Abstract
The perovskite CsPbBr3 attracts great attention due to its potential in optoelectronics. However, stability remains a major obstacle to achieving its effecting application. In this work, we prepared CsPbBr3 solids through a simple reaction and investigated reversible conversion between CsPbBr3, Cs4PbBr6, and CsPb2Br5. We found that CsPbBr3 can be respectively converted to Cs4PbBr6 or CsPb2Br5 by reacting with CsBr or PbBr2. Thermodynamic analysis demonstrated that the chemical reactions above were exothermic and occurred spontaneously. Moreover, the formed Cs4PbBr6 could be converted to CsPbBr3 reversely, and then progressively converted to Cs-deficient CsPb2Br5 by extraction of CsBr with water. The CsPb2Br5 was converted to CsPbBr3 reversely under thermal annealing at 400 °C. The thermodynamic processes of these conversions between the three compounds above were clarified. Our findings regarding the conversions not only provide a new method for controlled synthesis of the ternary Cs-Pb-Br materials but also clarify the underlying mechanism for the instability of perovskites CsPbBr3.
1. Introduction
All-inorganic cesium lead halide perovskite CsPbX3 (X = I, Br, Cl) nanocrystals (NCs) have attracted considerable attention owing to the outstanding photophysical properties, such as high photoluminescence quantum yields, narrow emission bandwidths, and tunable band gaps that covers the full visible range [1,2]. Since the pioneering work by the Kovalenko group in 2015, considerable progress in the preparation and application of CsPbBr3 NCs has been achieved within a very short time period [1]. CsPbBr3 NCs with a controllable morphology and composition have been fabricated by different methods, such as hot-injection [3], solvothermal synthesis [4], room-temperature precipitation [5], and chemical vapor deposition (CVD) [6]. Moreover, a variety of photoelectronic devices—such as photovoltaics [7], lasing [8], light-emitting diodes and photodetectors [9,10]—have been prepared from CsPbBr3 NC. In addition to CsPbBr3 NCs, other types of materials of the ternary Cs-Pb-Br system, such as Cs4PbBr6 and CsPb2Br5, have also been reported [11,12]. The compounds CsPbBr3, Cs4PbBr6, and CsPb2Br5 differ in the stacking of PbBr6 octahedra in their crystal structures. In CsPbBr3, the lead halide octahedra share all corners and are electronically coupled in three directions in space. However, in the Cs4PbBr6 lattice the octahedra do not share any corners [11]. One lead atom and eight bromine atoms make up a hendecahedron with edge sharing in CsPb2Br5 [12]. It is reasonable to suspect that the existence of multiple compounds of Cs-Pb-Br system is probably relevant to the unstable luminescent property of CsPbBr3, which is the main obstacle on the progress of CsPbBr3. The photoluminescence quantum yield (PLQY) of colloidal CsPbBr3 NCs of ~90% decreases dramatically to below ~20% when they are in the solid phase (such as in a thin film). Different mechanisms have been proposed to explain the luminescence quenching, such as loss of the high quality of the NC by aggregation, removal of the surface passivation, and chemical decomposition of the materials [11,13]. Therefore, investigation on the inter-conversion between the Cs-Pb-Br compounds above is rather important.
Very recently, it has been reported that these ternary Cs-Pb-Br compounds can be inter-converted by physical and chemical treatments. Conversion of pre-synthesized CsPbBr3 NC to Cs4PbBr6 NCs have been reported by the extraction of PbBr2 through amine- and thiol-mediation method [14,15]. Furthermore, a reverse conversion from Cs4PbBr6 to CsPbBr3 has been reported by the Manna group through extraction of CsBr with Prussian Blue [16]. However, all the conversions above were performed on Cs-Pb-Br NCs with ligands on their surface and they were realized with mediation by a ligand. Investigations on the inter-conversion between the bare compounds of the Cs-Pb-Br system without ligand mediation are rarely reported, which is essential to reveal instability mechanism of CsPbBr3.
