Mechanical Grinding Preparation and Characterization of TiO2-Coated Wollastonite Composite Pigments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
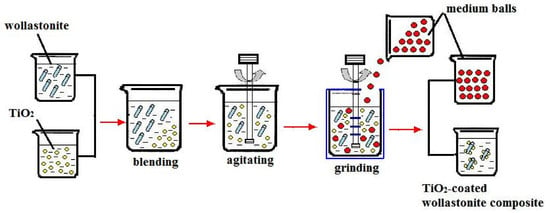
2.2. Preparation Method
2.3. Characterization
3. Results and Discussion
3.1. The Property and Microstructure of TiO2-Coated Wollastonite Composite Pigments
3.1.1. The Pigment Properties and Comparison of TiO2-Coated Wollastonite Composite Pigments
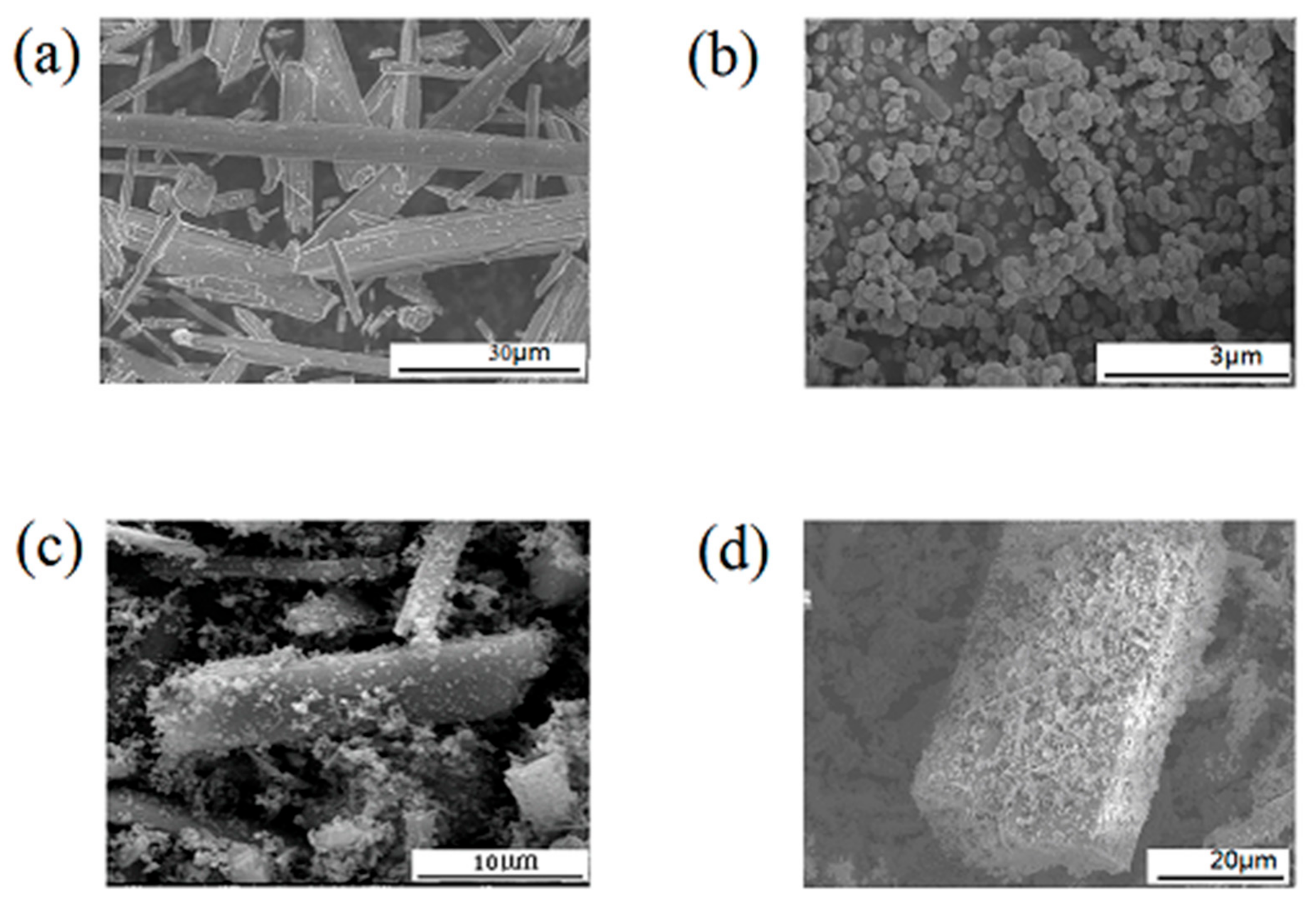
3.1.2. The Morphology of TiO2-Coated Wollastonite Composite Pigments
3.2. The Essence of Combination Reaction between Wollastonite and TiO2 Particles
3.2.1. Crystal Structure Analysis
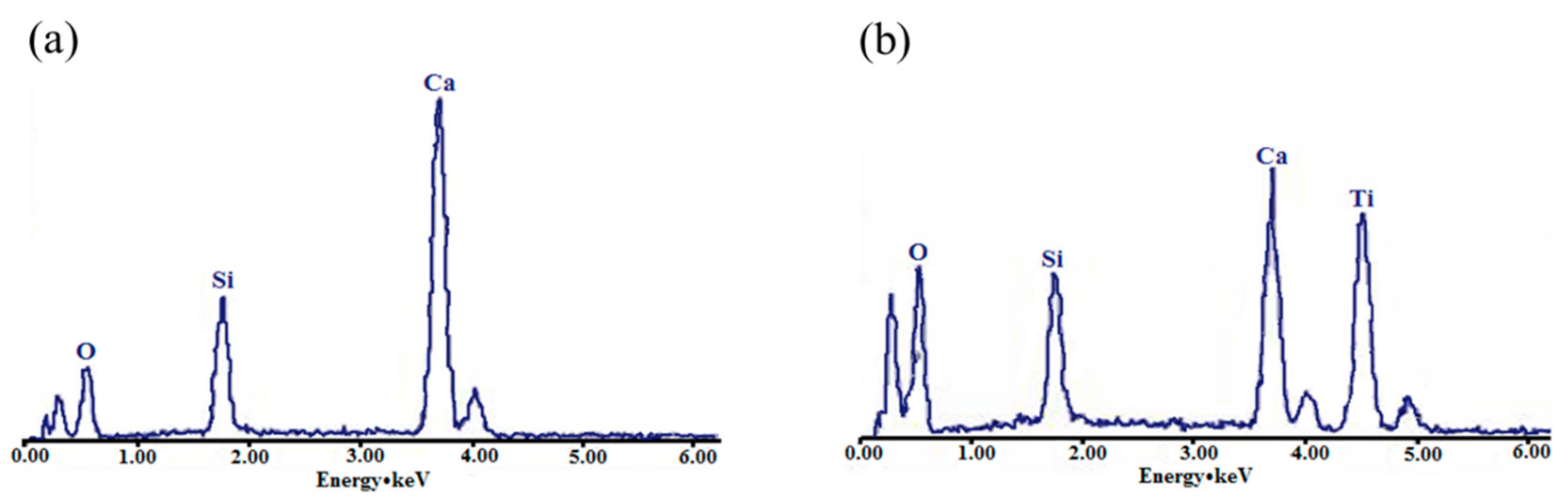
3.2.2. The Reaction Characteristics between Wollastonite and TiO2 Particles
