Thin Film Coating with Highly Dispersible Barium Titanate-Polyvinylpyrrolidone Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
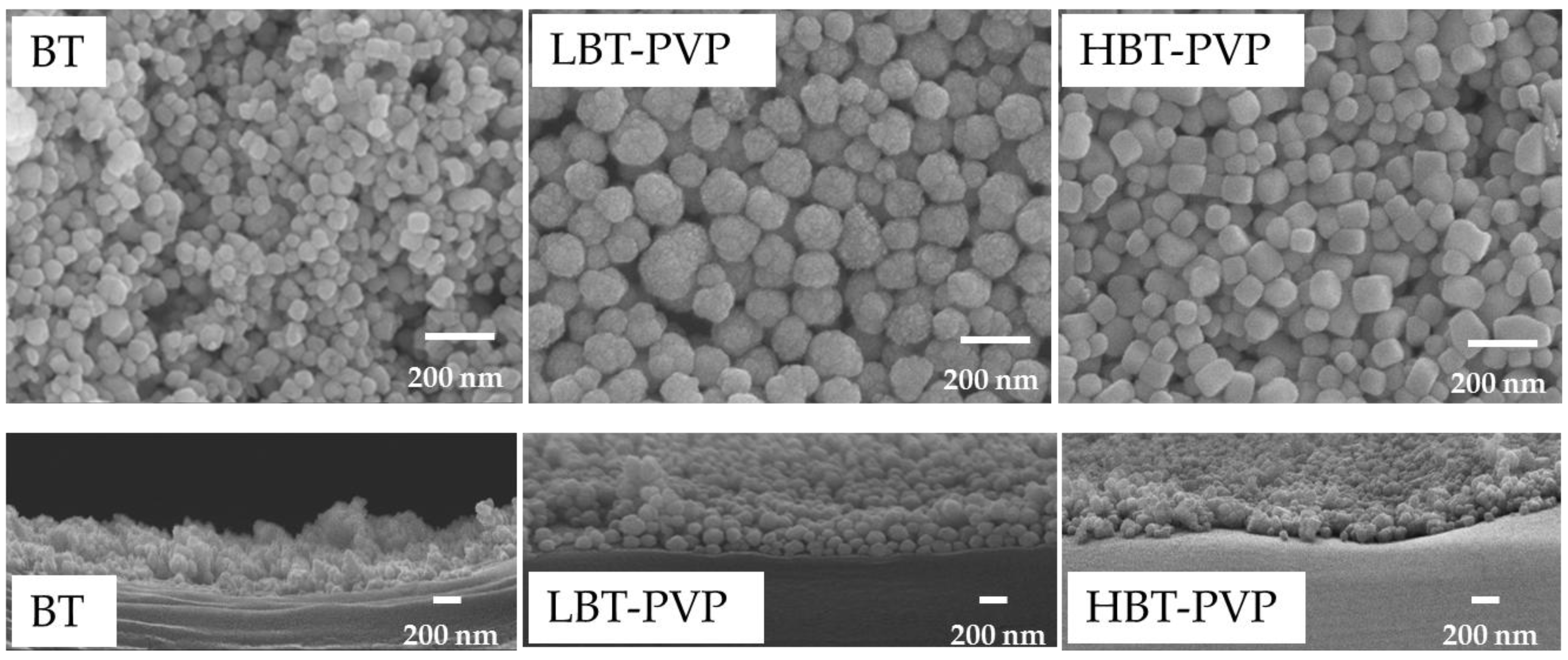
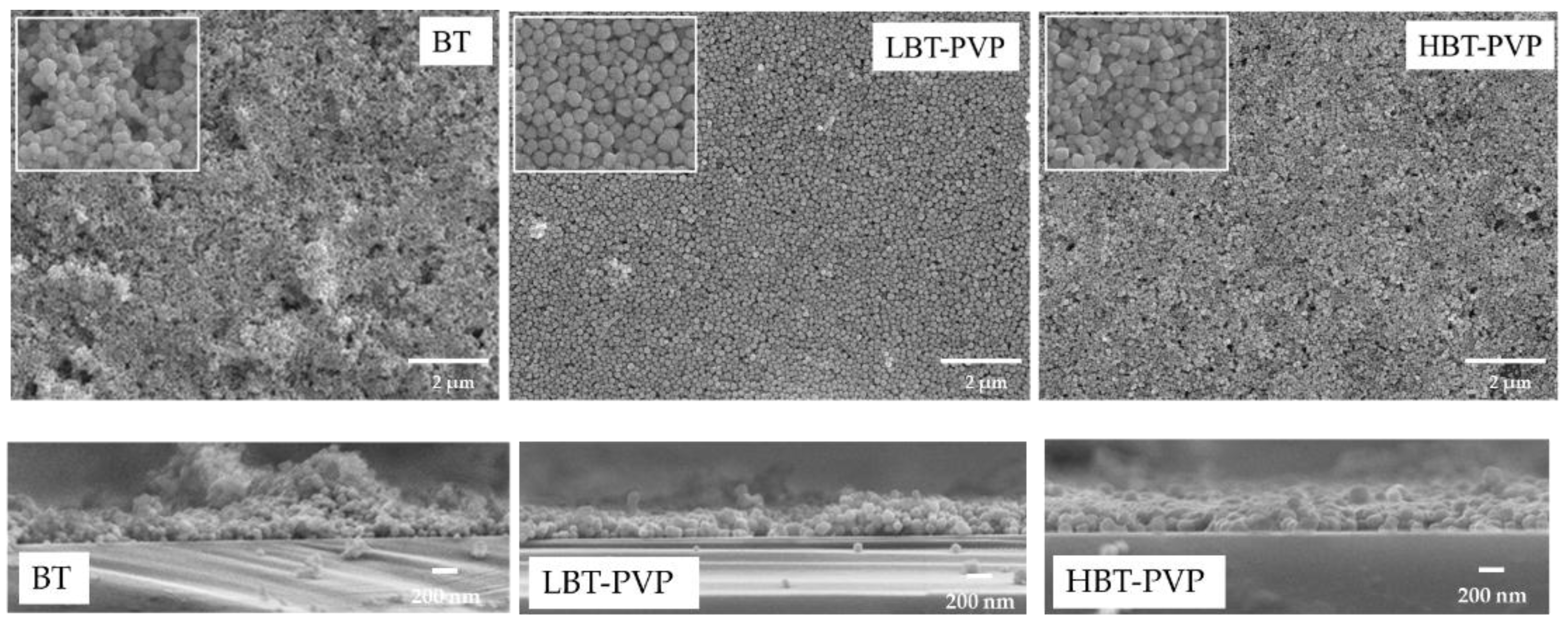
2.1. Properties of BT-PVPs Prepared by Different Synthesis Method
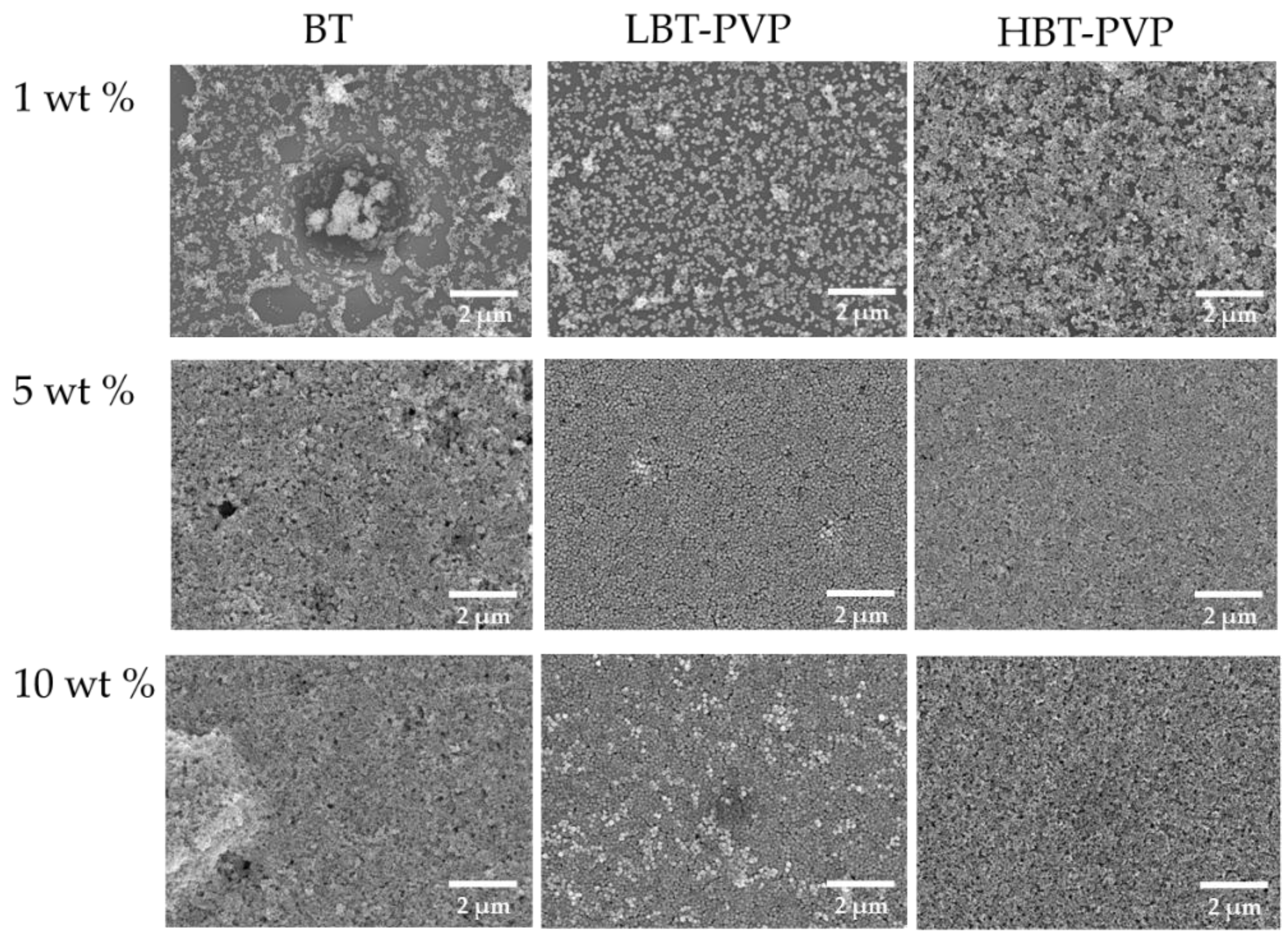
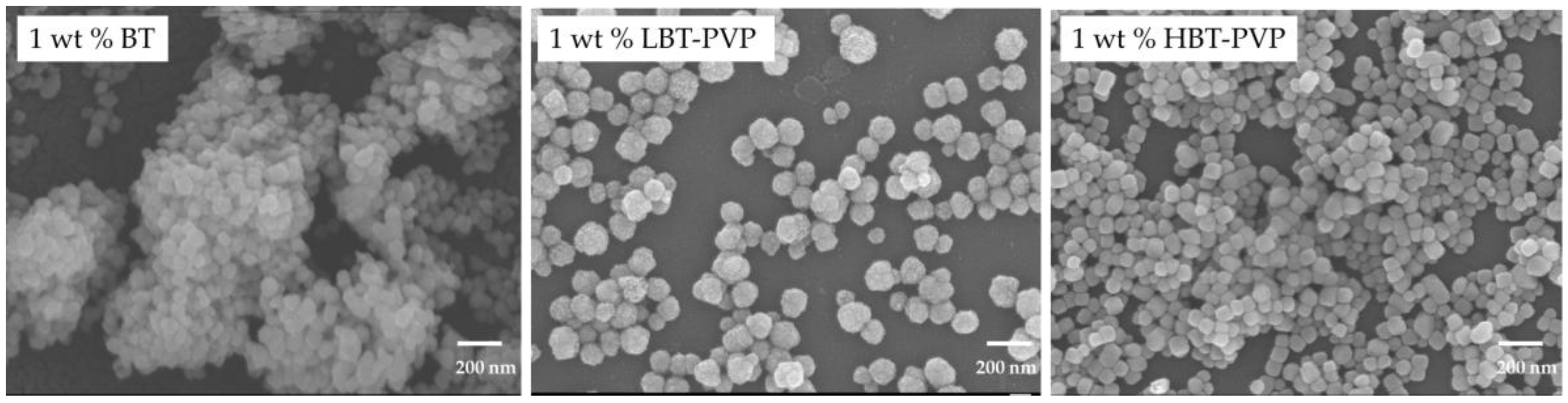
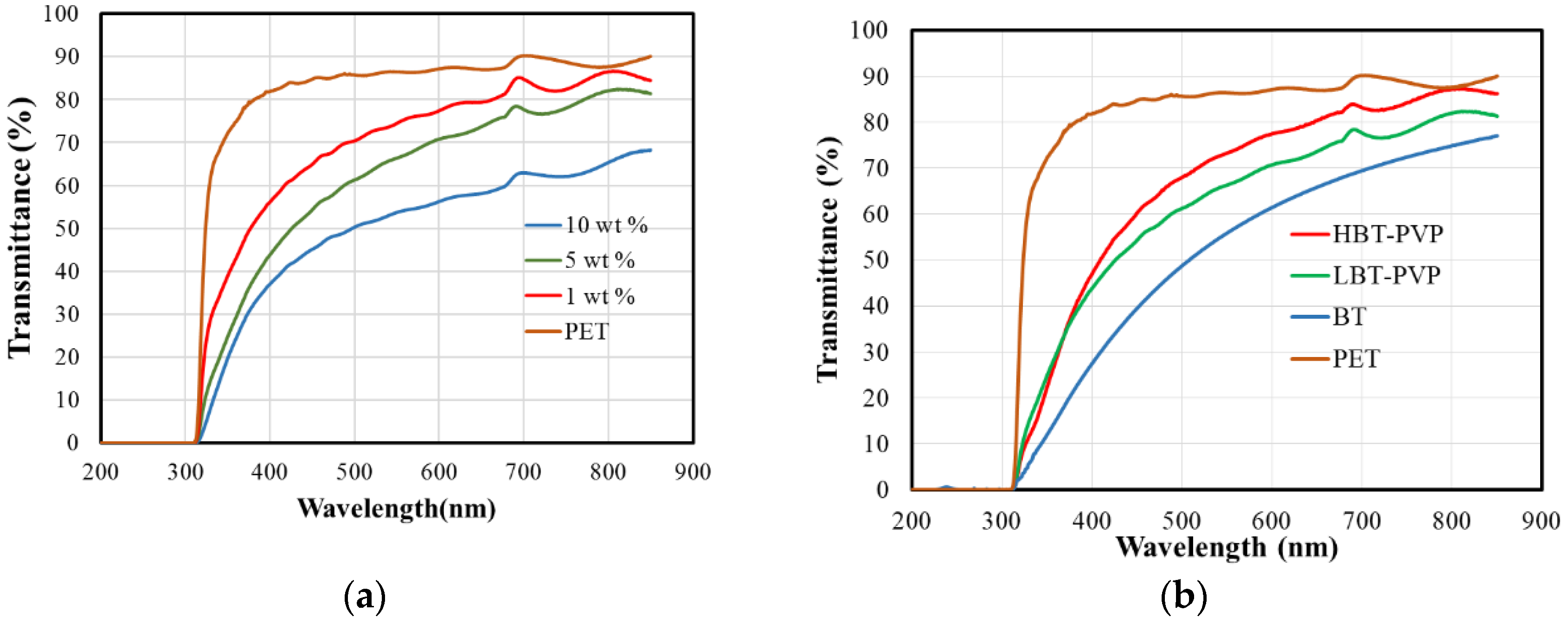
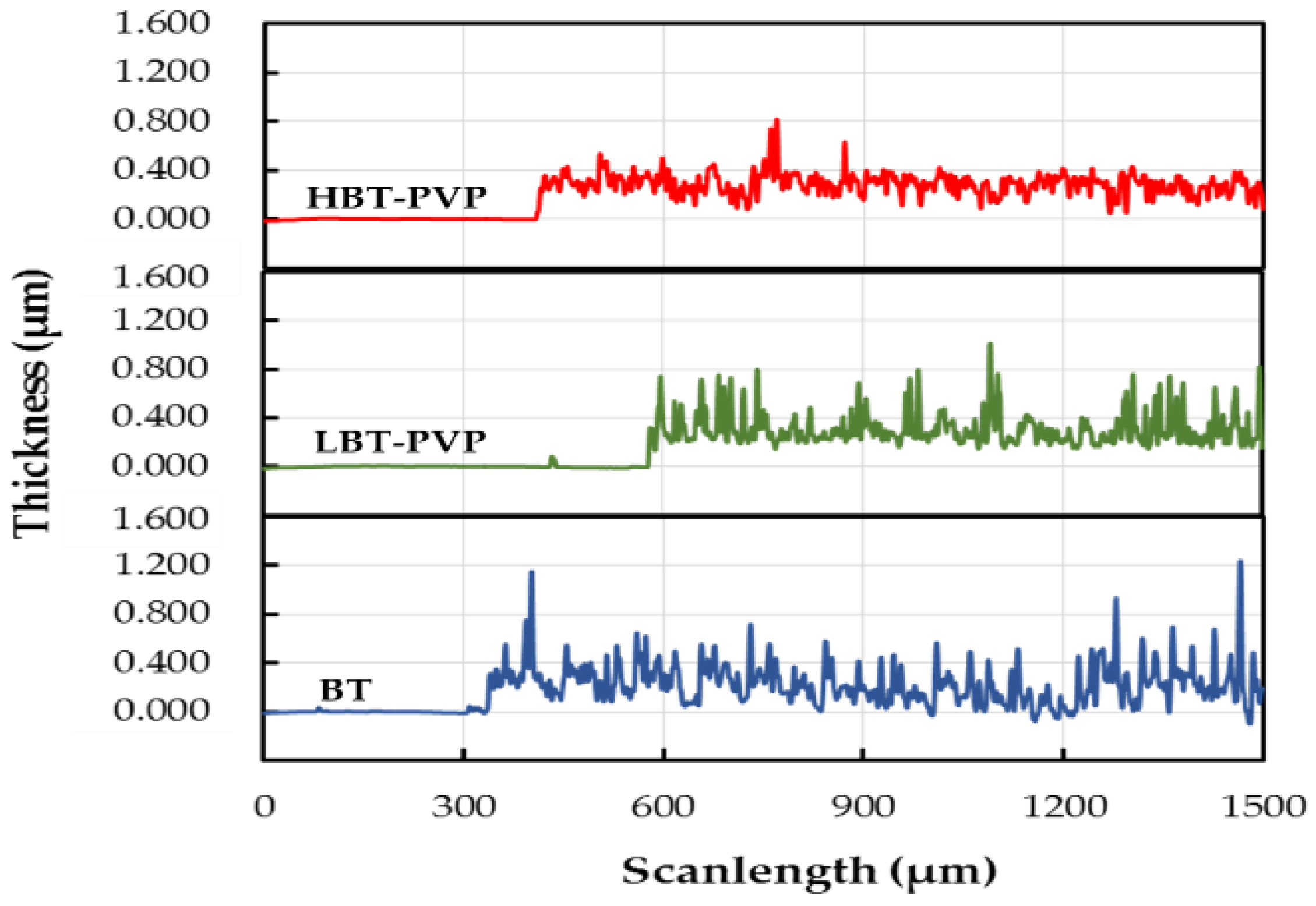
2.2. Properties of the BT-PVP Thin Film
3. Materials and Methods
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cross, L.E. Dielectric, Piezoelectric and Ferroelectric Components. Am. Ceram. Soc. Bull. 1984, 63, 586–590. [Google Scholar]
- Pandey, D.; Singh, A.P.; Tiwari, V.S. Developments in ferroelectric ceramics for capacitor applications. Bull. Mater. Sci. 1992, 15, 391–402. [Google Scholar] [CrossRef]
- Stachiotti, M. Ferroelectricity in BaTiO3 nanoscopic structures. Appl. Phys. Lett. 2004, 84, 251–253. [Google Scholar] [CrossRef]
