Electrochemical Impedance and Polarization Corrosion Studies of Tantalum Surface Modified by DC Plasma Electrolytic Oxidation
Abstract
:1. Introduction
2. Materials and Methods
- 0.5 M Ca(H2PO2)2 − molar ratio Ca:P = 1:2; sample label: Ca1P2
- 0.5 M Ca(H2PO2)2 + 1.15 M Ca(HCOO)2 − molar ratio Ca:P = 5:3; sample label: Ca5P3
- 0.5 M Ca(H2PO2)2 + 1.15 M Mg(HCOO)2 − molar ratio (CaMg):P = 5:3; sample label: (CaMg)5P3
- 5 h of open-circuit potential (EOC) stabilization; EOC drift rate was typically below 10 mV/h and did not exceed 16 mV/h for any sample
- Electrochemical impedance spectroscopy performed at EOC, frequency range 100 kHz–10 mHz and root mean square (RMS) amplitude of 10 mV; the measurement took up to 60 min
- After the potential was stabilized after the EIS experiment, a series of potentiostatic measurements (120 s) were carried out, starting at EOC and decreasing down to −30 mV vs. EOC with a step of 3 mV; then the potential was allowed to stabilize for 10 min prior to the analogical series of measurements up to +30 mV vs. EOC; the steady-state value of the measured current at each potential step was used to plot polarization curves near the EOC and extract polarization resistance (Rp) in the limit of ±10 mV vs. EOC
- Immediately after the potentiostatic experiments, the potentiodynamic polarization was conducted up to +2 V vs. SCE; the potential scan rate was 10 mV/min
3. Results and Discussion
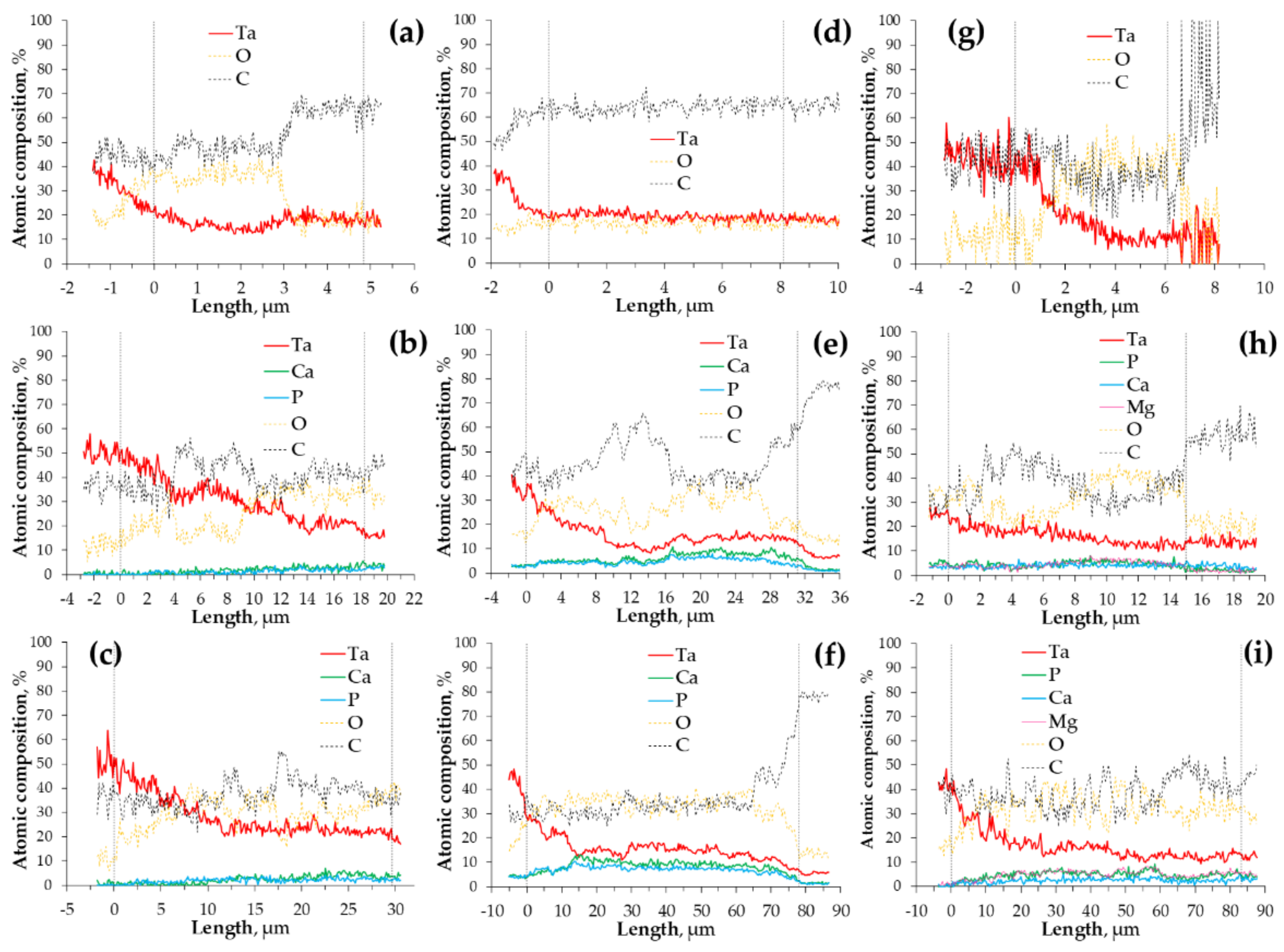
3.1. Surface Appearance and Morphology
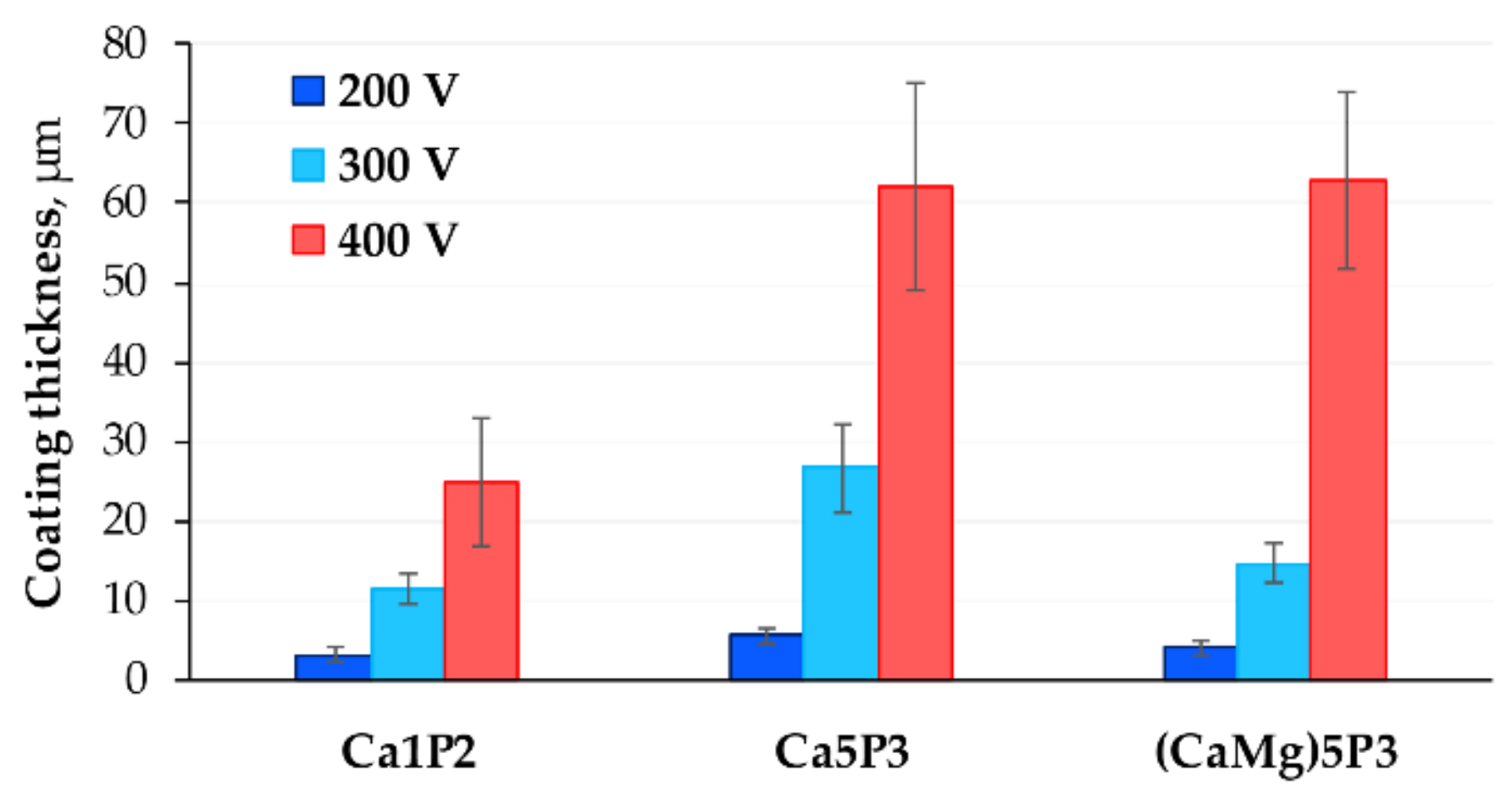
3.2. Coatings Structure and Thickness
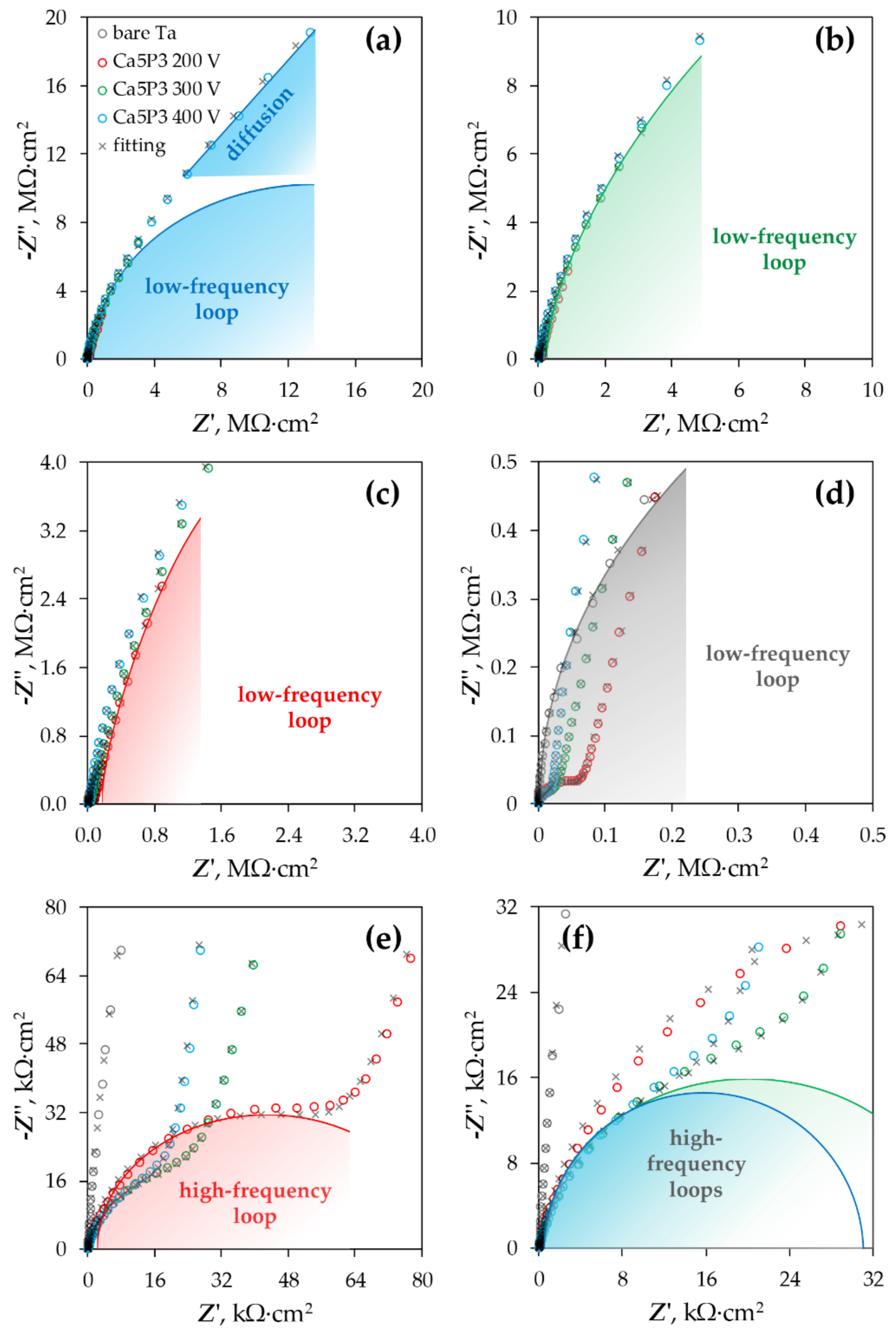
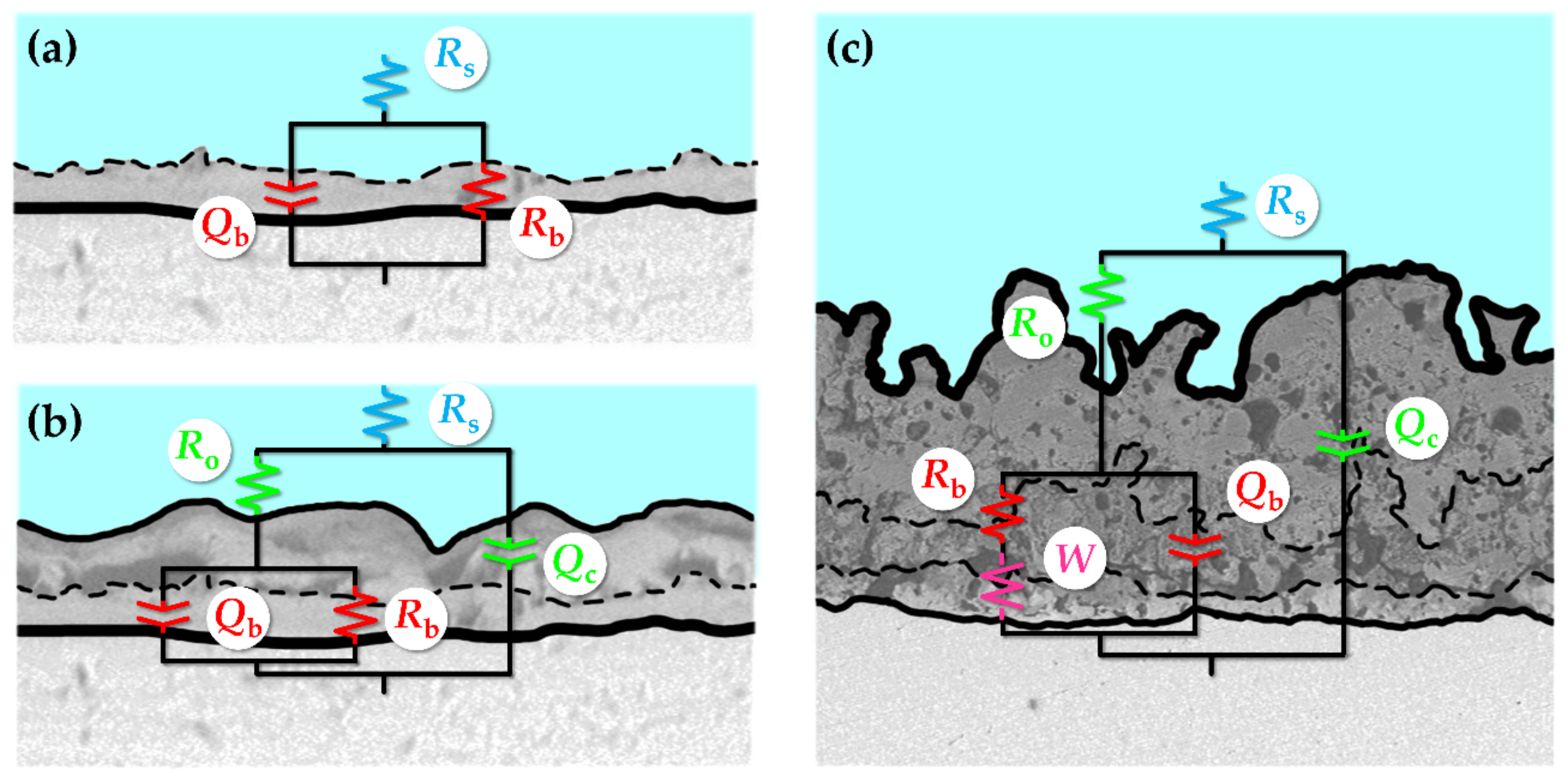
3.3. Electrochemical Impedance Spectroscopy
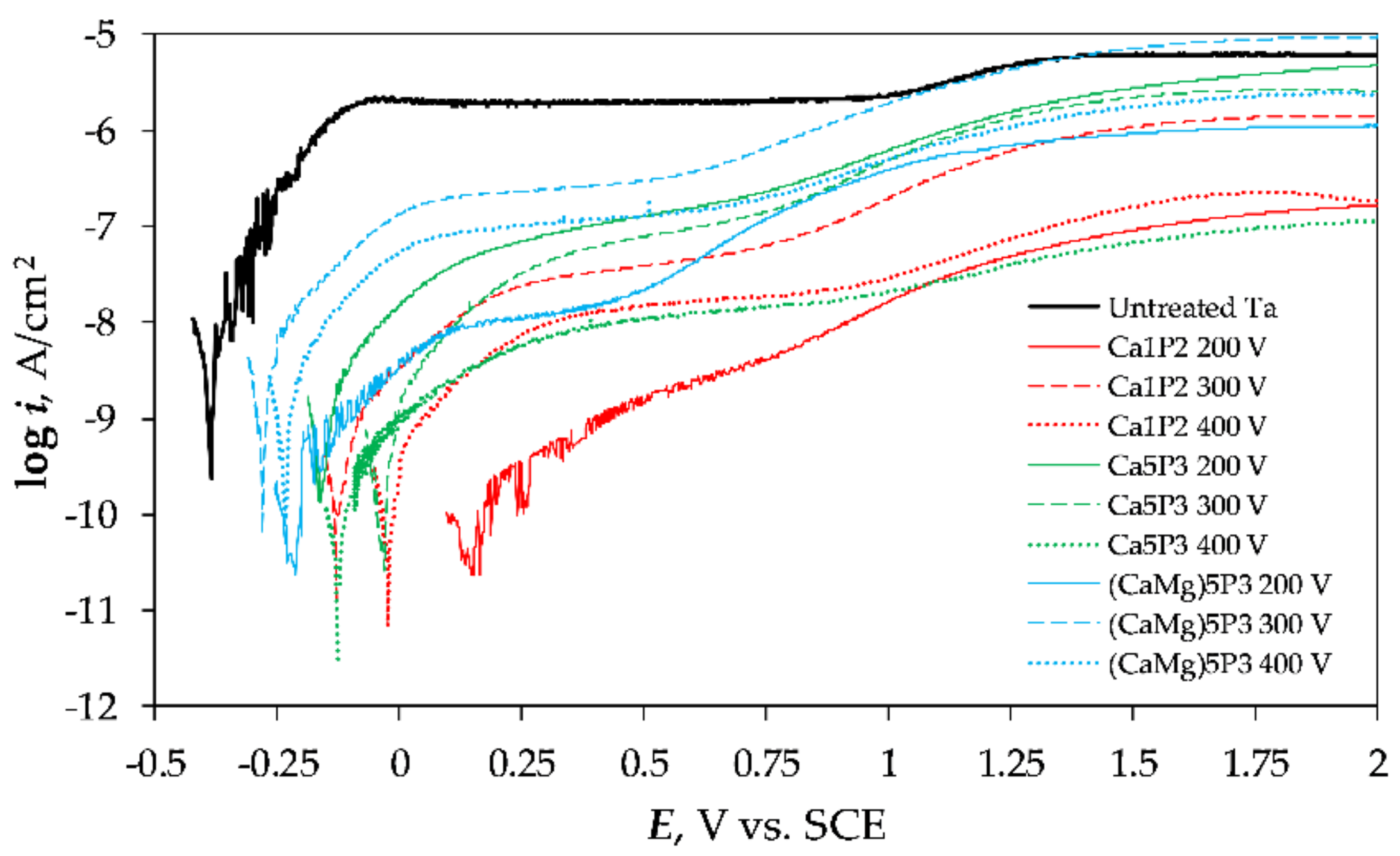
3.4. Polarization Experiments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burke, G.L. The Corrosion of Metals in Tissues; and an Introduction to Tantalum. Can. Med. Assoc. J. 1940, 43, 125–128. [Google Scholar] [PubMed]
