Effect of the Microstructure and Distribution of the Second Phase on the Stress Corrosion Cracking of Biomedical Mg-Zn-Zr-xSr Alloys
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Specimens
2.2. Degradation Behavior
2.3. Slow Strain Rate Tensile Test
3. Results
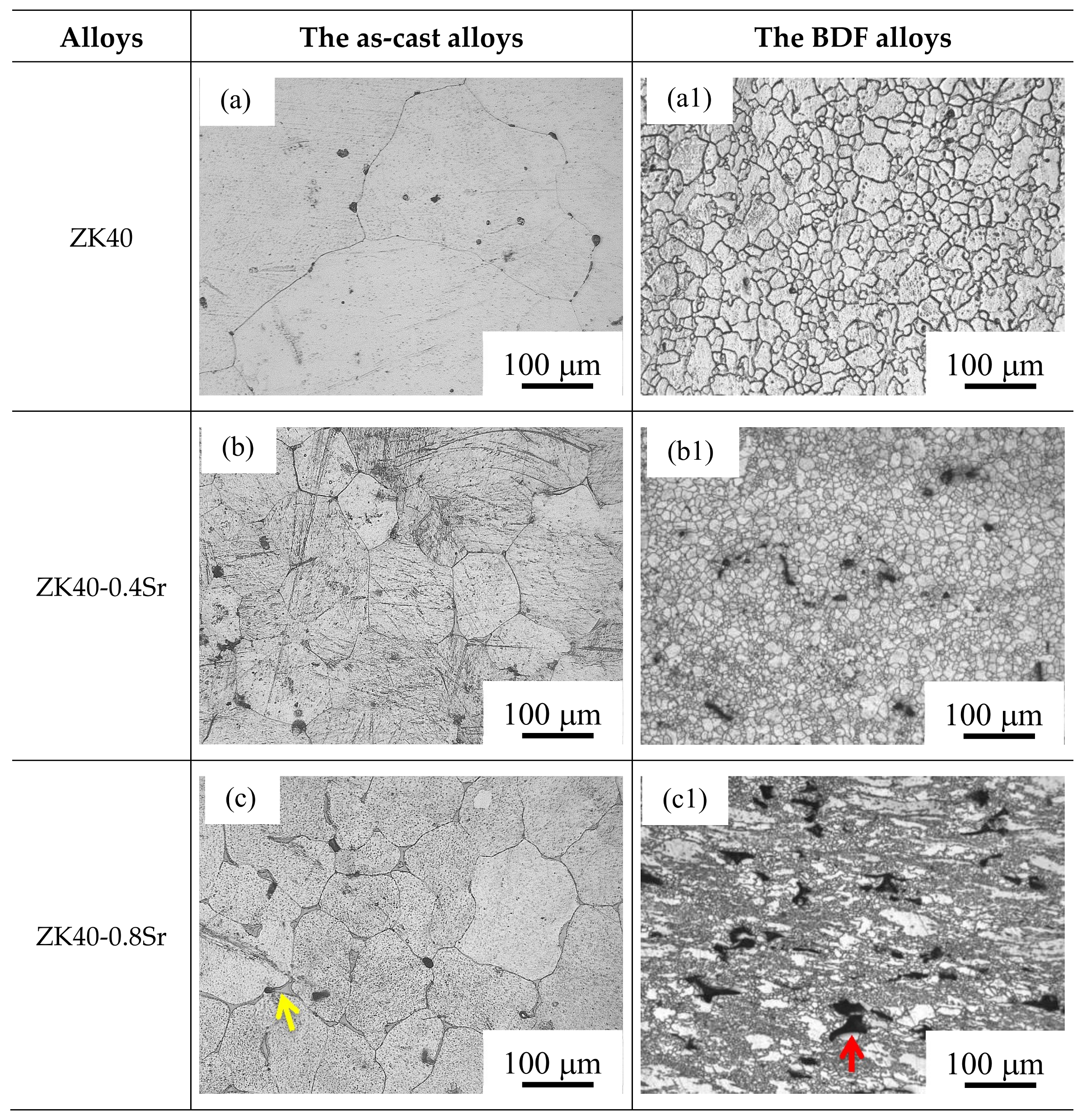
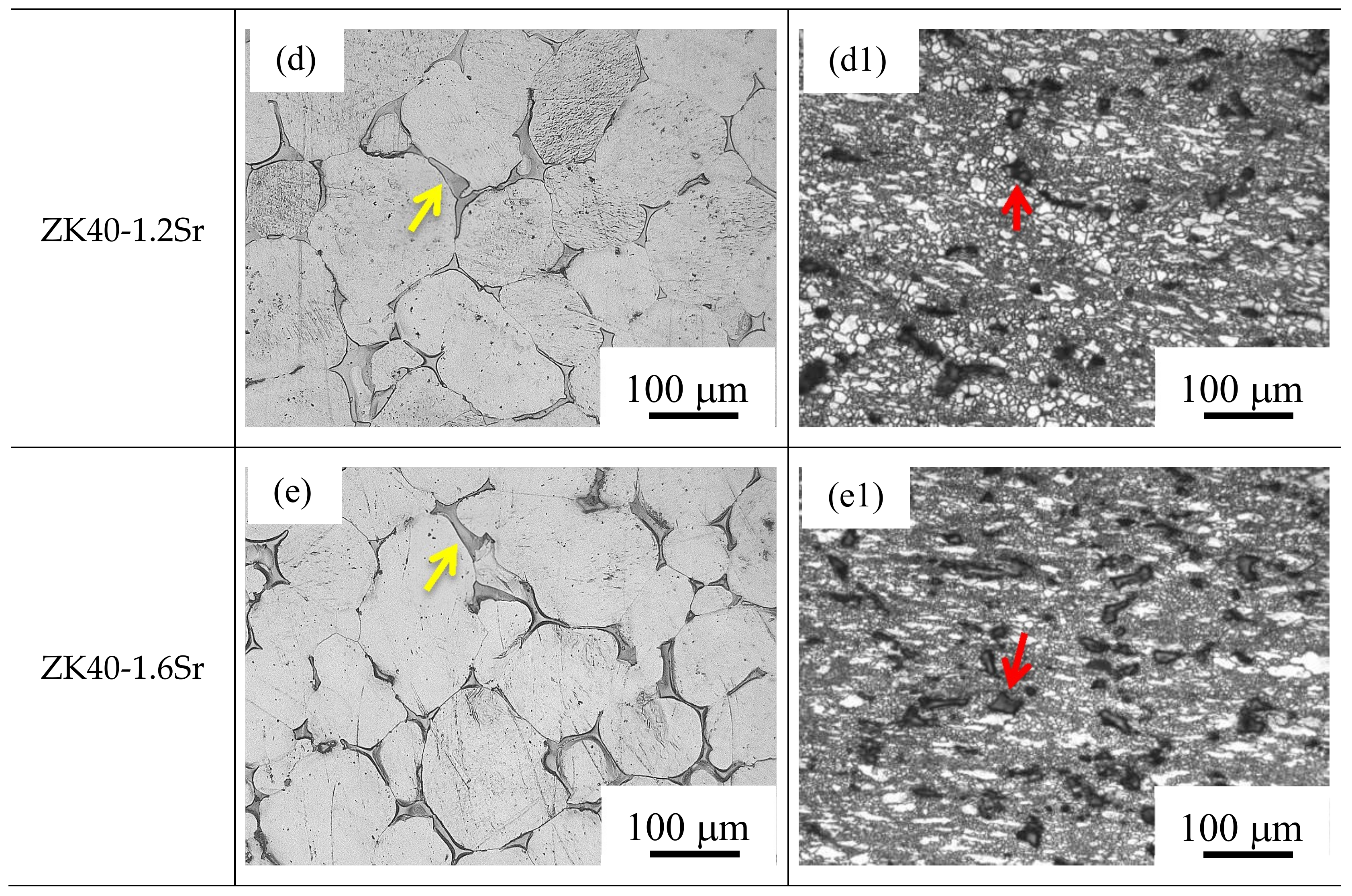
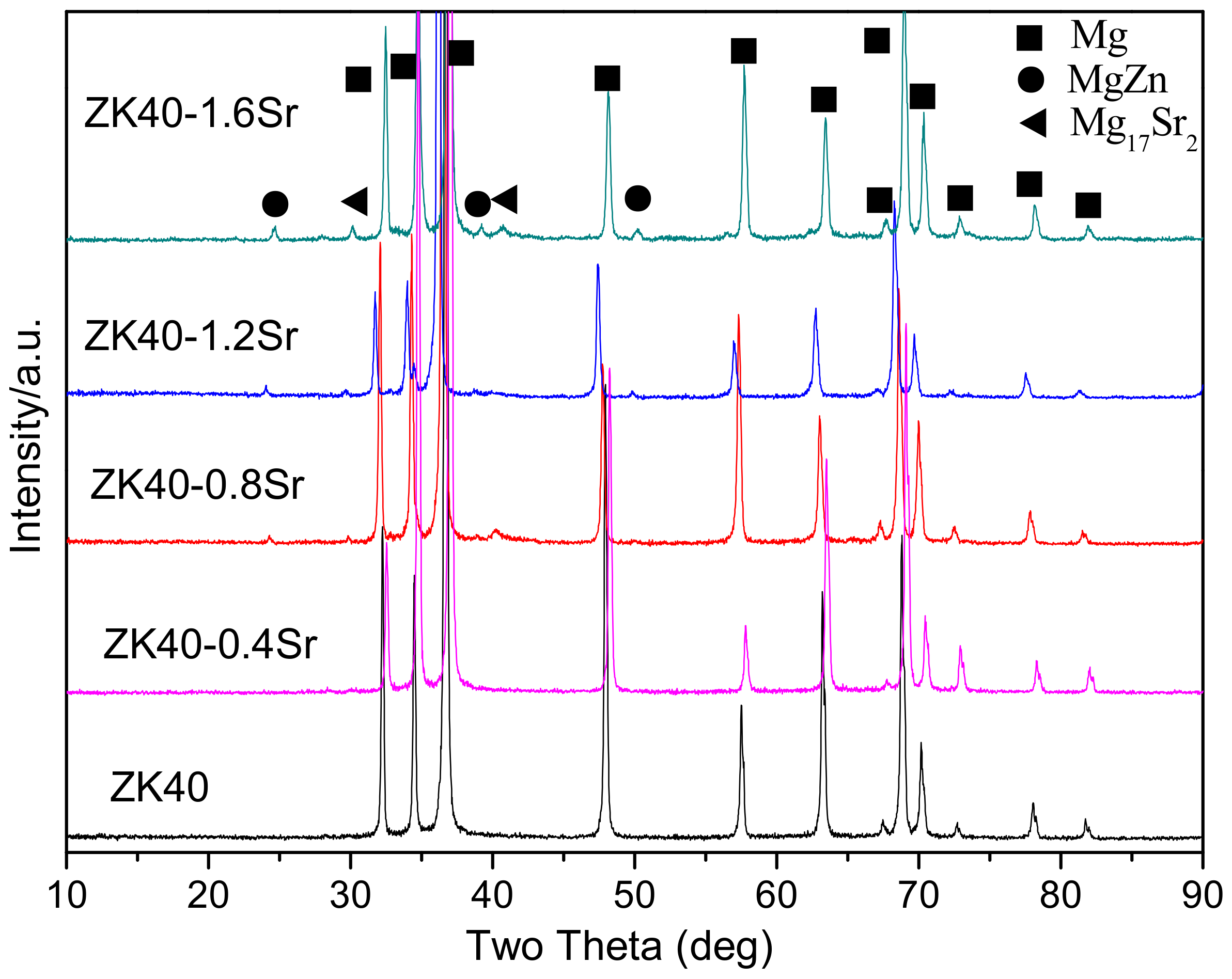
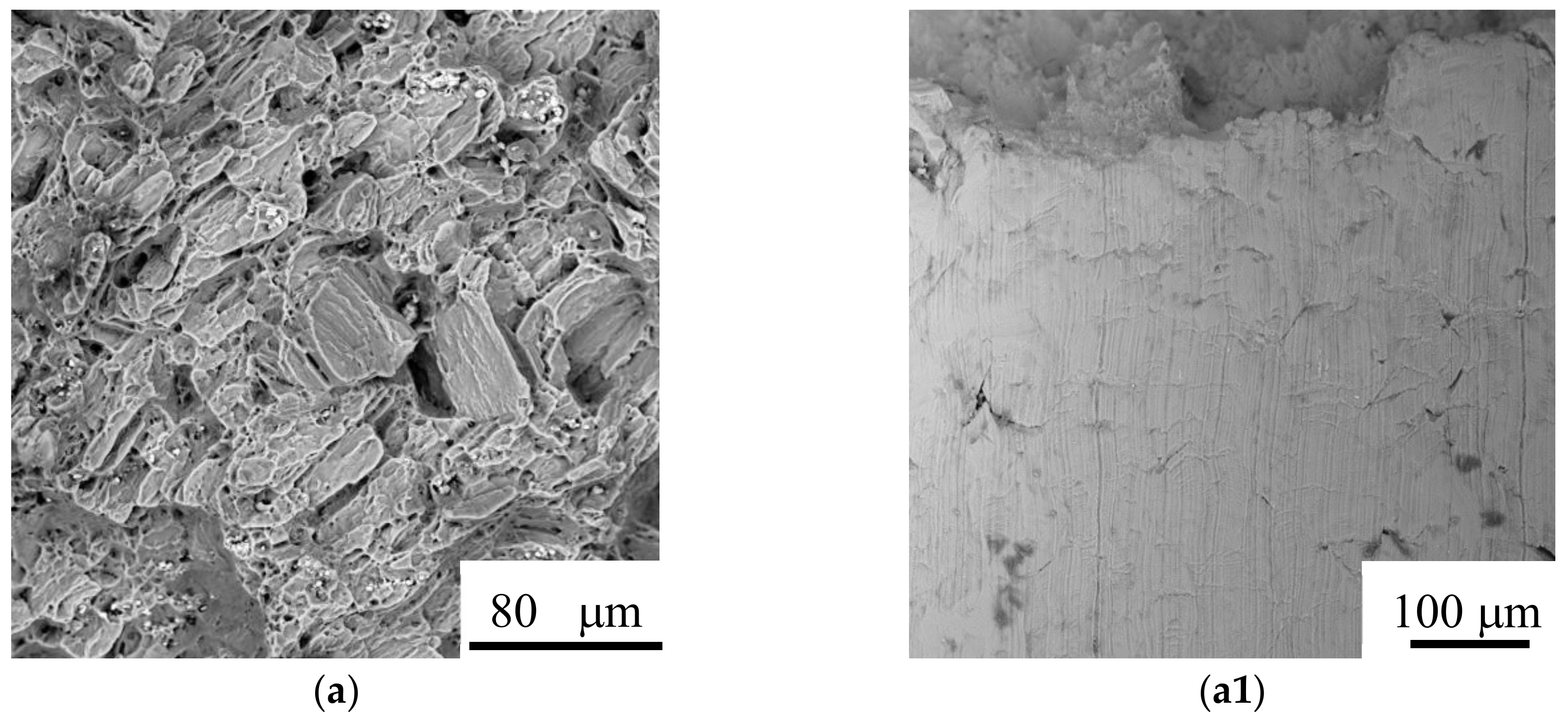
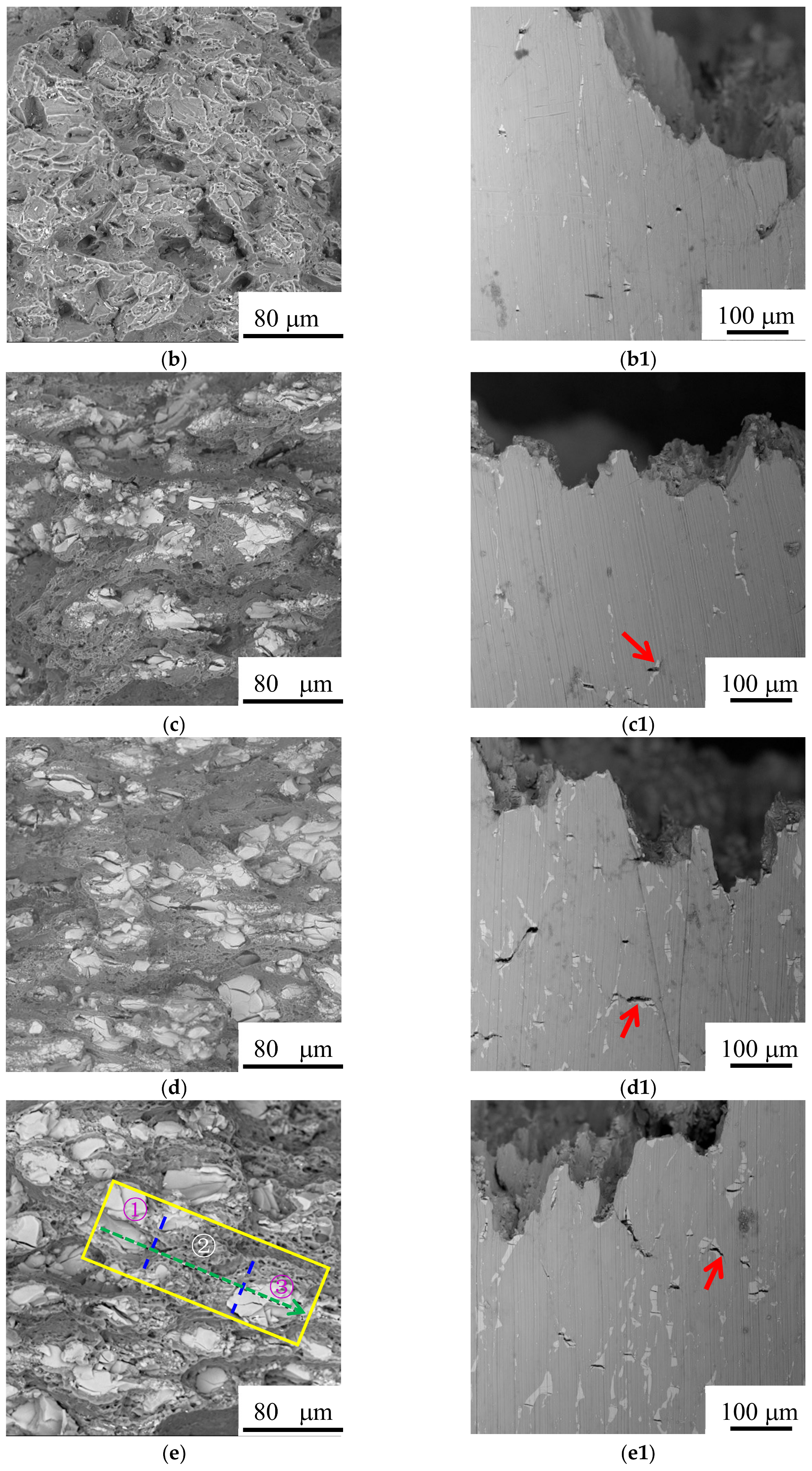
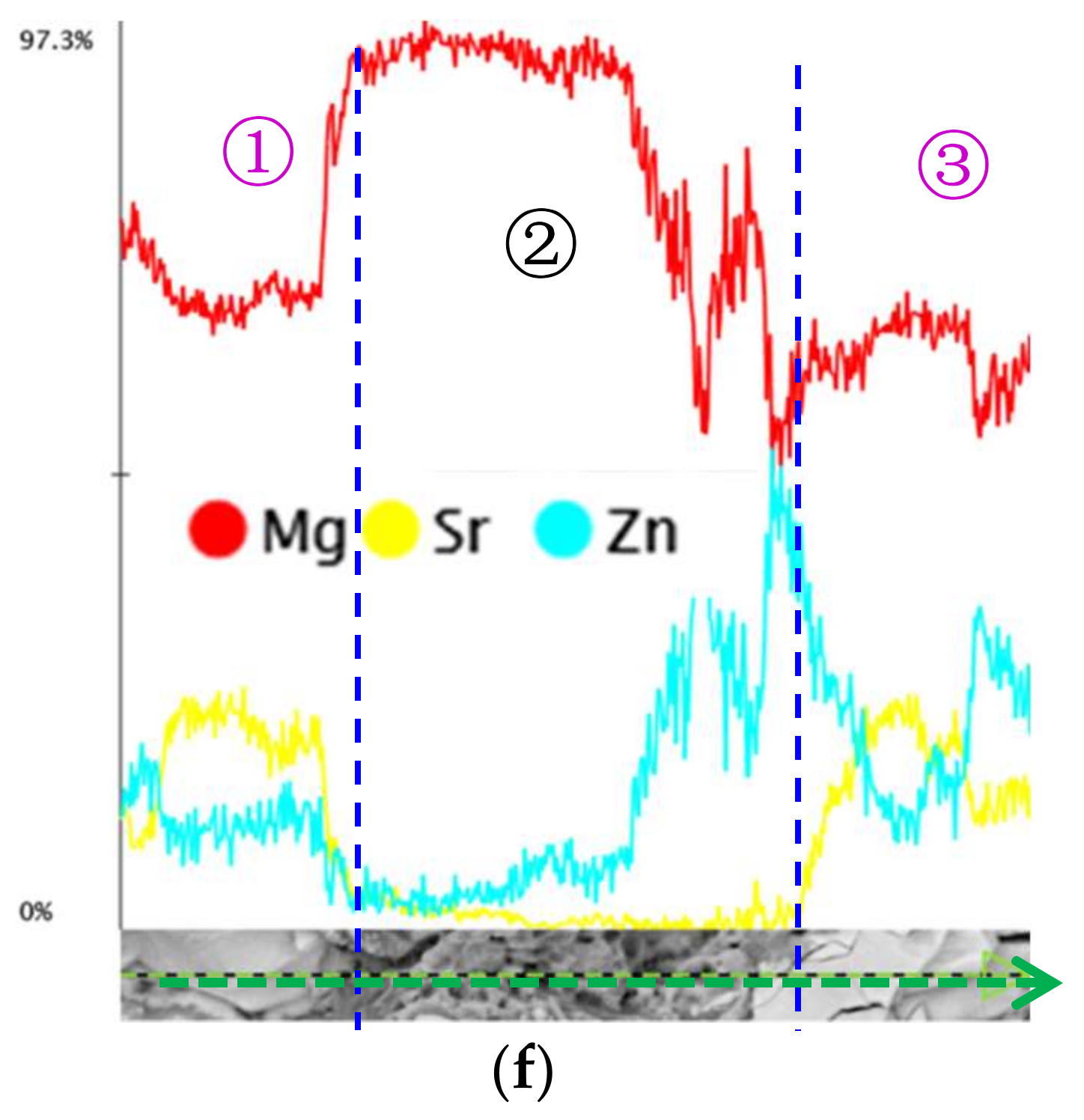
3.1. Microstructure and Compositions
3.2. Biodegradation Behavior