- Varghese, J.; Whatomore, R.W.; Holmes, J.D. Ferroelectric nanoparticles, wires and tubes: Synthesis, characterization and applications. J. Mater. Chem. 2013, 1, 2618–2638. [Google Scholar] [CrossRef]
- Berlincourt, D.; Jaffe, H. Elastic and piezoelectric coefficients of single-crystal barium titanate. Phys. Rev. 1958, 111, 143–148. [Google Scholar] [CrossRef]
- Novak, N.; Pirc, R.; Kutnjak, Z. Impact of critical point on piezoelectric and electrocaloric response in barium titanate. Phys. Rev. B 2013, 87, 104102–104107. [Google Scholar] [CrossRef]
- Wada, S.; Yamato, K.; Pulpan, P.; Kumada, N.; Lee, B.-Y.; Iijima, T.; Moriyoshi, C.; Kuroiwa, Y. Piezoelectric properties of high Curie temperature barium titanate–bismuth perovskite-type oxide system ceramics. J. Appl. Phys. 2010, 108, 094114. [Google Scholar] [CrossRef]
- Evangelou, E.K.; Konofaos, N.; Aslanoglou, X.; Kennou, S.; Thomas, C.B. Characterization of BaTiO3 thin films on p-Si. J. Mater. Sci. Semicond. Process. 2011, 4, 305–307. [Google Scholar] [CrossRef]
