Novel Calcium Zirconate Silicate Cement Biomineralize and Seal Root Canals
Abstract
1. Introduction
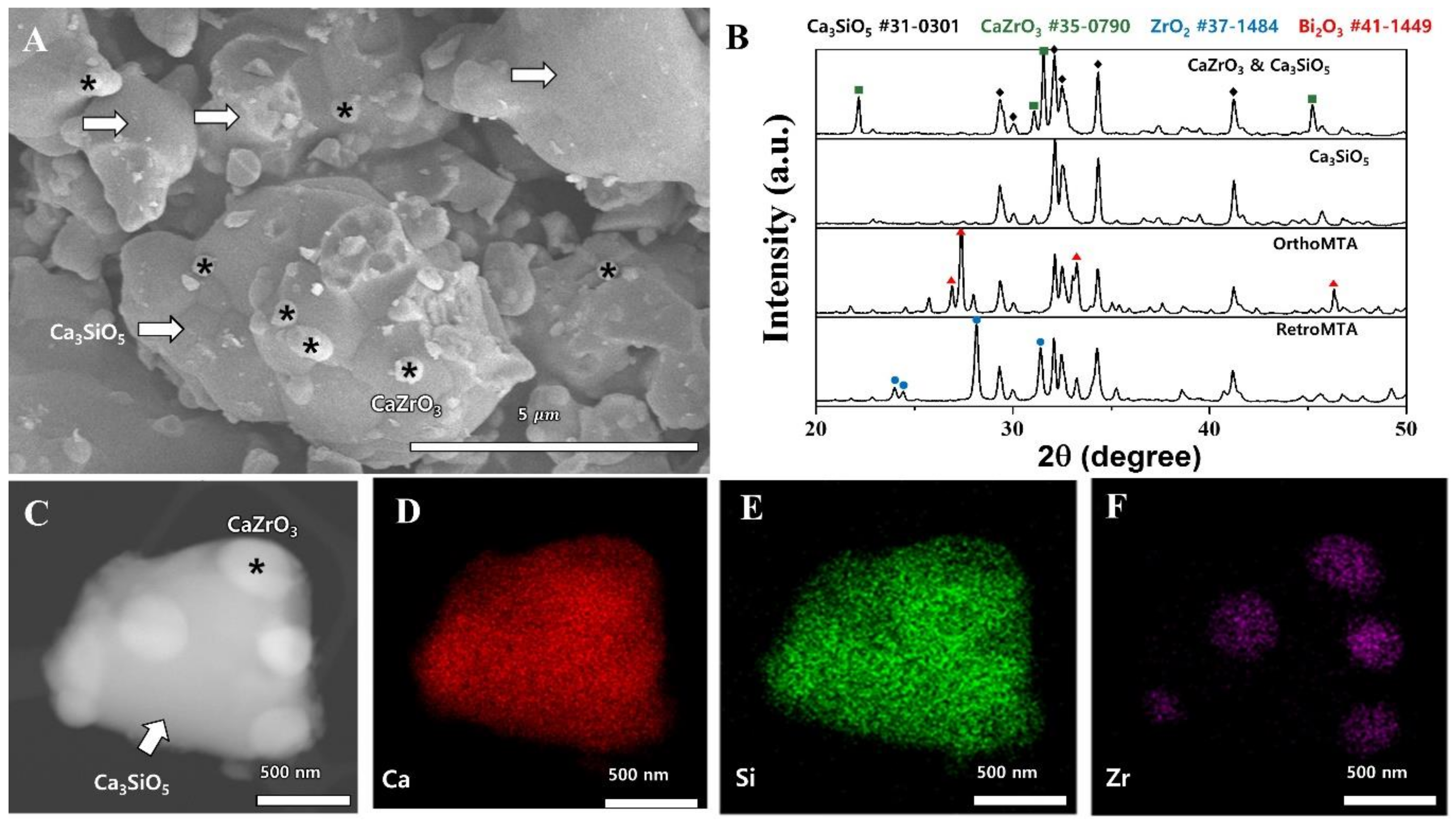
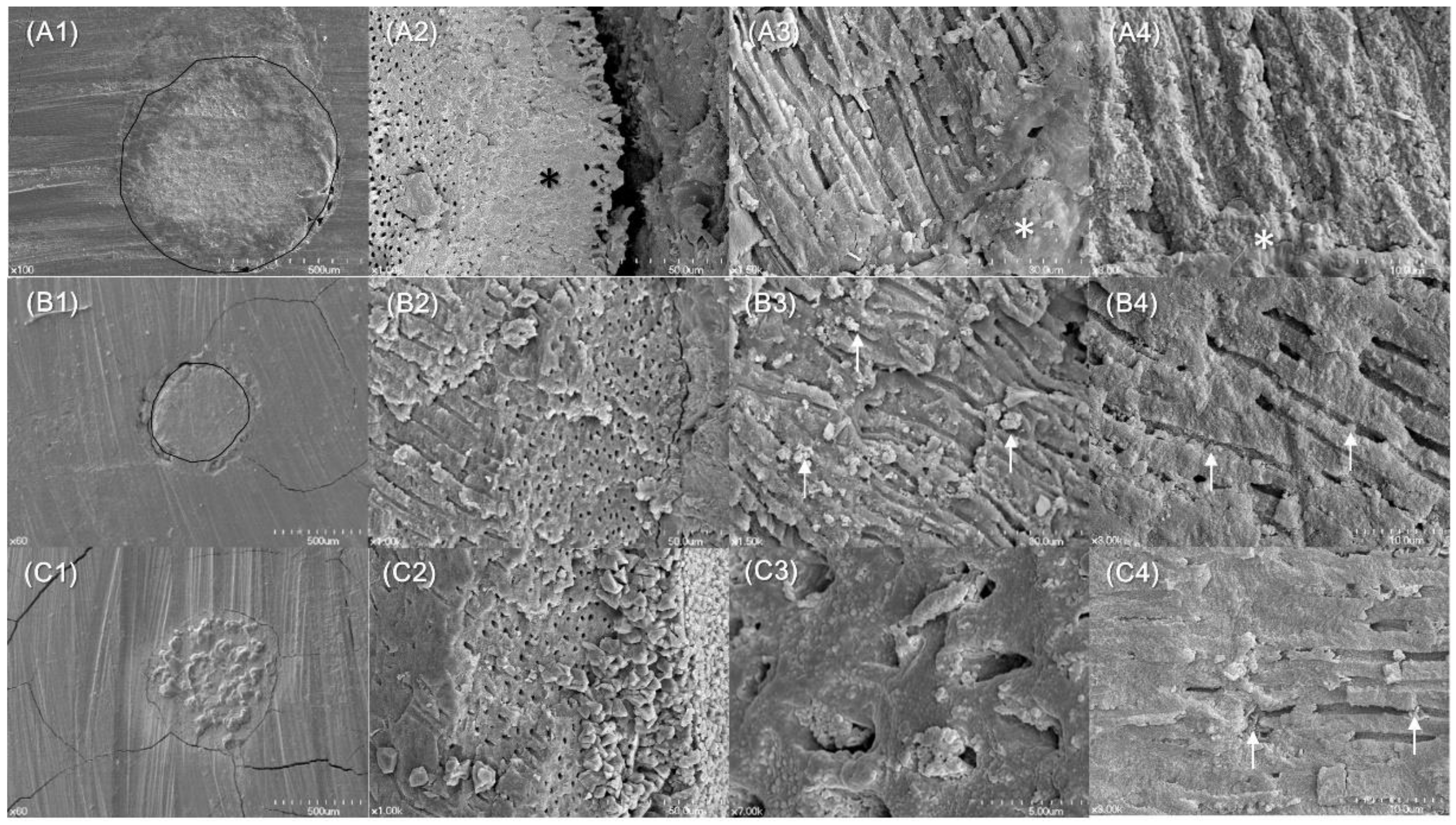
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthesis and Morphology Analysis of Calcium Zirconate Containing Calcium Silicate
4.2. Root Canal Preparation
4.3. Obturations
4.4. Endotoxin Leakage
4.5. Statistical Analysis
4.6. Biomineralization Identified
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Figdor, D.; Sundqvist, G. A big role for the very small—Understanding the endodontic microbial flora. Aust. Dent. J. 2007, 52, S38–S51. [Google Scholar] [CrossRef] [PubMed]
- Bouillaguet, S.; Shaw, L.; Barthelemy, J.; Krejci, I.; Wataha, J.C. Long-term sealing ability of pulp canal sealer, AH-plus, guttaflow and epiphany. Int. Endod. J. 2008, 41, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.S. Adhesive dentistry and endodontics. Part 2: Bonding in the root canal system-the promise and the problems: A review. J. Endod. 2006, 32, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Qualtrough, A.; Silikas, N. Extended setting shrinkage behavior of endodontic sealers. J. Endod. 2008, 34, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.A.; Nikonov, S.Y.; Looney, S.W.; Didato, A.; Niu, L.N.; Levin, M.D.; Rueggeberg, F.A.; Pashley, D.H.; Watanabe, I.; Tay, F.R. In vitro biocompatibility evaluation of a root canal filling material that expands on water sorption. J. Endod. 2013, 39, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Niu, L.N.; Zhang, W.; Olsen, M.; De-Deus, G.; Eid, A.A.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Ability of new obturation materials to improve the seal of the root canal system: A review. Acta Biomater. 2014, 10, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.J.; Baek, S.H.; Kum, K.Y.; Shon, W.J.; Woo, K.M.; Lee, W. Dynamic intratubular biomineralization following root canal obturation with pozzolan-based mineral trioxide aggregate sealer cement. Scanning 2016, 38, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Salles, L.P.; Gomes-Cornelio, A.L.; Guimaraes, F.C.; Herrera, B.S.; Bao, S.N.; Rossa-Junior, C.