Alloying and Hardness of Eutectics with Nbss and Nb5Si3 in Nb-silicide Based Alloys
Abstract
:1. Introduction
2. Methodology
3. Results and Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bewlay, B.P.; Jackson, M.R.; Gigliotti, M.F.X. Niobium Silicide High Temperature In-Situ Composites. In Intermetallic Compounds, Principles and Practice; Westbrook, J.H., Fleisher, R.L., Eds.; John Wiley and Sons: Chichester, UK, 2001; pp. 541–560. [Google Scholar]
- Broutman, L.J.; Krock, H.H. Modern Composite Materials; Addison Wesley: Boston, MA, USA, 1967; p. 442. [Google Scholar]
- Sharp, R.M.; Flemings, M.C. Growth of composites of off-eutectic alloys. In Proceedings of the Conference In-Situ Composites, Lakeville, CT, USA, 5–8 September 1972; National Materials Advisory Board: Washington, DC, USA, 1972; Volume 1, pp. 51–59. [Google Scholar]
- Mollard, F.R.; Flemings, M.C. Growth of composites from melt—Part I. TMS-AIME 1967, 239, 1534–1539. [Google Scholar]
- Yue, A.S.; Kaba, B.D. Fracture behaviour of unidirectionally solidified Ti-Ti5Si3 eutectic composites. ASTM STP 1975, 580, 504–514. [Google Scholar]
- Crossman, F.W.; Yue, A.S. Unidirectionally solidified Ti-TiB and Ti-Ti5Si3 eutectic composites. Metall. Trans. 1971, 2, 1545–1555. [Google Scholar]
- Jehanno, P.; Heilmaier, M.; Kestler, H.; Boning, M.; Venskutonis, A.; Bewlay, B.; Jackson, M. Assessment of a powder metallurgical route for refractory metal silicide alloys. Metall. Mater. Trans. A 2005, 36, 515–523. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; Okamoto, H.; Gokhale, A.B.; Abbaschian, R. The Nb-Si (Niobium-Silicon) system. J. Phase Equilib. 1993, 14, 502–5099. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Lipsitt, H.A.; Reeder, W.J.; Jackson, M.R.; Sutliff, J.A. Toughening mechanisms in directionally solidified Nb-Nb3Si-Nb3Si5 in-situ composites. In Processing and Fabrication of Advanced Materials III; Ravi, V.A., Srivatsan, T.S., Moore, J.J., Eds.; The Minerals Metals and Materials Society: Warrendale, PA, USA, 1994; pp. 547–565. [Google Scholar]