- Hache, F.; Richard, D.; Flytzanis, C. Optical nonlinearities of small metal particles: Surface-mediated resonance and quantum size effects. J. Opt. Soc. Am. 1986, B3, 1647–1655. [Google Scholar] [CrossRef]
- Vayunandana Reddy, Y.K.; Mergel, D.; Reuter, S.; Buck, V.; Sulkowski, M. Structural and optical properties of BaTiO3 thin films prepared by radio-frequency magnetron sputtering at various substrate temperatures. J. Phys. D Appl. Phys. 2006, 39, 1161–1168. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, J.; Huang, W.; Dai, S.; Wang, L. Preparation and optical properties of gold nanoparticles embedded in barium titanate thin films. J. Mater. Sci. 2003, 38, 1243–1248. [Google Scholar] [CrossRef]
- Hu, Z.G.; Wang, G.S.; Huang, Z.M.; Chu, J.H. Structure-related infrared optical properties of BaTiO3 thin films grown on Pt/Ti/SiO2/Si substrate. J. Phys. Chem. Solids 2003, 64, 2445–2450. [Google Scholar] [CrossRef]
- Kozuka, H. On ceramic thin film formation from gels: Evolution of stress, cracks and radiative striations. Jpn. J. Ceram. Soc. 2003, 111, 624–632. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Komeda, T.; Park, D.; Nishioka, Y. Comparative Study of Amorphous and Crystalline (Ba, Sr)TiO3 Thin Films Deposited by Laser Ablation. Jpn. J. Appl. Phys. 1993, 32, 4103–4106. [Google Scholar] [CrossRef]
- Yoon, S.G.; Lee, J.; Safari, A. Characterization of (Ba0.5, Sr0.5)TIO3 thin films by the laser ablation technique and their electrical properties with different electrodes. Integr. Ferroelectr. A 1995, 7, 329–339. [Google Scholar] [CrossRef]
- Tahan, D.; Safari, A.; Klin, L.C. Preparation and Characterization of BaxSr1−x, TiO3 Thin Films by a Sol-Gel Technique. J. Am. Ceram. Soc. C 1996, 79, 1593–1598. [Google Scholar] [CrossRef]
- Kozuka, H.; Kajimura, M. Single-Step Dip Coating of Crack-Free BaTiO3 Films >1 μm Thick: Effect of Poly(vinylpyrrolidone) on Critical Thickness. J. Am. Ceram. Soc. 2000, 83, 1056–1062. [Google Scholar] [CrossRef]
- Mimura, K.; Kato, K. Characteristics of Barium Titanate Nanocube Ordered Assembly Thin Films Fabricated by Dip-Coating Method. Jpn. J. Appl. Phys. 2013, 52, 09KC061-5. [Google Scholar] [CrossRef]