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. Mineral trioxide aggregate-based endodontic sealer stimulates hydroxyapatite nucleation in human osteoblast-like cell culture. J. Endod. 2012, 38, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.N.; Tay, K.C.; Garrett, L.V.; Mai, S.; Primus, C.M.; Gutmann, J.L.; Pashley, D.H.; Tay, F.R. Microscopic appearance and apical seal of root canals filled with gutta-percha and proroot endo sealer after immersion in a phosphate-containing fluid. Int. Endod. J. 2008, 41, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Jafari, S. Composition and physicochemical properties of calcium silicate based sealers: A review article. J. Clin. Exp. Dent. 2017, 9, e1249–e1255. [Google Scholar] [CrossRef] [PubMed]
- Candeiro, G.T.; Correia, F.C.; Duarte, M.A.; Ribeiro-Siqueira, D.C.; Gavini, G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J. Endod. 2012, 38, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.M.; Shen, Y.; Zheng, W.; Li, L.; Zheng, Y.F.; Haapasalo, M. Physical properties of 5 root canal sealers. J. Endod. 2013, 39, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Gupta, I.; Elshamy, F.M.; Boreak, N.; Homeida, H.E. In vitro comparison of antibacterial properties of bioceramic-based sealer, resin-based sealer and zinc oxide eugenol based sealer and two mineral trioxide aggregates. Eur. J. Dent. 2016, 10, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M. Mineral trioxide aggregate: A comprehensive literature review—Part ii: Leakage and biocompatibility investigations. J. Endod. 2010, 36, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.S.; Chang, S.W.; Oh, S.R.; Perinpanayagam, H.; Lim, S.M.; Yoo, Y.J.; Oh, Y.R.; Woo, S.B.; Han, S.H.; Zhu, Q.; et al. Bacterial entombment by intratubular mineralization following orthograde mineral trioxide aggregate obturation: A scanning electron microscopy study. Int. J. Oral Sci. 2014, 6, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Coomaraswamy, K.S.; Lumley, P.J.; Hofmann, M.P. Effect of bismuth oxide radioopacifier content on the material properties of an endodontic portland cement-based (MTA-like) system. J. Endod. 