In this work, we prepared CsPbBr3 particles without ligands through a simple low temperature method and realized reversible conversions between CsPbBr3 and Cs4PbBr6, and between CsPbBr3 and CsPb2Br5. We combined our experimental observations with calculations of the total energy of the three Cs-Pb-Br compounds, we found that conversions of CsPbBr3 to Cs4PbBr6 and to CsPb2Br5 could take place spontaneously. However, the reverse conversions required external intervention.
2. Materials and Methods
2.1. Materials
Lead(II) bromide (PbBr2, Aladdin, 99.999%), cesium bromide (CsBr, Aladdin, 99.999%), hydrobromic acid (HBr, ≥40.0%), and N,N-dimethylformamide (DMF, Aladdin, 99.9%), were used without any further purification.
2.2. Synthesis of CsPbBr3
The synthesis of CsPbBr3 was performed via a simple reaction and crystallization method. Briefly, 0.5 mmol of PbBr2 and 0.5 mmol of CsBr were dissolved in 10 mL of DMF and stirred until completely dissolved. The mixture was then placed in an oven at 40 °C to evaporate the solvent and induce the reaction to produce CsPbBr3 solids.
2.3. Experiments on the Inter-Conversion between the Compounds
2.3.1. Forward Conversion from CsPbBr3 to Cs4PbBr6 or CsPb2Br5
Cesium bromide (CsBr, 3 mmol) was first dissolved in hydrobromic acid (HBr, 2 mL). Pre-synthesized CsPbBr3 (1 mmol) was added to the solution and stirred to react with CsBr and produce Cs4PbBr6, which precipitated at the bottom of the mixture. The precipitate was collected by evaporating the HBr solvent. For the conversion to CsPb2Br5, lead(II) bromide (PbBr2, 1 mmol) was first dissolved in hydrobromic acid (HBr, 2 mL). Then the CsPbBr3 solid (1 mmol) was added to the solution with stirring to react with PbBr2. The CsPb2Br5 was produced and precipitated at the bottom of the mixture. The precipitate was collected by evaporating the HBr solvent.
2.3.2. Reverse Conversion from Cs4PbBr6 or CsPb2Br5 to CsPbBr3
A 0.25 mmol portion of Cs4PbBr6 was added to de-ionized water (1 mL) and stirred, to trigger the reverse conversion from Cs4PbBr6 to CsPbBr3. The conversion of CsPb2Br5 to CsPbBr3 was conducted by annealing the CsPb2Br5 solids for 4 h at 400 °C in air.
2.4. Materials Characterization
Crystal structures were measured with an X-ray diffractometer (Bruker D8 Advance, Karlsruhe, Baden-Wurttemberg, Germany) with Cu-Ka radiation (λ = 1.5406 Å). Scanning electron microscope (SEM) and energy dispersive spectrum (EDS) measurements were performed on a JSM7100F, Tokyo, Honshu, Japan. A transmission electron microscope (TEM) (FEI; Tecnai-G20 and 200 kV, Hillsboro, OR, USA) was used to characterize the microstructure of the CsPbBr3, Cs4PbBr6 and CsPb2Br5. Absorption spectra were measured on a UV-Visible-NIR spectrophotometer (SHIMADZU UV-3600, Kyoto, Honshu, Japan).
2.5. First-Principle Calculations
First-principle calculations were performed on the basis of density functional theory (DFT) as implemented in the QUANTUM ESPRESSO (QE) code. The exchange and correlation terms were described using the general gradient approximation (GGA) of Perdew-Burke-Ernzerhof (PBE). The energy cutoff for the plane wave basis set was 600 eV. The accuracy of the self-consistent field (SCF) energy convergence and the convergence accuracy of the internal stress of the crystal were less than 1.4 × 10−5 eV/atom and 0.05 Gpa, respectively. For the different alloy configurations, Monkhorst-Pack grids were determined automatically for the Brillouin zone integration and the KPPRA parameter was set to be 1000.
3. Results and Discussion
3.1. Synthesis of CsPbBr3 and Forward Conversion to Cs4PbBr6 and CsPb2Br5.
Synthesis of CsPbBr3 (PDF#18-0364) was performed via a simple reaction and crystallization method without the use of any ligands. Full details are described in the experimental section. Characterization results of the prepared solids by XRD and absorption spectroscopy, shown in Figure 1a,b, demonstrated that the product was pure monoclinic CsPbBr3.