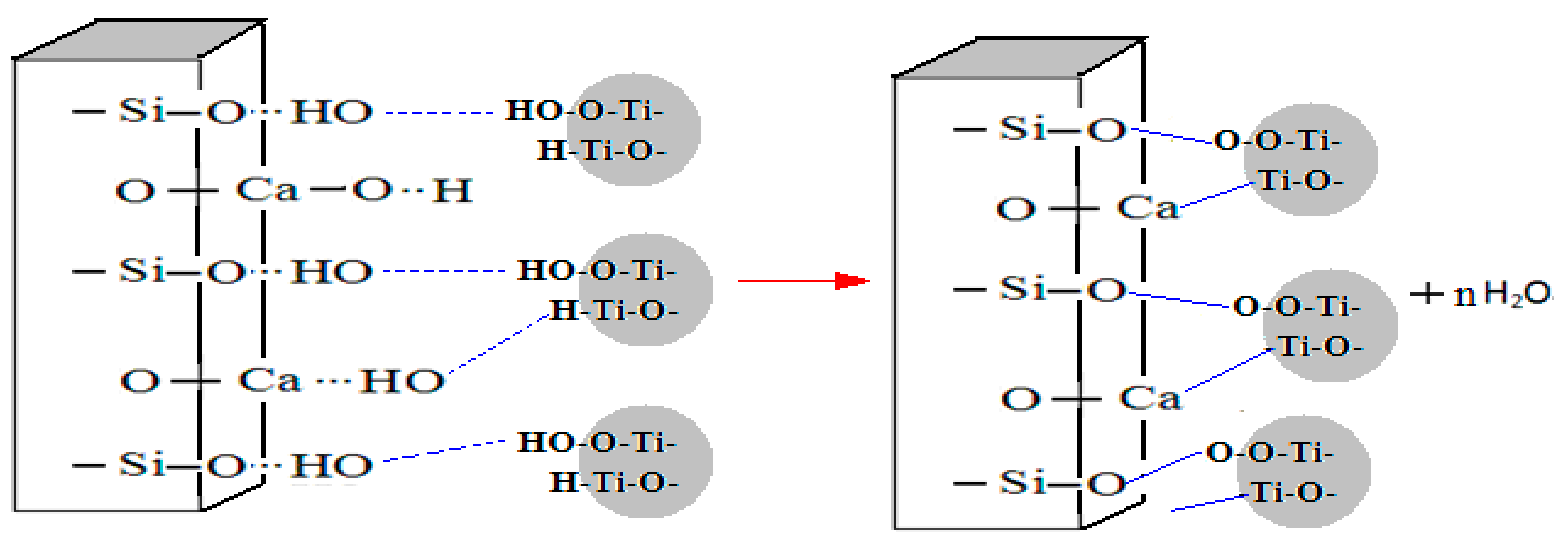
3.3. The Composite Model between Wollastonite and TiO2 Particles
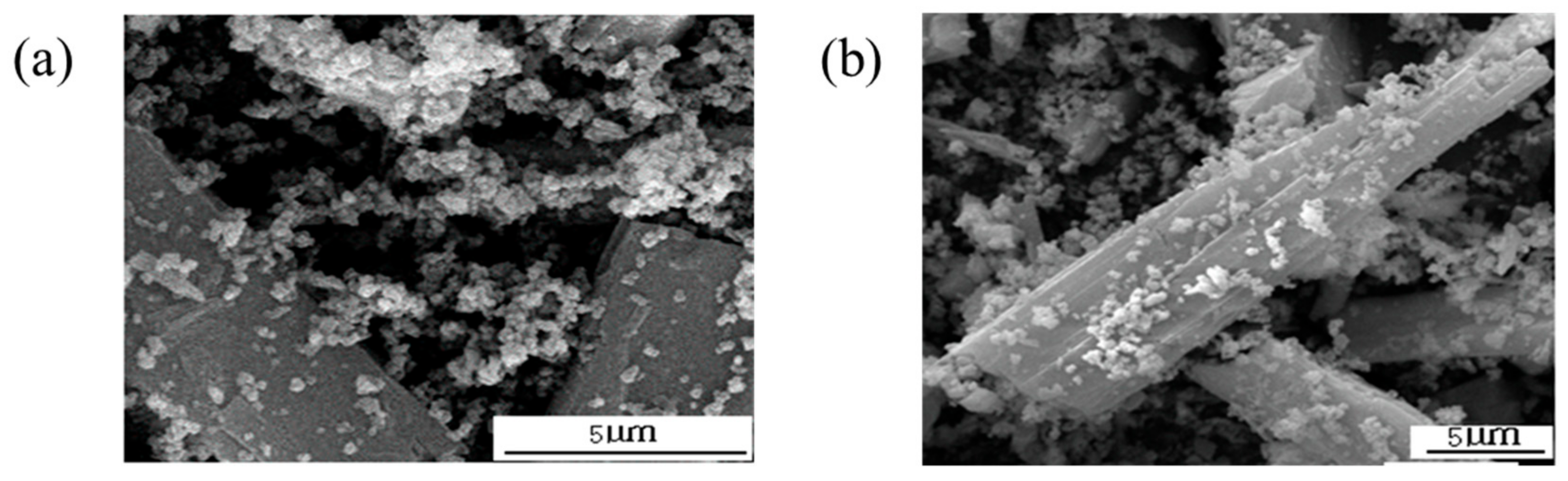
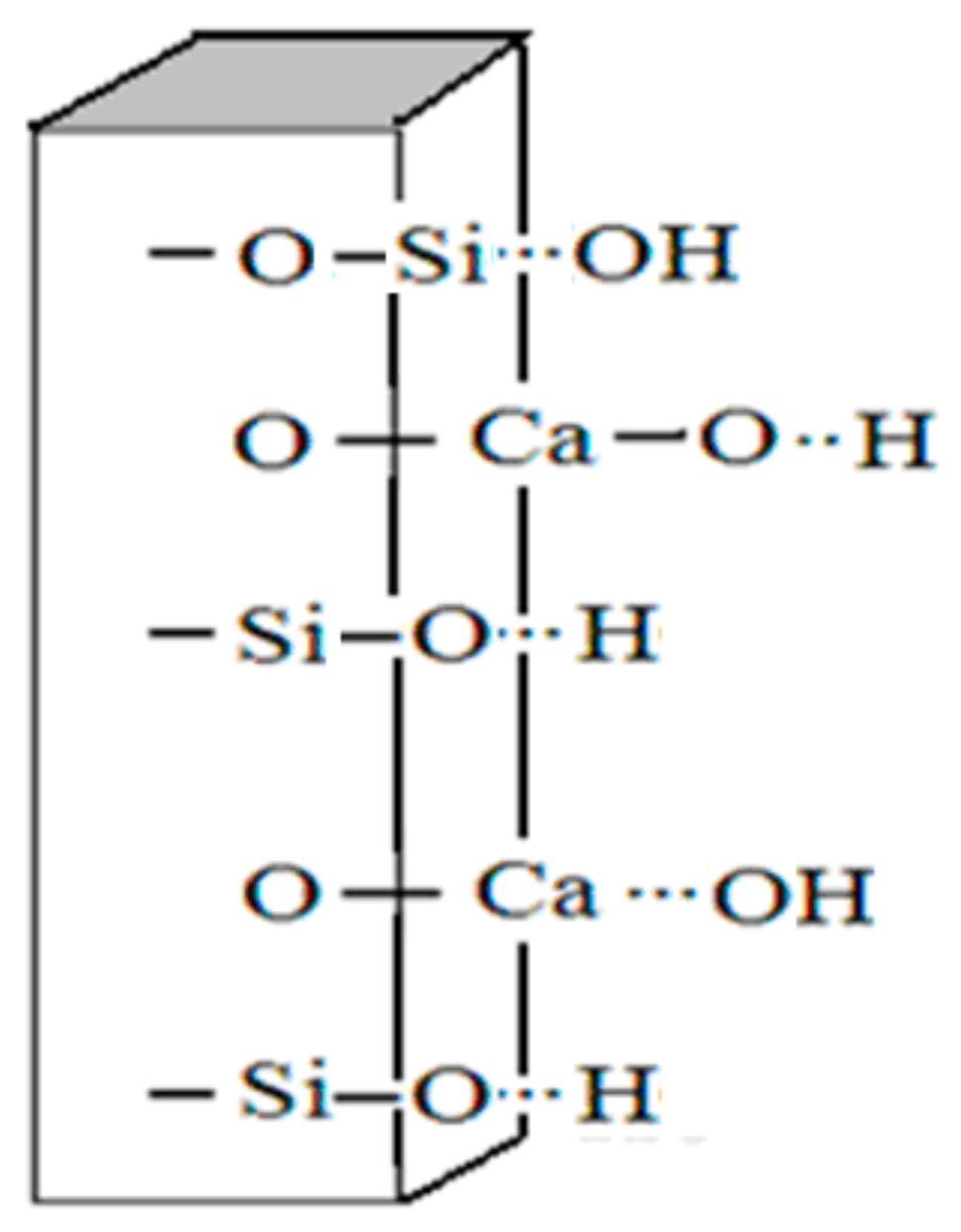
3.3.1. The Surface Morphology of Wollastonite Particles
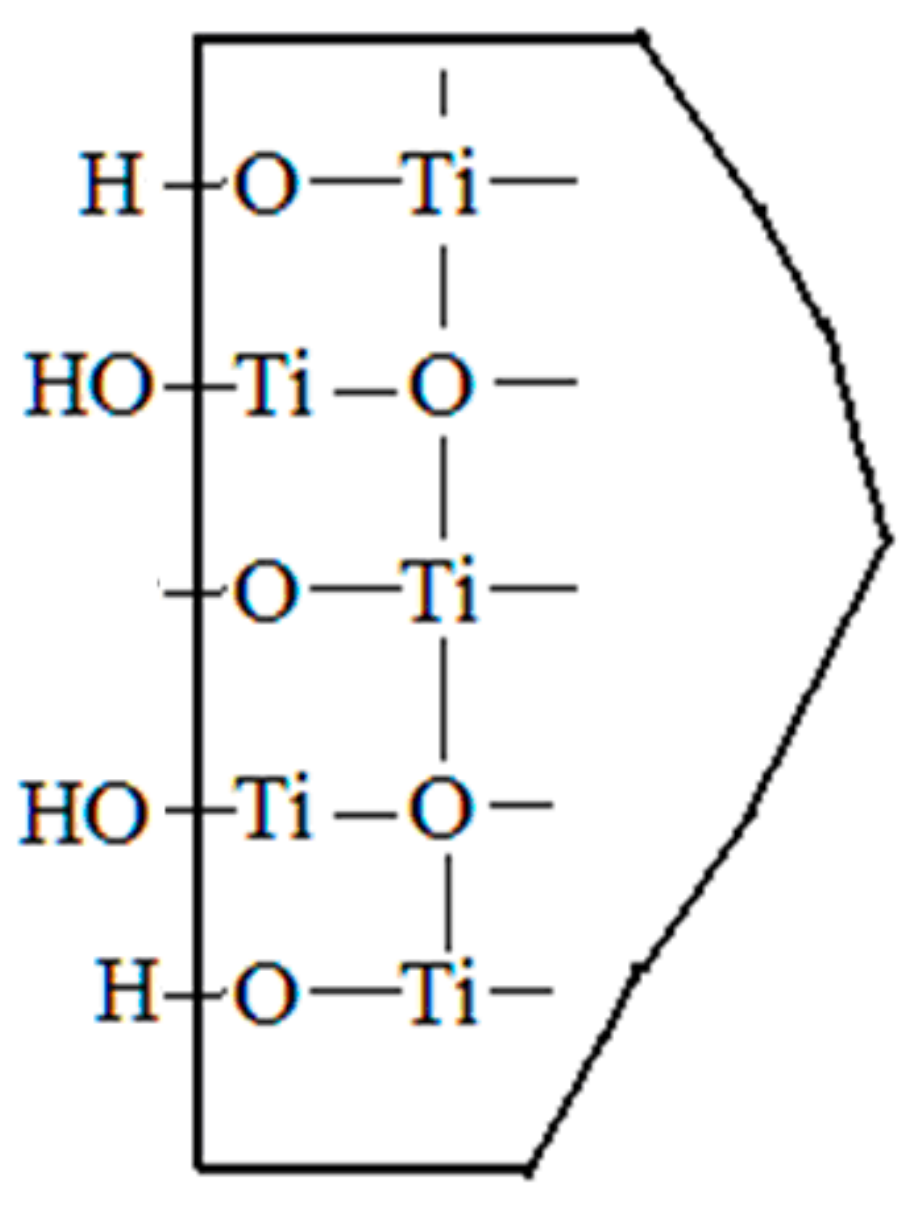
3.3.2. The Surface Morphology of TiO2 Particles
3.3.3. The Composite Model between Wollastonite and TiO2 Particles
4. Conclusions
- (1)
- TiO2-coated wollastonite composite pigments were successfully prepared by way of the mechano-chemical method. The composite pigment (contains 45% TiO2) has similar oil absorption to titanium dioxide. The hiding power is 17.97 g/m2, reaching 81.08% of titanium dioxide with an increase of 36.08% compared to the same amount of TiO2 used in composite particles.
