The Mechanical Properties and In Vitro Biocompatibility of PM-Fabricated Ti-28Nb-35.4Zr Alloy for Orthopedic Implant Applications
Abstract
:1. Introduction
2. Experimental and Methods
2.1. Materials and Sample Preparation
2.2. Materials Characterization
2.3. In Vitro Biocompatibility Testing
3. Results and Discussion
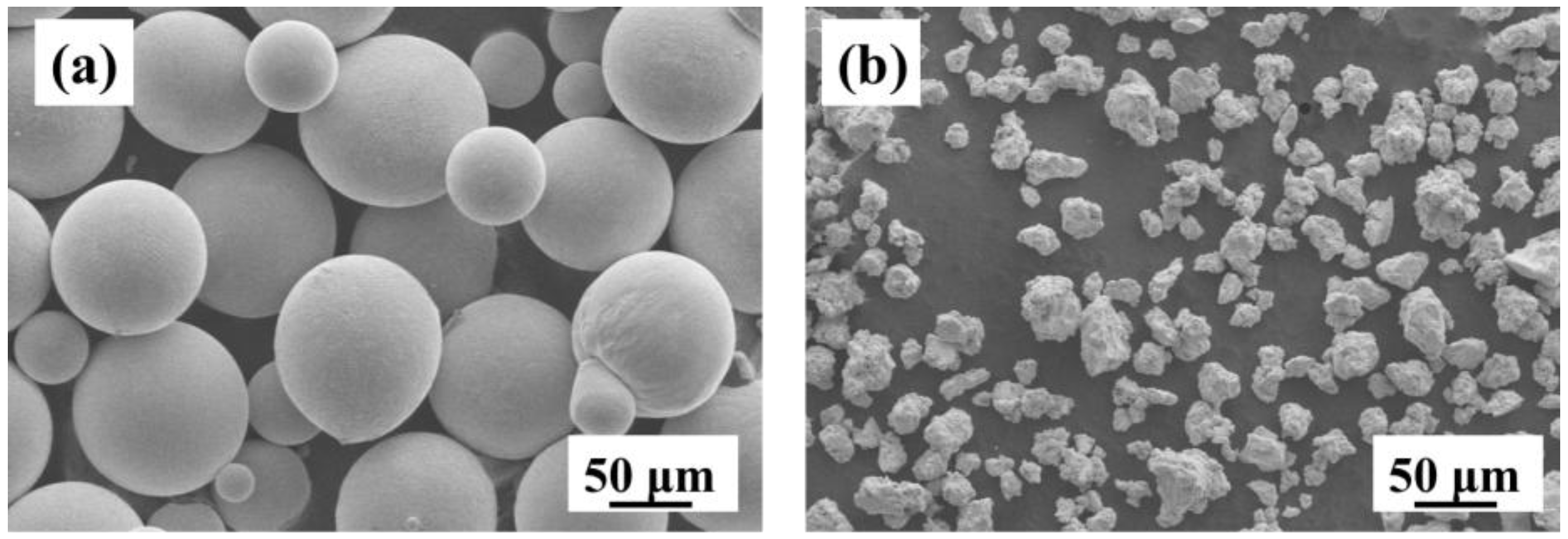
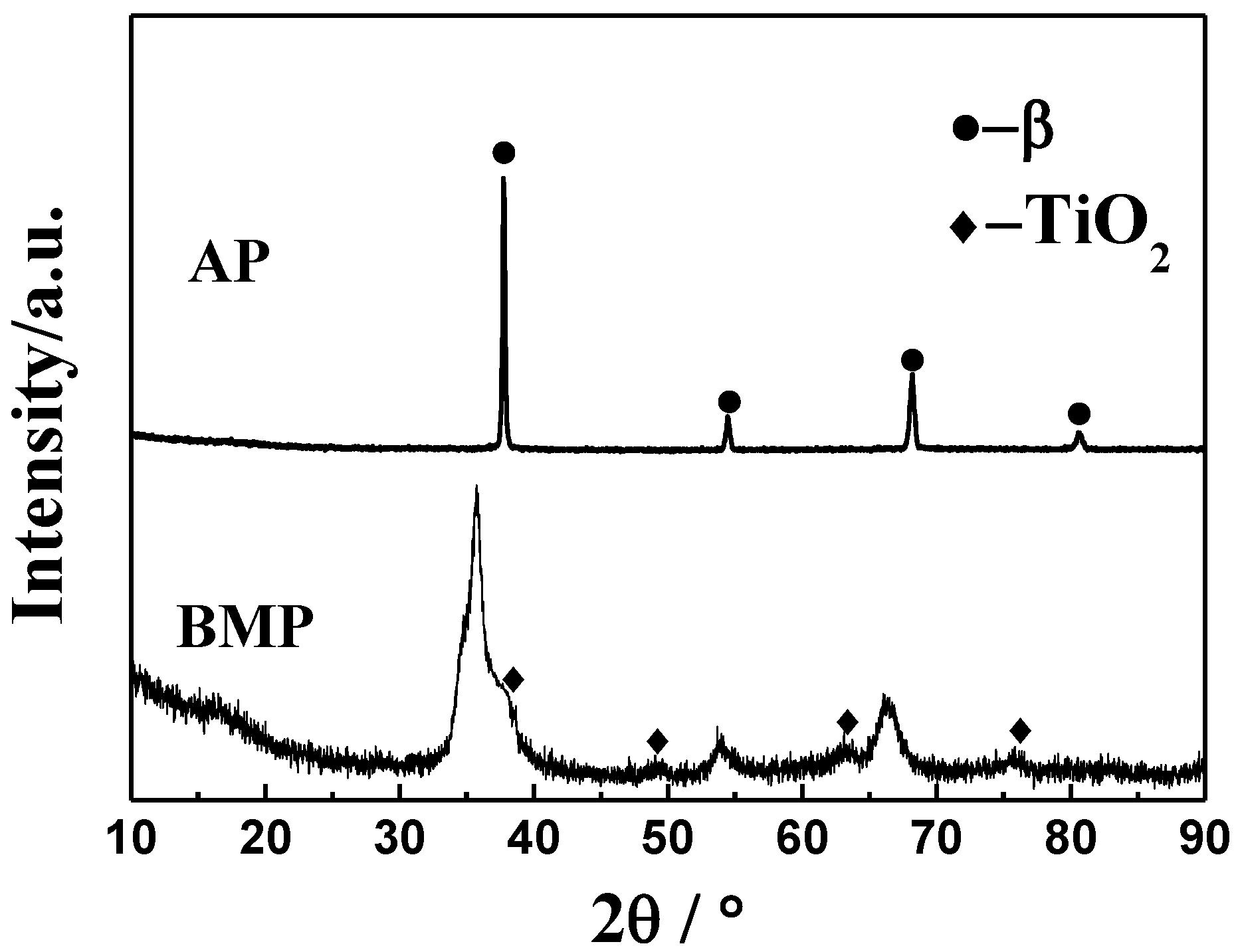
3.1. Raw Powder Characterization
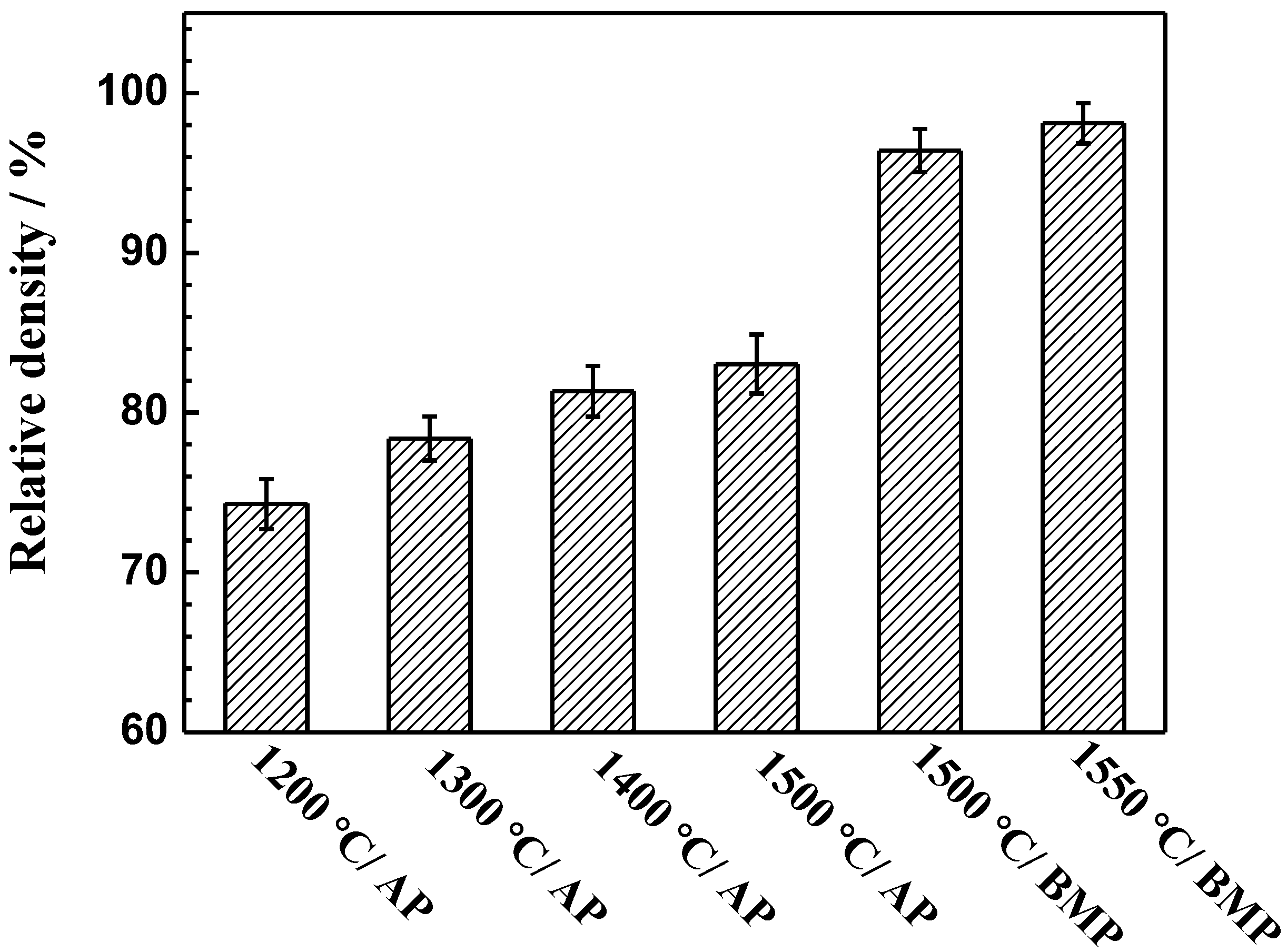
3.2. As-Sintered Density
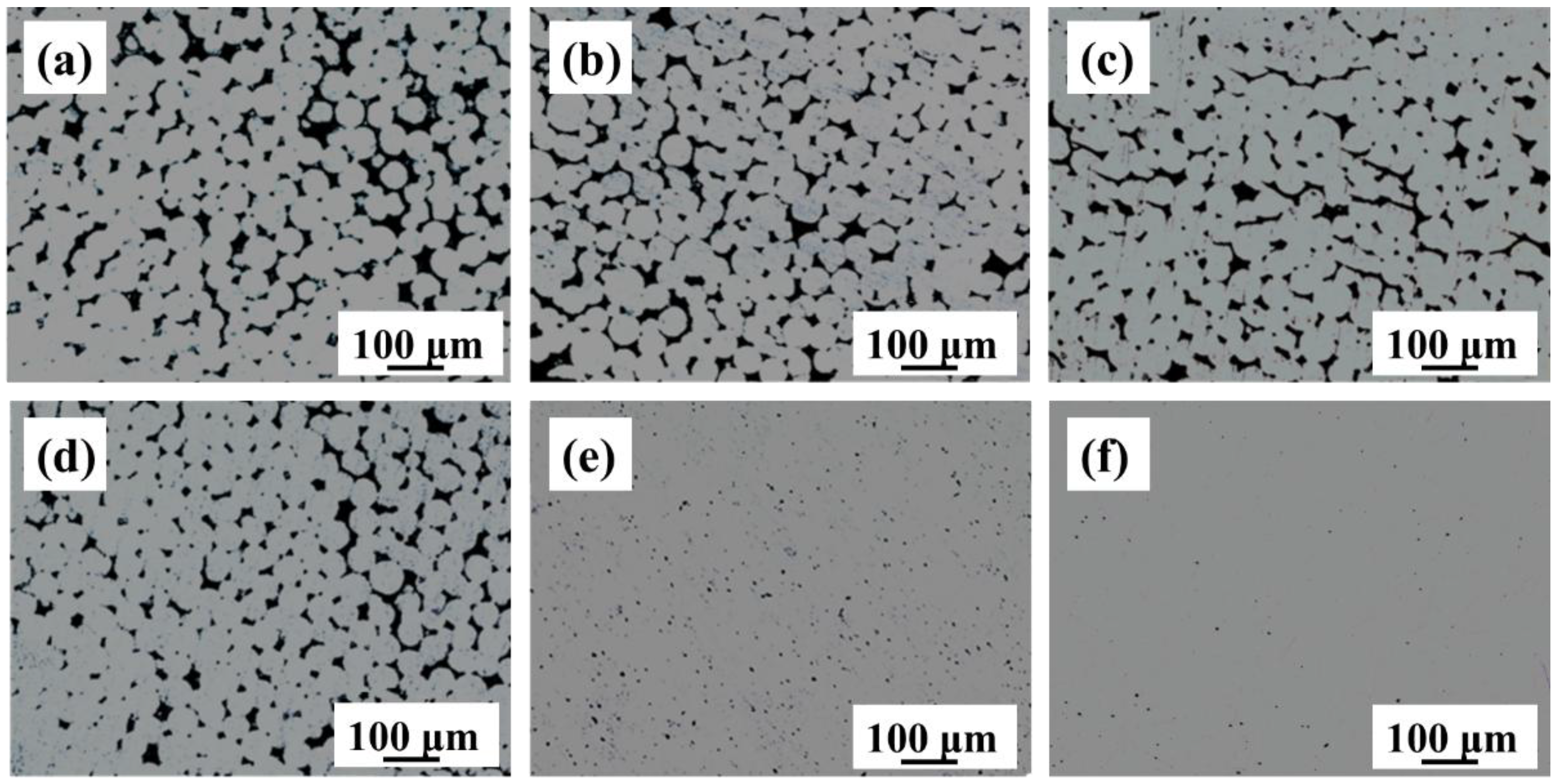
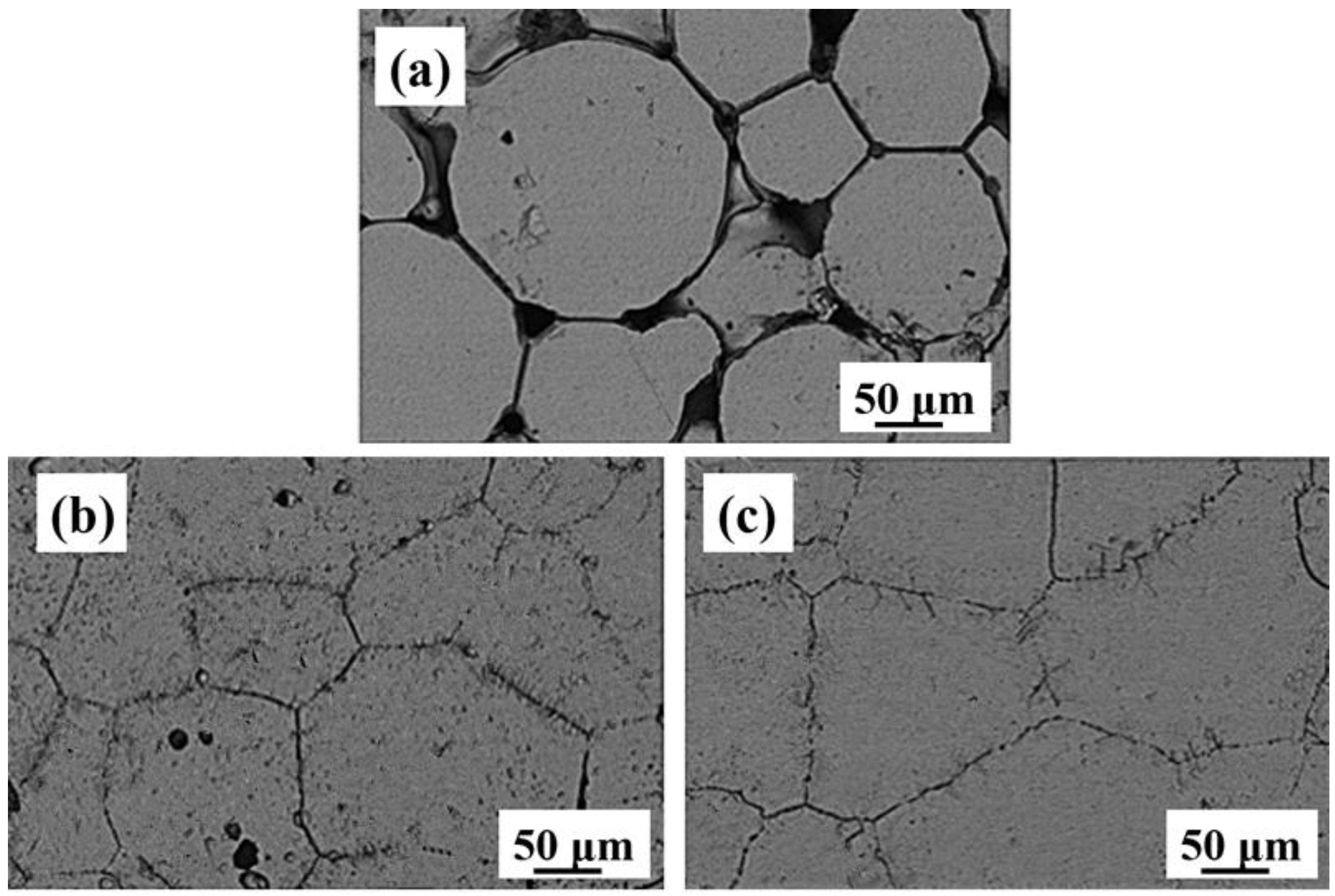
3.3. As-Sintered Microstructure
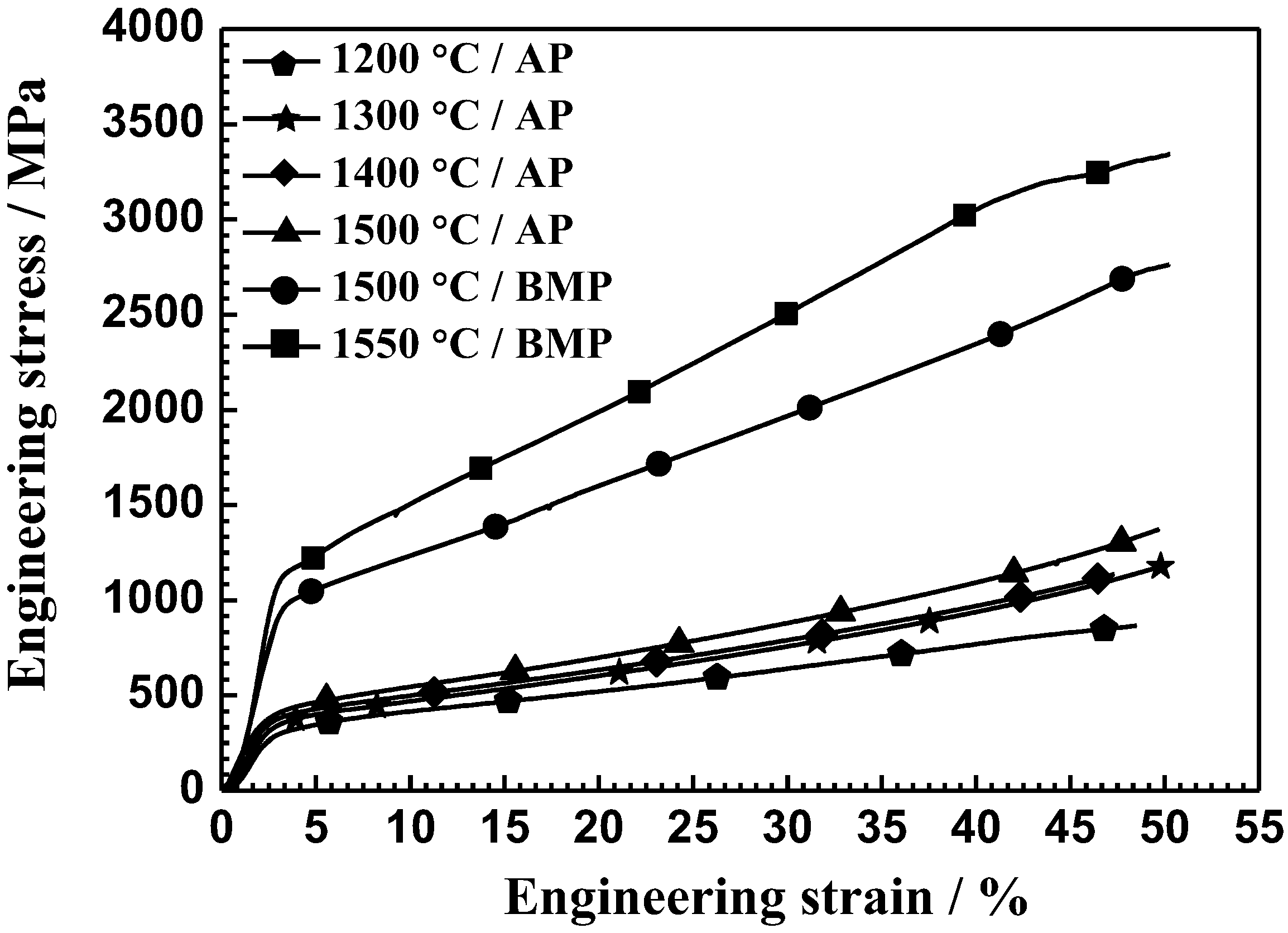
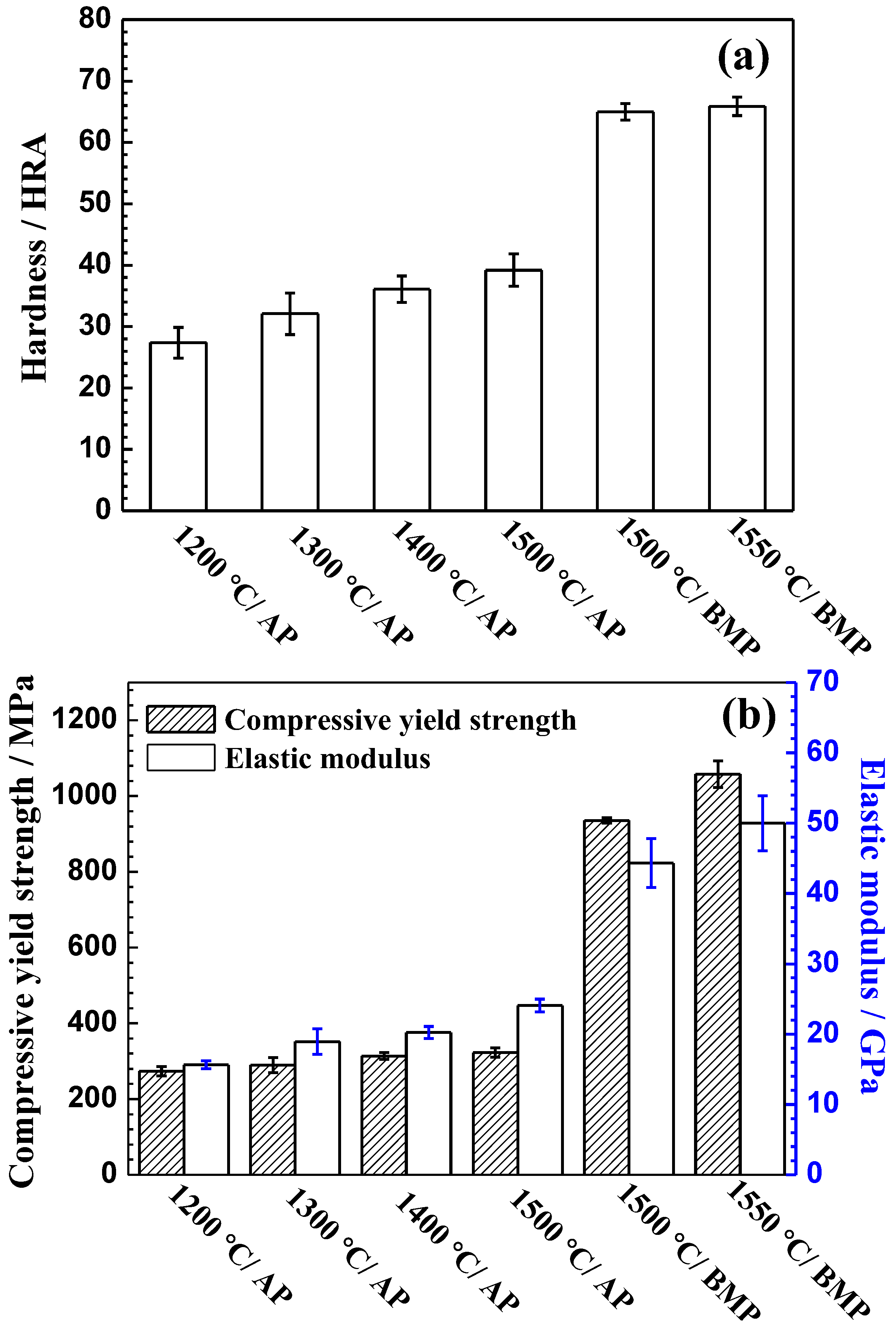
3.4. Room-Temperature Mechanical Properties
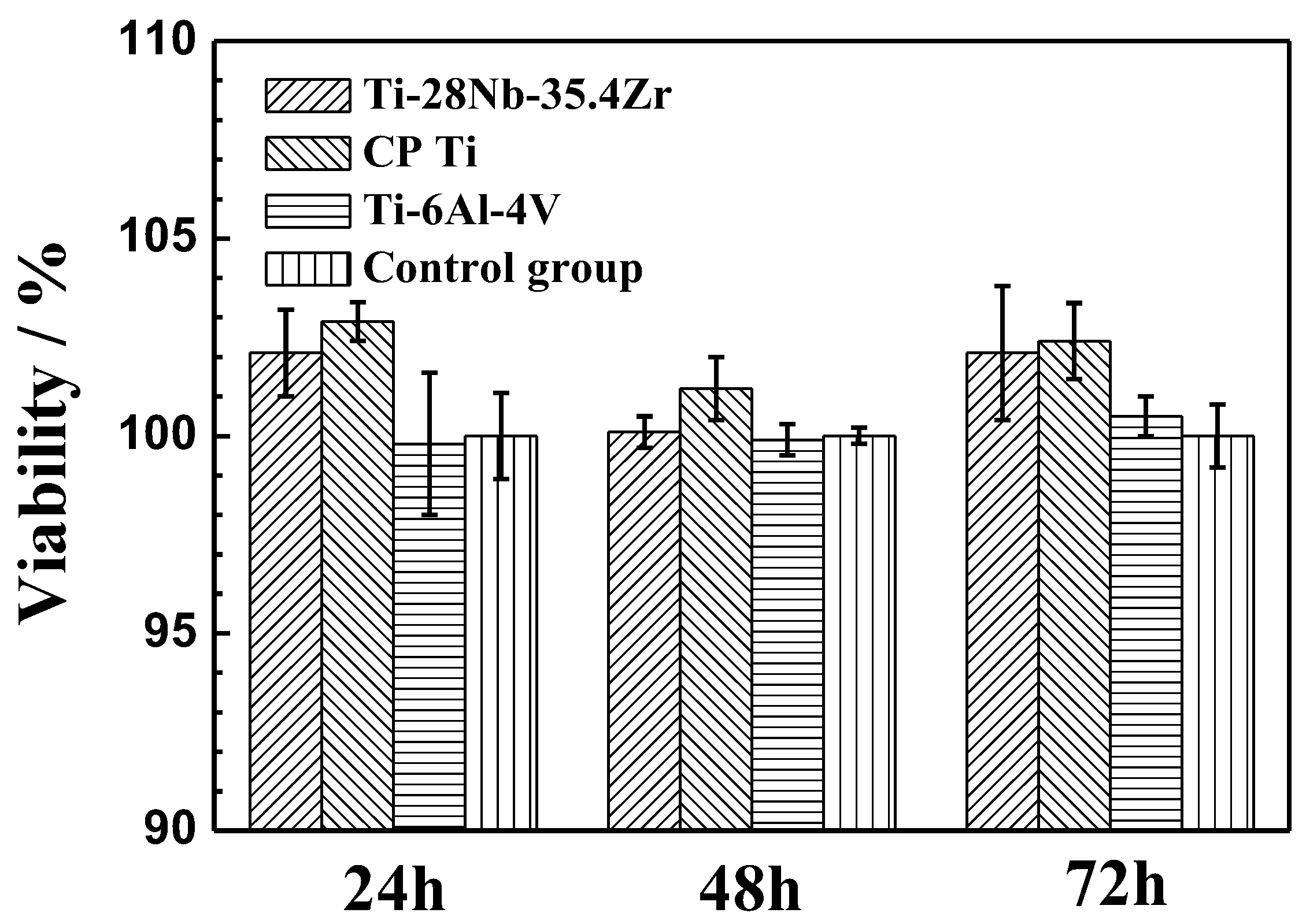
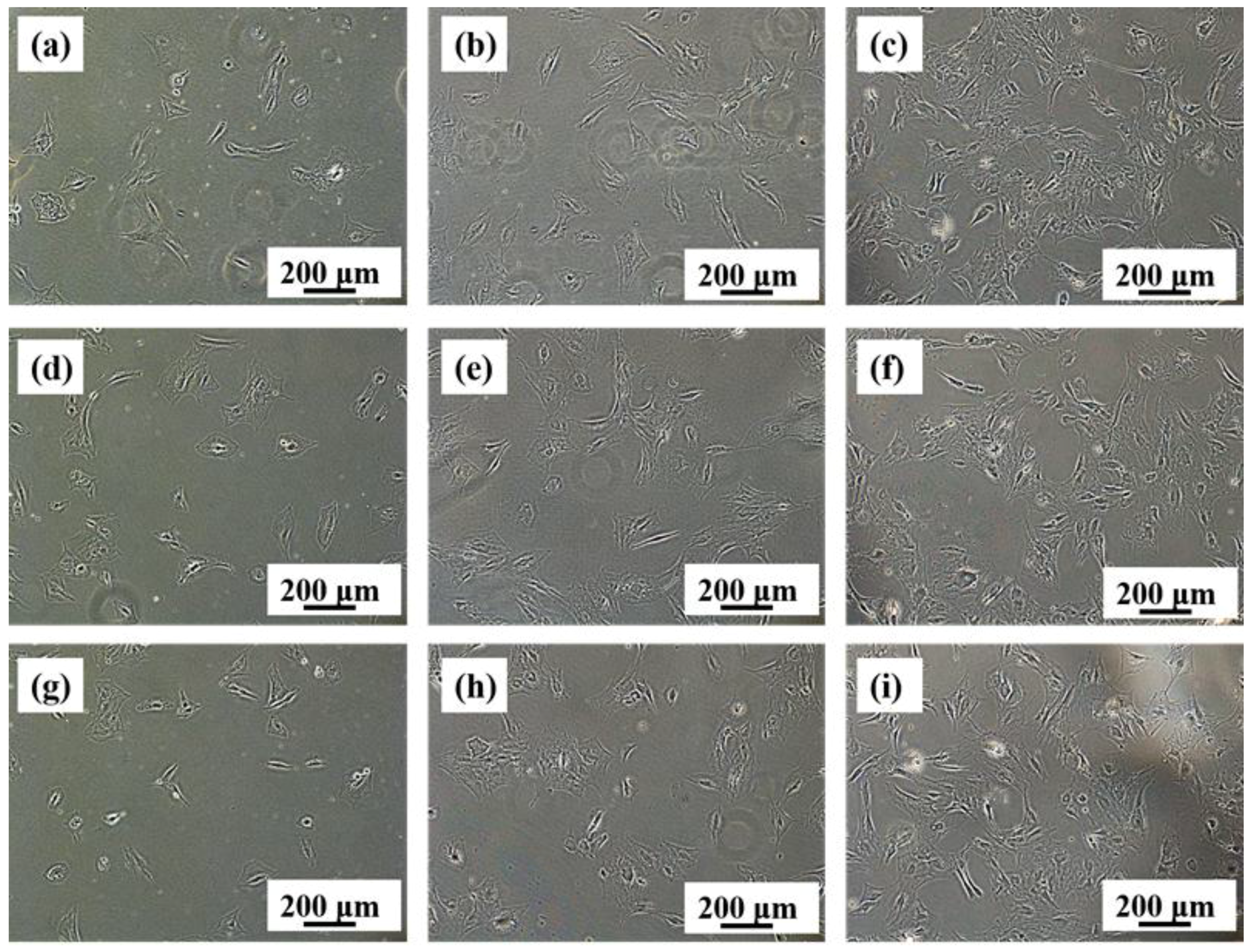
3.5. In Vitro Biocompatibility
4. Conclusions
- (1)
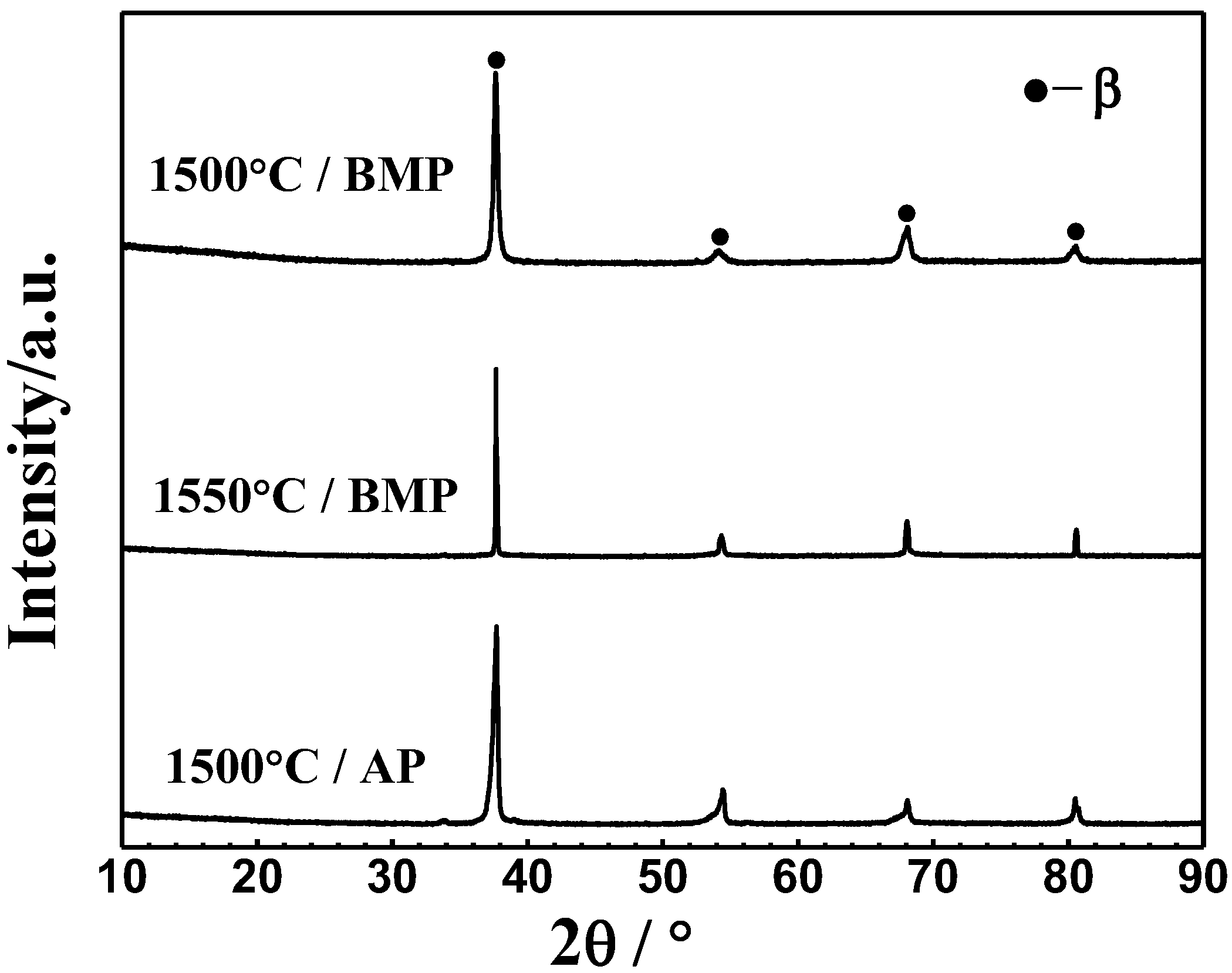
