Effect of Gypsum on the Early Hydration of Cubic and Na-Doped Orthorhombic Tricalcium Aluminate
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
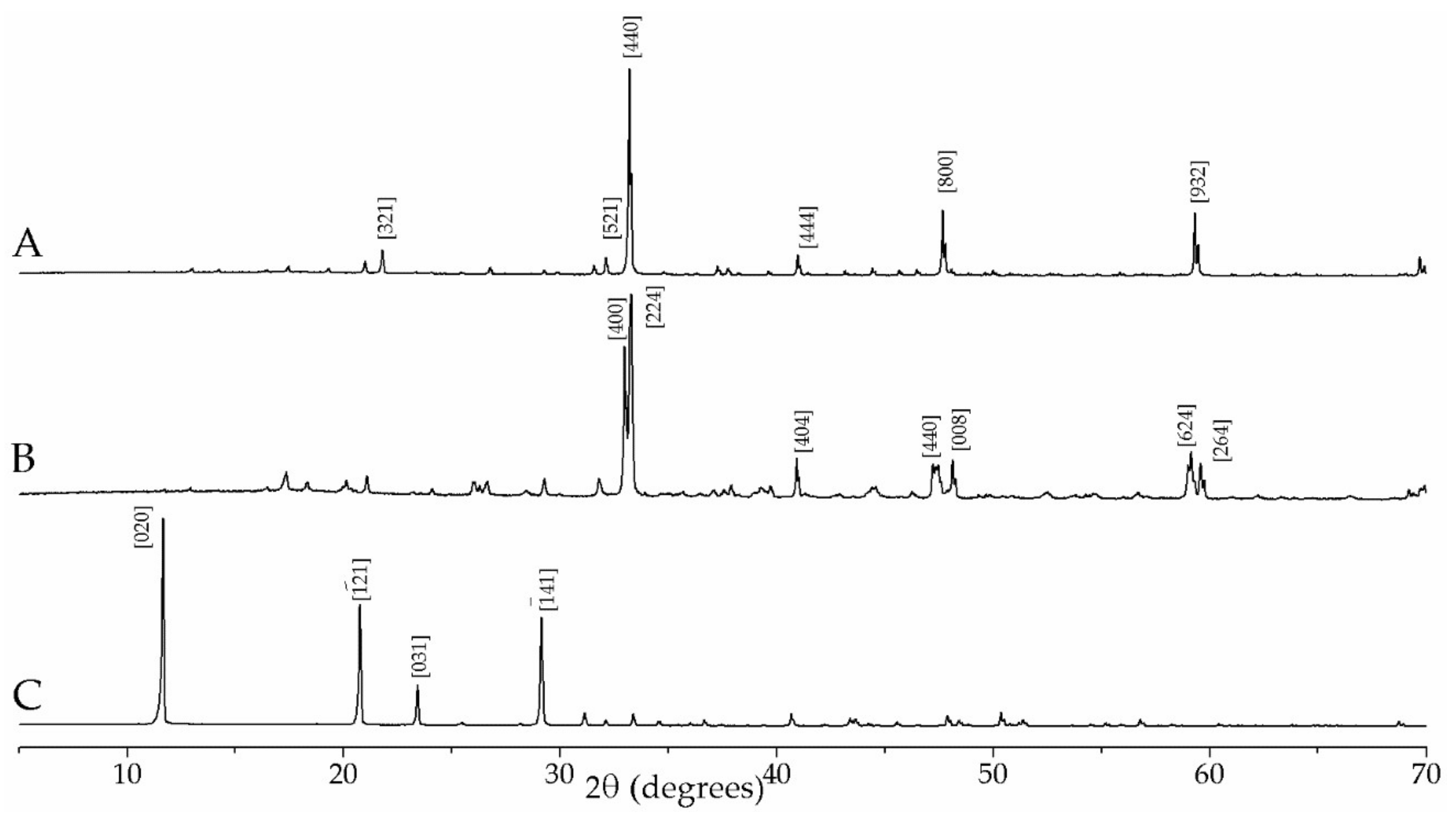
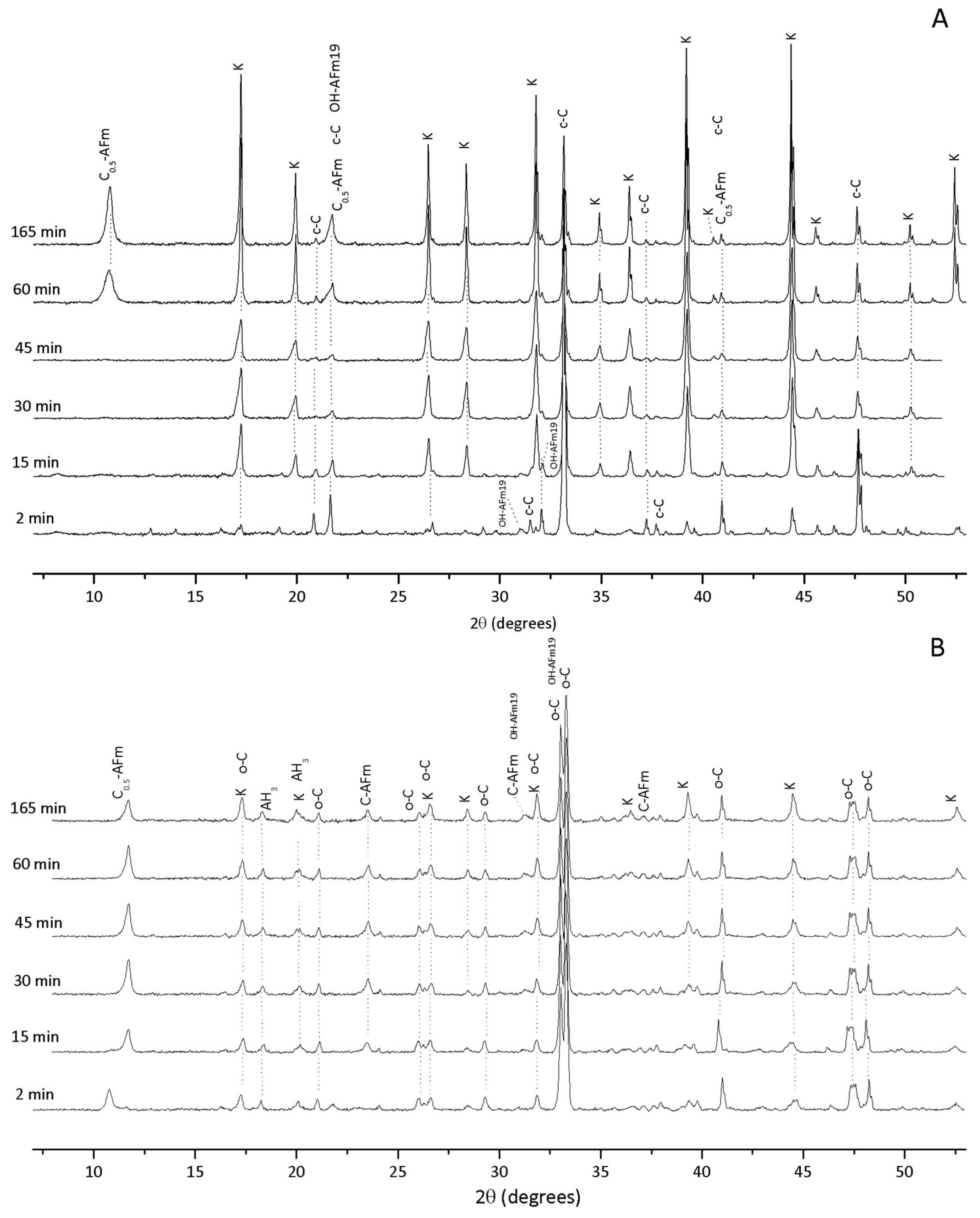
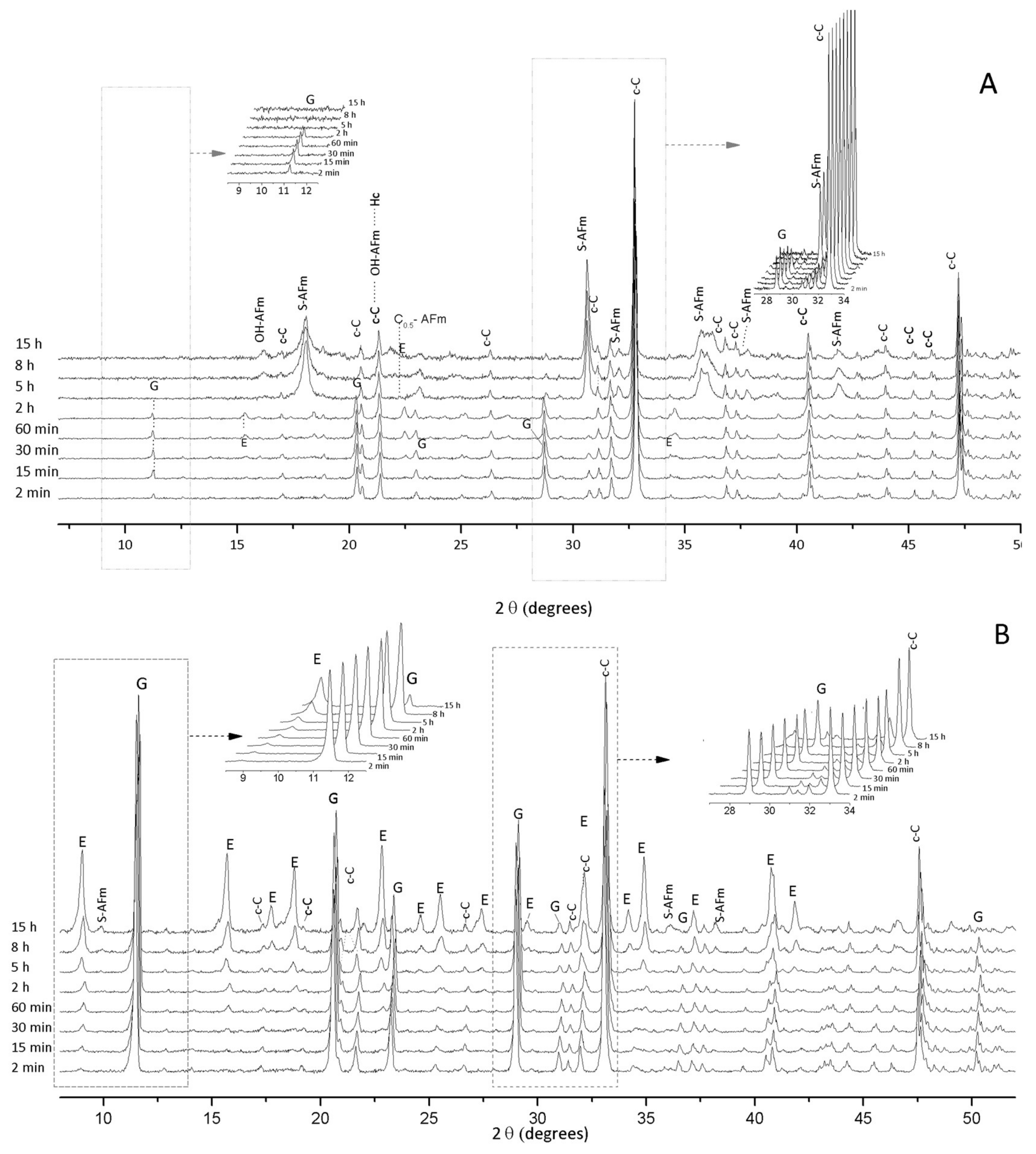
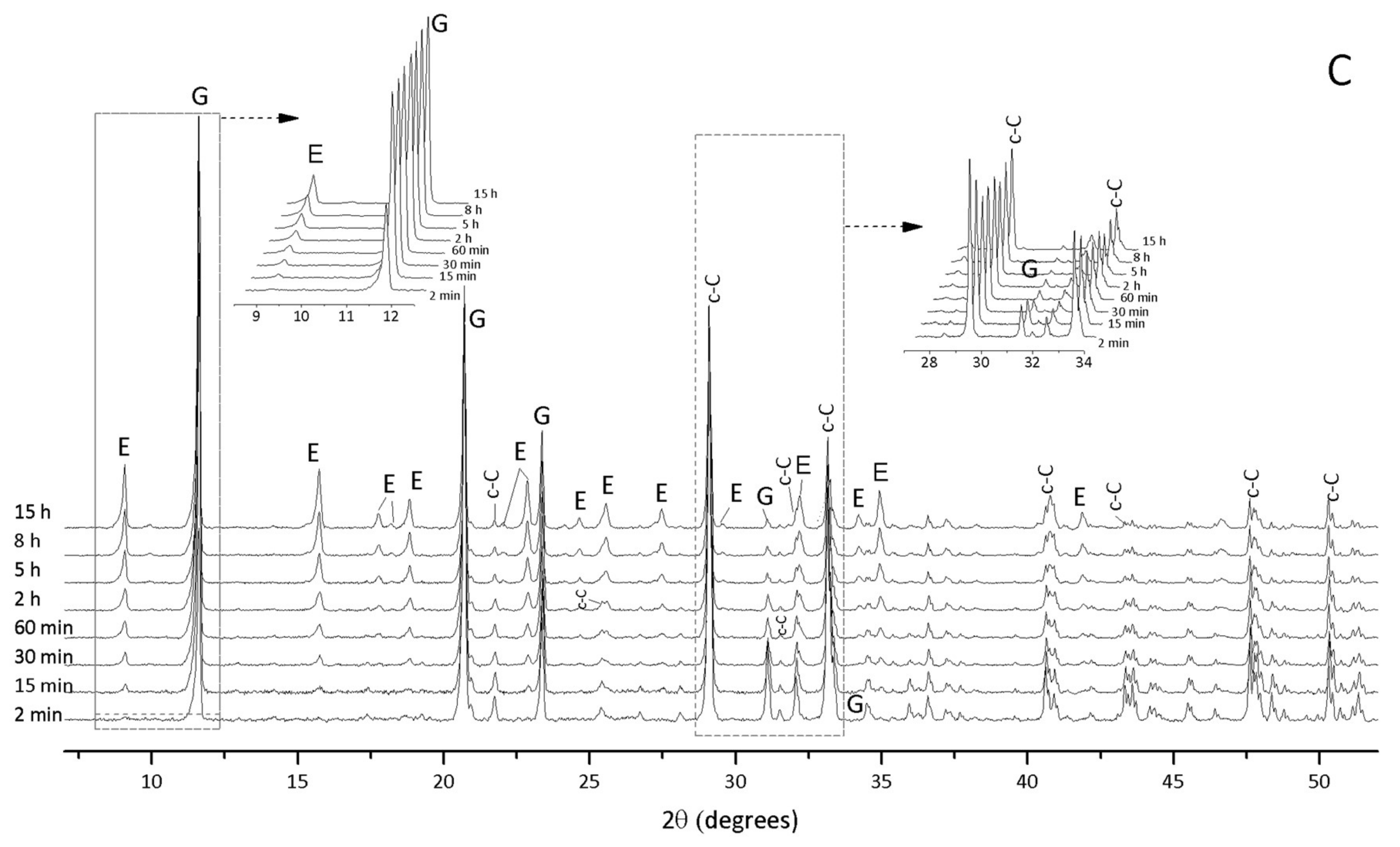
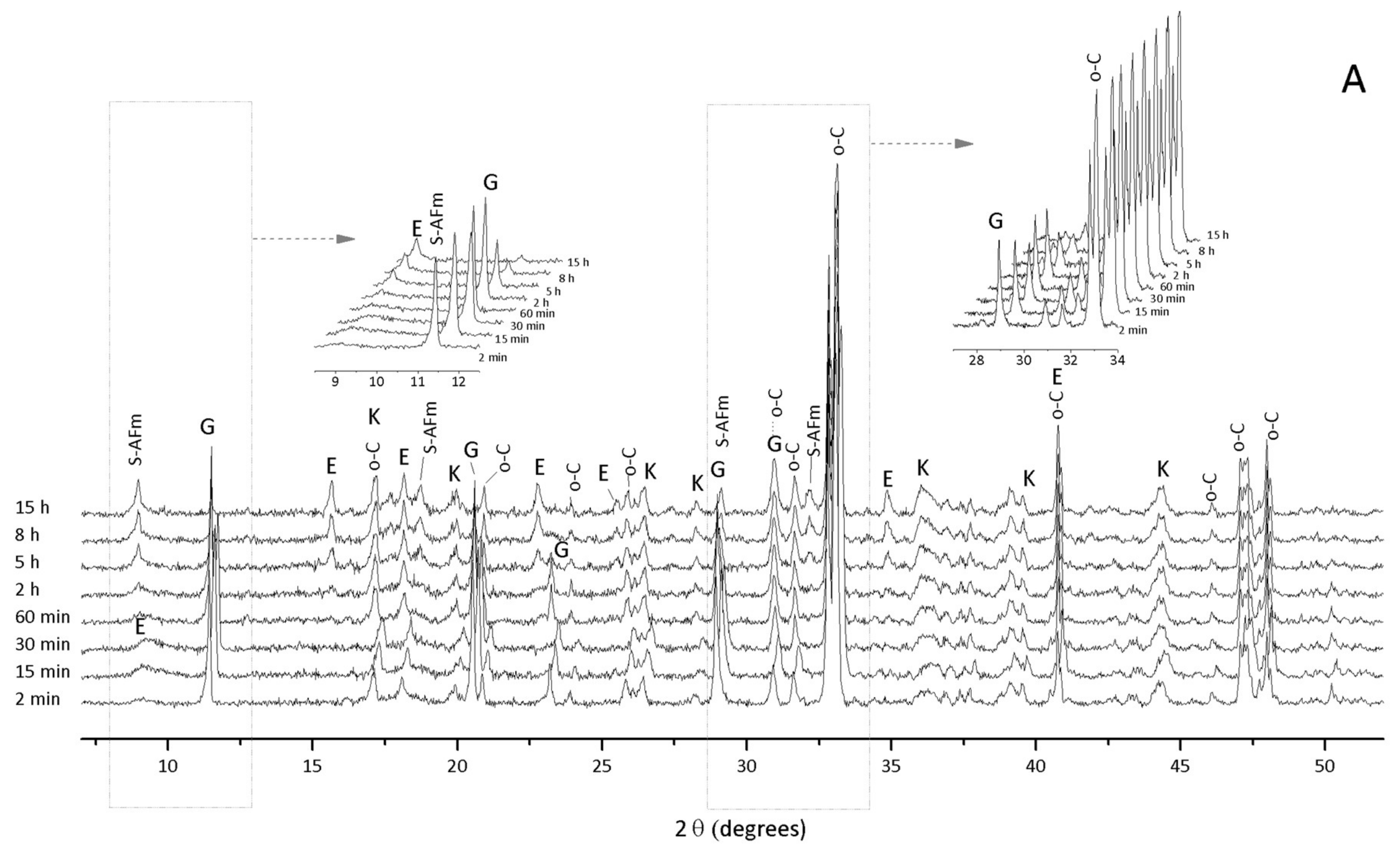
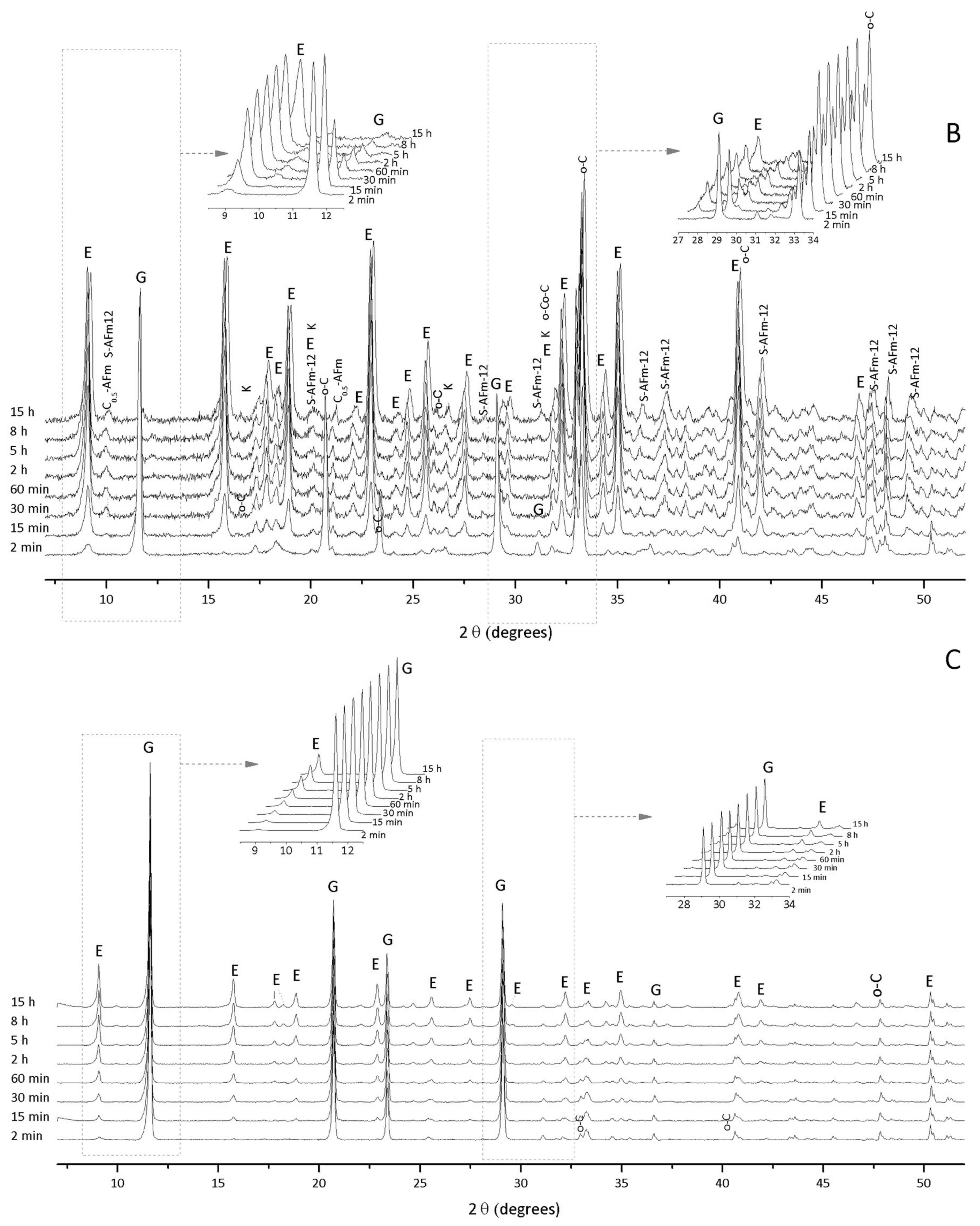
3.1. Hydration of C3A without Gypsum—In Situ XRD
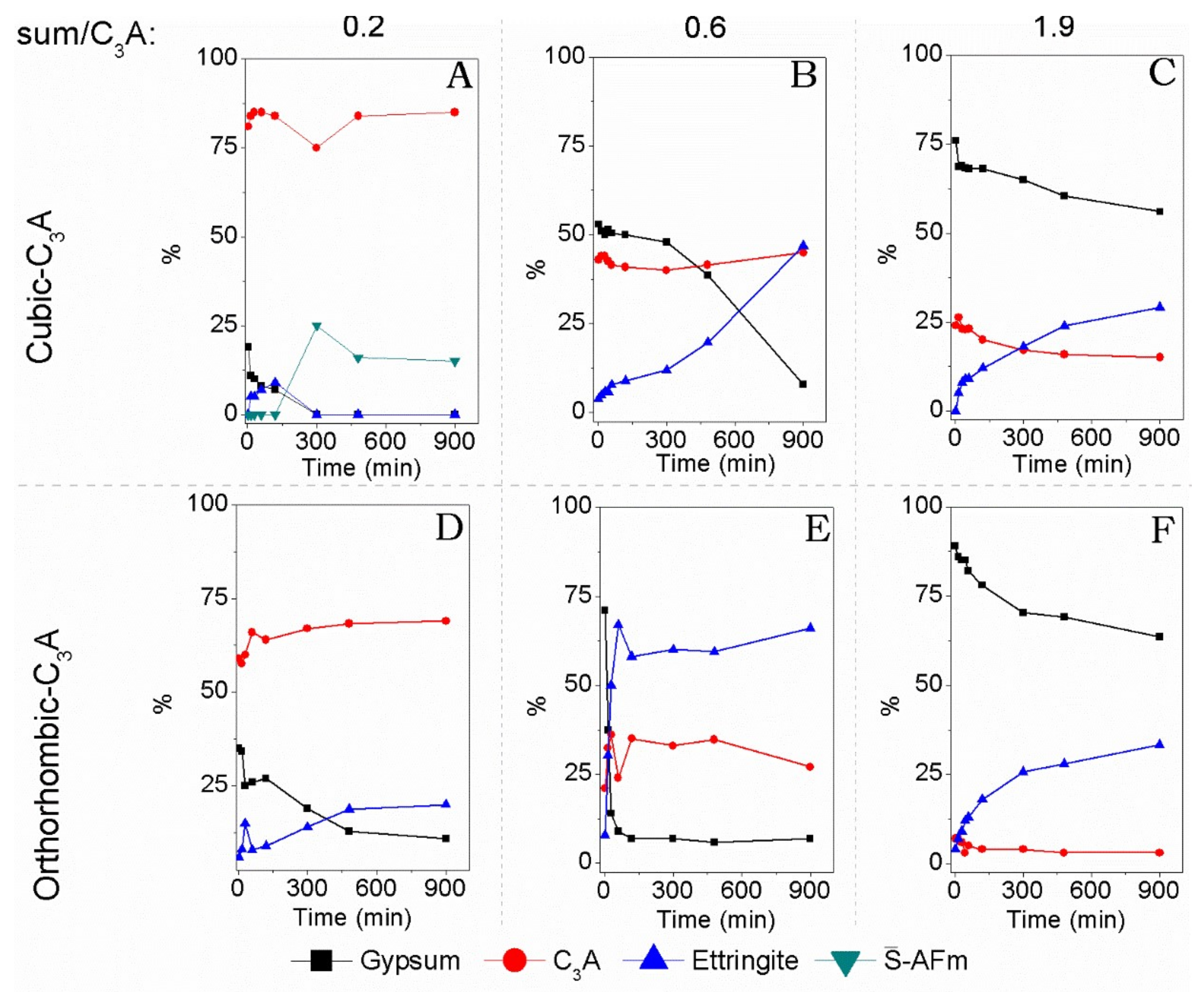
3.2. Hydration of C3A with Gypsum—In Situ XRD
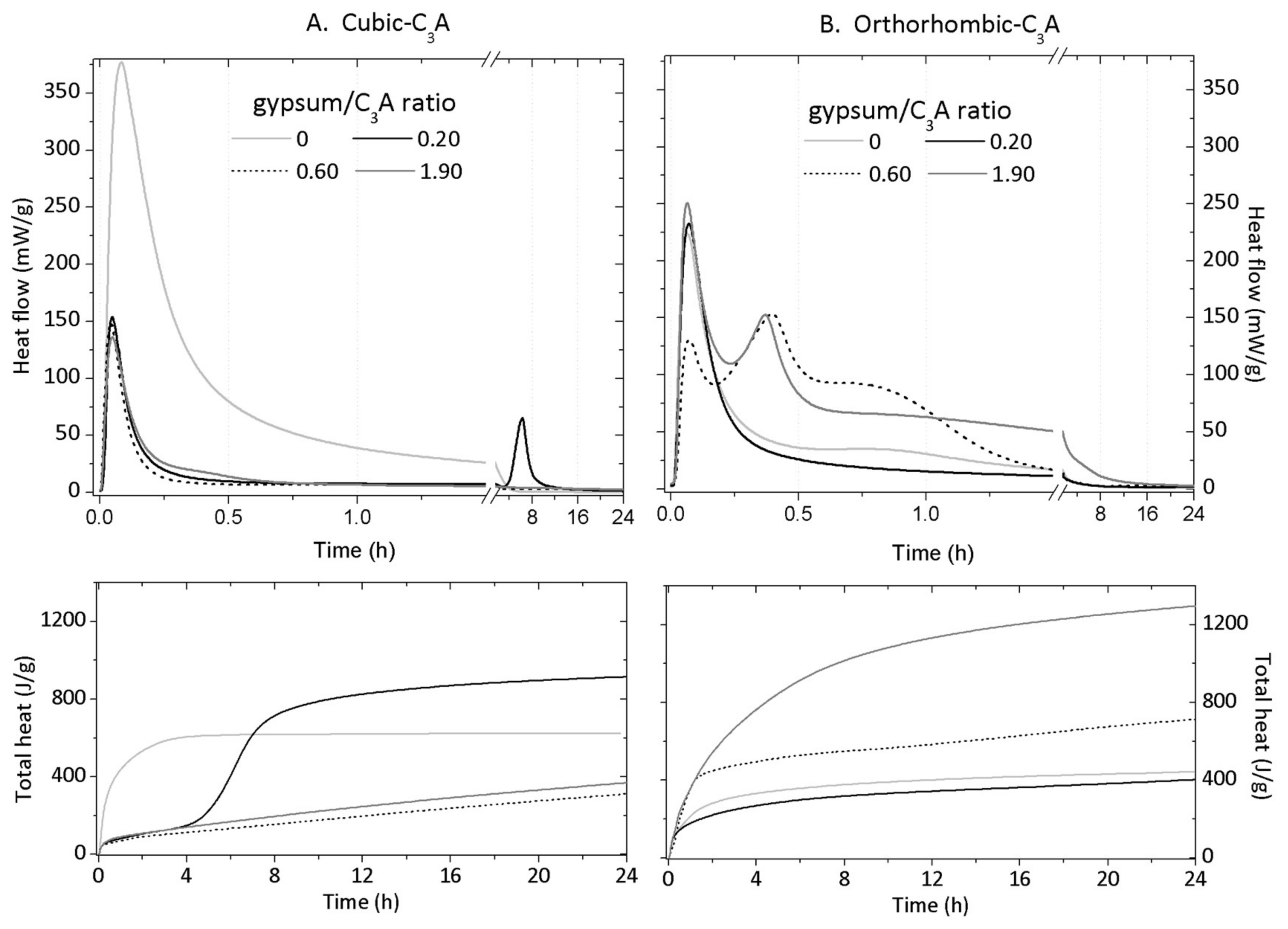
3.3. Isothermal Conduction Calorimetry
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Cement Chemistry Notation
- C: CaO
- S: SiO2
- Al: Al2O3
- H: H2O
- : CaCO3
- : SO3
- OH-AFm19: C4AH(7+x) (or OH-AFm(7+x))
- C0.5-AFm: Ca4Al2O6(CO3)0.5·11.5H2O
- -AFm: Ca4Al2O6(SO4)·14H2O
- -AFm: 3CaO·Al2O3·CaCO3·11H2O
Appendix B. Thermogravimetric Analysis (TGA)
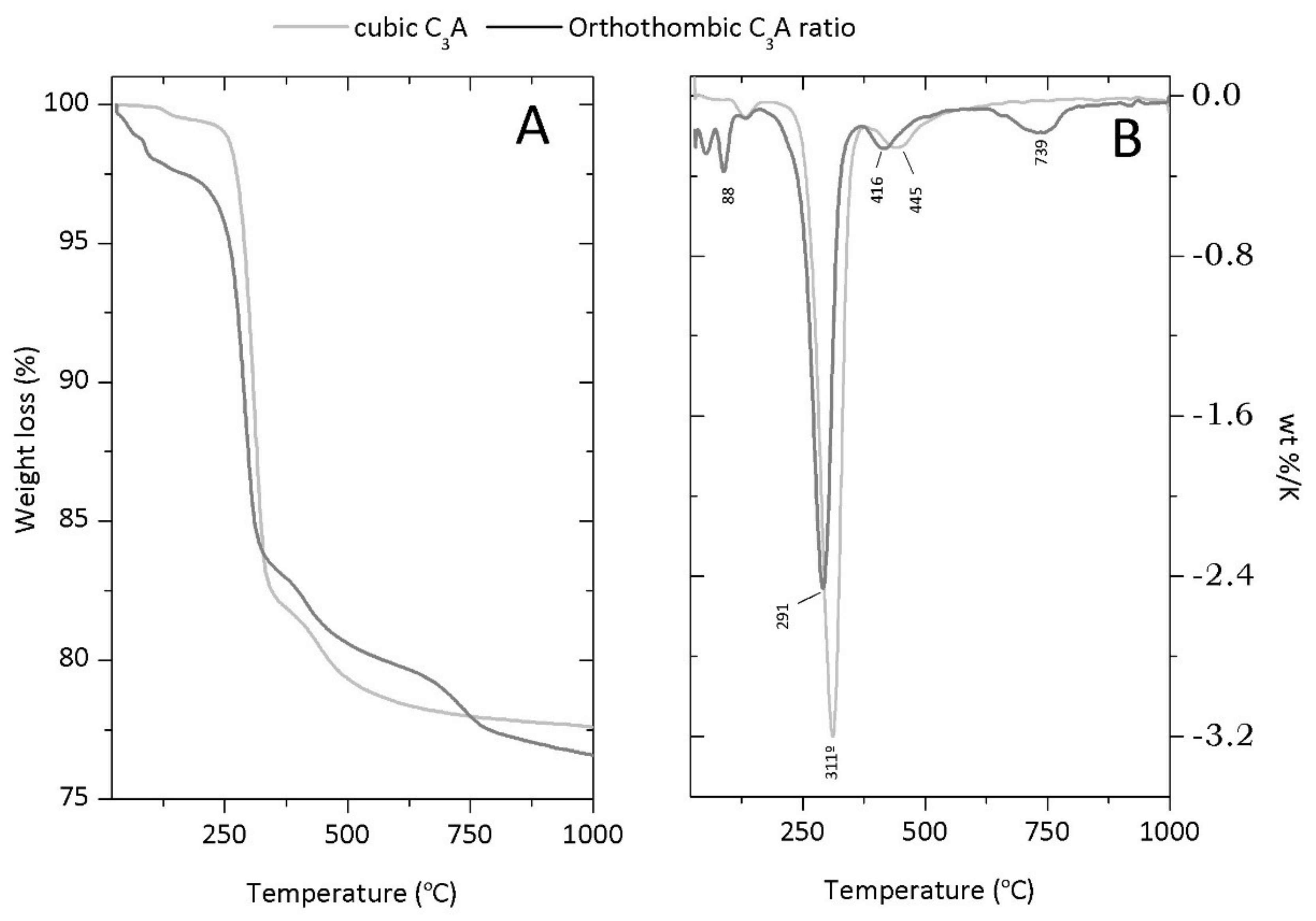
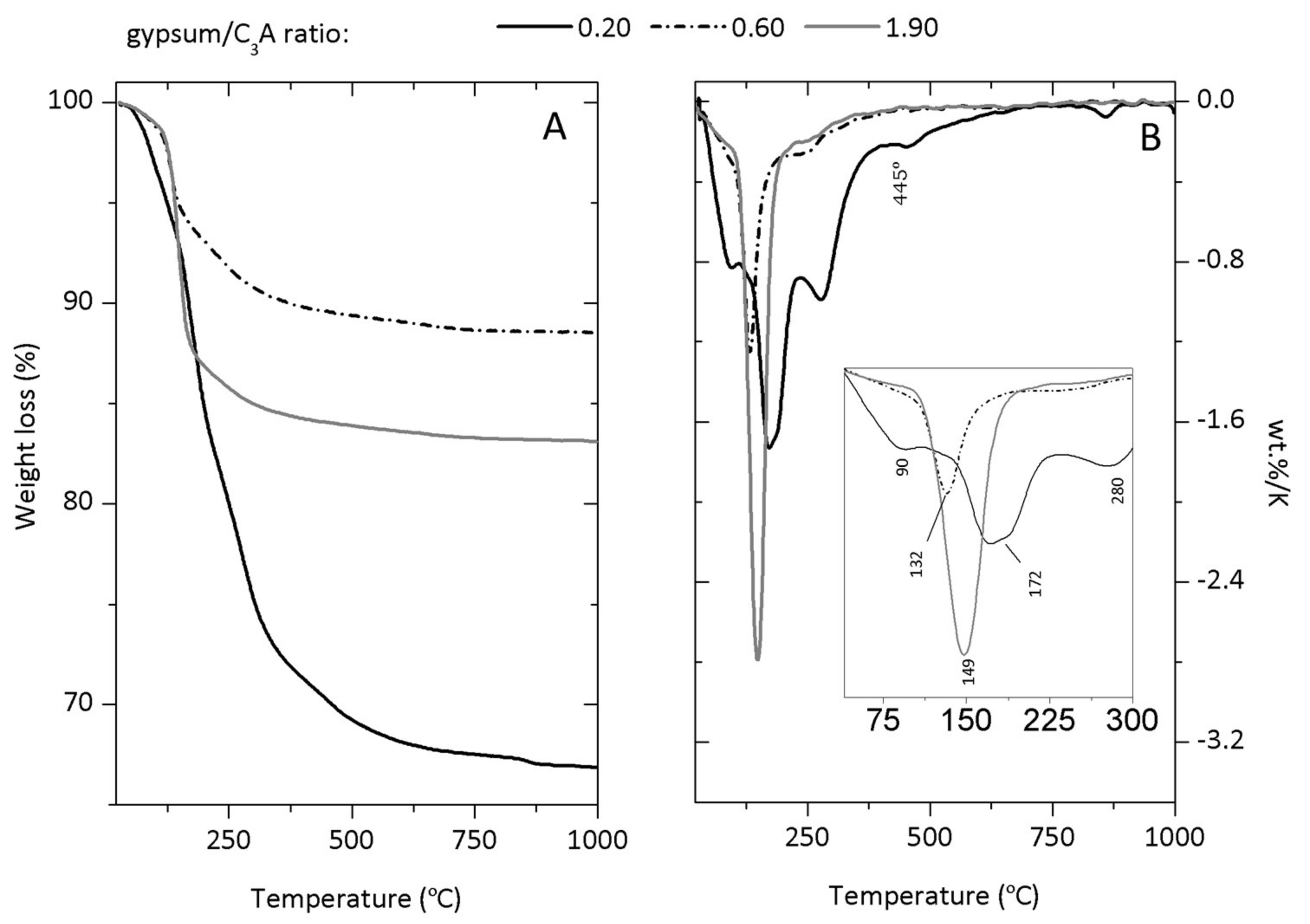

References
- Taylor, H.F.W. Cement Chemistry; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Scrivener, K.L.; Nonat, A. Hydration of cementitious materials, present and future. Cem. Concr. Res. 2011, 41, 651–665. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Mehta, K.; Monteiro, P.J.M. Concrete. Microstructure, Properties and Materials; Metha, P.K., Monteiro, P.J.M., Eds.; McGraw-Hill: New York, NY, USA, 2005; ISBN 9788578110796. [Google Scholar]