- (2)
- A firm combination between wollastonite and TiO2 particles is formed through a dehydroxylation reaction, leading the composite materials to have the structure of TiO2 coating on the wollastonite surface evenly and closely. The wollastinite-TiO2 composite materials have similar properties to titanium dioxide.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lu, Y.; Sathasivam, S.; Song, J.; Crick, C.; Carmalt, C.; Parkin, I. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Mei, B.; Pedersen, T.; Malacrida, P.; Bae, D.; Frydendal, R.; Hansen, O.; Vesborg, P.C.K.; Seger, B.; Chorkendorff, I. Crystalline TiO2: A Generic and Effective Electron Conducting Protection Layer for Photo-anodes and -Cathodes. J. Phys. Chem. C 2015, 26, e228. [Google Scholar] [CrossRef]
- Thomas, V.J.; Ramaswamy, S. Application of Graphene and Graphene Compounds for Environmental Remediation. Sci. Adv. Mater. 2016, 8, 477–500. [Google Scholar] [CrossRef]
- Driel, V.; Kooyman, P.J.; Berg, K.J.V.D.; Schmidt-Ott, A.; Dik, J. A quick assessment of the photocatalytic activity of TiO2 pigments—From lab to conservation studio! Microchem. J. 2016, 126, 162–171. [Google Scholar] [CrossRef]
- Teh, C.Y.; Wu, T.Y.; Juan, J.C. An application of ultrasound technology in synthesis of titania-based photocatalyst for degrading pollutant. Chem. Eng. J. 2017, 317, 586–612. [Google Scholar] [CrossRef]
- Ding, H.; Liu, Y.; Zhou, H. The effect of resources and environment on titanium dioxide production and countermeasures for sustainable development in China. Earth Sci. Front. 2014, 5, 30. [Google Scholar]
- Subcommittee, T.D. Analysis on the Economic Running Situation of China Titanium Dioxide Industry in 2011. China Coat. 2012, 3, 19–20. [Google Scholar]
- Zhou, H.; Wang, M.; Ding, H.; Du, G. Preparation and Characterization of Barite/TiO2 Composite Particles. Adv. Mater. Sci. Eng. 2015, 2015, 878594. [Google Scholar] [CrossRef]
- Yan, Q.; Lei, Y.; Yuan, J. Preparation of titanium dioxide compound pigments based on kaolin substrates. J. Coat. Technol. Res. 2010, 7, 229–237. [Google Scholar] [CrossRef]
- Ninness, B.J.; Bousfield, D.W.; Tripp, C.P. Formation of a thin TiO2 layer on the surfaces of silica and kaolin pigments through atomic layer deposition. Colloids Surf. A Physicochem. Eng. Asp. 2003, 214, 195–204. [Google Scholar] [CrossRef]
- Lu, Z.; Min, R.; Yin, H.; Wang, A.; Chen, G.; Zhang, Y.; Yu, L.; Jiang, T. Preparation of nanosized anatase TiO2-coated kaolin composites and their pigmentary properties. Powder Technol. 2009, 196, 122–125. [Google Scholar] [CrossRef]
- Mamulová, K.K.; Tokarský, J.; Kovář, P.; Vojtěšková, S.; Kovářová, A.; Smetana, B.; Kukutschová, J.P.Č.; Matějka, V. Preparation and characterization of photoactive composite kaolinite/TiO(2). J. Hazard. Mater. 2011, 188, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Wu, X.; Fan, Y. The effect of iron ions on the anatase–rutile phase transformation of titania (TiO2) in mica–titania pigments. Dyes Pigment. 2012, 95, 96–101. [Google Scholar] [CrossRef]
- Gao, Q.; Wu, X.; Fan, Y.; Zhou, X. Low temperature synthesis and characterization of rutile TiO2-coated mica–titania pigments. Dyes Pigment. 2012, 95, 534–539. [Google Scholar] [CrossRef]
- Eskelinen, P.; Ritala, M.; Leskelä, M. The Effect of Calcination on the Surface Composition and Structure of Titanium Dioxide Coated Mica Particles. J. Solid State Chem. 1993, 103, 160–169. [Google Scholar] [CrossRef]
- Wang, B.; Ding, H.; Wang, Y. Preparation of Barite/TiO2 Composite Particle and Interaction Mechanism between TiO2 and Barite Particles. Rare Met. Mater. Eng. 2011, 40, 193–197. [Google Scholar]
- Lin, H.; Dong, Y.; Jiang, L. Preparation of calcium carbonate particles coated with titanium dioxide. Int. J. Miner. Metall. Mater. 2009, 16, 592–597. [Google Scholar] [CrossRef]
- Ding, H. Minerals-Titanium Dioxide Micro-Nanometer Scale Particle Composition and Functionalization; Tsinghua University Press: Beijing, China, 2016. [Google Scholar]
- Liang, Y.; Ding, H.; Xue, Q. Characterization of brucite/TiO2 composite particle material prepared by mechano-chemical method. Surf. Rev. Lett. 2017, 1850085. [Google Scholar] [CrossRef]
- Wang, B.; Ding, H. Characterization of Calcined Kaolin/TiO2 Composite Particle Material Prepared by Mechano-Chemical Method. J. Wuhan Univ. Technol. 2010, 25, 765–769. [Google Scholar] [CrossRef]
- Hou, X.F.; Ding, H.; Du, G.X.; Yu, S.R.; Wang, Y.B.; Ye, C. Preparation of Sericite/TiO2 Composite Particle Material via Mechano-chemistry and Its Characterization. J. Beijing Univ. Technol. 2013, 39, 1413–1419. [Google Scholar]
- Hou, X.F.; Ding, H.; Zheng, Y.X.; Wang, M.M. Preparation and characterisation of amorphous silica/anatase composite through mechanochemical method. Mater. Res. Innov. 2013, 17 (Suppl. S1), 234–239. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Wang, L.; Yang, H.; Zhao, M. The synthesis and characterization of TiO2/wollastonite composite. Mater. Lett. 1998, 37, 149–155. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Z.; Zhao, J.; Xue, L.; Zhao, C.; Zhao, M. Contrasts of Structure and Properties of Two Nano-TiO2/Wollastonite Composites with Wollastonite Having Different Partical Sizes. Acta Sci. Nat. Univ. Jilinensis 2000, 2000, 73–76. [Google Scholar]
- General Methods of Test for Pigments and Extenders; GB/T 5211.15-2014; Standards Press of China: Beijing, China, 2014.