- Yuan, S.; Jia, L.; Ma, L.; Cui, R.; Su, L.; Zhang, H. The microstructure optimising of the Nb-14Si-22Ti-4Cr-2Al-2Hf alloy processed by directional solidification. Mater. Lett. 2012, 84, 124–127. [Google Scholar] [CrossRef]
- Su, L.; Jia, L.; Feng, Y.; Zhang, H.; Yuan, S.; Zhang, H. Microstructure and room temperature fracture toughness of directionally solidified Nb-Si-Ti-Cr-Al-Hf alloy. Mater. Sci. Eng. A 2013, 560, 672–677. [Google Scholar] [CrossRef]
- Wang, J.; Jia, L.; Ma, L.; Yuan, S.; Zhang, X.; Zhang, H. Microstructure optimisation of directionally solidified hypereutectic Nb-Si alloy. Trans. Nonferr. Met. Soc. China 2013, 23, 2874–2881. [Google Scholar] [CrossRef]
- Weng, J.F.; Jia, L.N.; Yuan, S.N.; Su, L.F.; Ding, F.; Zhang, H. Microstructure evolution of directionally solidified Nb-Si alloy. Mater. Sci. Technol. 2014, 30, 418–423. [Google Scholar] [CrossRef]
- Guo, H.; Guo, X. Microstructure evolution and room temperature fracture toughness of an integrally directionally solidified Nb-Ti-Si based ultrahigh temperature alloy. Scr. Mater. 2011, 64, 637–640. [Google Scholar] [CrossRef]
- Yuan, S.N.; Jia, L.N.; Su, L.F.; Ma, L.M.; Zhang, H. Microstructure evolution and solidification behaviour of Nb-16Si-22Ti-2Cr-2Al-6Hf alloy processed by directional solidification. Mater. Res. Innov. 2013, 17, 184–188. [Google Scholar] [CrossRef]
- Guo, B.H.; Guo, X.P. Microstructure of Nb-Ti-Si based ultrahigh temperature alloy processed by integrally directional solidification. Mater. Sci. Technol. 2015, 31, 231–236. [Google Scholar] [CrossRef]
- Sekido, N.; Kimura, Y.; Miura, S.; Wei, F.; Mishima, Y. Fracture toughness and high temperature strength of unidirectionally solidified Nb-Si binary and Nb-Ti-Si ternary alloys. J. Alloys Compd. 2006, 425, 223–229. [Google Scholar] [CrossRef]
- Sekido, N.; Kimura, Y.; Miura, S.; Mishima, Y. Microstructure development of unidirectionally solidified (Nb)/Nb3Si eutectic alloys. Mater. Sci. Eng. A 2007, 444, 51–57. [Google Scholar] [CrossRef]
- Hunag, Q.; Guo, X.; Kang, Y.; Song, J.; Qu, S.; Han, Y. Microstructures and mechanical properties of directionally solidified multi-element Nb-Si alloy. Prog. Nat. Sci. Mater. Int. 2011, 21, 146–152. [Google Scholar] [CrossRef]