- Minard, H.; Garrault, S.; Regnaud, L.; Nonat, A. Mechanisms and parameters controlling the tricalcium aluminate reactivity in the presence of gypsum. Cem. Concr. Res. 2007, 37, 1418–1426. [Google Scholar] [CrossRef]
- Pourchet, S.; Regnaud, L.; Perez, J.P.; Nonat, A. Early C3A hydration in the presence of different kinds of calcium sulfate. Cem. Concr. Res. 2009, 39, 989–996. [Google Scholar] [CrossRef]
- Samet, B.; Sarkar, L. The influence of calcium sulfate form on the initial hydration of clinkers containing different Alkali combinations. Cem. Concr. Compos. 1997, 27, 369–380. [Google Scholar] [CrossRef]
- Quennoz, A.; Scrivener, K.L. Hydration of C3A–gypsum systems. Cem. Concr. Res. 2012, 42, 1032–1041. [Google Scholar] [CrossRef]
- Uchikawa, H.; Uchida, S.; Ogawa, K.; Hanehara, S. Influence of on the initial hydration of clinker having different burning degree. Cem. Concr. Res. 1984, 14, 645–656. [Google Scholar] [CrossRef]
- Black, L.; Breen, C.; Yarwood, J.; Deng, C.-S.; Phipps, J.; Maitland, G. Hydration of tricalcium aluminate (C3A) in the presence and absence of gypsum—Studied by Raman spectroscopy and X-ray diffraction. J. Mater. Chem. 2006, 16, 1263. [Google Scholar] [CrossRef]
- Stephan, D.; Wistuba, S. Crystal structure refinement and hydration behaviour of doped tricalcium aluminate. Cem. Concr. Res. 2006, 36, 2011–2020. [Google Scholar] [CrossRef]
- Merlini, M.; Artioli, G.; Cerulli, T.; Cella, F.; Bravo, A. Tricalcium aluminate hydration in additivated systems. A crystallographic study by SR-XRPD. Cem. Concr. Res. 2008, 38, 477–486. [Google Scholar] [CrossRef]