- Ti-28Nb-35.4Zr alloy with a high relative density and uniform microstructure can be obtained from milled powder via PM. After milling, the average particle sizes of powders was 15.2 μm, and when sintered at 1550 °C, the relative density reaches 98.1 ± 1.2%. The alloy fabricated by milled powders at 1550 °C is characterized by the single β phase.
- (2)
- The alloys fabricated by the ball-milled powder at 1550 °C can achieve a high mechanical properties with the compressive yield strength of 1058 ± 35.1 MPa, the elastic modulus of 50.8 ± 3.9 GP, and the hardness of 65.8 ± 1.5 HRA. This is superior to the mechanical properties fabricated by the cold-crucible levitation melting technique.
- (3)
- From in vitro cytotoxicity tests, the Ti-28Nb-35.4Zr alloy fabricated by milled powder at 1550 °C has no adverse effects on cell proliferation, and the cytotoxicity level is ranked as 0 grade, which is similar to the existing biomedical materials of CP Ti or ELI Ti-6Al-4V.
- (4)
- Based on its demonstrated net-shape manufacturability by PM, high compressive yield strength, low elastic modulus and excellent in vitro biocompatibility, the PM-fabricated Ti-28Nb-35.4Zr can be considered as an attractive orthopedic implant alloy.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Okulov, I.V.; Volegov, A.S.; Attar, H.; Bönisch, M.; Ehtemam-Haghighi, S.; Calin, M.; Eckert, J. Composition optimization of low modulus and high-strength TiNb-based alloys for biomedical applications. J. Mech. Behav. Biomed. 2016, 65, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Attar, H.; Bönisch, M.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Selective laser melting of in situ titanium-titanium boride composites: Processing, microstructure and mechanical properties. Acta Mater. 2014, 76, 13–22. [Google Scholar] [CrossRef]
- Dai, N.W.; Zhang, J.X.; Chen, C.; Zhang, L.C. Heat treatment degrading the corrosion resistance of selective laser melted Ti-6Al-4V alloy. J. Electrochem. Soc. 2017, 164, C428–C434. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Lin, J.; Li, Y.; Ping, D.H.; Yamabe-Mitarai, Y.; Wen, C.E. Investigations into Ti-(Nb,Ta)-Fe alloys for biomedical applications. Acta Biomater. 