- Cheng, G.M.; He, L.L. Microstructure evolution and room temperature deformation of a unidirectionally solidified Nb-22Ti-16Si-3Ta-2Hf-7Cr-3Al-0.2Ho (at.%) alloy. Intermetallics 2011, 19, 196–201. [Google Scholar] [CrossRef]
- Li, Y.; Miura, S.; Ohsasa, K.; Ma, C.; Zhang, H. Ultra-high temperature Nbss/Nb5Si3 fully lamellar microstructure developed by directional solidification in OFZ furnace. Intermetallics 2011, 19, 460–469. [Google Scholar] [CrossRef]
- McCaughey, C. The Solidification of Niobium Silicides for Next Generation Gas Turbine Engines. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2016. [Google Scholar]
- Bendersky, L.; Biancaniello, F.S.; Boettinger, W.J.; Perepezko, J.H. Microstructural characterisation of rapidly solidified Nb-Si alloys. Mater. Sci. Eng. 1987, 89, 151–159. [Google Scholar] [CrossRef]
- Abbaschian, R.; Lipschutz, M.D. Eutectic solidification processing via bulk melt undercooling. Mater. Sci. Eng. A 1997, 226–228, 13–21. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, S.; Wei, B.; Li, W. Undercooling and rapid solidification of Nb-Si eutectic alloys studied by long drop tube. Trans. Nonferr. Met. Soc. China 2006, 16, s89–s92. [Google Scholar]
- Liang, H.; Chang, Y.A. Thermodynamic modelling of the Nb-Si-Ti ternary system. Intermetallics 1999, 7, 561–570. [Google Scholar] [CrossRef]
- Okamoto, H.; Gokhale, A.B.; Abbaschian, G.J. Binary Phase Diagrams, 2nd ed.; Massalski, T.B., Ed.; ASM International: Materials Park, OH, USA, 1990; pp. 2764–2768. [Google Scholar]
- Okamoto, H. Phase Diagrams for Binary Alloys: Desk Handbook; ASM International: Metals Park, OH, USA, 2000. [Google Scholar]
- Fernandes, P.B.; Coelho, G.C.; Ferreira, F.; Nunes, C.A.; Sudman, B. Thermodynamic modelling of the Nb-Si system. Intermetallics 2002, 10, 993–999. [Google Scholar] [CrossRef]
- ASM Alloy Phase Diagram Database; Villars, P.; Okamoto, H.; Cenzual, K. (Eds.) ASM International: Materials Park, OH, USA, 2016. [Google Scholar]