- Lagosz, A.; Malolepszy, J.; Garrault, S. Hydration of tricalcium aluminate in the presence of various amounts of calcium sulphite hemihydrate: Conductivity tests. Cem. Concr. Res. 2006, 36, 1016–1022. [Google Scholar] [CrossRef]
- Meredith, P.; Donald, A.M.; Meller, N.; Hall, C. Tricalcium aluminate hydration: Microstructural observations by in-situ electron microscopy. J. Mater. Sci. 2004, 39, 997–1005. [Google Scholar] [CrossRef]
- Pauri, M.; Collepardi, M. Combined effect of lignosulfonate and carbonate on pure Portland clinker compounds hydration. IV. Hydration of tricalcium aluminate-sodiu oxide solid solution. Cem. Concr. Res. 1983, 13, 61–68. [Google Scholar] [CrossRef]
- Collepardi, M.; Baldini, G.; Pauri, M.; Corradi, M. Tricalcium aluminate hydration in the presence of lime, gypsum or sodium sulfate. Cem. Concr. Res. 1978, 8, 571–580. [Google Scholar] [CrossRef]
- Pauri, M.; Baldini, G.; Collepardi, M. Combined effect of lignosulfonate and carbonate on pure Portland clinker compounds hydration. II. Tricalcium aluminate hydration. Cem. Concr. Res. 1982, 12, 271–277. [Google Scholar] [CrossRef]
- Pourchez, J.; Grosseau, P.; Ruot, B. Current understanding of cellulose ethers impact on the hydration of C3A and C3A-sulphate systems. Cem. Concr. Res. 2009, 39, 664–669. [Google Scholar] [CrossRef]