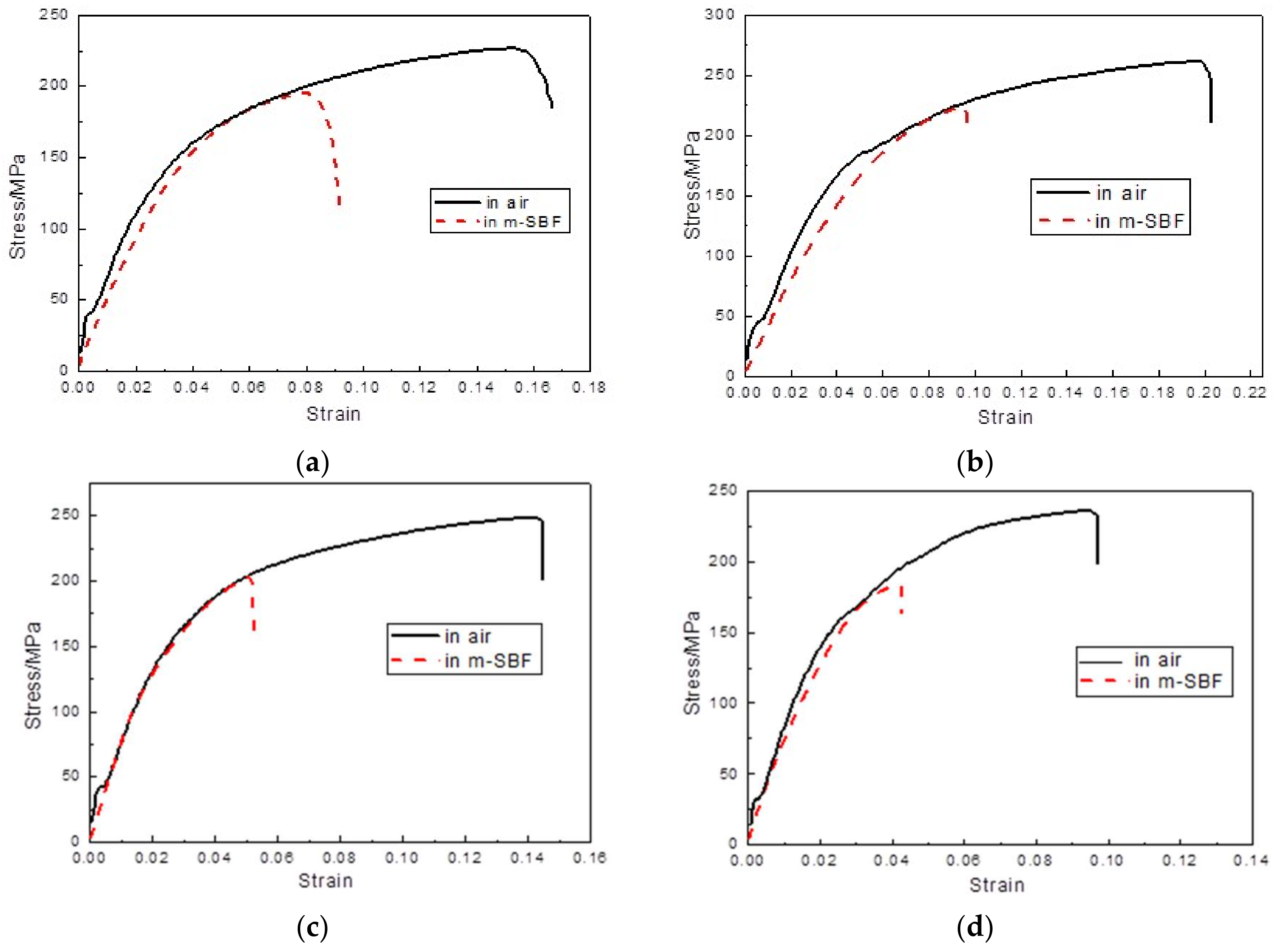
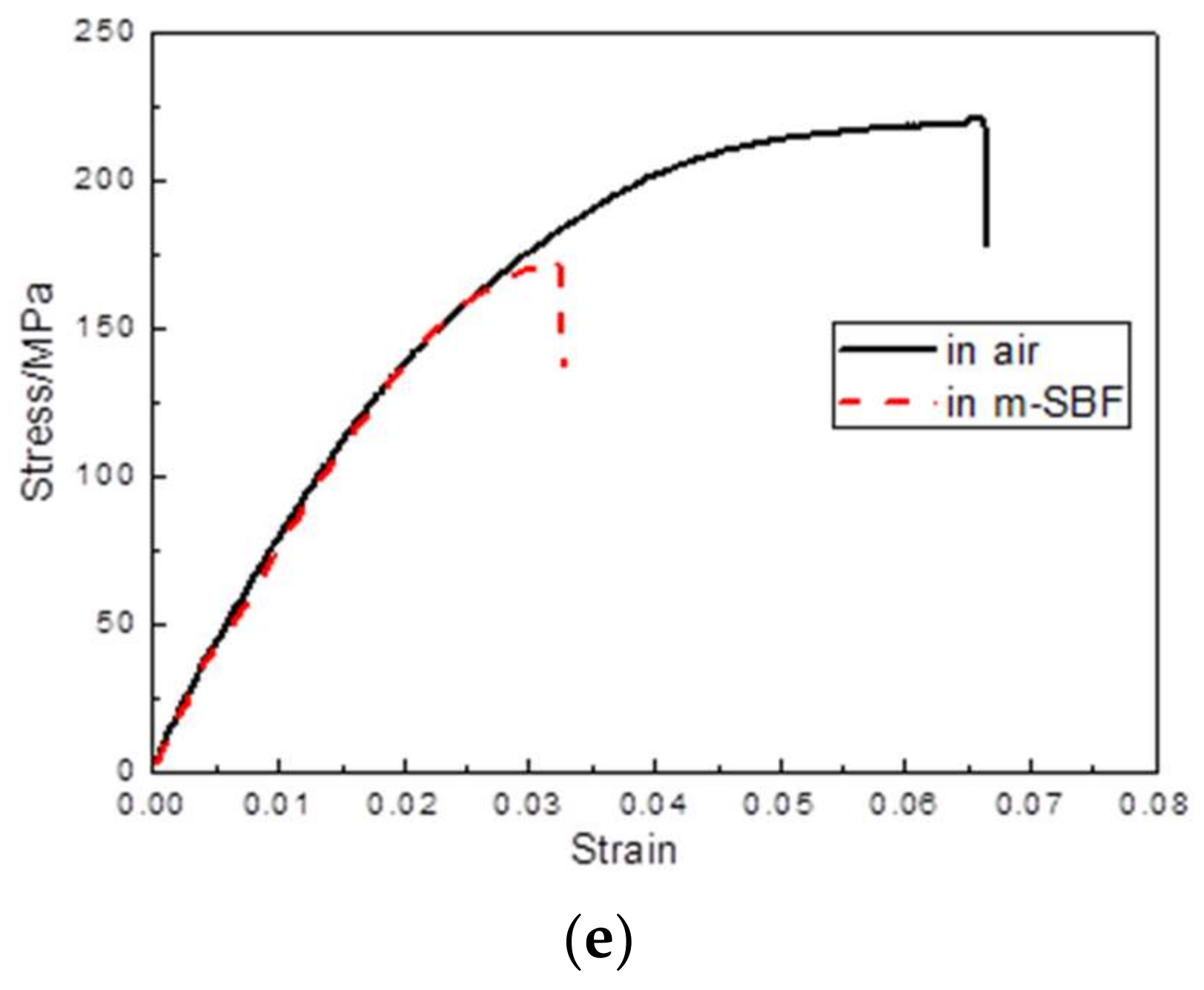
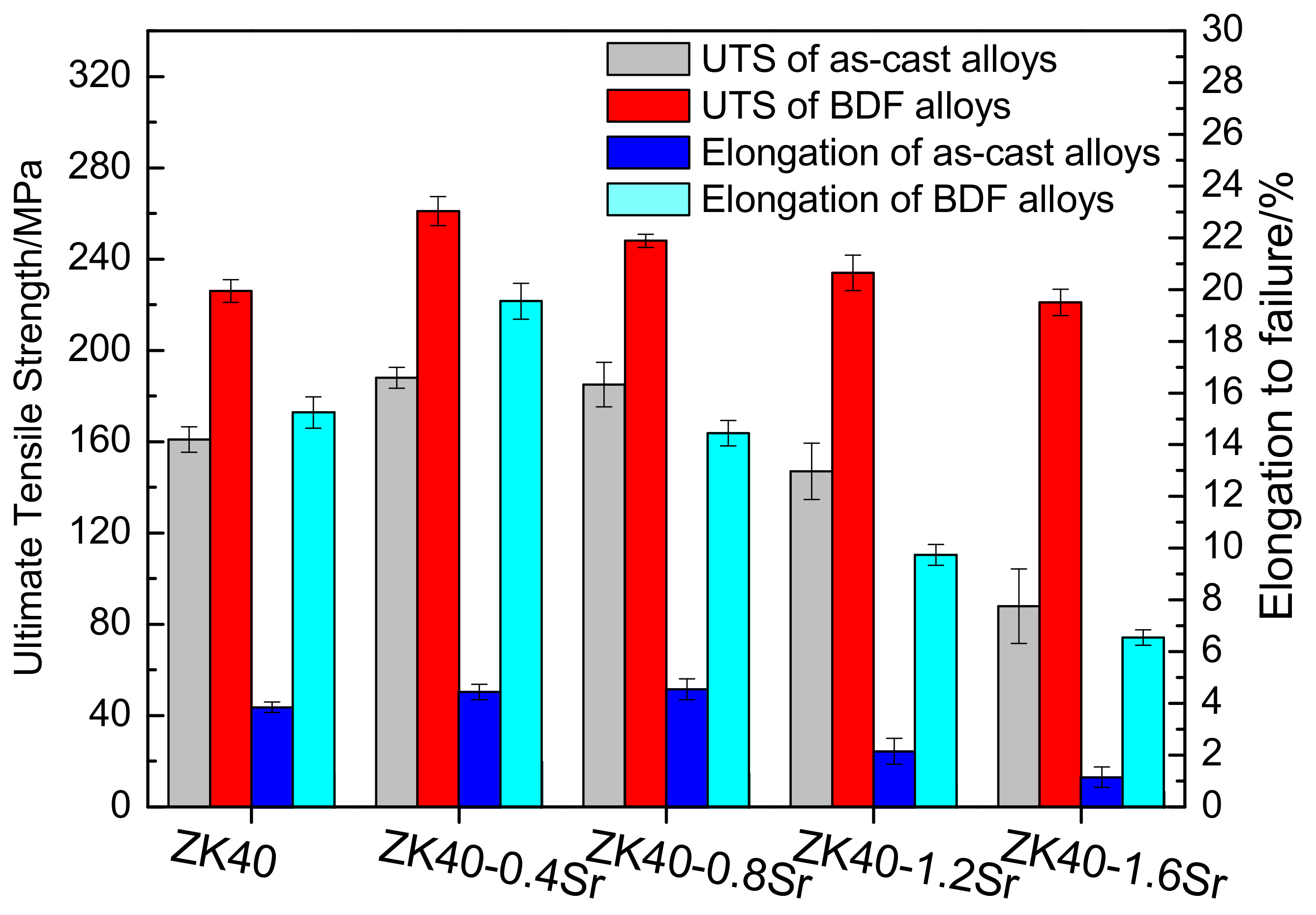
3.3. Slow Strain Rate Testing
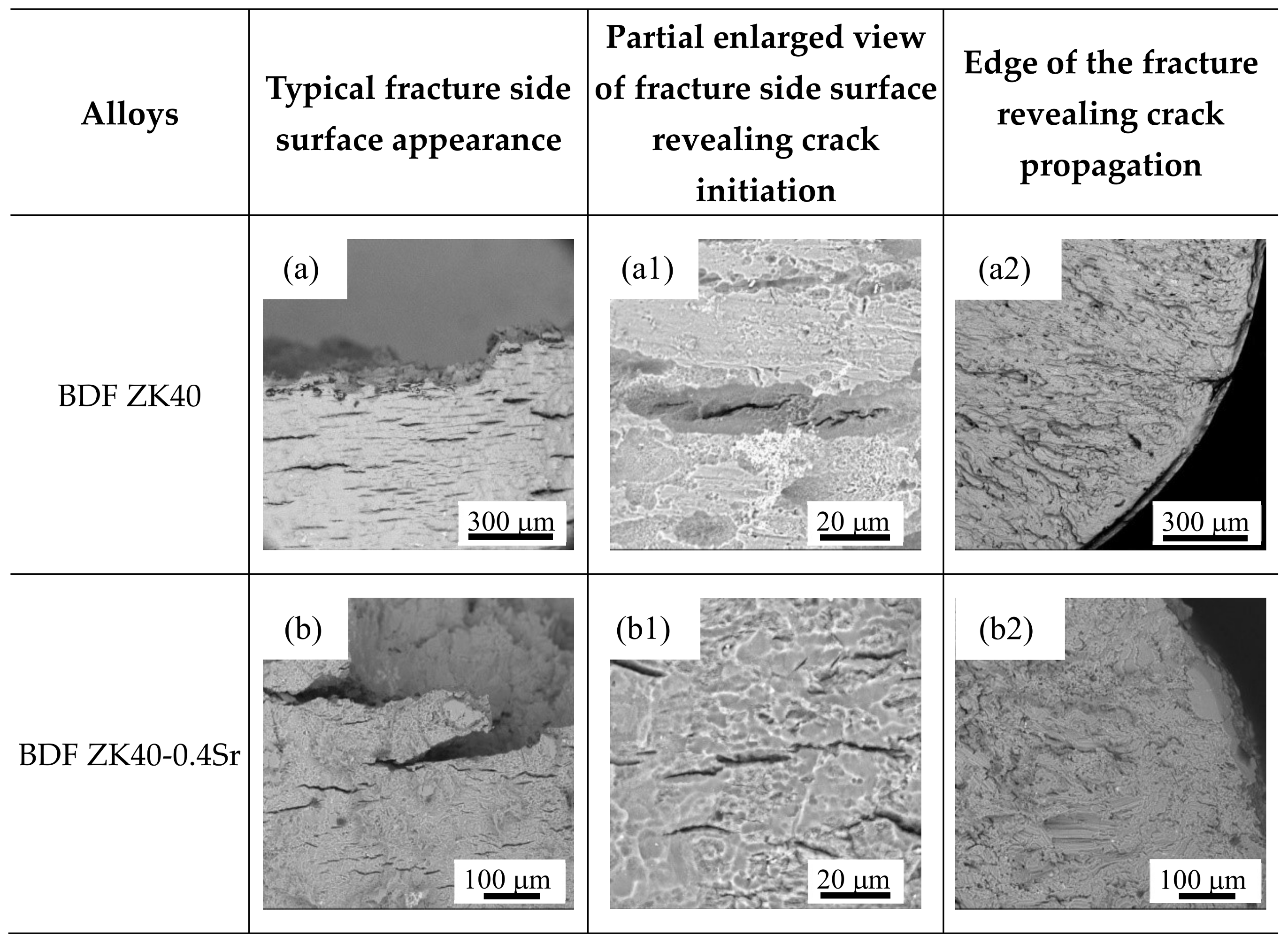
3.4. Surface Appearance and Fractography
4. Discussion
4.1. Degradation Behavior and Mechanical Properties