- Geng, T.; Li, C.; Bao, J.; Zhao, X.; Du, Z.; Guo, C. Thermodynamic assessment of the Nb-Si-Ti system. Intermetallics 2009, 17, 343–357. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Du, Z.; Guo, C.; Zhao, X. As-cast microstructures and solidification paths of the Nb-Si-Ti ternary alloys in Nb5Si3–Ti5Si3 region. Rare Met. 2015, 32, 502–511. [Google Scholar] [CrossRef]
- Gigolotti, J.C.J.; Coelho, G.C.; Nunes, C.A.; Suzuki, P.A.; Joubert, J. Experimental evaluation of the Nb-Si-Ti system from as-cast alloys. Intermetallics 2017, 82, 76–92. [Google Scholar] [CrossRef]
- Goldschmidt, H.J.; Brand, J.A. The constitution of the chromium–niobium–silicon system. J. Less-Common Met. 1961, 3, 34–43. [Google Scholar] [CrossRef]
- Zhao, C.; Jackson, M.R.; Peluso, L.A. Determination of the Nb-Cr-Si phase diagram using diffusion multiples. Acta Mater. 2003, 51, 6395–6405. [Google Scholar] [CrossRef]
- Geng, J.; Shao, G.; Tsakiropoulos, P. Study of three-phase equilibrium in the Nb rich corner of Nb-Si-Cr system. Intermetallics 2006, 14, 832–837. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Yang, Y.; Casey, R.L.; Jackson, M.R.; Chang, Y.A. Experimental study of the liquid-solid phase equilibria at the metal rich region of the Nb-Cr-Si system. Intermetallics 2009, 17, 120–127. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On the Nb silicide based alloys: Part I—The bcc Nb solid solution. J. Alloys Compd. 2017, 708, 961–971. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On the alloying and properties of tetragonal Nb5Si3 in Nb-silicide based alloys. Materials 2018, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Grammenos, I.; Tsakiropoulos, P. Study of the role of Al, Cr and Ti additions in the microstructure of Nb-18Si-5Hf base alloys. Intermetallics 2010, 18, 242–253. [Google Scholar] [CrossRef]
- Grammenos, I.; Tsakiropoulos, P. Study of the role of Mo and Ta additions in the microstructure of Nb-18Si-5Hf silicide based alloys. Intermetallics 2010, 18, 1524–1530. [Google Scholar] [CrossRef]