- Dalas, F.; Pourchet, S.; Rinaldi, D.; Nonat, A.; Sabio, S.; Mosquet, M. Modification of the rate of formation and surface area of ettringite by polycarboxylate ether superplasticizers during early C3A–CaSO4 hydration. Cem. Concr. Res. 2015, 69, 105–113. [Google Scholar] [CrossRef]
- Brown, P.W.; Liberman, L.O.; Frohnsdorff, G. Kinetics of the Early Hydration of Tricalcium Aluminate in Solutions Containing Calcium Sulfate. J. Am. Ceram. Soc. 1984, 67, 793–795. [Google Scholar] [CrossRef]
- Spierging, G.A.C.M.; Stein, H.N. The influence of Na2O on the hydration of C3A. II. Suspension hydration. Cem. Concr. Res. 1976, 6, 487–496. [Google Scholar]
- Regourd, M.; Guinier, A. Cristallochimie des constituants du clinker de ciment Portland. Rev. Des. Mater. Constr. 1975, 201–221. [Google Scholar]
- Manzano, H.; Dolado, J.S.; Ayuela, A. Structural, Mechanical, and Reactivity Properties of Tricalcium Aluminate Using First-Principles Calculations. J. Am. Ceram. Soc. 2009, 92, 897–902. [Google Scholar] [CrossRef]
- Fukuda, K.; Inoue, S.; Yoshida, H. Cationic substitution in tricalcium aluminate. Cem. Concr. Res. 2003, 33, 1771–1775. [Google Scholar] [CrossRef]
- Shin, G.Y.; Glasser, F.P. Interdependence of sodium and potassium substitution in tricalcium aluminate. Cem. Concr. Res. 1983, 13, 135–140. [Google Scholar] [CrossRef]
- Goetz-neunhoeffer, F.; Neubauer, J. Crystal Structure refinement of Na-substituted C3A by Rietveld analysis and quantification in OPC. In Proceedings of the 10th International Congress on the Chemistry of Cement, Gothenburg, Sweden, 2–6 June 1997; Volume 1. [Google Scholar]