2016, 32, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Ehtemam-Haghighi, S.; Prashanth, K.G.; Attar, H.; Chaubey, A.K.; Cao, G.H.; Zhang, L.C. Evaluation of mechanical and wear properties of Ti-xNb-7Fe alloys designed for biomedical applications. Mater. Des. 2016, 111, 592–599. [Google Scholar] [CrossRef]
- Lu, S.L.; Qian, M.; Tang, H.P.; Yan, M.; Wang, J.; StJohn, D.H. Massive transformation in Ti-6Al-4V additively manufactured by selective electron beam melting. Acta Mater. 2016, 104, 303–311. [Google Scholar] [CrossRef]
- Siqueira, R.P.; Sandim, H.R.Z.; Hayama, A.O.F.; Henriques, V.A.R. Microstructural evolution during sintering of the blended elemental Ti-5Al-2.5Fe alloy. J. Alloys Compd. 2009, 476, 130–137. [Google Scholar] [CrossRef]
- Polyakova, V.V.; Semenova, I.P.; Polyakov, A.V.; Magomedova, D.K.; Huang, Y.; Langdon, T.G. Influence of grain boundary misorientations on the mechanical behavior of a near-αTi-6Al-7Nb alloy processed by ECAP. Mater. Lett. 2017, 190, 256–259. [Google Scholar] [CrossRef]
- Flaten, T.P. Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res. Bull. 2001, 55, 187–196. [Google Scholar] [CrossRef]