2007, 33, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Grazziotin-Soares, R.; Nekoofar, M.H.; Davies, T.E.; Bafail, A.; Alhaddar, E.; Hubler, R.; Busato, A.L.; Dummer, P.M. Effect of bismuth oxide on white mineral trioxide aggregate: Chemical characterization and physical properties. Int. Endod. J. 2014, 47, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.C.; Lee, B.C.; Chang, H.S.; Lee, W.; Hong, C.U.; Min, K.S. Evaluation of the radiopacity and cytotoxicity of portland cements containing bismuth oxide. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, e54–e57. [Google Scholar] [CrossRef] [PubMed]
- Marciano, M.A.; Costa, R.M.; Camilleri, J.; Mondelli, R.F.; Guimaraes, B.M.; Duarte, M.A. Assessment of color stability of white mineral trioxide aggregate angelus and bismuth oxide in contact with tooth structure. J. Endod. 2014, 40, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Bosso-Martelo, R.; Guerreiro-Tanomaru, J.M.; Viapiana, R.; Berbert, F.L.; Duarte, M.A.; Tanomaru-Filho, M. Physicochemical properties of calcium silicate cements associated with microparticulate and nanoparticulate radiopacifiers. Clin. Oral Investig. 2016, 20, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.F.; Bosso, R.; Ferino, R.V.; Tanomaru-Filho, M.; Bernardi, M.I.; Guerreiro-Tanomaru, J.M.; Cerri, P.S. Microparticulated and nanoparticulated zirconium oxide added to calcium silicate cement: Evaluation of physicochemical and biological properties. J. Biomed. Mater. Res. A 2014, 102, 4336–4345. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N.J.; Li, Q. The impact of zirconium oxide radiopacifier on the early hydration behaviour of white portland cement. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Mineral trioxide aggregate: Present and future developments. Endod. Top. 2015, 32, 31–46. [Google Scholar] [CrossRef]
- Kum, K.Y.; Zhu, Q.; Safavi, K.; Gu, Y.; Bae, K.S.; Chang, S.W. Analysis of six heavy metals in ortho mineral trioxide aggregate and proroot mineral trioxide aggregate by inductively coupled plasma–optical emission spectrometry. Austral. Endod. J. 2013, 39, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Felsenfeld, A.J.; Llach, F. Aluminum administration in the rat separately affects the osteoblast and bone mineralization. J. Bone Miner. Res. 1990, 5, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.R.; Yamaguchi, M.; Setzer, F.C.; Karabucak, B. Spectrophotometric analysis of coronal tooth discoloration induced by various bioceramic cements and other endodontic materials. J. Endod. 