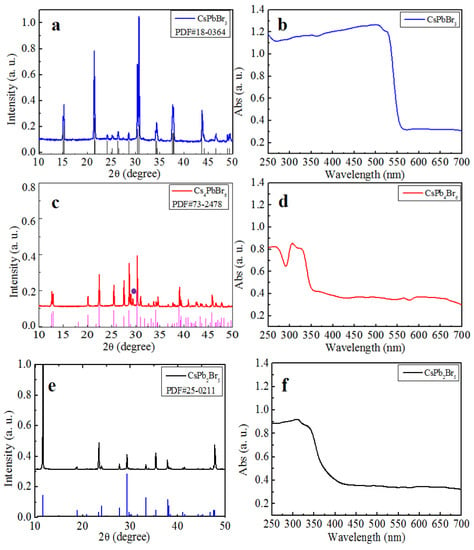
Figure 1.
The XRD pattern of (a) CsPbBr3, (c) Cs4PbBr6 and (e) CsPb2Br5. The absorption spectra of (b) CsPbBr3, (d) Cs4PbBr6, and (f) CsPb2Br5.
The as-synthesized CsPbBr3 solids were used to perform the forward conversion from CsPbBr3 to Cs4PbBr6 and CsPb2Br5. First, CsBr was dissolved in HBr, and a certain amount of the CsPbBr3 solid synthesized above (yellow) was added into the solution (CsPbBr3/CsBr = 1:3, mole ratio) with stirring. This approach ensured that only H was introduced into the reaction system, containing Cs, Pb, and Br, which simplified the analysis on the reaction. After stirring for several hours, a white precipitate formed, which was revealed to be rhombohedral Cs4PbBr6 by XRD, as shown in Figure 1c. To reveal whether CsPbBr3 remnants exist in the product since both CsPbBr3 and Cs4PbBr6 exhibit diffraction peaks near 27°, the absorption spectrum of Cs4PbBr6 product was measured and is shown in Figure 1d. A typical absorption peak at 315 nm was observed, which is characteristic of Cs4PbBr6, and no absorption peaks corresponding to CsPbBr3 appear [17]. This result further confirmed the conversion from CsPbBr3 to Cs4PbBr6. As far as the additional small diffraction peak near 29° denoted by purple dot in Figure 1c is concerned, it corresponds to CsBr. Because the ratio of the reactants CsPbBr3/CsBr was 1:3 and the only product was Cs4PbBr6, Equation (1) is proposed to describe the chemical reaction of the conversion. Except for operating as the solvent, the HBr also supplies abundance of Br+ and promotes the chemical reaction according to Equation (1).
CsPbBr3 + 3CsBr = Cs4PbBr6
The conversion from CsPbBr3 to CsPb2Br5 was realized by a similar reaction. First, PbBr2 was dissolved in HBr and CsPbBr3 solid was added into the solution (CsPbBr3/PbBr2 = 1:1, mole ratio). After several hours, white solids precipitated at the bottom of the mixture. XRD characterization, as shown in Figure 1e, of the precipitate revealed that it was pure tetragonal CsPb2Br5, indicating that the conversion from CsPbBr3 to CsPb2Br5 occurred. To confirm the conversion, the absorption spectra of the reactant and product were measured. As shown in Figure 1f, the absorption edge moved to 380 nm, indicating that CsPbBr3 was converted into CsPb2Br5. The conversion was believed to occur through Equation (2), as shown below, based on the fact that the ratio of the reactants CsPbBr3/PbBr2 was 1:1 and the only product formed was CsPb2Br5.