- Lima, P.D.; Vasconcellos, M.C.; Montenegro, R.C.; Bahia, M.O.; Costa, E.T.; Antunes, L.M.; Burbano, R.R. Genotoxic effects of aluminum, iron and manganese in human cells and experimental systems: A review of the literature. Hum. Exp. Toxicol. 2011, 30, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Xu, S.Q.; Zhou, S.W.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lu, X.; Zhang, B.; Liu, C.C.; Lv, S.M.; Yang, S.D.; Qu, X.H. Effects of Porosity on Mechanical Properties and Corrosion Resistances of PM-Fabricated Porous Ti-10Mo Alloy. Metals 2018, 8, 188. [Google Scholar] [CrossRef]
- Lee, T.; Park, K.T.; Dong, J.L.; Jeong, J.; Sang, H.O.; Kim, H.S.; Park, C.H.; Lee, C.S. Microstructural evolution and strain-hardening behavior of multi-pass caliber-rolled Ti-13Nb-13Zr. Mater. Sci. Eng. A 2015, 648, 359–366. [Google Scholar] [CrossRef]
- Niinomi, M. Fatigue performance and cyto-toxicity of low rigidity titanium alloy, Ti-29Nb-13Ta-4.6Zr. Biomaterials 2003, 24, 2673–2683. [Google Scholar] [CrossRef]