2015, 41, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.K.; Wesselink, P.R. Endodontic leakage studies reconsidered. Part i. Methodology, application and relevance. Int. Endod. J. 1993, 26, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, R.C.; Gomes, B.P.; Shah, H.N.; Ferraz, C.C.; Zaia, A.A.; Souza-Filho, F.J. Quantification of endotoxins in necrotic root canals from symptomatic and asymptomatic teeth. J. Med. Microbiol. 2005, 54, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.G.; Ferreira, N.S.; Martinho, F.C.; Nascimento, G.G.; Manhaes, L.R.; Rocco, M.A.; Carvalho, C.A.; Valera, M.C. Correlation between volume of apical periodontitis determined by cone-beam computed tomography analysis and endotoxin levels found in primary root canal infection. J. Endod. 2015, 41, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, N.; Krunic, J.; Popovic, B.; Stojicic, S.; Zivkovic, S. Prevalence of enterococcus faecalis and porphyromonas gingivalis in infected root canals and their susceptibility to endodontic treatment procedures: A molecular study. Srp. Arh. Celok. Lek. 2014, 142, 535–541. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ding, J.L.; Ho, B. Endotoxin detection—From limulus amebocyte lysate to recombinant factor C. Subcell. Biochem. 2010, 53, 187–208. [Google Scholar] [PubMed]
- Marinho, A.C.; Polay, A.R.; Gomes, B.P. Accuracy of turbidimetric limulus amebocyte lysate assay for the recovery of endotoxin interacted with commonly used antimicrobial agents of endodontic therapy. J. Endod. 2015, 41, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- De-Deus, G. Research that matters—Root canal filling and leakage studies. Int. Endod. J. 2012, 45, 1063–1064. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, F.; Sadek, F.T.; Schuster, G.S.; Volkmann, K.R.; Looney, S.W.; Ferrari, M.; Toledano, M.; Pashley, D.H.; Tay, F.R. Efficacy of two contemporary single-cone filling techniques in preventing bacterial leakage. J. Endod. 