- Gobbo, L.; Sant’Agostino, L.; Garcez, L. C3A polymorphs related to industrial clinker alkalies content. Cem. Concr. Res. 2004, 34, 657–664. [Google Scholar] [CrossRef]
- Kirchheim, A.P.; Dal Molin, D.C.; Fischer, P.; Emwas, A.-H.; Provis, J.L.; Monteiro, P.J.M. Real-time high-resolution X-ray imaging and nuclear magnetic resonance study of the hydration of pure and Na-doped C3A in the presence of sulfates. Inorg. Chem. 2011, 50, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Glasser, F.P.; Marinho, M.B. Early stages of the hydration of tricalcium aluminate and its sodium-containing solid solution. Proc. Br. Ceram. Soc. 1984, 35, 221–236. [Google Scholar]
- Kirchheim, A.P.; Fernàndez-Altable, V.; Monteiro, P.J.M.; Dal Molin, D.C.C.; Casanova, I. Analysis of cubic and orthorhombic C3A hydration in presence of gypsum and lime. J. Mater. Sci. 2009, 44, 2038–2045. [Google Scholar] [CrossRef]
- Stephan, D.; Wistuba, S. Crystal structure refinement and hydration behaviour of 3CaO·SiO2 solid solutions with MgO, Al2O3 and Fe2O3. J. Eur. Ceram. Soc. 2006, 26, 141–148. [Google Scholar] [CrossRef]
- Alonso, M.M.; Puertas, F. Adsorption of PCE and PNS superplasticisers on cubic and orthorhombic C3A. Effect of sulfate. Constr. Build. Mater. 2015, 78, 324–332. [Google Scholar] [CrossRef]