- Huo, W.T.; Zhao, L.Z.; Yu, S.; Yu, Z.T.; Zhang, P.X.; Zhang, Y.S. Significantly enhanced osteoblast response to nano-grained pure tantalum. Sci. Rep. 2017, 7, 40868. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Leng, Y.X.; Tian, X.B.; Wang, L.P.; Huang, N.; Chu, P.K.; Yang, P. Antithrombogenic investigation of surface energy and optical bandgap and hemocompatibility mechanism of Ti(Ta+5)O2 thin films. Biomaterials 2002, 23, 2545–2552. [Google Scholar] [CrossRef]
- Holt, G.E.; Christie, M.J.; Schwartz, H.S. Trabecular metal endoprosthetic limb salvage reconstruction of the lower limb. J. Arthroplast. 2009, 24, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Löfgren, H.; Engquist, M.; Hoffmann, P.; Sigstedt, B.; Vavruch, L. Clinical and radiological evaluation of Trabecular Metal and the Smith–Robinson technique in anterior cervical fusion for degenerative disease: A prospective, randomized, controlled study with 2-year follow-up. Eur. Spine J. 2010, 19, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Schildhauer, T.A.; Peter, E.; Muhr, G.; Koller, M. Activation of human leukocytes on tantalum trabecular metal in comparison to commonly used orthopedic metal implant materials. J. Biomed. Mater. Res. Part A 2009, 88, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fan, Z.; Han, Y. Formation and osteoblast behavior of HA Nano-rod/fiber patterned coatings on tantalum in porous and compact forms. J. Mater. Chem. B 2015, 5442, 5442–5454. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Elmkhah, H.; Ansari, G.; Attarzadeh, F.; Imantalab, O. A comparison of electrochemical behavior of coated nanostructured Ta on Ti substrate with pure uncoated Ta in Ringer’s physiological solution. J. Alloys Compd. 2018, 739, 918–925. [Google Scholar] [CrossRef]