- Zhang, L.C.; Klemm, D.; Eckert, J.; Hao, Y.L.; Sercombe, T.B. Manufacture by selective laser melting and mechanical behavior of a biomedical Ti-24Nb-4Zr-8Sn alloy. Scr. Mater. 2011, 65, 21–24. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.Y.; Wang, B.L.; Qiu, K.J.; Lin, W.J.; Li, L.; Wang, Y.B.; Nie, F.L.; Zheng, Y.F. Microstructure, corrosion behavior and cytotoxicity of Zr-Nb alloys for biomedical application. Mater. Sci. Eng. C 2012, 32, 851–857. [Google Scholar] [CrossRef]
- Li, S.J.; Yang, R.; Li, S.; Hao, Y.L.; Cui, Y.Y.; Niinomi, M.; Guo, Z.X. Wear characteristics of Ti-Nb-Ta-Zr and Ti-6Al-4V alloys for biomedical applications. Wear 2004, 257, 869–876. [Google Scholar] [CrossRef]
- Kobayashi, E.; Ando, M.; Tsutsumi, Y.; Doi, H.; Yoneyama, T.; Kobayashi, M.; Hanawa, T. Inhibition effect of zirconium coating on calcium phosphate precipitation of titanium to avoid assimilation with bone. Mater. Trans. 2007, 48, 301–306. [Google Scholar] [CrossRef]
- Hanawa, T.; Okuno, O.; Hamanaka, H. Compositional change in surface of Ti-Zr alloys in artificial bioliquid. J. Jpn. Inst. Met. Mater. 1992, 56, 1168–1173. [Google Scholar] [CrossRef]