- Grammenos, I.; Tsakiropoulos, P. Study of the role of Hf, Mo and W additions in the microstructure of Nb-20Si silicide based alloys. Intermetallics 2011, 19, 1612–1621. [Google Scholar] [CrossRef]
- Vellios, N.; Tsakiropoulos, P. The role of Sn and Ti additions in the microstructure of Nb-18Si based alloys. Intermetallics 2007, 15, 1518–1528. [Google Scholar] [CrossRef]
- Vellios, N.; Tsakiropoulos, P. The role of Fe and Ti additions in the microstructure of Nb-18Si-5Sn silicide based alloys. Intermetallics 2007, 15, 1529–1537. [Google Scholar] [CrossRef]
- Li, Z.; Tsakiropoulos, P. Study of the effects of Ge addition on the microstructure of Nb-18Si in situ composites. Intermetallics 2010, 18, 1072–1078. [Google Scholar]
- Li, Z.; Tsakiropoulos, P. Study of the effect of Ti and Ge in the microstructure of Nb-24Ti-18Si-5Ge in situ composite. Intermetallics 2011, 19, 1291–1297. [Google Scholar] [CrossRef]
- Li, Z.; Tsakiropoulos, P. Study of the effect of Cr and Ti additions in the microstructure of Nb-18Si-5Ge based in situ composites. Intermetallics 2012, 26, 18–25. [Google Scholar] [CrossRef]
- Li, Z.; Tsakiropoulos, P. The microstructures of Nb-18Si-5Ge-5Al and Nb-24Ti-18Si-5Ge-5Al in situ composites. J. Alloys Compd. 2013, 550, 553–560. [Google Scholar] [CrossRef]
- Zacharis, E.; Tsakiropoulos, P. Development of N Silicide Based Alloys with Hf and Sn Additions. University of Sheffield: Sheffield, UK, Unpublished Research. 2013. [Google Scholar]
- Zhao, J. The Role of Refractory Metals in Controlling Properties of Nb-silicide Based In Situ Composites. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2017. [Google Scholar]
- Xu, Z. The Effect of Sn on the Phase Stability and Oxidation Behaviour of Nb-silicide Based Alloys. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2016. [Google Scholar]
- Vellios, N.; Tsakiropoulos, P. Nb-silicide Based Alloys with Sn and Fe Additions. University of Surrey: Guildford, UK, Unpublished Research. 2007. [Google Scholar]
- Anazodo, B.; Tsakiropoulos, P. Nb-Si Alloys with Refractory Metal and Metalloid Element Additions. University of Sheffield: Sheffield, UK, Unpublished Research. 2013. [Google Scholar]