- Mimura, K.; Naka, T.; Shimura, T.; Sakamoto, W.; Yogo, T. Synthesis and dielectric properties of (Ba,Ca)(Zr,Ti)O3 thin films using metal-organic precursor solutions. Thin Solid Films 2008, 516, 8408–8413. [Google Scholar] [CrossRef]
- Lee, B.; Zhang, J. Preparation, structure evolution and dielectric properties of BaTiO3 thin films and powders by an aqueous sol–gel process. Thin Solid Films 2001, 388, 107–113. [Google Scholar] [CrossRef]
- Li, J.; Inukai, K.; Takahashi, Y.; Shin, W. Synthesis and size control of monodispersed BaTiO3–PVP nanoparticles. J. Asian Ceram. Soc. 2016, 4, 394–402. [Google Scholar] [CrossRef]
- Li, J.; Inukai, K.; Tsuruta, A.; Takahashi, Y.; Shin, W. Synthesis of highly disperse tetragonal BaTiO3 nanoparticles with core–shell by a hydrothermal method. J. Asian Ceram. Soc. 2017, 5, 444–451. [Google Scholar] [CrossRef]
- Itoh, T.; Uchida, T.; Izu, N.; Shin, W. Effect of Core-Shell Ceria/Poly(vinylpyrrolidone) (PVP) Nanoparticles Incorporated in Polymer Films and Their Optical Properties (2): Increasing the