- Rheinheimer, V.; Chae, S.; Geng, G.; Monteiro, P.; Kirchheim, A.; Rodríguez, E. A Scanning Transmission X-ray Microscopy Study of Cubic and Orthorhombic C3A and Their Hydration Products in the Presence of Gypsum. Materials 2016, 9, 745. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.J.; Geng, G.; Rodriguez, E.D.; da Rosa, P.; Kirchheim, A.P.; Monteiro, P.J.M. Solution chemistry of cubic and orthorhombic tricalcium aluminate hydration. Cem. Concr. Res. 2017, 100, 176–185. [Google Scholar] [CrossRef]
- Regourd, M.; Hornain, H. Hydratation de C3A dans des mélanges synthétiques et dans des ciments portland industriels. In Proceedings of the 7th International Congress on the Chemistry of Cement (ICCC), Paris, France, 30 June–5 July 1980; pp. 477–482. [Google Scholar]
- Chipera, S.J.; Bish, D.L. Fitting Full X-Ray Diffraction Patterns for Quantitative Analysis: A Method for Readily Quantifying Crystalline and Disordered Phases. Adv. Mater. Phys. Chem. 2013, 3, 47–53. [Google Scholar] [CrossRef]
- Dubina, E.; Plank, J.; Black, L. Impact of water vapour and carbon dioxide on surface composition of C3A polymorphs studied by X-ray photoelectron spectroscopy. Cem. Concr. Res. 2015, 73, 36–41. [Google Scholar] [CrossRef]
- Christensen, A.N.; Jensen, T.R.; Scarlett, N.V.Y.; Madsen, I.C.; Hanson, J.C. Hydrolysis of Pure and Sodium Substituted Calcium Aluminates and Cement Clinker Components Investigated by in Situ Synchrotron X-ray Powder Diffraction. J. Am. Ceram. Soc. 2004, 87, 1488–1493. [Google Scholar] [CrossRef]