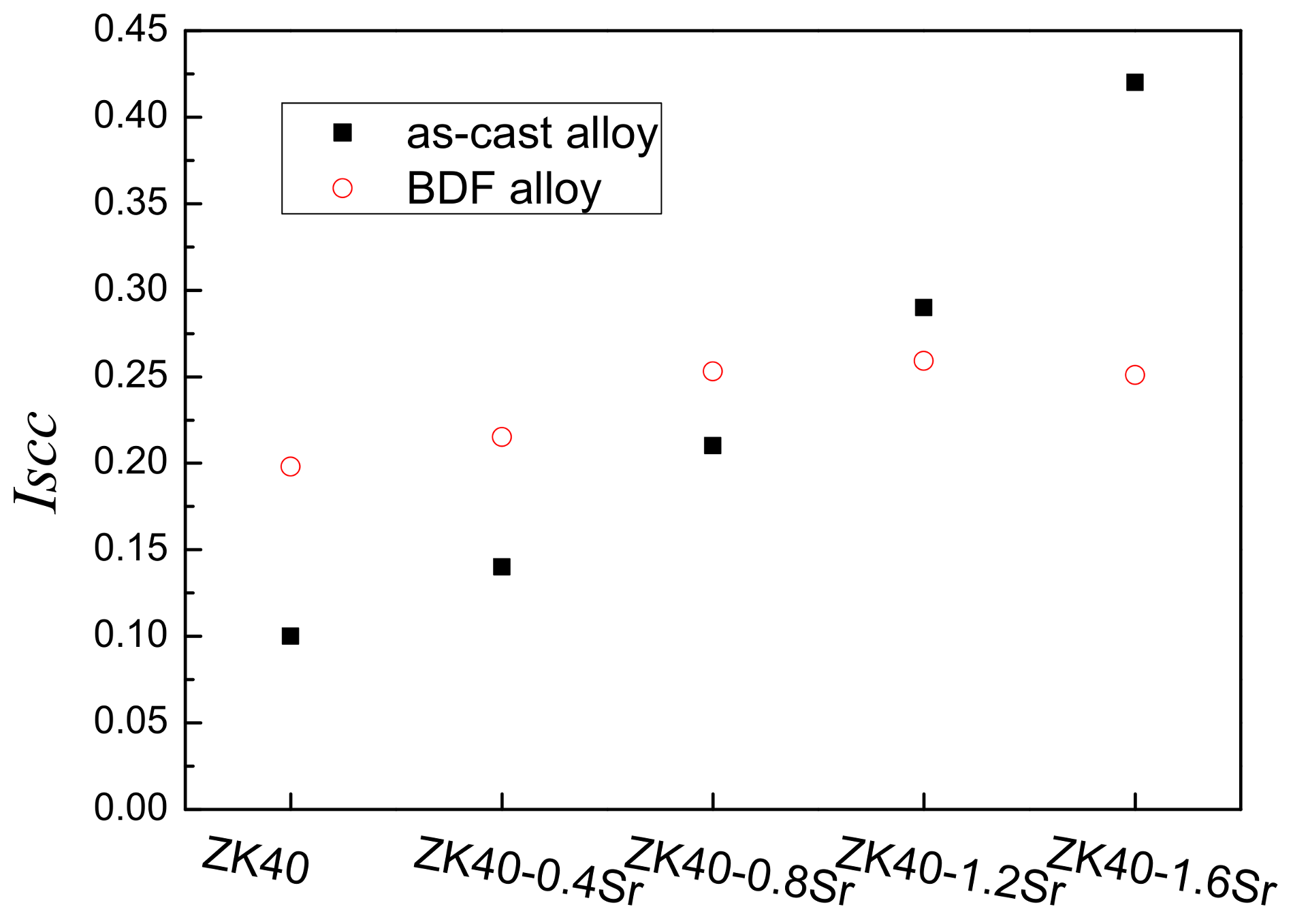
4.2. Stress Corrosion Cracking (SCC) Susceptibility
5. Conclusions
- (1)
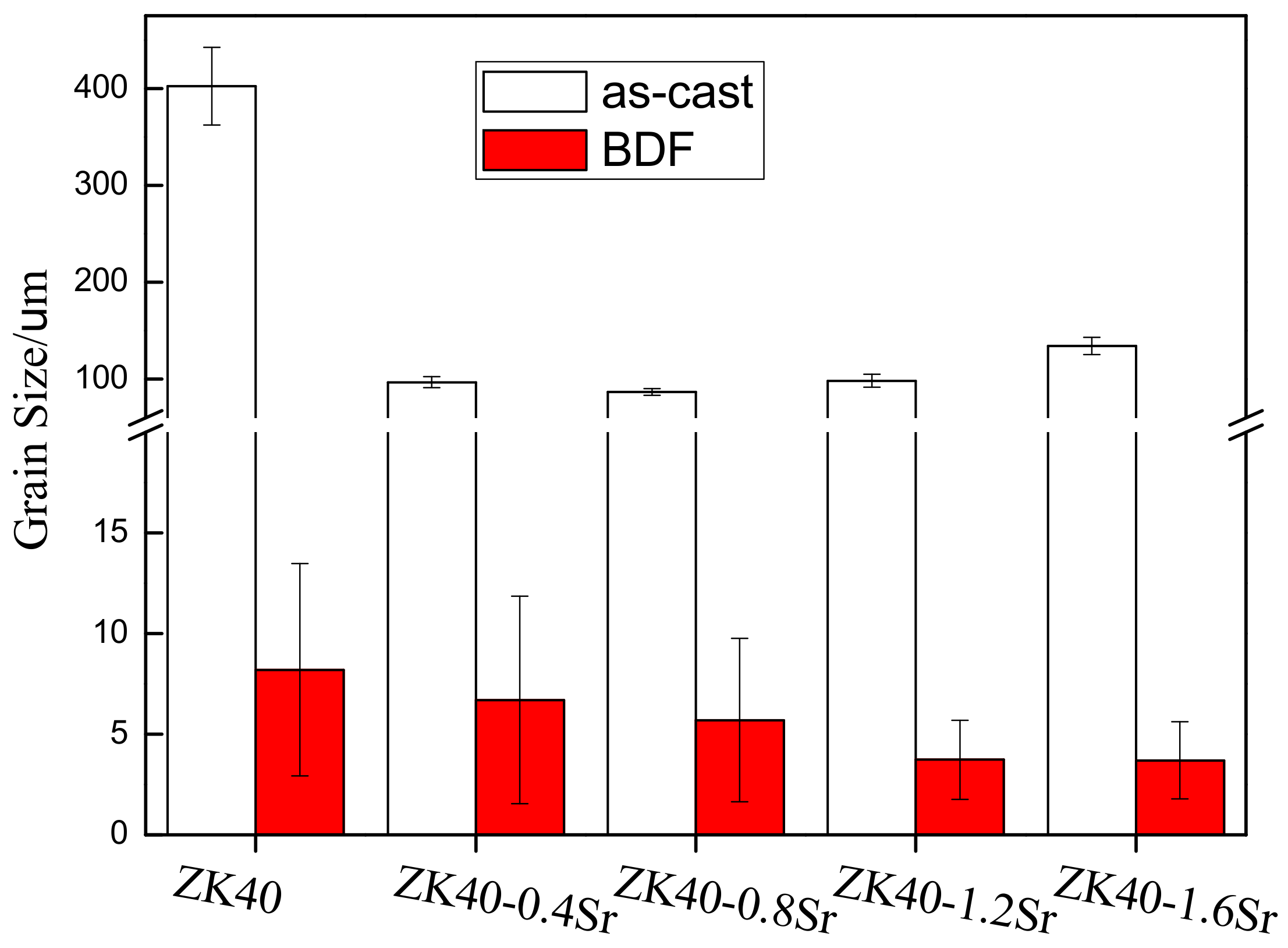
- The average grain size changed from about 100 µm (as-cast ZK40-xSr alloys) to below 10 µm after the forging process. The average grain size of the BDF ZK40-0, 0.4, 08, 1.2, and 1.6Sr alloys were 8.2 ± 5.3, 6.7 ± 5.3, 5.7 ± 4.3, 3.7 ± 2.0, and 3.7 ± 1.9 µm, respectively. The forging process also changed the distribution of the second phase from net-shape along the grain boundaries to uniformly island shape inside the Mg matrix.
- (2)
- The mechanical properties of the BDF ZK40-xSr alloys improved significantly, and the corrosion resistance enhanced after the forging process. These were attributed to the grain refinement and uniformly distribution of the second phase.