- Balla, V.K.; Banerjee, S.; Bose, S.; Bandyopadhyay, A. Direct laser processing of a tantalum coating on titanium for bone replacement structures. Acta Biomater. 2010, 6, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Pouilleau, J.; Devilliers, D.; Garrido, F.; Durand-Vidal, S.; Mahé, E. Structure and composition of passive titanium oxide films. Mater. Sci. Eng. B 1997, 47, 235–243. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Niinomi, M.; Akahori, T.; Fukui, H.; Toda, H. Corrosion resistance and biocompatibility of Ti-Ta alloys for biomedical applications. Mater. Sci. Eng. A 2005, 398, 28–36. [Google Scholar] [CrossRef]
- Brunski, J.B. Metals. An Introduction to Materials in Medicine. In Biomaterials Science; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 37–50. [Google Scholar]
- Kokubo, T.; Kim, H.M.; Kawashita, M.; Nakamura, T. Bioactive metals: Preparation and properties. J. Mater. Sci. Mater. Med. 2004, 15, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate coatings, films and layers. Prog. Biomater. 2012, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Krząkała, A.; Kazek-Kęsik, A.; Simka, W. Application of plasma electrolytic oxidation to bioactive surface formation on titanium and its alloys. RSC Adv. 2013, 3, 19725–19743. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T. Comparative SEM and EDX analysis of surface coatings created on niobium and titanium alloys after plasma electrolytic oxidation (PEO). Teh. Vjesn. 2017, 24, 465–472. [Google Scholar]
- Sankara Narayanan, T.S.N.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Malorny, W. Characterization of porous coatings obtained on materials by plasma electrolytic oxidation. Mater. Sci. Forum 2016, 862, 86–95. [Google Scholar] [CrossRef]
- Shchedrina, I.; Rakoch, A.G.; Henrion, G.; Martin, J. Non-destructive methods to control the properties of MAO coatings on the surface of 2024 aluminium alloy. Surf. Coat. Technol. 2014, 238, 27–44. [Google Scholar] [CrossRef]
- Sowa, M.; Woszczak, M.; Kazek-Kęsik, A.; Dercz, G.; Korotin, D.M.; Zhidkov, I.S.; Kurmaev, E.Z.; Cholakh, S.O.; Basiaga, M.; Simka, W. Influence of process parameters on plasma electrolytic surface treatment of tantalum for biomedical applications. Appl. Surf. Sci. 2017, 407, 52–63. [Google Scholar] [CrossRef]