CsPbBr3 + PbBr2 = CsPb2Br5
The morphology of each material was examined by scanning electron microscope (SEM) imaging, as shown in Figure S1. Energy dispersive spectroscopy (EDS) results, also shown in Figure S1, indicated that the molar ratios of Cs/Pb/Br were 1.18/1/2.89, 1/1.87/5.57, and 4.18/1/6.36 respectively, which agreed well with the stoichiometries of CsPbBr3, CsPb2Br5, and Cs4PbBr6. The microstructure of each Cs-Pb-Br ternary compound was characterized by high resolution TEM (HRTEM), as shown in Figure 2. Well-resolved lattice fringes were observed in the HRTEM images. In Figure 2b, the separation between the fringes was 0.588 nm, which corresponded to the (001) plane of CsPbBr3. In the HRTEM images of CsPb2Br5 and Cs4PbBr6, the (110) and (220) planes were clearly observed, with lattice separations of 0.609 and 0.451 nm, respectively. These EDS and HRTEM results further confirmed that the conversions had occurred.
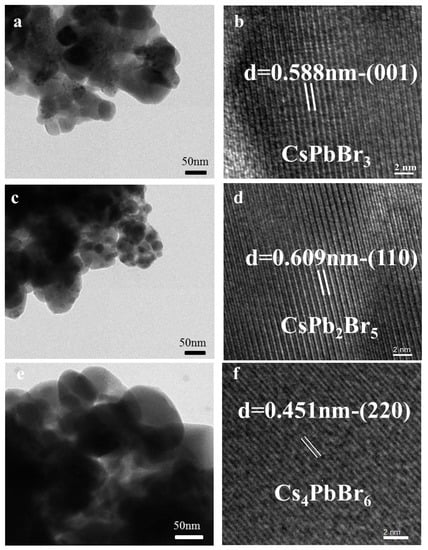
Figure 2.
(a,c,e) TEM images; and (b,d,f) high-resolution lattice resolved TEM images of a representative CsPbBr3, CsPb2Br5, and Cs4PbBr6, respectively.
In the conversions above, we did not use high temperature, high pressure or a catalyst to trigger the reactions. Hence, the Equations (1) and (2) are thermodynamically controlled process and the driving force, described by the free energy should be negative. Therefore, we calculated the total energy (Et) of the Equations (1) and (2) by first principles. The changes of the total energy (ΔEt) for Equations (1) and (2) were −9508.07 eV and −15019.13 eV, respectively, indicating that the chemical reactions were exothermic and could occur spontaneously. The total energy of each materials is shown in Table S1 in the supporting information. These results explain why this simple method can successfully realize the conversion of CsPbBr3 into Cs4PbBr6 or CsPb2Br5.
3.2. Reverse Conversion by Water Extraction and Thermal Annealing
For the reverse conversion from Cs4PbBr6 to CsPbBr3, we used the water extraction method proposed by the Sun group [18]. By mixing a Cs4PbBr6 quantum dot dispersion in nonpolar hexane with water, Sun et al. found that CsBr could be extracted from Cs4PbBr6 owing to the high solubility of CsBr in water. This effect led to a conversion from Cs4PbBr6 to CsPbBr3.
Here, we found that water could also extract CsBr from Cs4PbBr6 solids without ligands on their surface. However, the reaction we observed was much more vigorous and quick. These differences between our observations and those of Sun et al. could be attributed to the absence of protective ligands on the surface of our NCs, unlike the Cs4PbBr6 quantum dot dispersion reported by Sun [18]. When the Cs4PbBr6 solids were added into deionized water, the color of the precipitate changed to yellow immediately, and returned to white again over a longer time, suggesting that chemical reactions occurred in two stages. The precipitate at different stages was removed from the deionized water and the composition was measured by XRD.
As shown in Figure 3a, most of the Cs4PbBr6 transformed to CsPbBr3 within 1 min. However, CsPbBr3 was not the final product. The conversion into CsPb2Br5 proceeded within 5 min, and the major product was CsPb2Br5 after 1 h. Therefore, we suggest that the water not only extracted CsBr from Cs4PbBr6, but also extracted CsBr from CsPbBr3 to produce CsPb2Br5. The composition of the solution was also investigated to clarify the nature of the chemical transformation. To confirm the water extraction mechanism for the conversions, we evaporated the water and performed XRD measurements on the solid obtained, which was determined to be CsBr, as shown in the supporting information Figure S2. Therefore, we propose the following Equations for the chemical reactions occurring at each stage of the transformation as:
Cs4PbBr6 = CsPbBr3 + 3CsBr,
2CsPbBr3 = CsPb2Br5 + CsBr
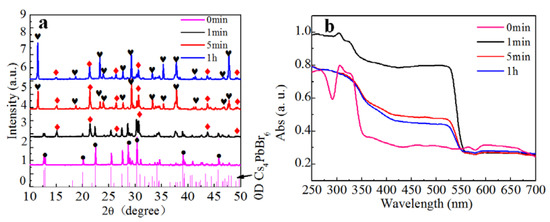
Figure 3.