Refractive Index. Materials 2017, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Kedia, A.; Kumar, P.S. Solvent-adaptable poly(vinylpyrrolidone) binding induced anisotropic shape control of gold nanostructures. J. Phys. Chem. C 2012, 116, 23721–23728. [Google Scholar] [CrossRef]
- Itoh, T.; Uchida, T.; Izu, N.; Shin, W. Effect of Core-Shell Ceria/Poly(vinylpyrrolidone) (PVP) Nanoparticles Incorporated in Polymer Films and Their Optical Properties. Materials 2013, 6, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Maneeshya, L.V.; Anitha, V.S.; Thomas, P.V.; Joy, K. Thickness dependence of structural, optical and luminescence properties of BaTiO3 thin films prepared by RF magnetron sputtering. J. Mater. Sci. Mater. Electron. 2015, 26, 2947–2954. [Google Scholar] [CrossRef]
- Warusawithana, M.P.; Cen, C.; Sleasman, C.R.; Woicki, J.C.; Li, Y.; Kourkoutis, L.F.; Klug, J.A.; Li, H.; Ryan, P.; Wang, L.-P.; et al. A ferroelectric oxide made directly on silicon. Science 2009, 324, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Stojanovica, B.D.; Foschinic, C.R.; Pejovicd, V.Z.; Pavlovice, V.B.; Varelaa, J.A. Electrical Properties of Screen Printed BaTiO3 Thick Films. J. Eur. Ceram. Soc. 2004, 24, 1467–1471. [Google Scholar] [CrossRef]
- Kwon, S.; Park, B.; Choi, K.; Choi, E.; Nam, S.; Kim, J.; Kim, J. Solvothermally synthesized tetragonal barium titanate powders using H2O/EtOH solvent. J. Eur. Ceram. Soc. 2006, 26, 1401–1404. [Google Scholar] [CrossRef]
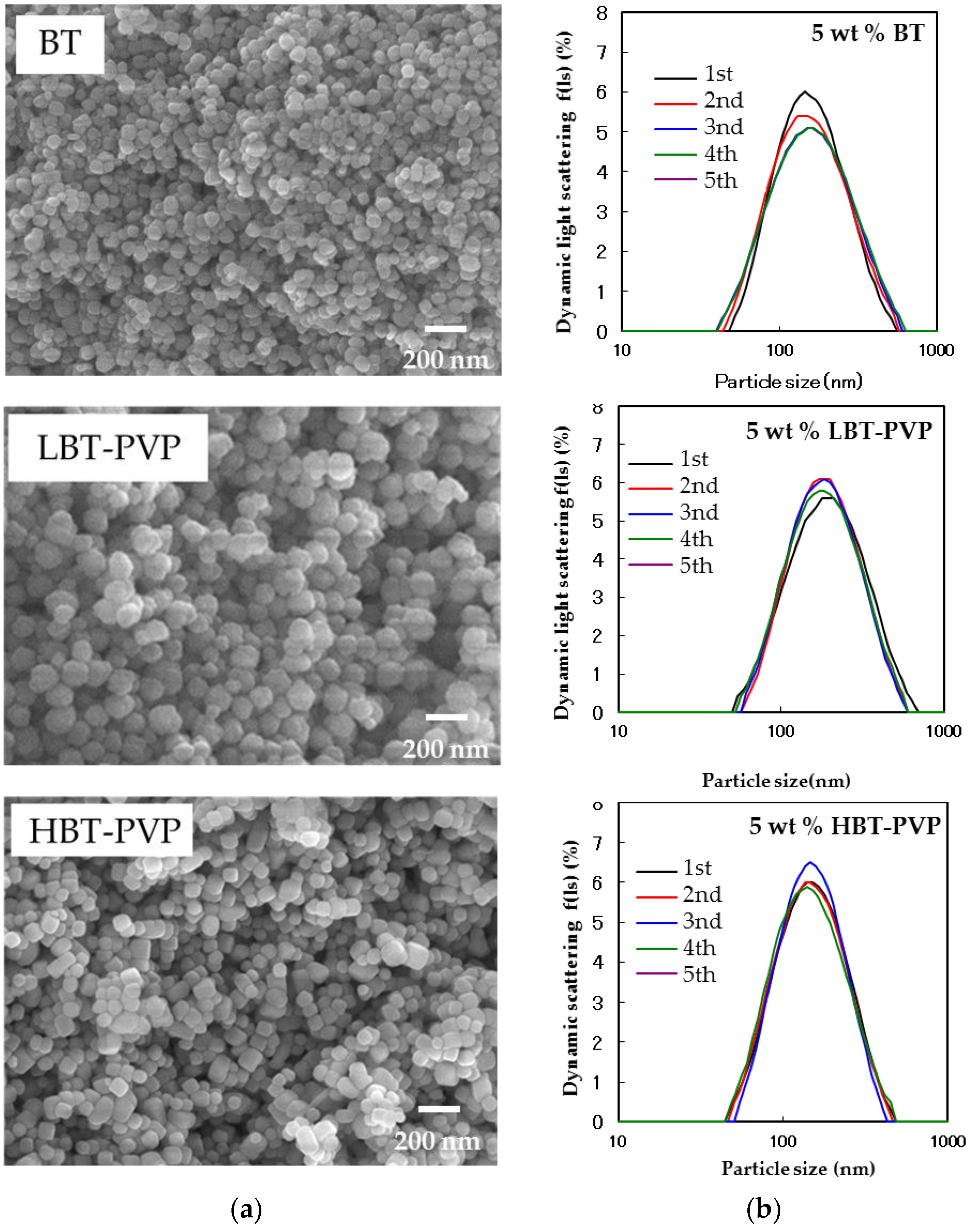
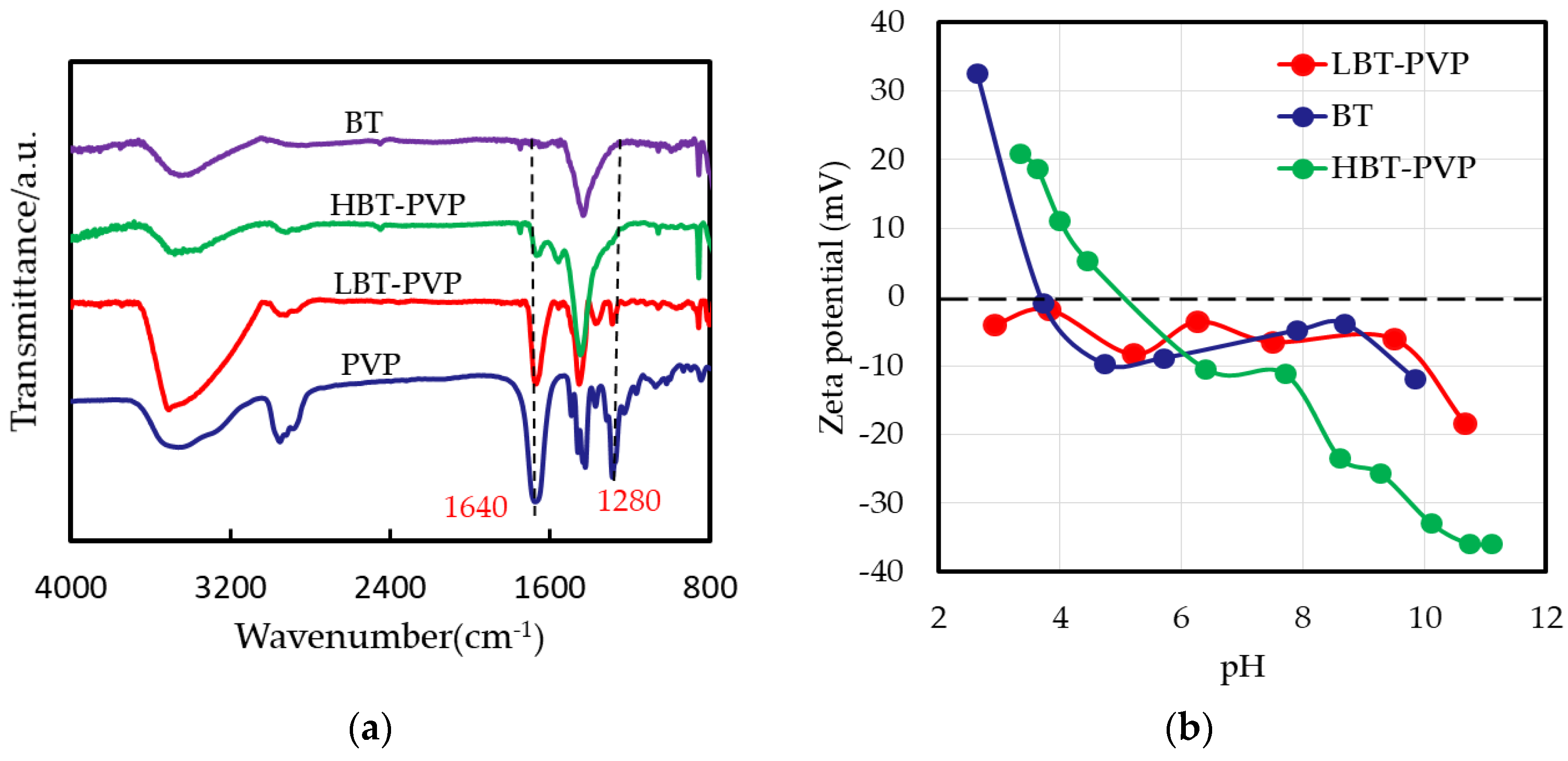
| Particles | Particle Size (nm) | Tetragonality (c/a) | |||
|---|---|---|---|---|---|
| SEM | CV* (%) | DLS | CV* (%) | ||
| BT | 81.0 | 20.1 | 141 | 55.2 | 1.0008 |
| LBT-PVP | 128 | 16.6 | 164 | 48.2 | 1.0005 |
| HBT-PVP | 106 | 20.7 | 131 | 47.2 | 1.0058 |
| Particles | Thickness (nm) | Transmittance * (%) | Haze (%) |
|---|---|---|---|
| BT | 268.2 | 55.91 | 34.89 |
| LBT-PVP | 307.9 | 66.33 | 24.70 |
| HBT-PVP | 266.8 | 73.31 | 20.53 |
| Particles | Film Thickness (nm) | Ra* (nm) | |
|---|---|---|---|
| SEM | Profiler | ||