- (3)
- The micro-galvanic corrosion along the grain boundaries was the major factor affecting the SCC susceptibility in high Sr ZK40-xSr alloys. The hydrogen embrittlement increased the SCC susceptibility of the low Sr alloys (BDF ZK40 and ZK40-0.4Sr). However, the forging process can suppress the adverse effect of Sr on the SCC susceptibility of the magnesium alloy, owing to the uniformly distributed second phase and refined grains.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, S. Biodegradable Mg and Mg alloys: Corrosion and biocompatibility. Mater. Sci. Eng. B 2011, 176, 1600–1608. [Google Scholar] [CrossRef]
- Lee, J.W.; Han, H.S.; Han, K.J.; Park, J.; Jeon, H.; Ok, M.R.; Seok, H.K.; Ahn, J.P.; Lee, K.E.; Lee, D.H. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. PNAS 2016, 113, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.; Pietak, A.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Ye, C.R.; Mei, K.Y.; O’laughlin, M.; Wise, H.; Di, C.; Li, T.; Shi, D.; Wang, J. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials 2016, 81, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Lafont, A.; Yang, Y. Magnesium stent scaffolds: DREAMS become reality. Lancet 2016, 387, 3–4. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Rössig, C.; Angrisani, N.; Helmecke, P.; Besdo, S.; Seitz, J.M.; Welke, B.; Fedchenko, N.; Kock, H.; Reifenrath, J. In vivo evaluation of a magnesium-based degradable intramedullary nailing system in a sheep model. Acta Biomater. 2015, 25, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, G.; Niu, J.; Fu, P.; Ding, W. Microstructure, mechanical properties, biocorrosion behavior, and cytotoxicity of as-extruded Mg-Nd-Zn-Zr alloy with different extrusion ratios. J. Mech. Behav. Biomed. Mater. 2012, 9, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.N.; Xie, X.H.; Li, N.; Zheng, Y.F.; Qin, L. In vitro and in vivo studies on a Mg–Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ibrahim, J.M.; Chu, P.K. Surface design of biodegradable magnesium alloys—A review. Surf. Coat. Technol. 2013, 233, 2–12. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.W.; Zheng, Y.; Chu, P.K. Design of Magnesium Alloys with Controllable Degradation for Biomedical Implants: From Bulk to Surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.N.; Zhou, W.R.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Zhong, S.P.; Xi, T.F.; Chen, L.J. Corrosion fatigue behaviors of two biomedical Mg alloys–AZ91D and WE43–In simulated body fluid. Acta Biomater. 2010, 6, 4605–4613. [Google Scholar] [CrossRef] [PubMed]
- Kannan, M.B.; Raman, R.S. Evaluating the stress corrosion cracking susceptibility of Mg–Al–Zn alloy in modified-simulated body fluid for orthopaedic implant application. Scr. Mater. 2008, 59, 175–178. [Google Scholar] [CrossRef]
- Koo, Y.; Jang, Y.; Yun, Y. A study of long-term static load on degradation and mechanical integrity of Mg alloys-based biodegradable metals. Mater. Sci. Eng. B 2017, 219, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.R.; William, M.M.; Christopher, G.M. AO Principles of Fracture Management; Thieme: Stuttgart, German, 2007. [Google Scholar]
- Winzer, N.; Atrens, A.; Song, G.; Ghali, E.; Dietzel, W.; Kainer, K.; Hort, N.; Blawert, C. A Critical Review of the Stress Corrosion Cracking (SCC) of Magnesium Alloys. Adv. Eng. Mater. 2005, 7, 659–693. [Google Scholar] [CrossRef]
- Jafari, S.; Harandi, S.E.; Raman, R.S. A Review of Stress-Corrosion Cracking and Corrosion Fatigue of Magnesium Alloys for Biodegradable Implant Applications. J. Miner. Met. Mater. 2015, 67, 1143–1153. [Google Scholar] [CrossRef]
- Chen, J.; Ai, M.R.; Wang, J.Q.; Han, E.H.; Ke, W. Stress corrosion cracking behaviors of AZ91 magnesium alloy in deicer solutions using constant load. Mater. Sci. Eng. A 2009, 515, 79–84. [Google Scholar] [CrossRef]
- Raman, R.S.; Jafari, S.; Harandi, S.E. Corrosion fatigue fracture of magnesium alloys in bioimplant applications: A review. Eng. Fract. Mech. 2015, 137, 97–108. [Google Scholar] [CrossRef]
- Sivakumar, M.; Rajeswari, S. Investigation of failures in stainless steel orthopaedic implant devices: Pit-induced stress corrosion cracking. J. Mater. Sci. Lett. 1992, 11, 1039–1042. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Bombara, G.; Cavallini, M. Stress corrosion cracking of bone implants. Corros. Sci. 1977, 17, 77–85. [Google Scholar] [CrossRef]
- Choudhary, L.; Raman, R.S. Magnesium alloys as body implants: Fracture mechanism under dynamic and static loadings in a physiological environment. Acta Biomater. 2012, 8, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Winzer, N.; Atrens, A.; Dietzel, W.; Raja, S.V. Song Characterisation of stress corrosion cracking (SCC) of Mg-Al alloys. Mater. Sci. Eng. A 2008, 488, 339–351. [Google Scholar] [CrossRef]
- Lokesh, C.; Joelle, H.; Uggowitzer, P.J. In-vitro characterization of stress corrosion cracking of aluminium-free magnesium alloys for temporary bio-implant applications. Mater. Sci. Eng. C 2014, 42, 629–636. [Google Scholar] [CrossRef]
- Jafari, S.; Raman, R.K.; Davies, C.H.; Hofstetter, J.; Uggowitzer, P.J.; Löffler, J.F. Stress corrosion cracking and corrosion fatigue characterisation of MgZn1Ca0.3 (ZX10) in a simulated physiological environment. J. Mech. Behav. Biomed. Mater. 2016, 65, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, L.; Raman, R.S. Mechanical integrity of magnesium alloys in a physiological environment: Slow strain rate testing based study. Eng. Fract. Mech. 2013, 103, 94–102. [Google Scholar] [CrossRef]
- Chen, L.; Bin, Y.; Zou, W.; Wang, X.; Li, W. The influence of Sr on the microstructure, degradation and stress corrosion cracking of the Mg alloys - ZK40xSr. J. Mech. Behav. Biomed. Mater. 2016, 66, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Gazzano, M. Strontium-substituted hydroxyapatite nanocrystals. Inorg. Chim. Acta 2007, 360, 1009–1016. [Google Scholar] [CrossRef]
- Dahl, S.G.; Marie, P.J.; Delmas, P.D. Incorporation and distribution of strontium in bone. Bone 2001, 28, 446–453. [Google Scholar] [CrossRef]