- Rokosz, K.; Hryniewicz, T.; Chapon, P.; Raaen, S.; Ricardo Zschommler Sandim, H. XPS and GDOES characterization of porous coating enriched with copper and calcium obtained on tantalum via plasma electrolytic oxidation. J. Spectrosc. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Mohedano, M.; Matykina, E.; Arrabal, R.; Pardo, A.; Merino, M.C. Metal release from ceramic coatings for dental implants. Dent. Mater. 2014, 30, e28–e40. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.O.; Nie, X.; Northwood, D.O. An investigation of ceramic coating growth mechanisms in plasma electrolytic oxidation (PEO) processing. Electrochim. Acta 2013, 112, 111–119. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Scurr, D.J.; Baron, A.; Skeldon, P.; Thompson, G.E. Investigation of the mechanism of plasma electrolytic oxidation of aluminium using 18O tracer. Corros. Sci. 2010, 52, 1070–1076. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Chen, D.; Wang, R.; Li, D.; Guo, C.; Jiang, G.; Shen, D.; Yu, S.; Nash, P. Micro-structures and growth mechanisms of plasma electrolytic oxidation coatings on aluminium at different current densities. Surf. Coat. Technol. 2017, 321, 236–246. [Google Scholar] [CrossRef]
- Simchen, F.; Sieber, M.; Lampke, T. Electrolyte influence on ignition of plasma electrolytic oxidation processes on light metals. Surf. Coat. Technol. 2017, 315, 205–213. [Google Scholar] [CrossRef]
- Fatkullin, A.R.; Parfenov, E.V.; Yerokhin, A.; Lazarev, D.M.; Matthews, A. Effect of positive and negative pulse voltages on surface properties and equivalent circuit of the plasma electrolytic oxidation process. Surf. Coat. Technol. 2015, 284, 427–437. [Google Scholar] [CrossRef]
- Gao, Y.; Yerokhin, A.; Matthews, A. Effect of current mode on PEO treatment of magnesium in Ca- and P-containing electrolyte and resulting coatings. Appl. Surf. Sci. 2014, 316, 558–567. [Google Scholar] [CrossRef]