- Ghadyani, M. Study of the Microstructure and Oxidation of Alloys on the Al-Hf-Nb-Si-Ti System. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2017. [Google Scholar]
- Tweddle, A.; Tsakiropoulos, P. Nb-Si Based Alloys with Ge Additions. University of Sheffield: Sheffield, UK, Unpublished Research. 2015. [Google Scholar]
- Nelson, J. Study of the Effects of Cr, Hf and Sn with Refractory Metal Additions on the Microstructure and Properties of Nb-silicide Based Alloys. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2015. [Google Scholar]
- Kim, W.; Tanaka, H.; Kasama, A.; Hanada, S. Microstructure and room temperature fracture toughness of Nbss/Nb5Si3 in situ composites. Intermetallics 2001, 9, 827–834. [Google Scholar] [CrossRef]
- Kashyap, S.; Tiwary, C.S.; Chattopadhyay, K. Effect of Gallium on microstructure and mechanical properties of Nb-Si eutectic alloy. Intermetallics 2011, 19, 1943–1952. [Google Scholar] [CrossRef]
- Zelenitsas, K.; Tsakiropoulos, P. Study of the role of Al and Cr additions in the microstructure of Nb-Ti-Si in situ composites. Intermetallics 2005, 13, 1079–1095. [Google Scholar] [CrossRef]
- Zelenitsas, K.; Tsakiropoulos, P. Study of the role of Ta and Cr additions in the microstructure of Nb-Ti-Si-Al in situ composites. Intermetallics 2006, 14, 639–659. [Google Scholar] [CrossRef]
- Grammenos, I.; Tsakiropoulos, P. Development of Creep Resistant Nb Silicide Based Alloys. University of Surrey: Guildford, UK, Unpublished Research. 2007. [Google Scholar]
- Tsakiropoulos, P. On Nb silicide based alloys: Part II. J. Alloys Compd. 2018, 748, 569–576. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. Alloying and properties of C14-NbCr2 and A15-Nb3X (X = Al, Ge, Si, Sn) in Nb-silicide based alloys. Materials 2018, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, J.H. Intermetallic Compounds; R E Krieger Publishing Company: New York, NY, USA, 1997; p. 471. [Google Scholar]
- Tiwari, R.; Herman, H.; Sampath, S. Vacuum plasma spraying of MoSi2 and its composites. Mater. Sci. Eng. A 1992, 155, 95–100. [Google Scholar] [CrossRef]