(a) PXRD pattern of Cs4PbBr6 solids after water treatment for different time, the red diamonds represent CsPbBr3, the black hearts represents CsPb2Br5, the black dots represents Cs4PbBr6; (b) Absorption spectra of PXRD pattern of Cs4PbBr6 solids after water treatment for different times.
Figure 3b shows the absorption spectrum measured from the precipitate samples removed from deionized water after different reaction times. A strong characteristic absorption edge at 560 nm appeared after 1 min and its intensity decreased at longer reaction times. This result indicates that the CsPbBr3 was produced within 1 min and converted to CsPb2Br5 over longer reaction times. These findings are consistent with the XRD results.
To confirm the two-step transformation model suggested above, we added pure CsPbBr3 into deionized water and investigated the conversion. We found that the transformation in Equation (4) occurred and a white precipitate was formed quickly. As shown in the XRD pattern obtained from the precipitate in Figure 4a, the diffraction peaks related to CsPbBr3 became weak and strong diffraction peaks corresponding to CsPb2Br5 were observed after 1 min. Moreover, the intensity of CsPb2Br5 gradually increased as the reaction progressed. The absorption spectra in Figure 4b, show that the absorption peak at 560 nm from CsPbBr3 became progressively weaker. We note that the absorption peak of CsPbBr3 did not completely disappear, even after 1 h of reaction, indicating that a small amount of CsPbBr3 persisted. The XRD and absorption results clearly demonstrated that CsPbBr3 could be converted to CsPb2Br5 through extraction of CsBr by water. The conversion induced by water extraction is undoubtedly one of the reasons leading to the unstable luminescent property of CsPbBr3. The water vapor in the air can extract CsBr from CsPbBr3 and trigger the conversion into CsPb2Br5, which subsequently results in the degradation of luminescence.
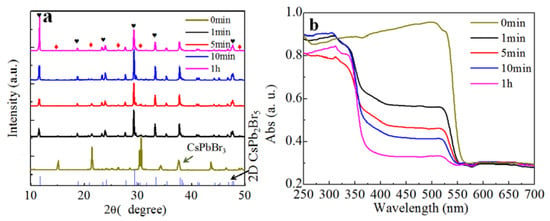
Figure 4.
(a) XRD patterns and (b) absorption spectra of CsPbBr3 solids after water treatment for different times. Red diamonds represent CsPbBr3 and black hearts denote the diffraction peaks of CsPb2Br5.
We realized a conversion from CsPb2Br5 to CsPbBr3, using a previously reported annealing method [19]. We annealed the CsPb2Br5 solids at 400 °C in air for 4 h and monitored the associated XRD and absorption properties. Figure 5a shows XRD data of the CsPb2Br5 before and after annealing. The corresponding diffraction peaks before annealing were indexed to CsPb2Br5. After annealing, the main product corresponded to CsPbBr3 and PbBr2, and a small amount of CsPb2Br5 remained. The decomposition is depicted by the Equation:
CsPb2Br5 = CsPbBr3 + PbBr2
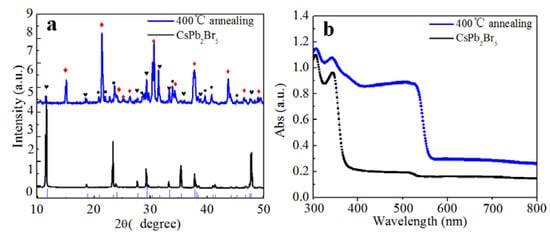
Figure 5.