2007, 33, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Celikten, B.; Uzuntas, C.F.; Orhan, A.I.; Orhan, K.; Tufenkci, P.; Kursun, S.; Demiralp, K.O. Evaluation of root canal sealer filling quality using a single-cone technique in oval shaped canals: An in vitro micro-CT study. Scanning 2016, 38, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Holland, R.; de Souza, V.; Nery, M.J.; Otoboni Filho, J.A.; Bernabe, P.F.; Dezan Junior, E. Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. J. Endod. 1999, 25, 161–166. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Prati, C. MTA and F-doped MTA cements used as sealers with warm gutta-percha. Long-term study of sealing ability. Int. Endod. J. 2010, 43, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Fridland, M.; Rosado, R. Mineral trioxide aggregate (MTA) solubility and porosity with different water-to-powder ratios. J. Endod. 2003, 29, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Jho, W.; Park, J.W.; Kim, E.; Song, M.; Seo, D.G.; Yang, D.K.; Shin, S.J. Comparison of root canal filling quality by mineral trioxide aggregate and gutta percha cones/ah plus sealer. Dent. Mater. J. 2016, 35, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, S.; Park, J.W.; Jung, I.Y.; Shin, S.J. Comparison of the percentage of voids in the canal filling of a calcium silicate-based sealer and gutta percha cones using two obturation techniques. Materials 2017, 10, 1170. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Chung, J.; Na, H.S.; Park, E.; Kwak, S.; Kim, H.C. Comparison of bacterial leakage resistance of various root canal filling materials and methods: Confocal laser-scanning microscope study. Scanning 2015, 37, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Xuereb, M.; Vella, P.; Damidot, D.; Sammut, C.V.; Camilleri, J. In situ assessment of the setting of tricalcium silicate-based sealers using a dentin pressure model. J. Endod. 2015, 41, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Evaluation of the effect of intrinsic material properties and ambient conditions on the dimensional stability of white mineral trioxide aggregate and portland cement. J. Endod. 2011, 37, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Loushine, B.A.; Bryan, T.E.; Looney, S.W.; Gillen, B.M.; Loushine, R.J.; Weller, R.N.; Pashley, D.H.; Tay, F.R. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J. Endod. 2011, 37, 673–677. [Google Scholar] [CrossRef] [PubMed]
