- Wu, X.; Wen, Z.; Xu, X.; Wang, X.; Lin, J. Synthesis and characterization of Li4SiO4nano-powders by a water-based sol-gel process. J. Nucl. Mater. 2009, 392, 471–475. [Google Scholar] [CrossRef]
- Hoshino, T.; Oikawa, F. Trial fabrication tests of advanced tritium breeder pebbles using sol-gel method. Fusion Eng. Des. 2011, 86, 2172–2175. [Google Scholar] [CrossRef]

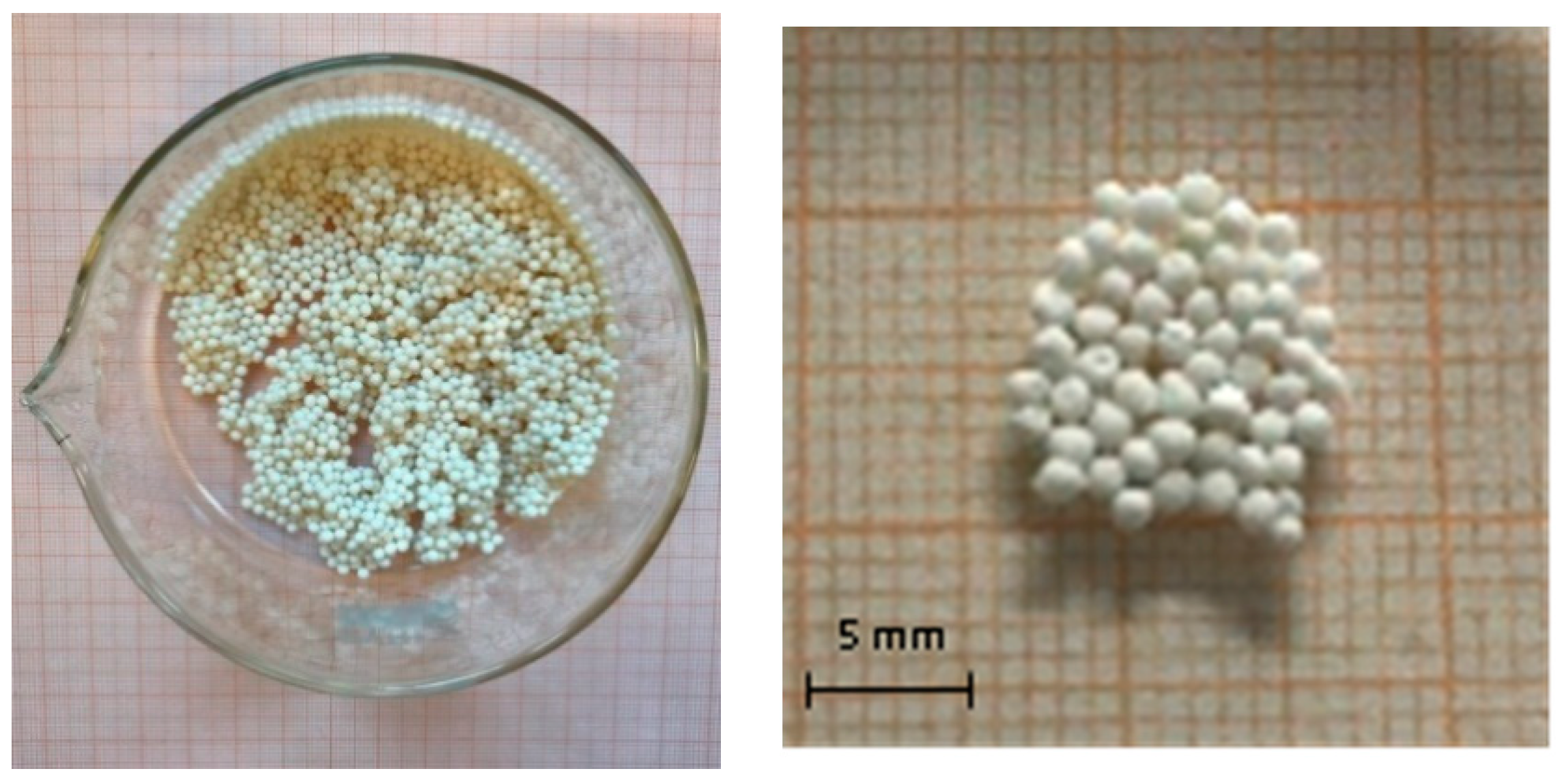
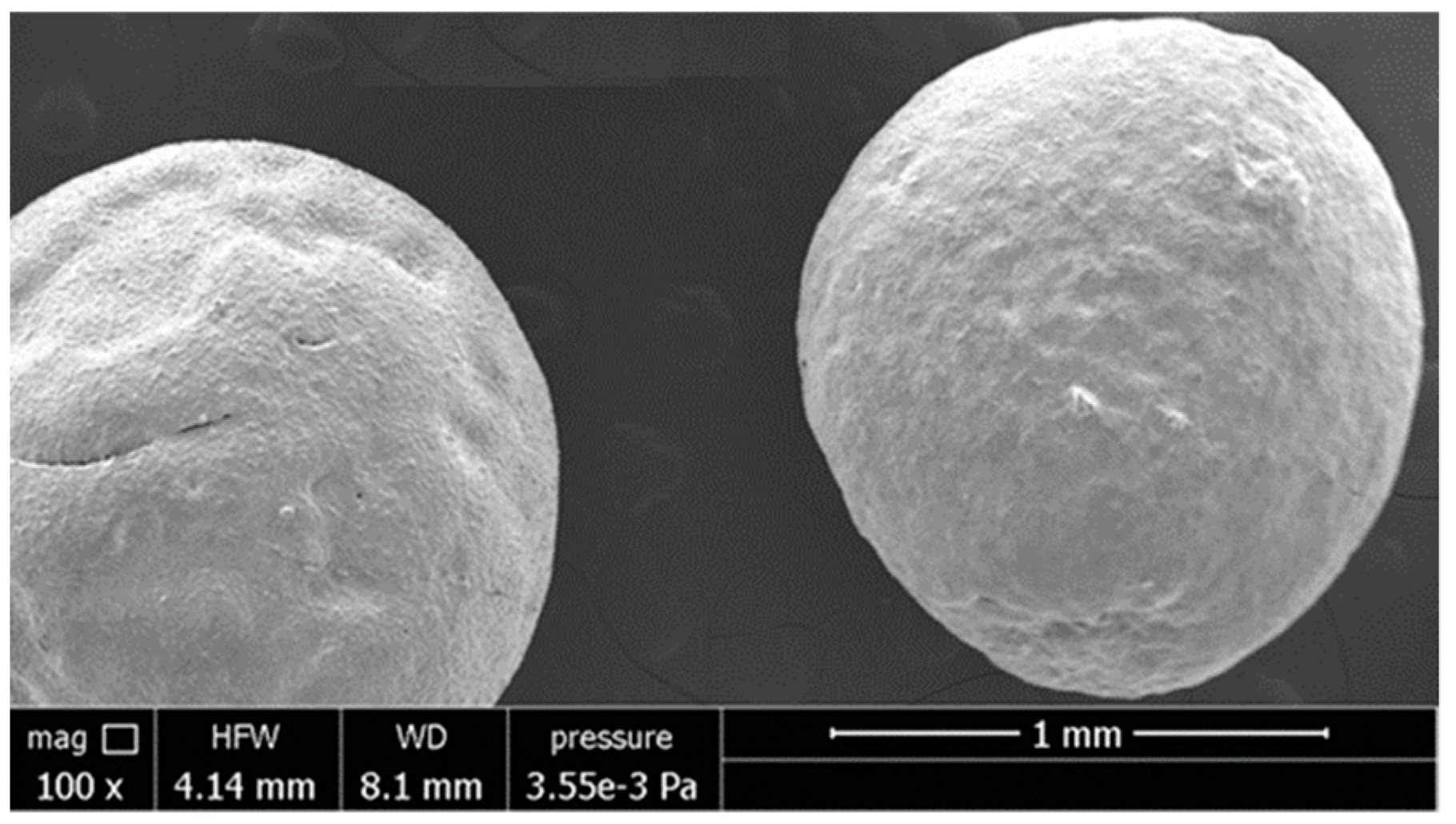
| Fabrication Process | Raw Materials | Main Process Phases | Diameter and Density |
|---|---|---|---|
| Melt-spraying [15,16,17,18] | LiOH + SiO2 | 1. Melting | Φ: 0.25 ÷ 0.63 mm (50%) ρ > 0.90 TD |
| 2. Dropping | |||
| 3. Quenching | |||
| 4. Annealing (2 h at 1000 °C) | |||
| Li2CO3 + SiO2 | 1. Melting | Φ: 0.8 ÷ 1.0 mm (50%) ρ > 0.90 TD | |
| 2. Dropping | |||
| 3. Quenching | |||
| 4. Annealing (2 h at 1000 °C) | |||
| Sol-gel [19,20] | LiOH in citric acid suspension (C6H8O7) + SiO2 (aerosol) | 1. LiOH + citric acid + water | Φ: 1.2 mm ρ = 0.74 TD |
| 2. Silica addition | |||
| 3. Vaporizing at 70 °C | |||
| 4. Dropping into acetone | |||
| 5. Calcining + sintering (4 h at 900 °C) | |||
| Capillary-based microfluidic [21] | C2H3O2Li·2H2O + SiO2 lithium acetate dihydrate | 1. Stirring raw materials. | Φ: 0.84 mm ρ = 0.82 TD |