- Yilmaz, F.; Elliot, R. The microstructure and mechanical properties of unidirectionally solidified Al-Si alloys. J. Mater. Sci. 1989, 24, 2065–2070. [Google Scholar] [CrossRef]
- Mason, D.P.; van Aken, D.C. The effect of microstructural scale on hardness of MoSi2-Mo5Si3 eutectics. Scr. Met. 1993, 28, 185–189. [Google Scholar] [CrossRef]
- Boldt, P.H.; Embury, J.D.; Weatherly, G.C. Room temperature micro-indentation of single crystal MoSi2. Mater. Sci. Eng. A 1992, 155, 251–258. [Google Scholar] [CrossRef]
- Gavens, A.J.; van Heerden, D.; Foecke, T.; Weihs, T.P. Fabrication and evaluation of Nb/Nb5Si3 micro-laminate foils. Metall. Mater. Trans. A 1999, 30, 2959–2965. [Google Scholar] [CrossRef]
- Ji, H.; Was, G.S.; Jones, J.W. Layered Materials for Structural, Applications. In Proceedings of the Materials Research Society Symposia, San Francisco, CA, USA, 8–10 April 1996; Materials Research Society: Pittsburgh, PA, USA, 1996; Volume 434, pp. 153–158. [Google Scholar]
- Shang, J.X.; Guan, K.; Wang, F.H. Atomic structure and adhesion of the Nb(001)/αNb5Si3(001) interface: A first-principles study. J. Phys. Condens. Matter. 2010, 22, 085004. [Google Scholar] [CrossRef] [PubMed]
- Guan, K.; Jia, L.; Kong, B.; Yuan, S.; Zhang, H. Improvement of fracture toughness of directionally solidified Nb-silicide in situ composites using artificial neural network. Mater. Sci. Eng. A 2016, 663, 98–107. [Google Scholar] [CrossRef]
- Gilman, J.J. Physical chemistry of intrinsic hardness. Mater. Sci. Eng. A 1996, 209, 74–81. [Google Scholar] [CrossRef]
- Teter, D.M. Computational alchemy: The search for new superhard materials. MRS Bull. 1998, 23, 22–27. [Google Scholar] [CrossRef]
- Jhi, S.; Ihm, J.; Loule, S.G.; Cohen, M.L. Electronic mechanism of hardness enhancement in transition-metal carbo-nitrides. Nature 1999, 399, 132–134. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y. Dependence of elastic stiffness on electronic band structure of nanolaminate M2AlC (M = Ti, V, Nb and Cr) ceramics. Phys. Rev. B 2004, 69, 214111. [Google Scholar] [CrossRef]