- Bandara, J.; Mielczarski, J.A.; Kiwi, J. Molecular Mechanism of Surface Recognition. Azo Dyes Degradation on Fe, Ti, and Al Oxides through Metal Sulfonate Complexes. Langmuir 2006, 15, 7670–7679. [Google Scholar] [CrossRef]

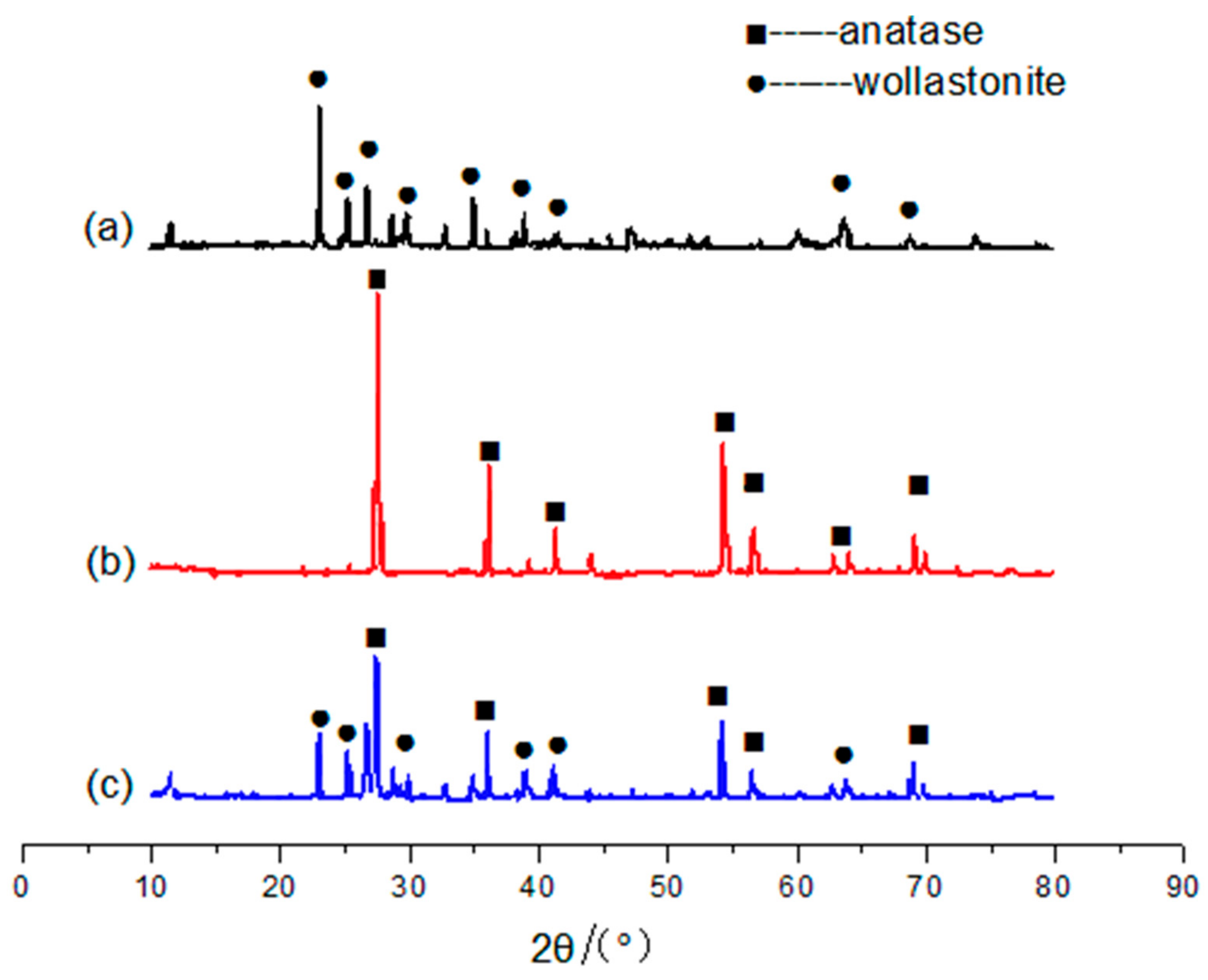
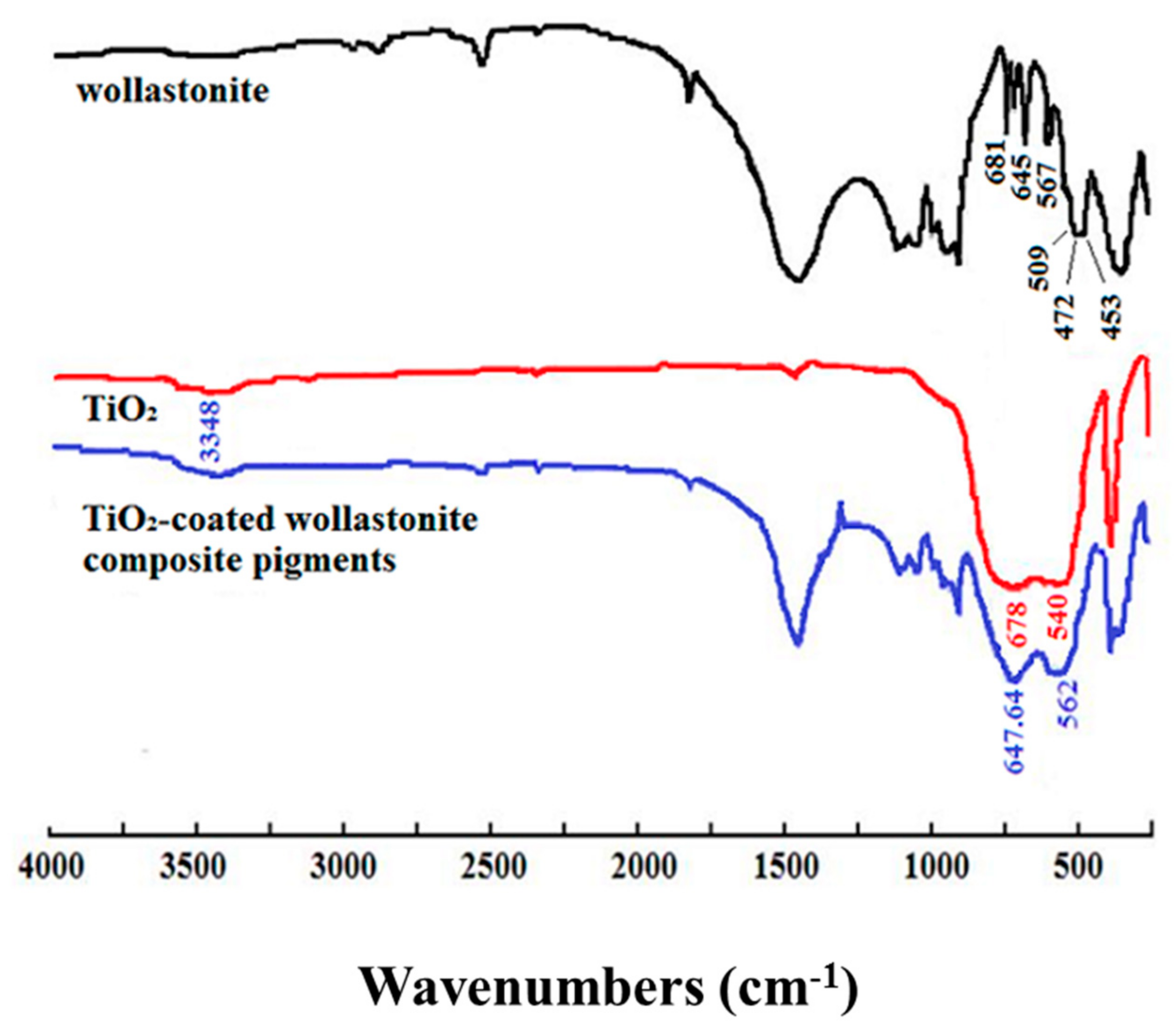
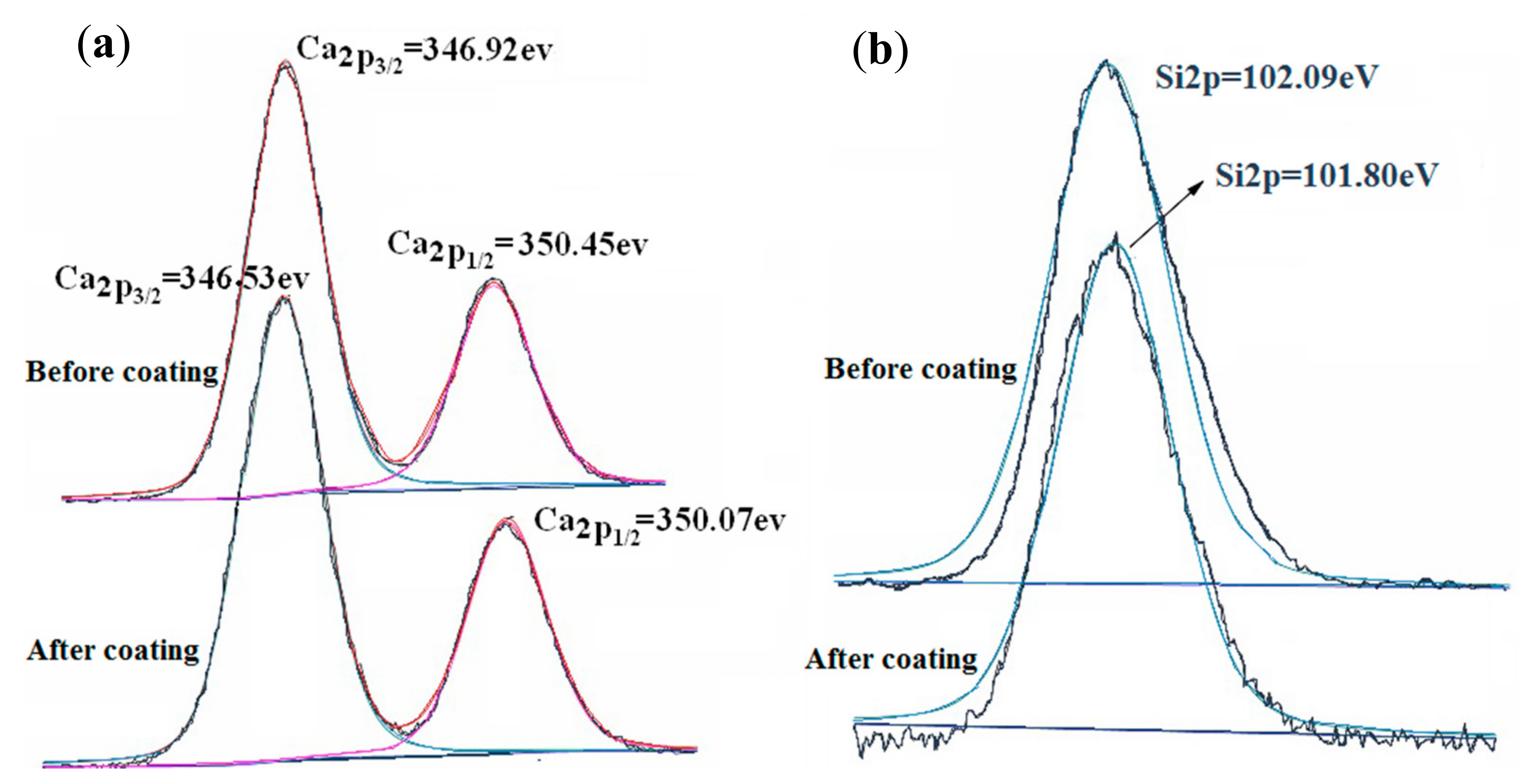
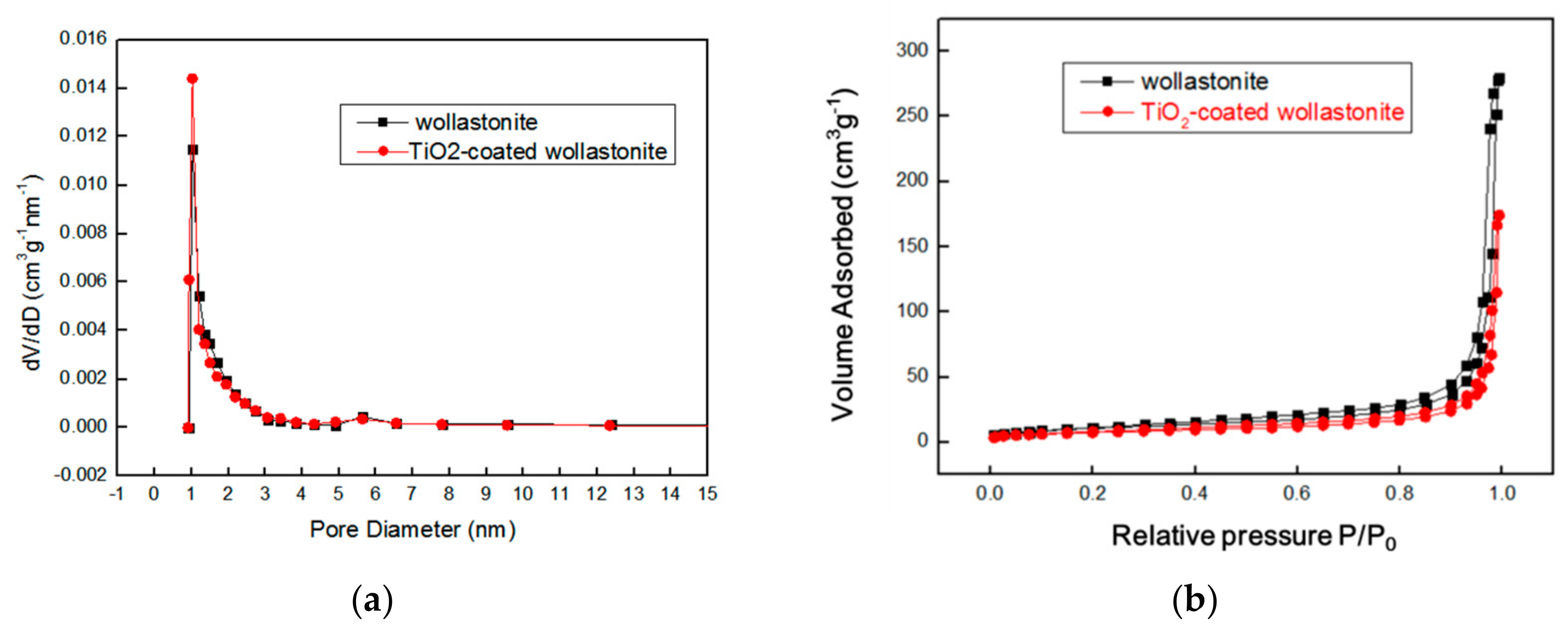
| Samples | Oil Absorption/(g/100 g) | Whiteness/% | Hiding Power/(g/m2) | Relative Hiding Power (E)/% | ΔE(E − E0)/% |
|---|---|---|---|---|---|
| TiO2-coated wollastonite composite pigments | 22.72 | 96.6 | 17.97 | 81.08 | 36.08 |