| 2. Inlet T junction with silicon oil | |||
| 3. Vaporization and drying | |||
| 4. Silicon oil removal (72 h at 120 °C) | |||
| 5. Calcining + sintering (4 h at 750 °C) | |||
| Wet process with substitution [15] | LMT powder | 1. Dropping LMT + sodium alginate + 4HF in zinc chloride | Φ: 0.238 mm ρ = 0.89 TD |
| 2. Calcining | |||
| 3. Sintering |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Frano, R.; Puccini, M.; Stefanelli, E.; Del Serra, D.; Malquori, S. Manufacturing Technology of Ceramic Pebbles for Breeding Blanket. Materials 2018, 11, 718. https://doi.org/10.3390/ma11050718
Lo Frano R, Puccini M, Stefanelli E, Del Serra D, Malquori S. Manufacturing Technology of Ceramic Pebbles for Breeding Blanket. Materials. 2018; 11(5):718. https://doi.org/10.3390/ma11050718
Chicago/Turabian StyleLo Frano, Rosa, Monica Puccini, Eleonora Stefanelli, Daniele Del Serra, and Stefano Malquori. 2018. "Manufacturing Technology of Ceramic Pebbles for Breeding Blanket" Materials 11, no. 5: 718. https://doi.org/10.3390/ma11050718
APA StyleLo Frano, R., Puccini, M., Stefanelli, E., Del Serra, D., & Malquori, S. (2018). Manufacturing Technology of Ceramic Pebbles for Breeding Blanket. Materials, 11(5), 718. https://doi.org/10.3390/ma11050718