- Tonstad, S.; Farsang, C.; Klaene, G.; Lewis, K.; Manolis, A.; Perruchoud, A.P.; Silagy, C.; Van Spiegel, P.I.; Astbury, C.; Hider, A. Bupropion SR for smoking cessation in smokers with cardiovascular disease: A multicentre, randomised study. ACC Curr. J. Rev. 2003, 12, 28–29. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Y.; Pan, H.; Lin, K.; Liu, X.; Darvell, B.W.; Lu, W.W.; Jiang, C.; Deng, L.; Wang, D. Effects of strontium in modified biomaterials. Acta Biomater. 2011, 7, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Strontium ranelate: A physiological approach for optimizing bone formation and resorption. Bone 2006, 38, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Argade, G.R.; Yuan, W.; Kandasamy, K.; Mishra, R.S. Stress corrosion cracking susceptibility of ultrafine grained AZ31. J. Mater. Sci. 2012, 47, 6812–6822. [Google Scholar] [CrossRef]
- Cao, F.; Shi, Z.; Song, G.L.; Liu, M.; Dargusch, M.S.; Atrens, A. Stress corrosion cracking of several hot-rolled binary Mg–X alloys. Corros. Sci. 2015, 98, 6–19. [Google Scholar] [CrossRef]
- Oyane, A.; Onuma, K.; Ito, A.; Kim, H.M.; Kokubo, T.; Nakamura, T. Formation and growth of clusters in conventional and new kinds of simulated body fluids. J. Biomed. Mater. Res. Part A 2003, 64, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Aung, N.N.; Zhou, W. Effect of grain size and twins on corrosion behaviour of AZ31B magnesium alloy. Corros. Sci. 2010, 52, 589–594. [Google Scholar] [CrossRef]
- IOS 7539-7. Corrosion of Metals and Alloys—Stress Corrosion Testing—Part 7: Method for Slow Strain Rate Testing; Beuth Verlag GmbH: Berlin, Germany, 2005. [Google Scholar]
- Rhodes, P.R. Environment-Assisted Cracking of Corrosion-Resistant Alloys in Oil and Gas Production Environments: A Review. Corrosion 2001, 57, 923–966. [Google Scholar] [CrossRef]
- Brar, H.S.; Wong, J.; Manuel, M.V. Investigation of the mechanical and degradation properties of Mg-Sr and Mg-Zn-Sr alloys for use as potential biodegradable implant materials. J. Mech. Behav. Biomed. Mater. 2012, 7, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.G.; Cipriano, A.F.; Zhao, Z.Y.; Lock, J.; Di, T.; Zhao, T.; Cui, T.; Liu, H. Development and evaluation of a magnesium–zinc–strontium alloy for biomedical applications—Alloy processing, microstructure, mechanical properties, and biodegradation. Mater. Sci. Eng. C 2013, 33, 3661–3669. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, Q.; Li, X.; Li, K.; Han, Z.; Fang, D. Microstructures, mechanical and cytocompatibility of degradable Mg–Zn based orthopedic biomaterials. Mater. Des. 2014, 58, 43–51. [Google Scholar] [CrossRef]
- Kubota, K.; Mabuchi, M.; Higashi, K. Review Processing and mechanical properties of fine-grained magnesium alloys. J. Mater. Sci. 1999, 34, 2255–2262. [Google Scholar] [CrossRef]
- Makar, G.L.; Kruger, J.; Sieradzki, K. Stress corrosion cracking of rapidly solidified magnesium-aluminum alloys. Corros. Sci. 1993, 34, 1311–1323. [Google Scholar] [CrossRef]
- Ben-hamu, G.; Eliezer, D.; Dietzel, W.; Shin, K.S. Stress corrosion cracking of new Mg–Zn–Mn wrought alloys containing Si. Corros. Sci. 2008, 50, 1505–1517. [Google Scholar] [CrossRef]
- Kannan, M.B.; Dietzel, W.; Blawert, C.; Atrens, A.; Lyon, P. Stress corrosion cracking of rare-earth containing magnesium alloys ZE41, QE22 and Elektron 21 (EV31A) compared with AZ80. Mater. Sci. Eng. A 2008, 480, 529–539. [Google Scholar] [CrossRef]
- Song, R.G.; Blawert, C.; Dietzel, W.; Atrens, A. A study on stress corrosion cracking and hydrogen embrittlement of AZ31 magnesium alloy. Mater. Sci. Eng. A 2005, 399, 308–317. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A. Understanding Magnesium Corrosion—A Framework for Improved Alloy Performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Hakimi, O.; Aghion, E.; Goldman, J. Improved stress corrosion cracking resistance of a novel biodegradable EW62 magnesium alloy by rapid solidification, in simulated electrolytes. Mater. Sci. Eng. C 2015, 51, 226–232. [Google Scholar] [CrossRef] [PubMed]
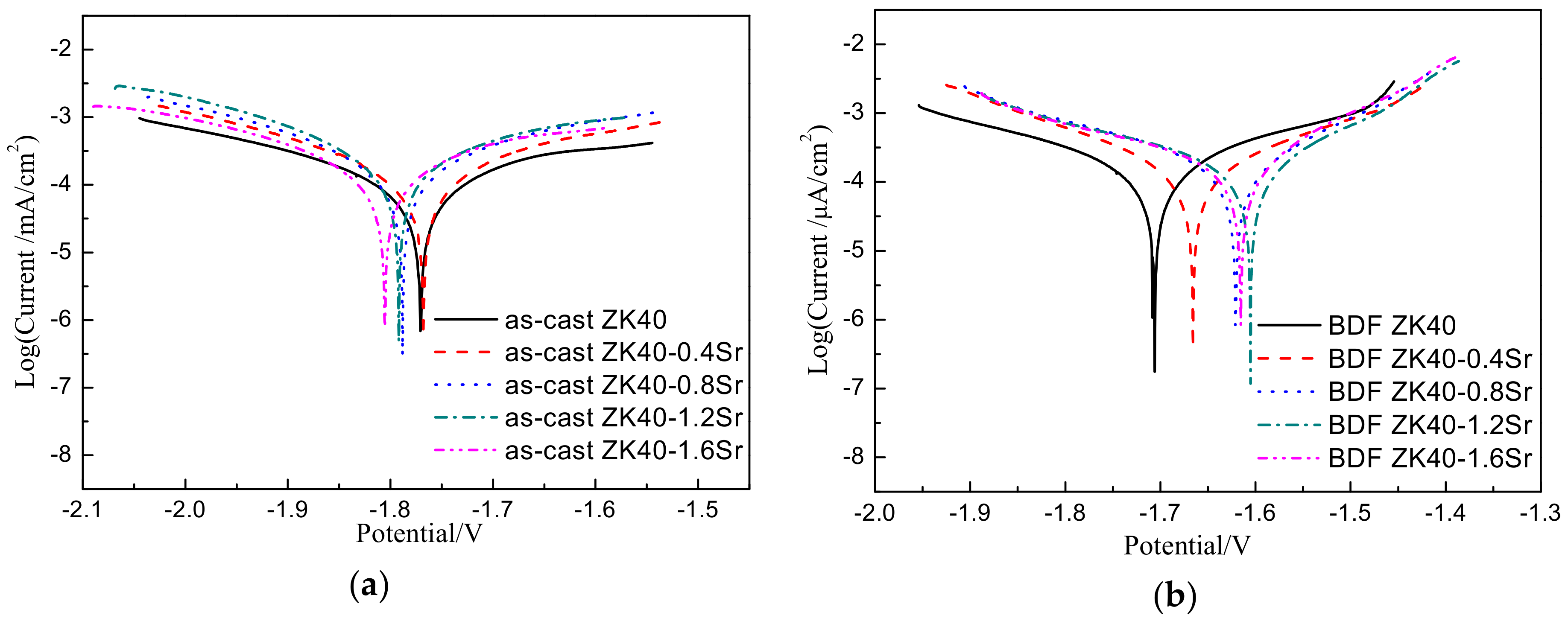

| Materials | Corrosion Potential, Ecorr (V) | Corrosion Current Density, icorr (mA·cm−2) | Degradation Rate (mm·y−1) by polarization CURVES | Degradation Rate (mm·y−1) by H2 Evolution | |
|---|---|---|---|---|---|
| As-cast | ZK40 | −1.770 | 0.255 | 5.83 ± 0.27 | 4.59 ± 0.30 |
| ZK40-0.4Sr | −1.767 | 0.266 | 6.08 ± 0.22 | 4.78 ± 0.21 | |
| ZK40-0.8Sr | −1.788 | 0.343 | 7.84 ± 0.31 | 6.47 ± 0.38 | |
| ZK40-1.2Sr | −1.791 | 0.445 | 10.17 ± 0.34 | 8.31 ± 0.31 | |
| ZK40-1.6Sr | −1.805 | 0.558 | 12.75 ± 0.41 | 10.34 ± 0.29 | |
| BDF | ZK40 | −1.706 | 0.207 | 4.73 ± 0.20 | 3.82 ± 0.13 |
| ZK40-0.4Sr | −1.665 | 0.195 | 4.46 ± 0.18 | 3.51 ± 0.19 | |
| ZK40-0.8Sr | −1.620 | 0.216 | 4.94 ± 0.14 | 3.99 ± 0.17 | |
| ZK40-1.2Sr | −1.605 | 0.236 | 5.39 ± 0.21 | 4.34 ± 0.13 | |
| ZK40-1.6Sr | −1.615 | 0.242 | 5.53 ± 0.16 | 4.61 ± 0.15 | |
| Alloys | εin air/% | εin m-SBF/% | UTS in air/MPa | UTS in m-SBF/MPa | ISCC |
|---|---|---|---|---|---|
| BDF ZK40 | 15.3 ± 0.6 | 7.8 ± 0.3 | 226.3 ± 4.9 | 194.5 ± 6.1 | ≈0.20 |
| BDF ZK40-0.4Sr | 19.6 ± 0.7 | 9.5 ± 0.5 | 261.1 ± 6.3 | 224.2 ± 7.7 | ≈0.22 |
| BDF ZK40-0.8Sr | 14.5 ± 0.5 | 5.3 ± 0.2 | 248.5 ± 2.8 | 202.1 ± 9.4 | ≈0.25 |
| BDF ZK40-1.2Sr | 9.8 ± 0.4 | 4.2 ± 0.4 | 234.2 ± 7.8 | 183.3 ± 2.1 | ≈0.26 |
| BDF ZK40-1.6Sr | 6.6 ± 0.3 | 3.2 ± 0.3 | 221.4 ± 5.7 | 171.3 ± 4.3 | ≈0.25 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Sheng, Y.; Wang, X.; Zhao, X.; Liu, H.; Li, W. Effect of the Microstructure and Distribution of the Second Phase on the Stress Corrosion Cracking of Biomedical Mg-Zn-Zr-xSr Alloys. Materials 2018, 11, 551. https://doi.org/10.3390/ma11040551
Chen L, Sheng Y, Wang X, Zhao X, Liu H, Li W. Effect of the Microstructure and Distribution of the Second Phase on the Stress Corrosion Cracking of Biomedical Mg-Zn-Zr-xSr Alloys. Materials. 2018; 11(4):551. https://doi.org/10.3390/ma11040551
Chicago/Turabian StyleChen, Lianxi, Yinying Sheng, Xiaojian Wang, Xueyang Zhao, Hui Liu, and Wei Li. 2018. "Effect of the Microstructure and Distribution of the Second Phase on the Stress Corrosion Cracking of Biomedical Mg-Zn-Zr-xSr Alloys" Materials 11, no. 4: 551. https://doi.org/10.3390/ma11040551
APA StyleChen, L., Sheng, Y., Wang, X., Zhao, X., Liu, H., & Li, W. (2018). Effect of the Microstructure and Distribution of the Second Phase on the Stress Corrosion Cracking of Biomedical Mg-Zn-Zr-xSr Alloys. Materials, 11(4), 551. https://doi.org/10.3390/ma11040551