| Wollastonite and TiO2 dry mixtures | 19.70 | 96.0 | 23.04 | 63.24 | 18.24 |
| Wollastonite and TiO2 wet mixtures | 20.12 | 96.3 | 21.56 | 67.58 | 22.58 |
| Anatase TiO2 | 25.03 | 96.2 | 14.57 | 100 | - |
| Wollastonite | 11.20 | 94.0 | 272.65 | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Liang, Y.; Hou, X.; Zhang, J.; Ding, H.; Sun, S.; Cao, H. Mechanical Grinding Preparation and Characterization of TiO2-Coated Wollastonite Composite Pigments. Materials 2018, 11, 593. https://doi.org/10.3390/ma11040593
Chen W, Liang Y, Hou X, Zhang J, Ding H, Sun S, Cao H. Mechanical Grinding Preparation and Characterization of TiO2-Coated Wollastonite Composite Pigments. Materials. 2018; 11(4):593. https://doi.org/10.3390/ma11040593
Chicago/Turabian StyleChen, Wanting, Yu Liang, Xifeng Hou, Jing Zhang, Hao Ding, Sijia Sun, and Hu Cao. 2018. "Mechanical Grinding Preparation and Characterization of TiO2-Coated Wollastonite Composite Pigments" Materials 11, no. 4: 593. https://doi.org/10.3390/ma11040593
APA StyleChen, W., Liang, Y., Hou, X., Zhang, J., Ding, H., Sun, S., & Cao, H. (2018). Mechanical Grinding Preparation and Characterization of TiO2-Coated Wollastonite Composite Pigments. Materials, 11(4), 593. https://doi.org/10.3390/ma11040593