| BT | 209.4 | 226.2 | 104.6 |
| LBT-PVP | 292.1 | 308.4 | 91.6 |
| HBT-PVP | 293.2 | 283.1 | 56.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Inukai, K.; Takahashi, Y.; Tsuruta, A.; Shin, W. Thin Film Coating with Highly Dispersible Barium Titanate-Polyvinylpyrrolidone Nanoparticles. Materials 2018, 11, 712. https://doi.org/10.3390/ma11050712
Li J, Inukai K, Takahashi Y, Tsuruta A, Shin W. Thin Film Coating with Highly Dispersible Barium Titanate-Polyvinylpyrrolidone Nanoparticles. Materials. 2018; 11(5):712. https://doi.org/10.3390/ma11050712
Chicago/Turabian StyleLi, Jinhui, Koji Inukai, Yosuke Takahashi, Akihiro Tsuruta, and Woosuck Shin. 2018. "Thin Film Coating with Highly Dispersible Barium Titanate-Polyvinylpyrrolidone Nanoparticles" Materials 11, no. 5: 712. https://doi.org/10.3390/ma11050712
APA StyleLi, J., Inukai, K., Takahashi, Y., Tsuruta, A., & Shin, W. (2018). Thin Film Coating with Highly Dispersible Barium Titanate-Polyvinylpyrrolidone Nanoparticles. Materials, 11(5), 712. https://doi.org/10.3390/ma11050712