(a) XRD pattern, and (b) absorption spectra of CsPb2Br5 particles after annealing at 400 °C temperature. In the XRD pattern, the red diamonds denotes the diffraction peak of CsPbBr3, the black dots denotes the diffraction peak of PbBr2 and black hearts denote the diffraction peaks of CsPb2Br5.
The absorption spectra in Figure 5b show that the absorption peak relevant to CsPbBr3 was considerably enhanced, indicating the generation of CsPbBr3, which is consistent with the XRD results shown in Figure 5a. As far as the mechanism of the Equation (5) is concerned, it is ascribed to the decompositon of CsPb2Br5 energetically driven by high temperature annealing.
4. Conclusions
In conclusion, we examined a reversible conversion between CsPbBr3, Cs4PbBr6, and CsPb2Br5. First, CsPbBr3 solids were synthesized through a simple reaction of CsBr and PbBr2 in HBr. Addition of the prepared CsPbBr3 solids into the CsBr/PbBr2 solution in HBr, resulted in its conversion into Cs4PbBr6 and CsPb2Br5, through Equations (1) and (2), respectively. Thermodynamic analysis revealed that the transformations above were exothermic and occurred spontaneously. Moreover, we found that when added into the water Cs4PbBr6 converted to CsPbBr3 first, and then to Cs-deficient CsPb2Br5. These results are attributed to the ionic nature of the Cs-Pb-Br system and the high solubility of CsBr in water, which led to extraction of CsBr by water. The CsPb2Br5 was converted to CsPbBr3 through thermal annealing at 400 °C. Our results on the inter-conversion of the Cs-Pb-Br compounds sheds a light on understanding the mechanism and developing new solutions for the instability problem of the Cs-Pb-Br compounds. Moreover, it supplies important information on the controllable preparation of the Cs-Pb-Br materials.
Supplementary Materials
The following are available online at http://www.mdpi.com/1996-1944/11/5/717/s1, Figure S1: Scanning electron microscope (SEM) and Energy dispersive X-spectroscopy (EDS); Figure S2: The XRD pattern of CsBr (PDF#73-0391) obtained from the solvent of water by evaporation; Table S1. The total energy of each materials is calculated based on the optimized structure using DFT.
Author Contributions
J.L., H.Z., and S.W. performed the experiments; D.L. and M.L. performed the calculation; D.W. and T.Z. design this study and analyzed the data; all authors read and approved the final manuscript.
Funding
This research was funded by National Natural Science Foundation of China (nos. 11174071, 11304088 and 51372180) and Special Technical Innovation Project of Hubei Province (no. 2016AAA035).
Acknowledgments
We thank Zhijun Ma in Hubei University for his help on the XRD measurement and analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, J.; Yang, Y.; Deng, H.; Farooq, U.; Yang, X.K.; Kan, J.; Tang, J.; Song, H.S. High quantum yield blue emission from lead-free inorganic antimony halide perovskite colloidal quantum dot. ACS Nano 2017, 11, 9294–9302. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; He, B.; Fan, X.; Liu, Z.; Urban, J.J.; Alivisatos, A.P.; He, L.; Liu, Y. Insight into the ligand-mediated synthesis of colloidal CsPbBr3 perovskite nanocrystals: the role of organic acid, base, and cesium precursors. ACS Nano 2016, 10, 7943–7954. [Google Scholar] [CrossRef] [PubMed]
- Rakita, Y.; Kedem, N.; Gupta, S.; Sadhanala, A.; Kalchenko, V.; Böhm, M.L.; Kulbak, M.; Friend, R.H.; Cahen, D.; Hodes, G. Low-temperature solution-grown CsPbBr3 single crystals and their characterization. Cryst. Growth Des. 2016, 16, 5717–5725. [Google Scholar] [CrossRef]