- Terleeva, O.P.; Sharkeev, Y.P.; Slonova, A.I.; Mironov, I.V.; Legostaeva, E.V.; Khlusov, I.A.; Matykina, E.; Skeldon, P.; Thompson, G.E. Effect of microplasma modes and electrolyte composition on micro-arc oxidation coatings on titanium for medical applications. Surf. Coat. Technol. 2010, 205, 1723–1729. [Google Scholar] [CrossRef]
- Frankel, G.S.; Papavinasam, S.; Berke, N.; Brossia, S.; Dean, S.W. Electrochemical techniques in corrosion: Status, limitations, and needs. J. ASTM Int. 2008, 5, 1–27. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 111820994X. [Google Scholar]
- Hee, A.C.; Martin, P.J.; Bendavid, A.; Jamali, S.S.; Zhao, Y. Tribo-corrosion performance of filtered-arc-deposited tantalum coatings on Ti-13Nb-13Zr alloy for bio-implants applications. Wear 2018, 400–401, 31–42. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Pourmahmoud, M. Passive and semiconducting properties assessment of commercially pure tantalum in Hank’s physiological solution. J. Mater. Eng. Perform. 2018, 27, 116–123. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Rameshbabu, N.; Sreekanth, D.; Sandhyarani, M.; Bose, A.C.; Muthupandi, V.; Subramanian, S. Role of electrolyte chemistry on electronic and in vitro electrochemical properties of micro-arc oxidized titania films on Cp Ti. Electrochim. Acta 2013, 105, 468–480. [Google Scholar]
- Shokouhfar, M.; Dehghanian, C.; Montazeri, M.; Baradaran, A. Preparation of ceramic coating on Ti substrate by plasma electrolytic oxidation in different electrolytes and evaluation of its corrosion resistance: Part II. Appl. Surf. Sci. 2012, 258, 2416–2423. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Rameshbabu, N.; Sreekanth, D.; Bose, A.C.; Muthupandi, V.; Subramanian, S. Fabrication and characterization of micro-arc oxidized fluoride containing titania films on Cp Ti. Ceram. Int. 2013, 39, 801–812. [Google Scholar] [CrossRef]
- Montazeri, M.; Dehghanian, C.; Shokouhfar, M.; Baradaran, A. Investigation of the voltage and time effects on the formation of hydroxyapatite-containing titania prepared by plasma electrolytic oxidation on Ti-6Al-4V alloy and its corrosion behavior. Appl. Surf. Sci. 2011, 257, 7268–7275. [Google Scholar] [CrossRef]