- Myers, R.J.; Geng, G.; Li, J.; Rodríguez, E.D.; Ha, J.; Kidkhunthod, P.; Sposito, G.; Lammers, L.N.; Kirchheim, A.P.; Monteiro, P.J.M. Role of Adsorption Phenomena in Cubic Tricalcium Aluminate Dissolution. Langmuir 2016. [Google Scholar] [CrossRef] [PubMed]
- Boikova, A.I.; Domansky, A.I.; Paramonova, V.A.; Stavitskaja, G.P.; Nikushchenko, V.M. The influence of Na2O on the structure and properties of 3CaO·Al2O3. Cem. Concr. Res. 1977, 7, 483–492. [Google Scholar] [CrossRef]
- Ramachandran, V.S.; Beaudoin, J.J. Handbook of Analytical Techniques in Concrete Science and Technology, 1st ed.; William Andrew: New York, NY, USA, 2000; ISBN 0815514379. [Google Scholar]
- Radwan, M.M.; Heikal, M. Hydration characteristics of tricalcium aluminate phase in mixes containing β-hemihydate and phosphogypsum. Cem. Concr. Res. 2005, 35, 1601–1608. [Google Scholar] [CrossRef]
- Lothenbach, B.; Durdziński, P.; De Weerdt, K. Chapter 5: Thermogravimetric analysis. In A Practical Guide to Microstructural Analysis of Cementitious Materials; Scrivener, K., Snellings, R., Lothenbach, B., Eds.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2015; pp. 178–208. ISBN 978-1-4987-3867-5. [Google Scholar]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; RC Press: Boca Raton, FL, USA, 2016; ISBN 9781498738675. [Google Scholar]
- Möschner, G.; Lothenbach, B.; Winnefeld, F.; Ulrich, A.; Figi, R.; Kretzschmar, R. Solid solution between Al-ettringite and Fe-ettringite (Ca6[Al1−xFex(OH)6]2(SO4)3·26H2O). Cem. Concr. Res. 2009, 39, 482–489. [Google Scholar] [CrossRef]