- Papadimitriou, I.; Utton, C.; Tsakiropoulos, P. The impact of Ti and temperature on the stability of Nb5Si3 phases: A first-principles study. Sci. Technol. Adv. Mater. 2017, 18, 467–479. [Google Scholar] [CrossRef] [PubMed]
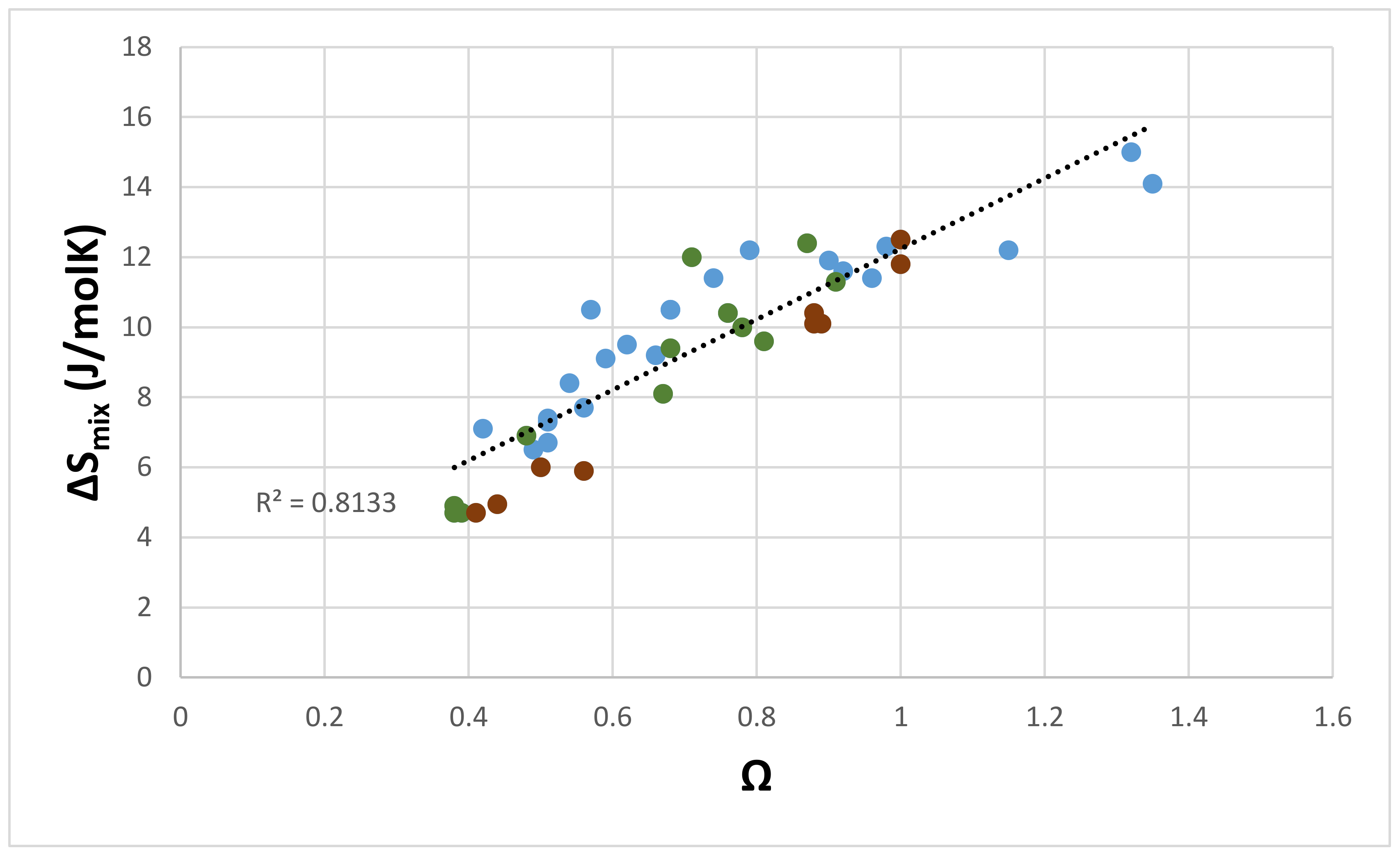
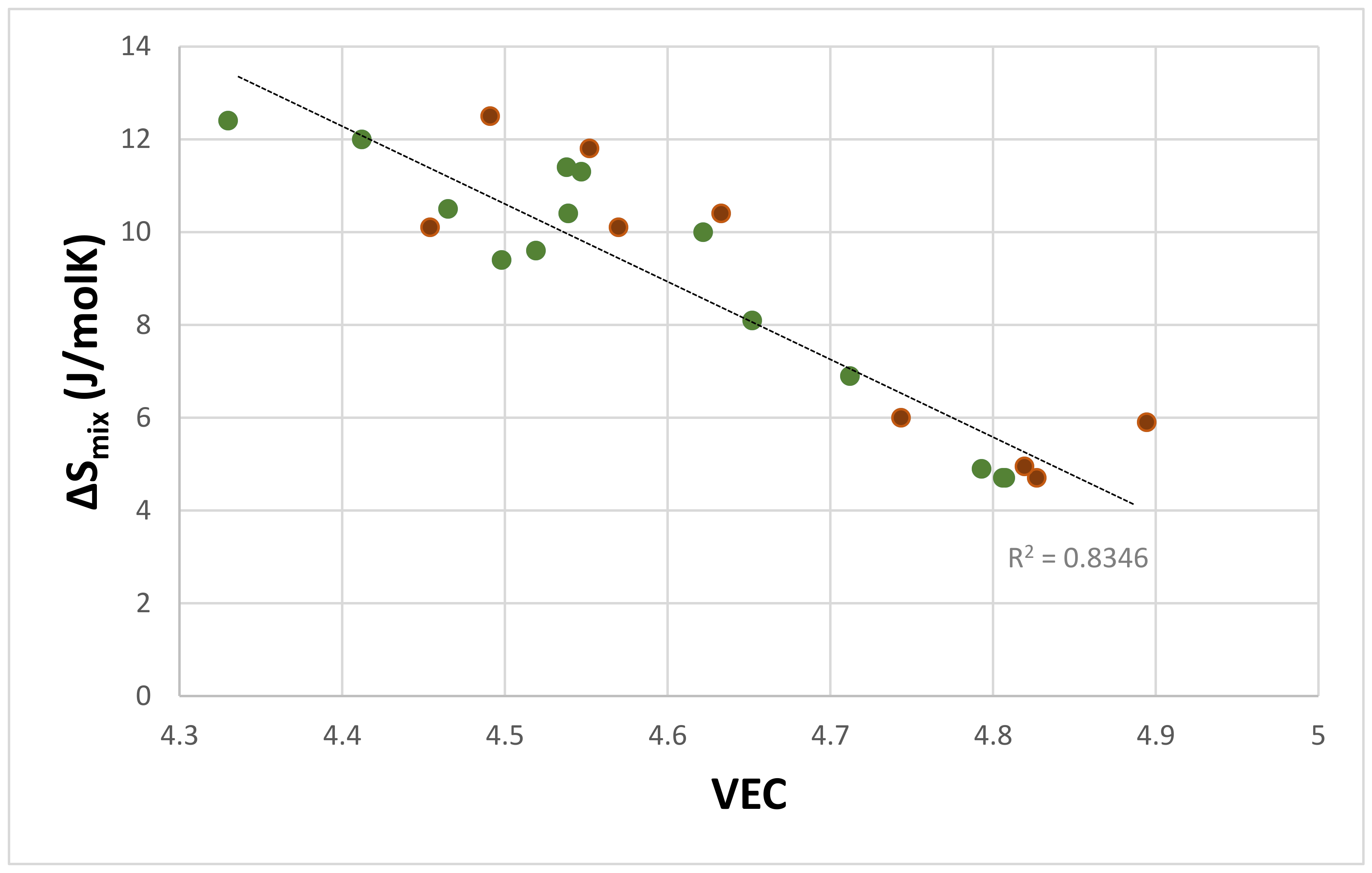
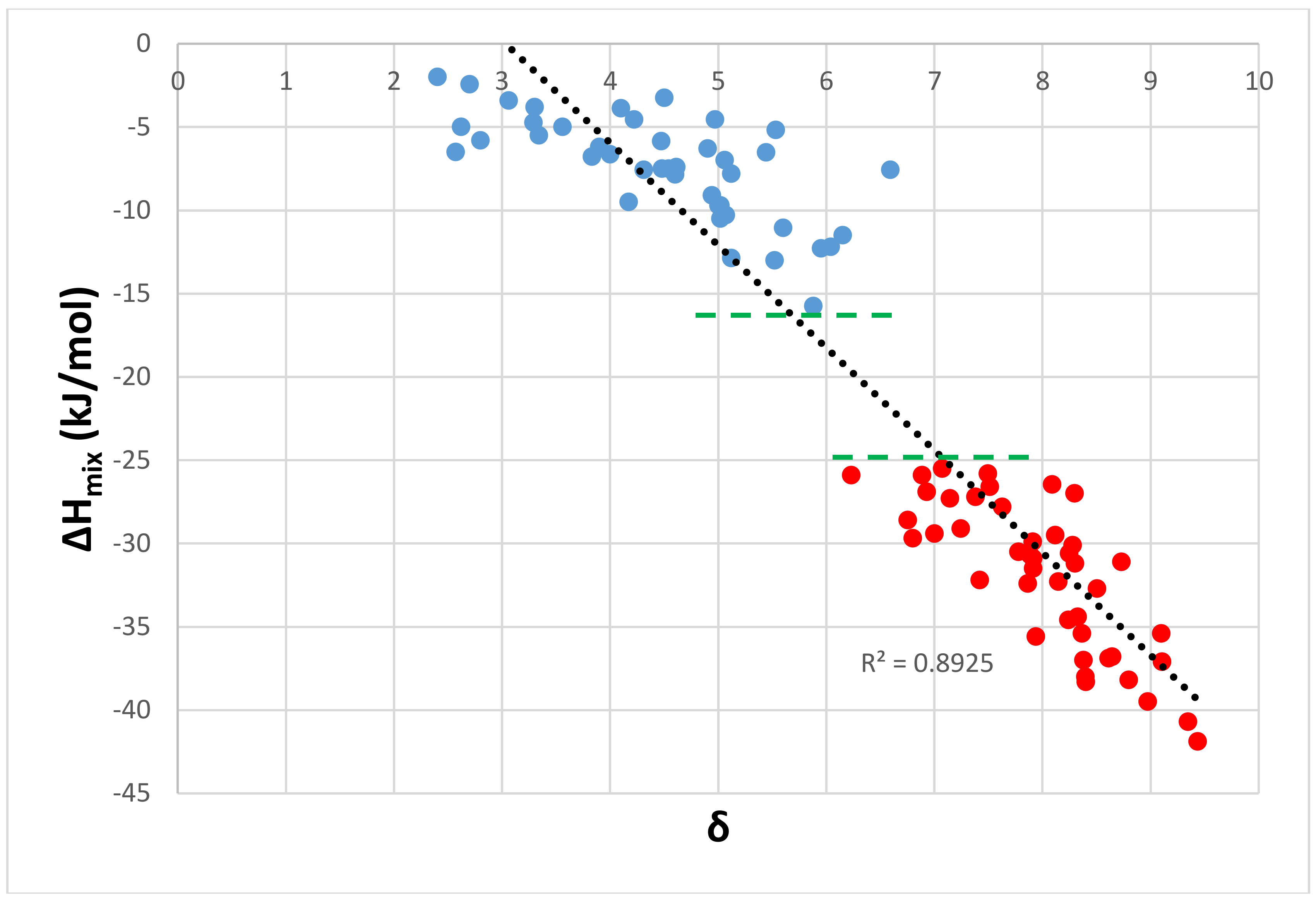
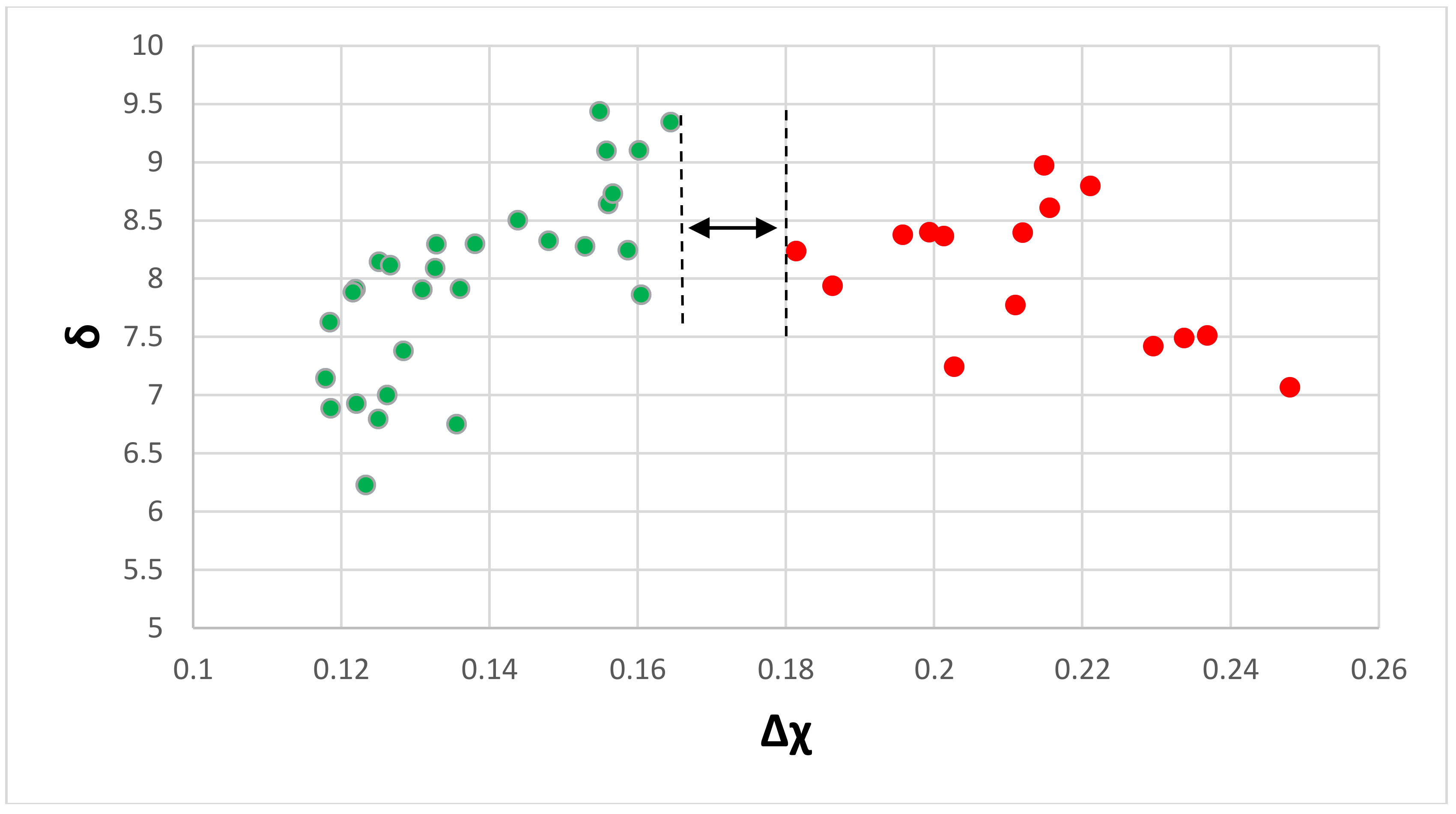

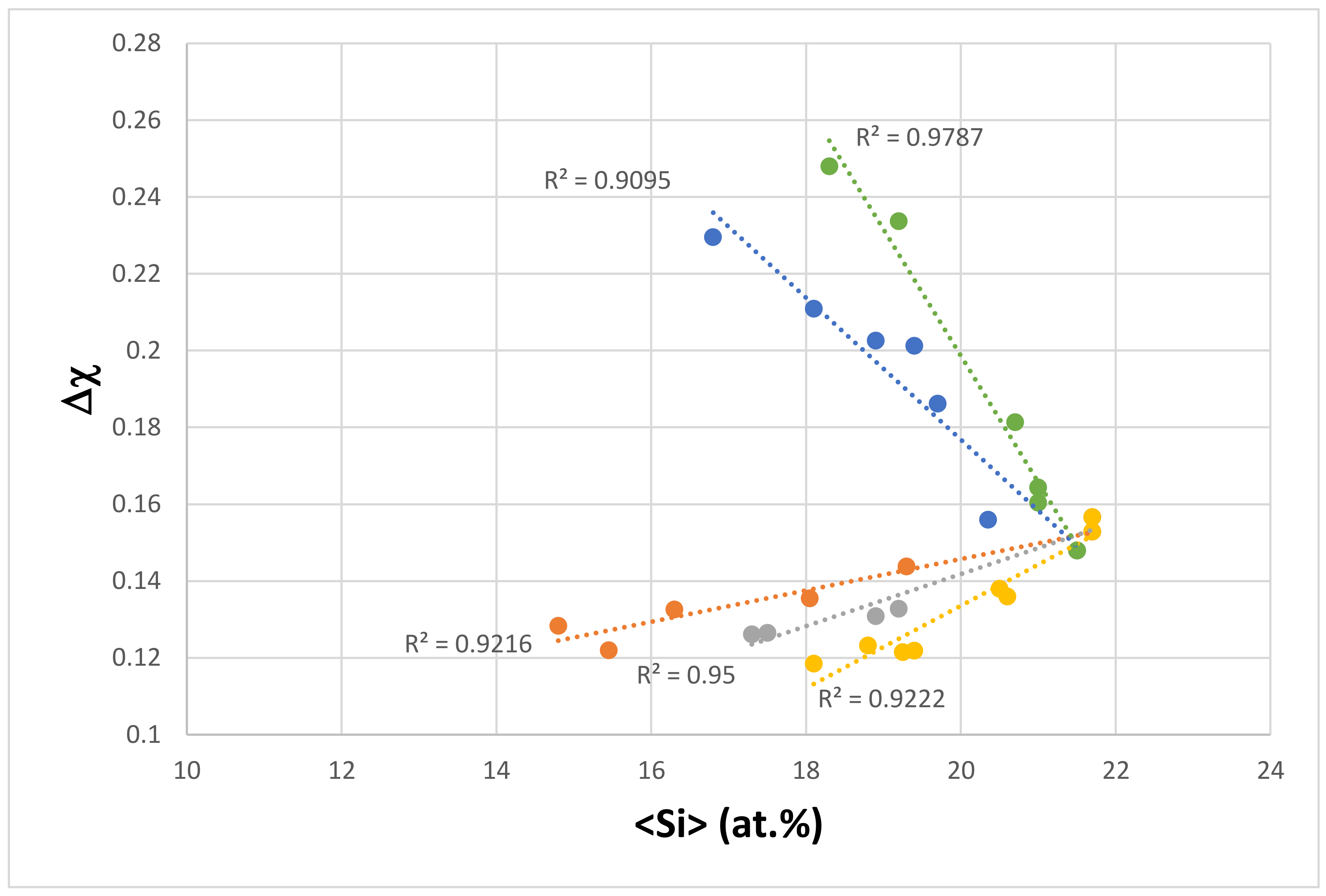
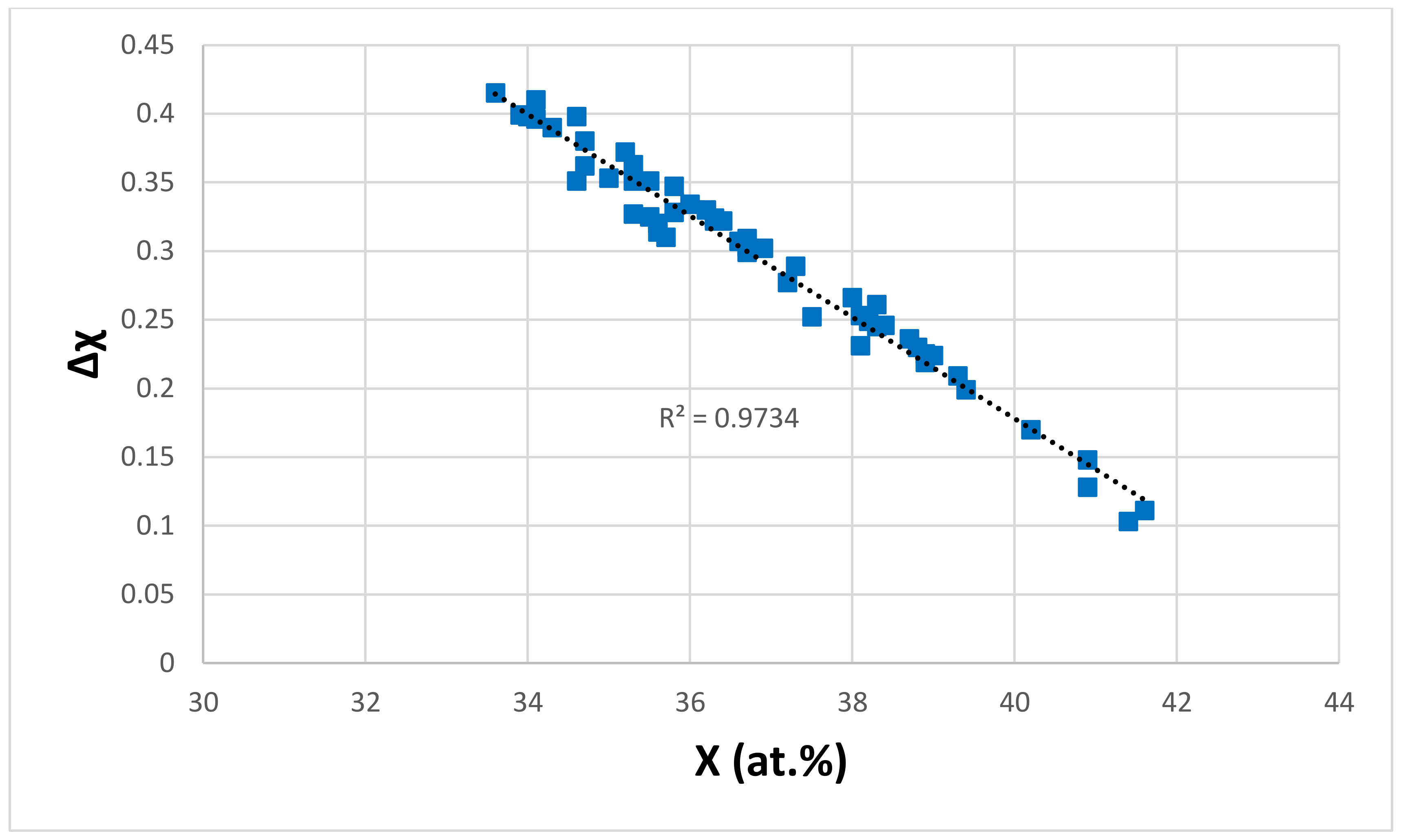
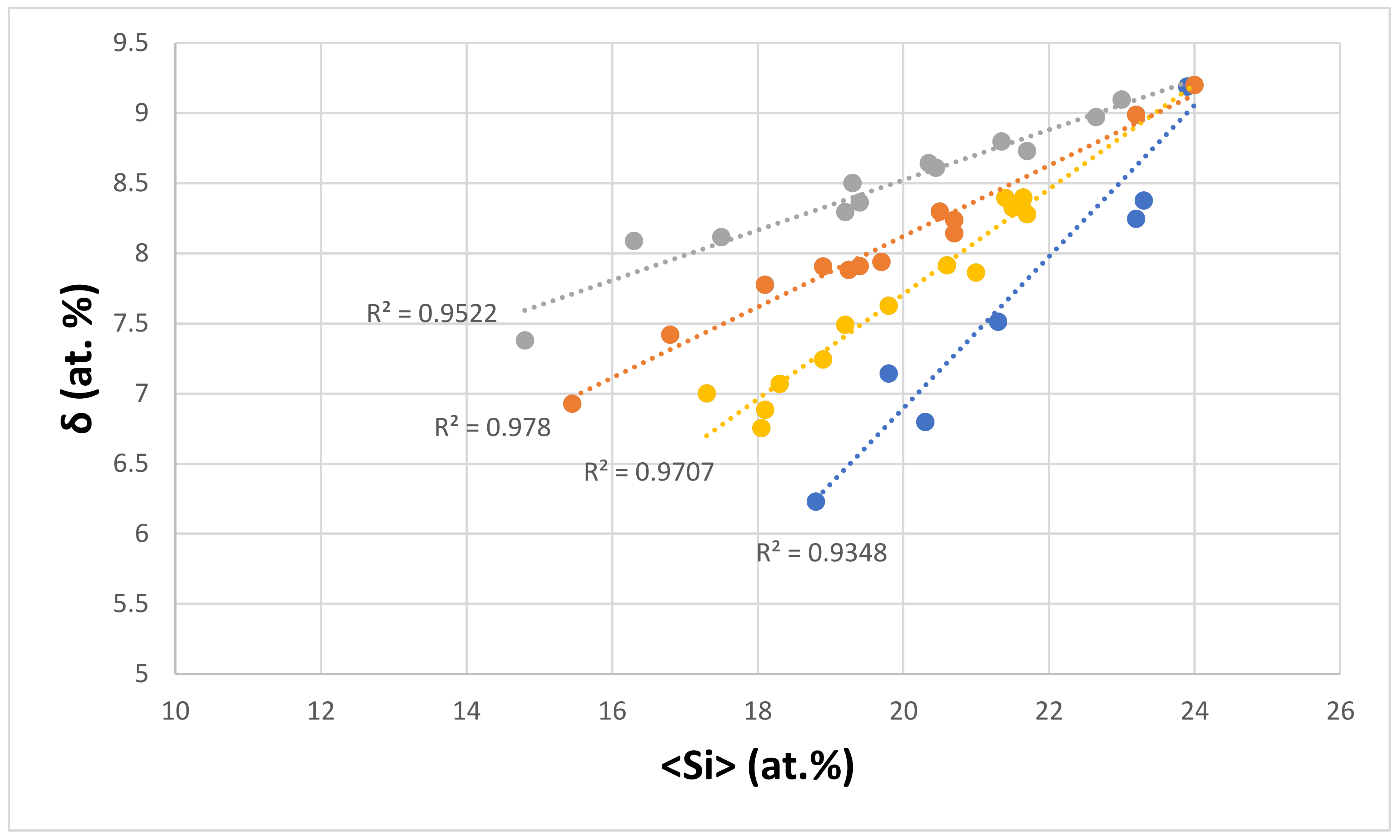
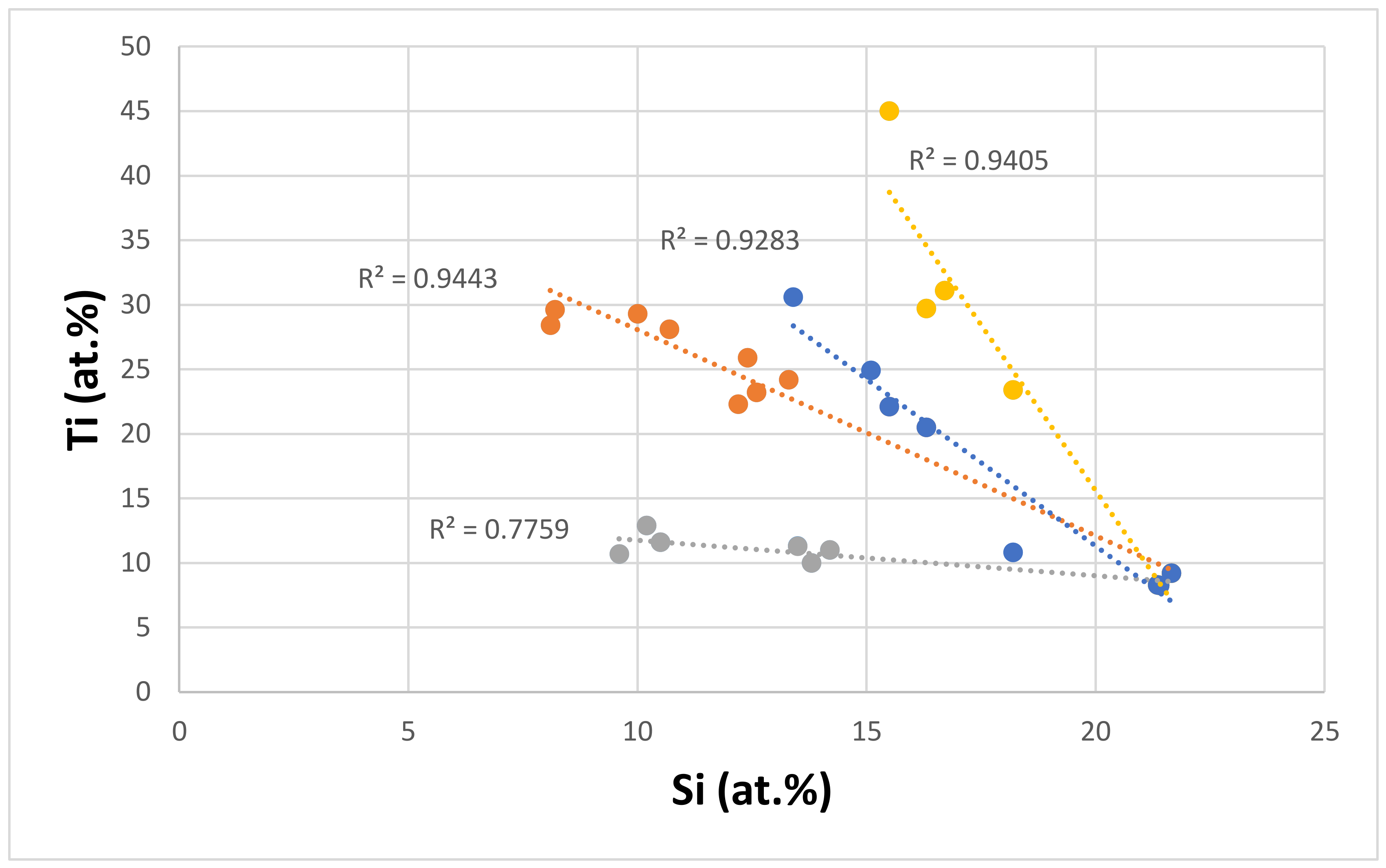
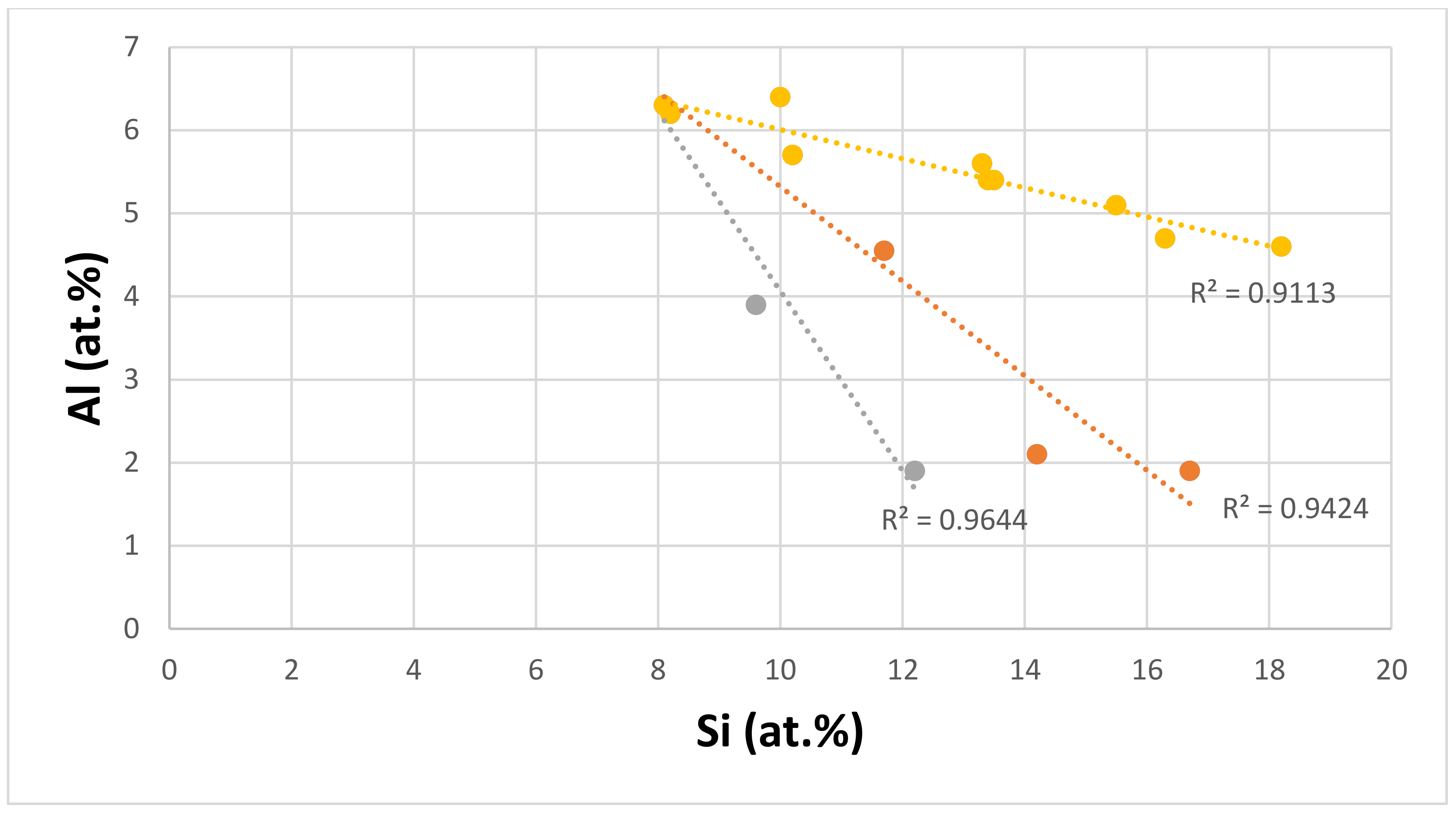

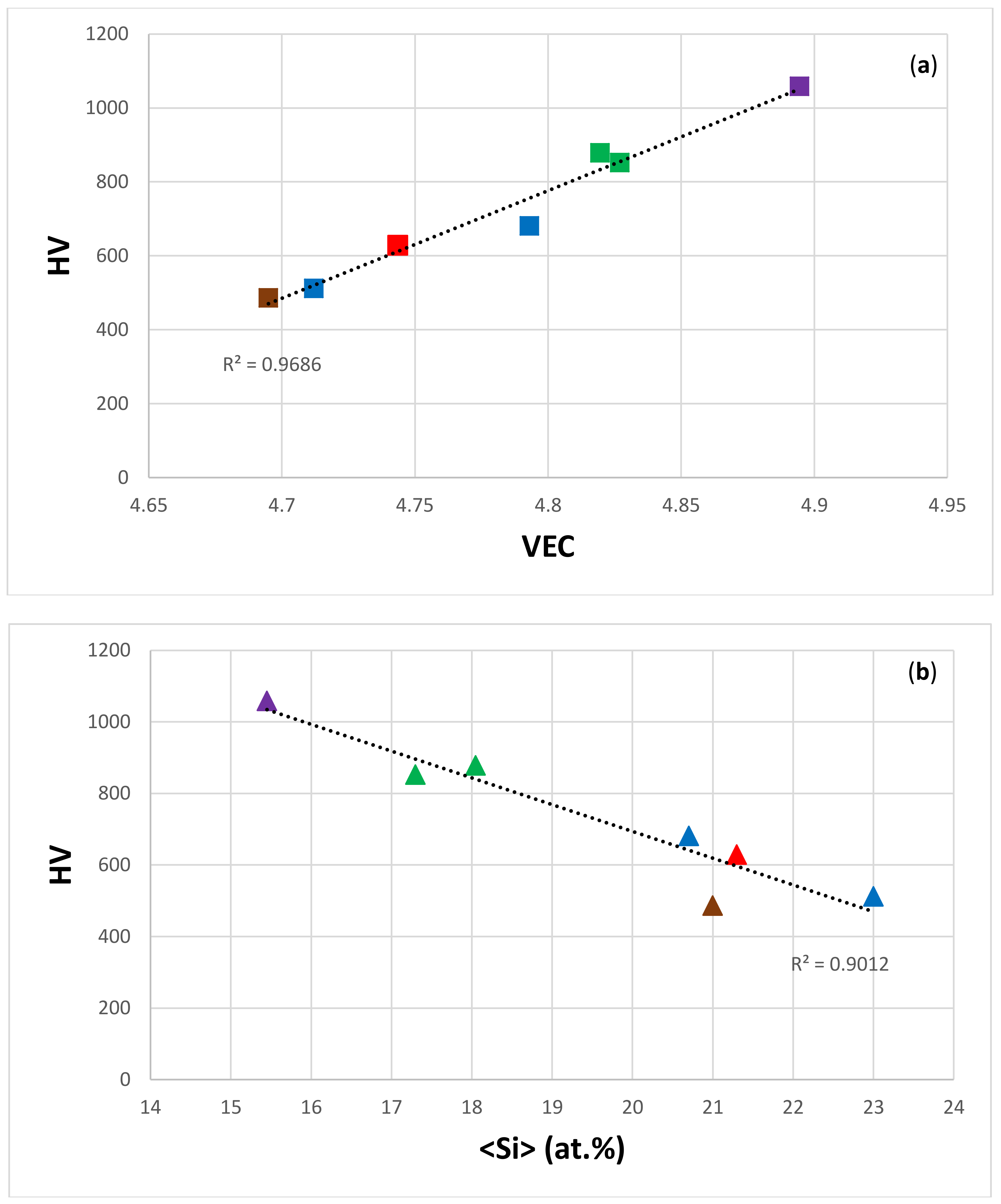
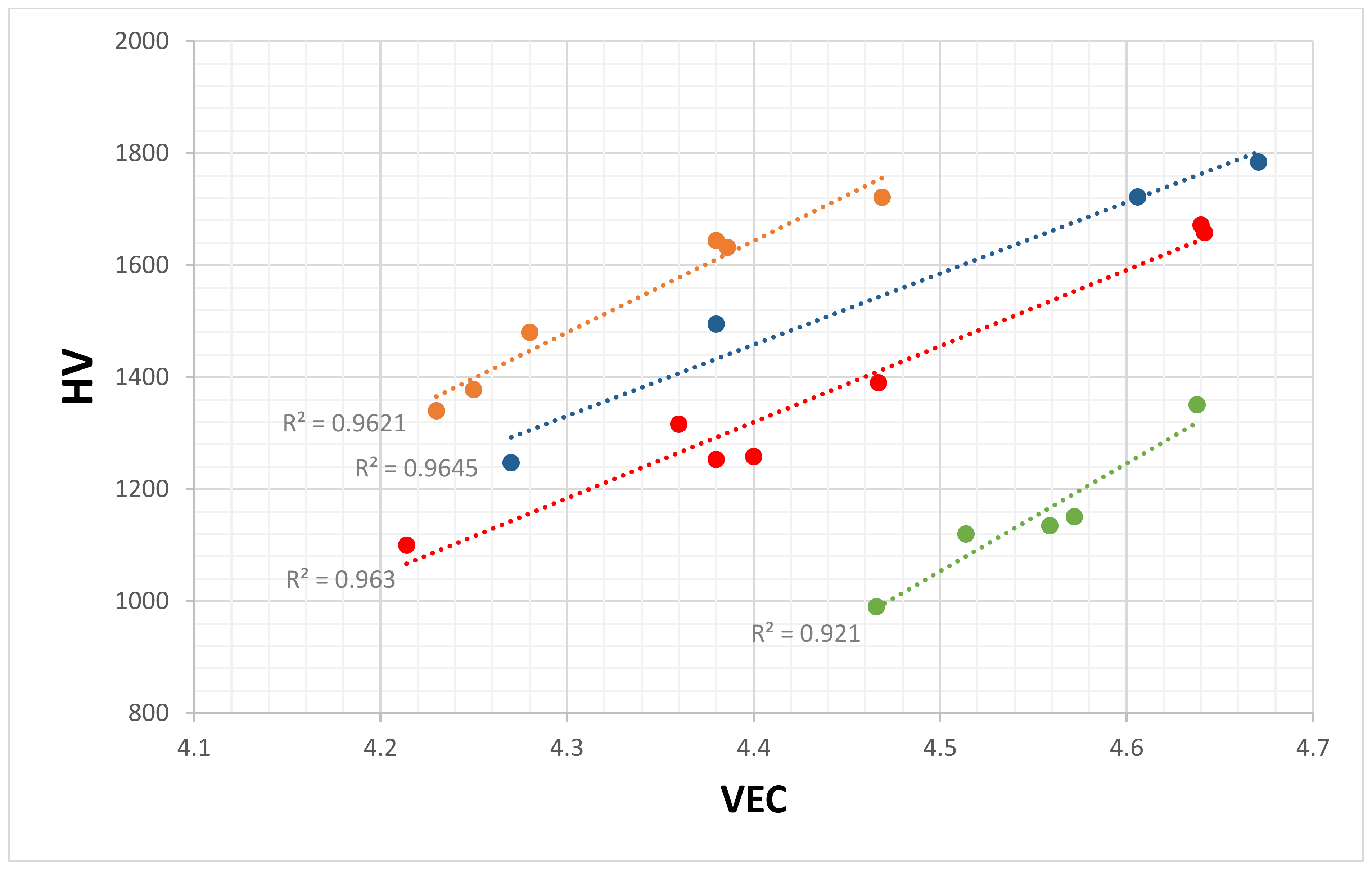
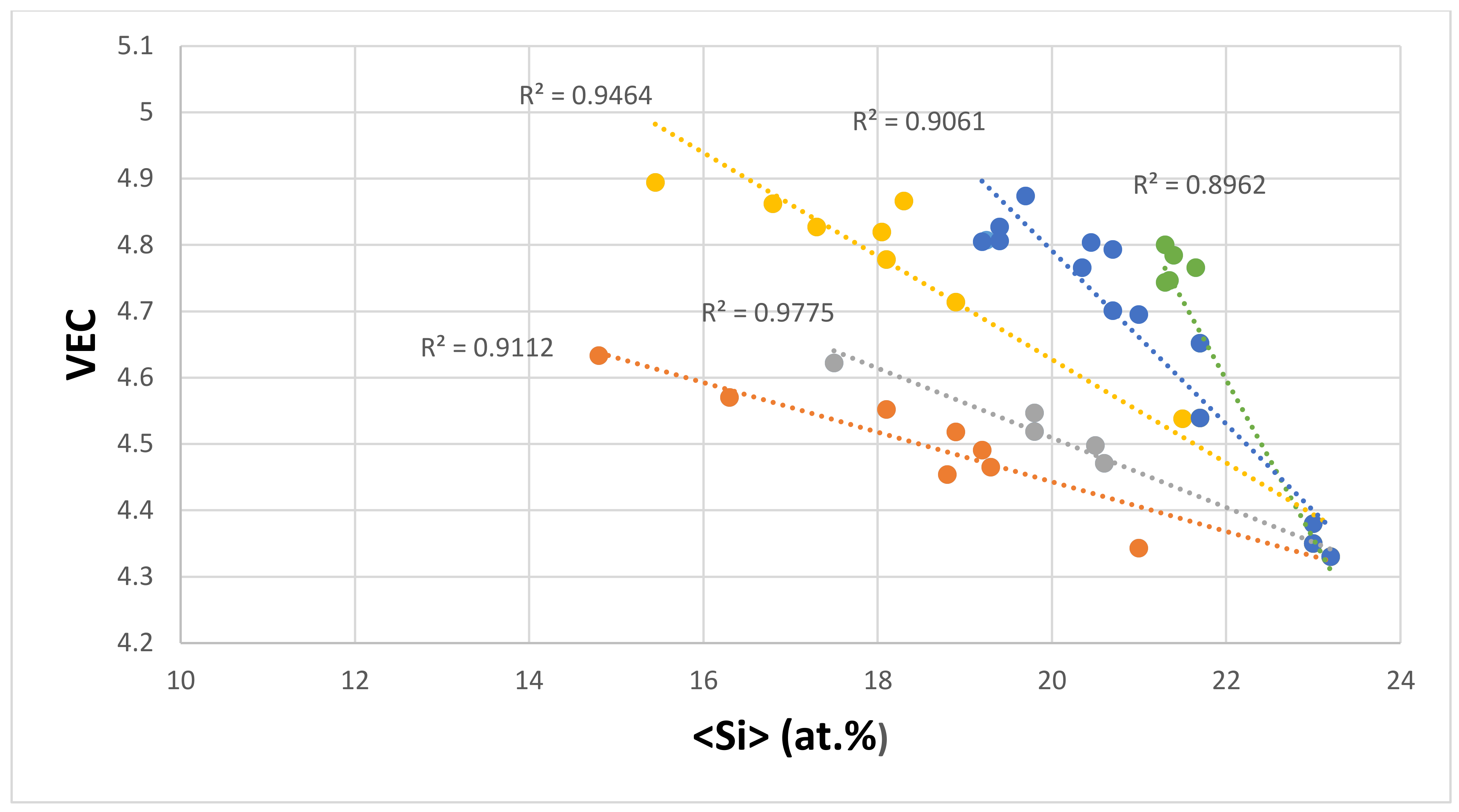
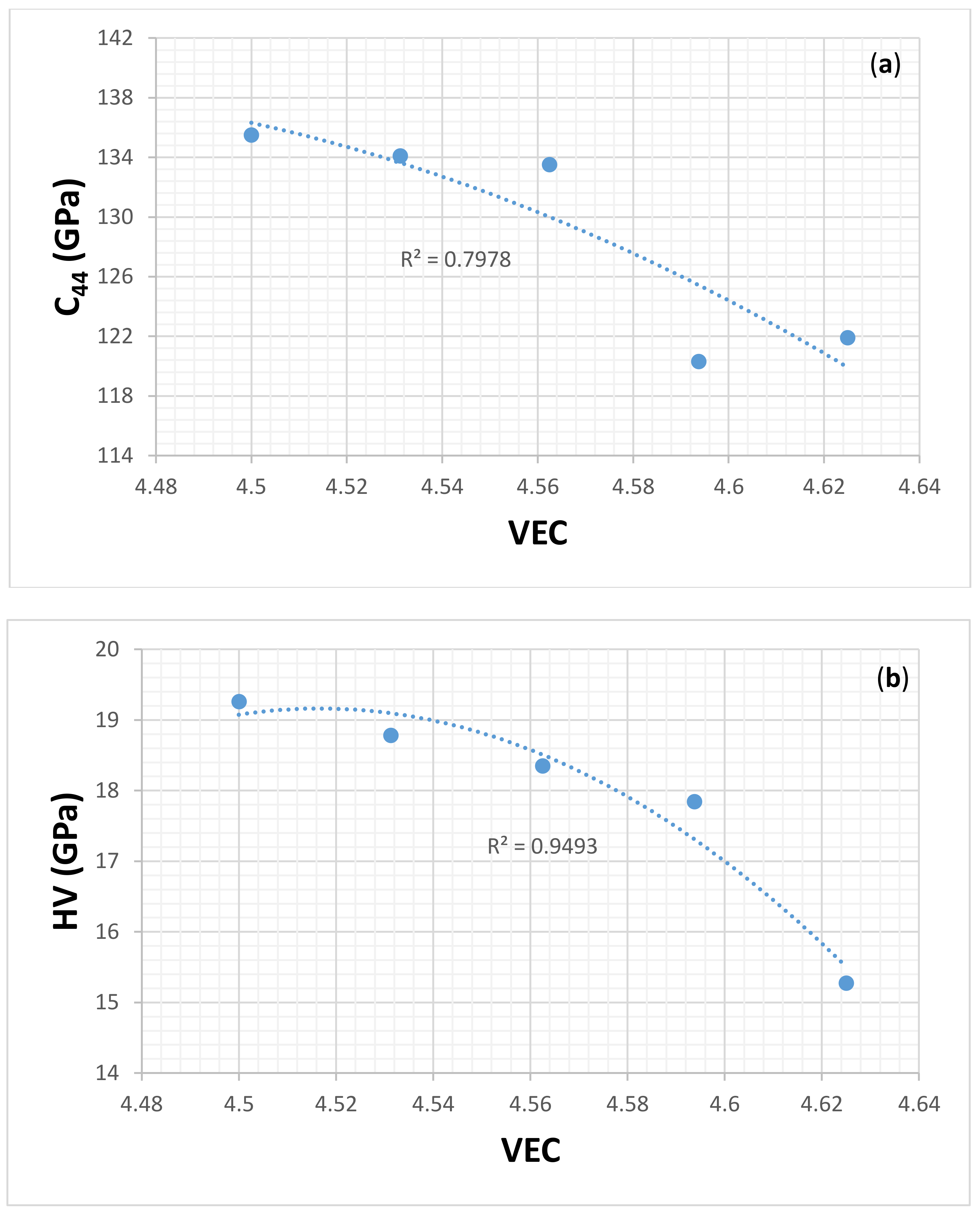
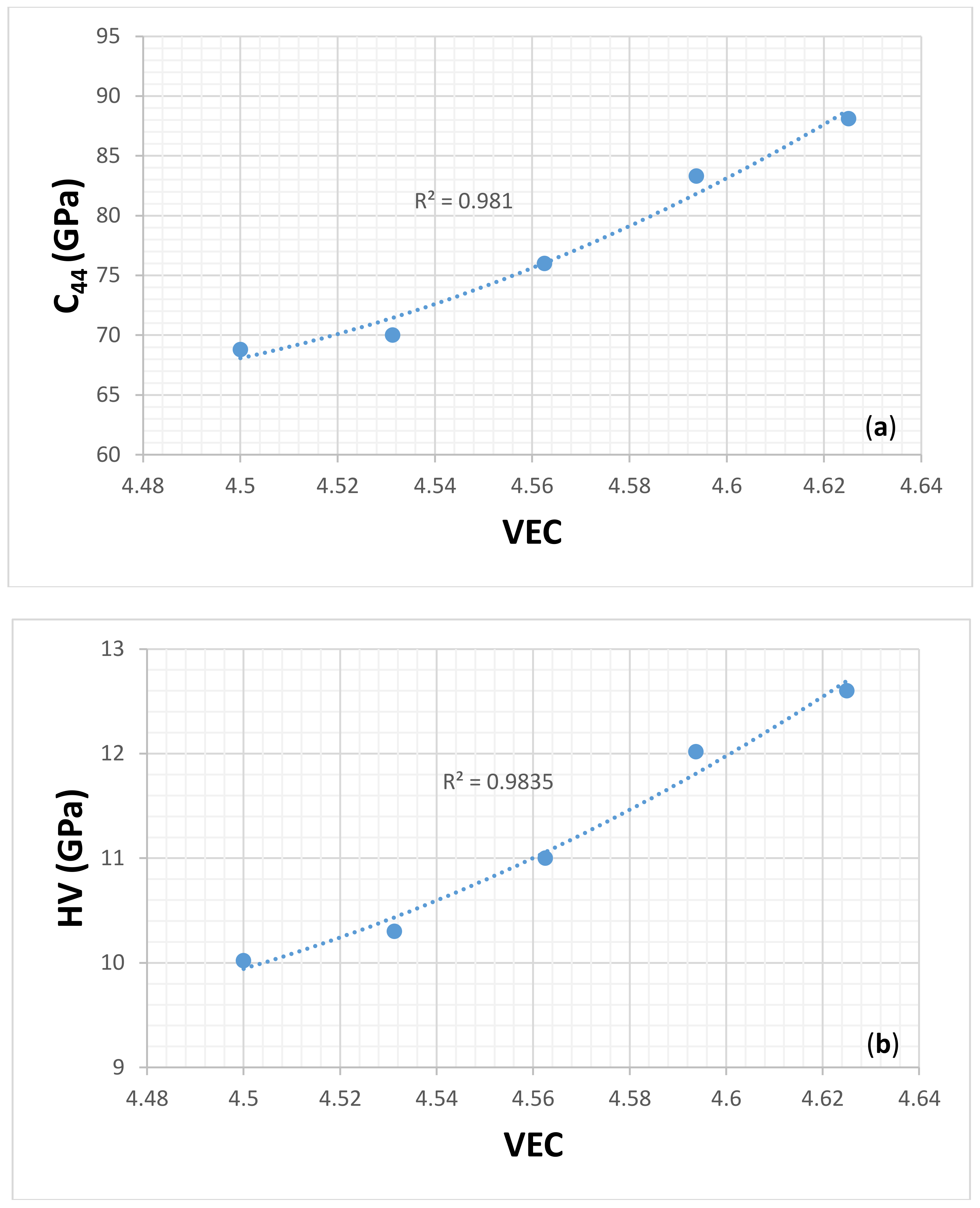
| Material | Parameter | |||||
|---|---|---|---|---|---|---|
| ΔHmix /(kJ/mol) | ΔSmix (J/molK) | VEC | δ | Δχ | Ω + | |
| Nb-silicide based alloys [62] | −32.7 to –44.8 | 8.3–14.7 | 4.37–4.9 | 8.1–14.3 | 0.12–0.237 | 0.57–0.95 |
| Nbss in Nb-silicide based alloys [38] | −2 to –15.9 | 5.8–14.5 | 4.4–5.4 | 2.4–9.7 | 0.039–0.331 with a gap in the range 0.13 to 0.179 | 1.55–8.9 |
| Eutectics with Nbss and Nb5Si3 | −25.5 to –41.9 | 4.7–15 | 4.33–4.89 | 6.23–9.44 | 0.118–0.248 with a gap in the range 0.164 to 0.181 | 0.38–1.35 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsakiropoulos, P. Alloying and Hardness of Eutectics with Nbss and Nb5Si3 in Nb-silicide Based Alloys. Materials 2018, 11, 592. https://doi.org/10.3390/ma11040592
Tsakiropoulos P. Alloying and Hardness of Eutectics with Nbss and Nb5Si3 in Nb-silicide Based Alloys. Materials. 2018; 11(4):592. https://doi.org/10.3390/ma11040592
Chicago/Turabian StyleTsakiropoulos, Panos. 2018. "Alloying and Hardness of Eutectics with Nbss and Nb5Si3 in Nb-silicide Based Alloys" Materials 11, no. 4: 592. https://doi.org/10.3390/ma11040592
APA StyleTsakiropoulos, P. (2018). Alloying and Hardness of Eutectics with Nbss and Nb5Si3 in Nb-silicide Based Alloys. Materials, 11(4), 592. https://doi.org/10.3390/ma11040592