- Hwang, I.-J.; Choe, H.-C.; Brantley, W.A. Electrochemical characteristics of Ti-6Al-4V after plasma electrolytic oxidation in solutions containing Ca, P, and Zn ions. Surf. Coat. Technol. 2017, 320, 458–466. [Google Scholar] [CrossRef]
- Sowa, M.; Worek, J.; Dercz, G.; Korotin, D.M.; Kukharenko, A.I.; Kurmaev, E.Z.; Cholakh, S.O.; Basiaga, M.; Simka, W. Surface characterisation and corrosion behaviour of niobium treated in a Ca- and P-containing solution under sparking conditions. Electrochim. Acta 2016, 198, 91–103. [Google Scholar] [CrossRef]
- Duarte, L.T.; Biaggio, S.R.; Rocha-Filho, R.C.; Bocchi, N. Preparation and characterization of biomimetically and electrochemically deposited hydroxyapatite coatings on micro-arc oxidized Ti-13Nb-13Zr. J. Mater. Sci. Mater. Med. 2011, 22, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Veys-Renaux, D.; Ait El Haj, Z.; Rocca, E. Corrosion resistance in artificial saliva of titanium anodized by plasma electrolytic oxidation in Na3PO4. Surf. Coat. Technol. 2016, 285, 214–219. [Google Scholar] [CrossRef]
- Fakhr Nabavi, H.; Aliofkhazraei, M.; Sabour Rouhaghdam, A. Morphology and corrosion resistance of hybrid plasma electrolytic oxidation on CP-Ti. Surf. Coat. Technol. 2017, 322, 59–69. [Google Scholar] [CrossRef]
- Alves, A.C.; Wenger, F.; Ponthiaux, P.; Celis, J.-P.; Pinto, A.M.; Rocha, L.A.; Fernandes, J.C.S. Corrosion mechanisms in titanium oxide-based films produced by anodic treatment. Electrochim. Acta 2017, 234, 16–27. [Google Scholar] [CrossRef]
- Fazel, M.; Salimijazi, H.R.; Golozar, M.A.; Garsivaz Jazi, M.R. A comparison of corrosion, tribocorrosion and electrochemical impedance properties of pure Ti and Ti6Al4V alloy treated by micro-arc oxidation process. Appl. Surf. Sci. 2015, 324, 751–756. [Google Scholar] [CrossRef]