- Borrachero, M.V.; Payá, J.; Bonilla, M.; Monzó, J. The use of thermogravimetric analysis technique for the characterization of construction materials. J. Therm. Anal. Calorim. 2008, 91, 503–509. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirchheim, A.P.; Rodríguez, E.D.; Myers, R.J.; A. Gobbo, L.; M. Monteiro, P.J.; C. C. Dal Molin, D.; De Souza, R.B.; Cincotto, M.A. Effect of Gypsum on the Early Hydration of Cubic and Na-Doped Orthorhombic Tricalcium Aluminate. Materials 2018, 11, 568. https://doi.org/10.3390/ma11040568
Kirchheim AP, Rodríguez ED, Myers RJ, A. Gobbo L, M. Monteiro PJ, C. C. Dal Molin D, De Souza RB, Cincotto MA. Effect of Gypsum on the Early Hydration of Cubic and Na-Doped Orthorhombic Tricalcium Aluminate. Materials. 2018; 11(4):568. https://doi.org/10.3390/ma11040568
Chicago/Turabian StyleKirchheim, Ana Paula, Erich D. Rodríguez, Rupert J. Myers, Luciano A. Gobbo, Paulo J. M. Monteiro, Denise C. C. Dal Molin, Rui B. De Souza, and Maria Alba Cincotto. 2018. "Effect of Gypsum on the Early Hydration of Cubic and Na-Doped Orthorhombic Tricalcium Aluminate" Materials 11, no. 4: 568. https://doi.org/10.3390/ma11040568
APA StyleKirchheim, A. P., Rodríguez, E. D., Myers, R. J., A. Gobbo, L., M. Monteiro, P. J., C. C. Dal Molin, D., De Souza, R. B., & Cincotto, M. A. (2018). Effect of Gypsum on the Early Hydration of Cubic and Na-Doped Orthorhombic Tricalcium Aluminate. Materials, 11(4), 568. https://doi.org/10.3390/ma11040568