- De-Deus, G.; Brandao, M.C.; Leal, F.; Reis, C.; Souza, E.M.; Luna, A.S.; Paciornik, S.; Fidel, S. Lack of correlation between sealer penetration into dentinal tubules and sealability in nonbonded root fillings. Int. Endod. J. 2012, 45, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Timpawat, S.; Amornchat, C.; Trisuwan, W.R. Bacterial coronal leakage after obturation with three root canal sealers. J. Endod. 2001, 27, 36–39. [Google Scholar] [CrossRef] [PubMed]
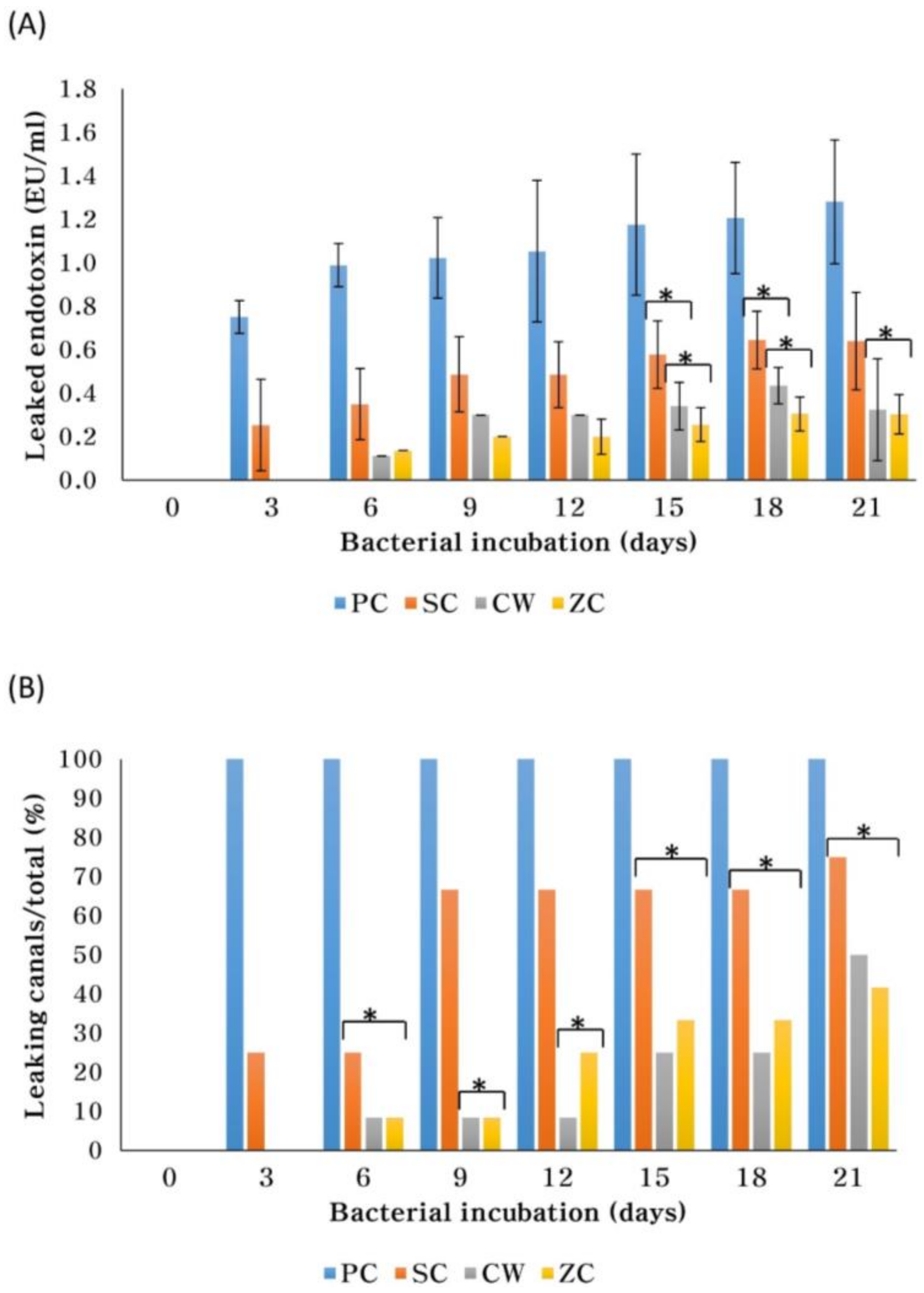
| Group | NC | PC | CW | SC | ZC |
|---|---|---|---|---|---|
| Amount of leaked endotoxin (Mean ± SD) (EU/mL) | <0.01 | 1.281 ± 0.284 | 0.324 ± 0.235 | 0.641 ± 0.225 | 0.303 ± 0.091 |
| (% of PC) * | (<1%) | (100%) | (25%) | (50%) | (24%) |
| Number leaked/total (%) | 0/6 (0%) | 6/6 (100%) | 6/12 (50%) | 9/12 (75%) | 5/12 (42%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.; Cho, S.-I.; Perinpanayagam, H.; You, J.; Hong, S.-H.; Yoo, Y.-J.; Chang, S.W.; Shon, W.-J.; Yoo, J.-S.; Baek, S.-H.; et al. Novel Calcium Zirconate Silicate Cement Biomineralize and Seal Root Canals. Materials 2018, 11, 588. https://doi.org/10.3390/ma11040588
Oh S, Cho S-I, Perinpanayagam H, You J, Hong S-H, Yoo Y-J, Chang SW, Shon W-J, Yoo J-S, Baek S-H, et al. Novel Calcium Zirconate Silicate Cement Biomineralize and Seal Root Canals. Materials. 2018; 11(4):588. https://doi.org/10.3390/ma11040588
Chicago/Turabian StyleOh, Soram, Sung-In Cho, Hiran Perinpanayagam, Jinsu You, Seong-Hyeon Hong, Yeon-Jee Yoo, Seok Woo Chang, Won-Jun Shon, Jun-Sang Yoo, Seung-Ho Baek, and et al. 2018. "Novel Calcium Zirconate Silicate Cement Biomineralize and Seal Root Canals" Materials 11, no. 4: 588. https://doi.org/10.3390/ma11040588
APA StyleOh, S., Cho, S.-I., Perinpanayagam, H., You, J., Hong, S.-H., Yoo, Y.-J., Chang, S. W., Shon, W.-J., Yoo, J.-S., Baek, S.-H., & Kum, K.-Y. (2018). Novel Calcium Zirconate Silicate Cement Biomineralize and Seal Root Canals. Materials, 11(4), 588. https://doi.org/10.3390/ma11040588