- Wang, B.L.; Zheng, Y.F.; Zhao, L.C. Electrochemical corrosion behavior of biomedical Ti-22Nb and Ti-22Nb-6Zr alloys in saline medium. Mater. Corros. 2009, 60, 788–794. [Google Scholar] [CrossRef]
- Yeum, B. EChem Software Tech Note 24—Pseudocapacitance associated with CPE; EChem software: Ann Arbor, MI, USA, 2002. [Google Scholar]
- Mosiałek, M.; Nawrat, G.; Szyk-Warszyńska, L.; Żak, J.; Maciej, A.; Radwański, K.; Winiarski, A.; Szade, J.; Nowak, P.; Simka, W. Anodic oxidation of the Ti–13Nb–13Zr alloy. J. Solid State Electrochem. 2014, 18, 3073–3080. [Google Scholar] [CrossRef]



| Solution | - | Ca1P2 | Ca5P3 | (CaMg)5P3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UL, V | Untreated | 200 | 300 | 400 | 200 | 300 | 400 | 200 | 300 | 400 |
| Rs, Ω·cm2 | 32.0 ± 1.3 | 35.4 ± 6.1 | 27.7 ± 2.2 | 34.1 ± 1.0 | 32.4 ± 1.4 | 31.2 ± 1.8 | 34.0 ± 2.0 | 30.3 ± 5.7 | 34.6 ± 4.2 | 27.8 ± 5.9 |
| Qc, sn/(GΩ·cm2) | - | 143 ± 55 | 102 ± 16 | 60.0 ± 1.4 | 226 ± 68 | 178 ± 80 | 83.4 ± 10.5 | 471 ± 65 | 3060 ± 370 | 538 ± 207 |
| nc | - | 0.94 ± 0.02 | 0.92 ± 0.01 | 0.93 ± 0.00 | 0.89 ± 0.02 | 0.89 ± 0.03 | 0.90 ± 0.01 | 0.86 ± 0.01 | 0.75 ± 0.02 | 0.79 ± 0.02 |
| Ro, kΩ·cm2 | - | 57.0 ± 20.4 | 89.3 ± 8.8 | 64.3 ± 6.4 | 70.8 ± 3.8 | 38.4 ± 6.0 | 31.4 ± 3.4 | 60.8 ± 28.5 | 90.4 ± 16.9 | 31.7 ± 1.6 |
| Qb, sn/(GΩ·cm2) | 24,600 ± 3200 | 138 ± 38 | 1160 ± 170 | 243 ± 24 | 4190 ± 1270 | 1050 ± 280 | 228 ± 38 | 39.8 ± 9.0 | 3970 ± 1670 | 2030 ± 450 |
| nb | 0.97 ± 0.00 | 0.82 ± 0.01 | 0.83 ± 0.01 | 0.90 ± 0.02 | 0.84 ± 0.01 | 0.84 ± 0.03 | 0.89 ± 0.06 | 1.00 ± 0.00 | 0.95 ± 0.04 | 0.78 ± 0.03 |
| Rb, MΩ·cm2 | 1.67 ± 0.22 | 186 ± 88 | 41.0 ± 10.5 | 5.04 ± 3.98 | 14.1 ± 4.0 | 32.9 ± 5.3 | 18.1 ± 5.2 | 82.7 ± 14.9 | 5.34 ± 2.17 | 25.4 ± 7.3 |
| σ, MΩ·cm2/s0.5 | - | - | - | 5.75 ± 0.93 | - | - | 5.91 ± 2.09 | - | - | - |
| χ2 × 104 | <8.81 | <5.16 | <14.5 | <8.78 | <40.5 | <11.2 | <11.7 | <4.17 | <6.42 | <15.6 |
| Solution | UL, V | Ceff,1, nF/cm2 | Ceff,2, nF/cm2 |
|---|---|---|---|
| Ca1P2 | 200 | 177 ± 83 | 156 ± 66 |
| 300 | 116 ± 20 | 98.0 ± 15.1 | |