- Sun, S.; Yuan, D.; Xu, Y.; Wang, A.; Deng, Z. Ligand-mediated synthesis of shape-controlled cesium lead halide perovskite nanocrystals via reprecipitation process at room temperature. ACS Nano 2016, 10, 3648–3657. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guan, X.; Li, D.; Cheng, H.-C.; Duan, X.; Lin, Z.; Duan, X. Chemical vapor deposition growth of single-crystalline cesium lead halide microplatelets and heterostructures for optoelectronic applications. Nano Res. 2017, 10, 1223–1233. [Google Scholar] [CrossRef]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot–induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.W.; Lai, M.; Gibson, N.A.; Wong, A.B.; Dou, L.; Ma, J.; Wang, L.-W.; Leone, S.R.; Yang, P. Lasing in robust cesium lead halide perovskite nanowires. Proc. Natl. Acad. Sci. USA 2016, 113, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, P.; Lim, D.-H.; Kim, B.; Lee, S.-H.; Lee, M.-S.; Lee, J.-S. All-inorganic cesium lead halide perovskite nanocrystals for photodetector applications. Chem. Commun. 2016, 52, 2067–2070. [Google Scholar] [CrossRef] [PubMed]
- Saidaminov, M.I.; Almutlaq, J.; Sarmah, S.; Dursun, I.; Zhumekenov, A.A.; Begum, R.; Pan, J.; Cho, N.; Mohammed, O.F.; Bakr, O.M. Pure Cs4PbBr6: Highly luminescent zero-dimensional perovskite solids. ACS Energy Lett. 2016, 1, 840–845. [Google Scholar] [CrossRef]
- Wang, K.H.; Wu, L.; Li, L.; Yao, H.B.; Qian, H.S.; Yu, S.H. Large-scale synthesis of highly luminescent perovskite-related CsPb2Br5 nanoplatelets and their fast anion exchange. Angew. Chem. Int. Ed. 2016, 55, 8328–8332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Saidaminov, M.I.; Dursun, I.; Yang, H.; Murali, B.; Alarousu, E.; Yengel, E.; Alshankiti, B.A.; Bakr, O.M.; Mohammed, O.F. Zero-dimensional Cs4PbBr6 perovskite nanocrystals. J Phys. Chem. Lett. 2017, 8, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bekenstein, Y.; Ye, X.; Nguyen, S.C.; Swabeck, J.; Zhang, D.; Lee, S.-T.; Yang, P.; Ma, W.; Alivisatos, A.P. Ligand mediated transformation of cesium lead bromide perovskite nanocrystals to lead depleted Cs4PbBr6 nanocrystals. J Am. Chem. Soc. 2017, 139, 5309–5312. [Google Scholar] [CrossRef] [PubMed]
- Palazon, F.; Almeida, G.; Akkerman, Q.A.; De Trizio, L.; Dang, Z.; Prato, M.; Manna, L. Changing the dimensionality of cesium lead bromide nanocrystals by reversible postsynthesis transformations with Amines. Chem. Mater. 2017, 29, 4167–4171. [Google Scholar] [CrossRef] [PubMed]
- Palazon, F.; Urso, C.; De Trizio, L.; Akkerman, Q.; Marras, S.; Locardi, F.; Nelli, I.; Ferretti, M.; Prato, M.; Manna, L. Postsynthesis Transformation of insulating Cs4PbBr6 nanocrystals into bright perovskite CsPbBr3 through physical and chemical extraction of CsBr. ACS Energy Lett. 2017, 2, 2445–2448. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.N.; Quintero-Bermudez, R.; Voznyy, O.; Walters, G.; Jain, A.; Fan, J.Z.; Zheng, X.; Yang, Z.; Sargent, E.H. Highly emissive green perovskite nanocrystals in a solid state crystalline matrix. Adv. Mater. 2017, 29, 1605945. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Hu, H.; Xu, Y.; Jiang, S.; Chen, M.; Zhong, Q.; Yang, D.; Liu, Q.; Zhao, Y.; Sun, B. From nonluminescent Cs4PbX6 (X = Cl, Br, I) nanocrystals to highly luminescent CsPbX3 nanocrystals: water-triggered transformation through a CsX-stripping mechanism. Nano Lett. 2017, 17, 5799–5804. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Wang, S.; Long, D.; Li, M.; Guo, Y.; Zhong, Z.; Wu, K.; Wang, D.; Zhang, T. Synthesis of all-inorganic CsPb2Br5 perovskite and determination of its luminescence mechanism. RSC Adv. 2017, 7, 54002–54007. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).