| 400 | 66.5 ± 5.0 | 57.7 ± 4.9 | |
| Ca5P3 | 200 | 262 ± 97 | 208 ± 70 |
| 300 | 231 ± 125 | 180 ± 89 | |
| 400 | 103 ± 16 | 85.9 ± 12.2 | |
| (CaMg)5P3 | 200 | 864 ± 190 | 639 ± 131 |
| 300 | 7570 ± 600 | 4110 ± 730 | |
| 400 | 1070 ± 540 | 658 ± 296 |
| Solution | UL, V | Ecor, mV vs. SCE | Rp,LPR, MΩ·cm2 | Rp,EIS, MΩ·cm2 | ipeak, µA/cm2 |
|---|---|---|---|---|---|
| Untreated | 0 | −371.1 ± 58.5 | 3.52 ± 0.41 | 1.67 ± 0.22 | 5.91 ± 3.67 |
| Ca1P2 | 200 | 142.7 ± 97.7 | 593 ± 143 | 186 ± 88 | 0.341 ± 0.257 |
| 300 | −102.3 ± 52.8 | 53.1 ± 17.2 | 41.1 ± 10.5 | 1.59 ± 0.18 | |
| 400 | −69.9 ± 41.0 | 137 ± 48 | 63.4 ± 13.4* | 0.169 ± 0.069 | |
| Ca5P3 | 200 | −166.6 ±18.5 | 26.2 ± 17.6 | 14.2 ± 4.0 | 4.17 ± 0.56 |
| 300 | −81.2 ± 53.8 | 37.5 ± 7.4 | 33.0 ± 5.8 | 2.04 ± 1.19 | |
| 400 | −78.7 ± 89.4 | 216 ± 153 | 84.2 ± 33.2* | 0.155 ± 0.129 | |
| (CaMg)5P3 | 200 | −184.2 ± 45.4 | 225 ± 73 | 83.7 ± 10.7 | 1.96 ± 1.69 |
| 300 | −307.2 ± 56.6 | 7.04 ± 1.28 | 5.43 ± 2.16 | 7.15 ± 5.03 | |
| 400 | −202.6 ± 48.7 | 15.6 ± 4.4 | 25.4 ± 7.3 | 3.24 ± 0.76 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sowa, M.; Simka, W. Electrochemical Impedance and Polarization Corrosion Studies of Tantalum Surface Modified by DC Plasma Electrolytic Oxidation. Materials 2018, 11, 545. https://doi.org/10.3390/ma11040545
Sowa M, Simka W. Electrochemical Impedance and Polarization Corrosion Studies of Tantalum Surface Modified by DC Plasma Electrolytic Oxidation. Materials. 2018; 11(4):545. https://doi.org/10.3390/ma11040545
Chicago/Turabian StyleSowa, Maciej, and Wojciech Simka. 2018. "Electrochemical Impedance and Polarization Corrosion Studies of Tantalum Surface Modified by DC Plasma Electrolytic Oxidation" Materials 11, no. 4: 545. https://doi.org/10.3390/ma11040545
APA StyleSowa, M., & Simka, W. (2018). Electrochemical Impedance and Polarization Corrosion Studies of Tantalum Surface Modified by DC Plasma Electrolytic Oxidation. Materials, 11(4), 545. https://doi.org/10.3390/ma11040545