- Lee, C.M.; Ju, C.P.; Lin, J.H.C. Structure-property relationship of cast Ti-Nb alloys. J. Oral Rehabil. 2002, 29, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.F.; Chen, W.K.; Wu, S.C.; Hsu, H.C. Structure, mechanical properties, and grindability of dental Ti-Zr alloys. J. Mater. Sci. 2008, 19, 3179–3186. [Google Scholar] [CrossRef]
- Ozan, S.; Lin, J.X.; Li, Y.C.; Ipek, R.; Wen, C.E. Development of Ti-Nb-Zr alloys with high elastic admissible strain for temporary orthopedic devices. Acta Biomater. 2015, 20, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, D.K.; Matsushita, T.; Doi, K.; Takadama, H.; Nakamura, T.; Kokubo, T. Effects of oxygen content of porous titanium metal on its apatite-forming ability and compressive strength. Mater. Sci. Eng. C 2009, 29, 1974–1978. [Google Scholar] [CrossRef]
- Niu, W.J.; Bai, C.G.; Qiu, G.B.; Wang, Q. Processing and properties of porous titanium using space holder technique. Mater. Sci. Eng. C 2009, 506, 148–151. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.F.; Tang, H.P.; Liu, C.T.; Liu, B.; Huang, B.Y. Design of powder metallurgy titanium alloys and composites. Mater. Sci. Eng. A 2006, 418, 25–35. [Google Scholar] [CrossRef]
- Kipouros, G.J.; Caley, W.F.; Bishop, D.P. On the advantages of using powder metallurgy in new light metal alloy design. Metall. Mater. Trans. A 2006, 37, 3429–3436. [Google Scholar] [CrossRef]
- Sharma, B.; Vaipai, S.K.; Ameyama, K. Microstructure and properties of beta Ti-Nb alloy prepared by powder metallurgy route using titanium hydride powder. J. Alloys Compd. 2012, 656, 978–986. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, K.; Liu, L.; Wu, F.Y. Hot deformation behavior and processing map of a powder metallurgy Ti-22Al-25Nb alloy. J. Alloys Compd. 2014, 600, 215–221. [Google Scholar] [CrossRef]
- Mendes, M.W.D.; Ágreda, C.G.; Bressiani, A.H.; Bressiani, J.C. A new titanium based alloy Ti-27Nb-13Zr produced by powder metallurgy with biomimetic coating for use as a biomaterial. Mater. Sci. Eng. C 2016, 63, 671–677. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5. Biological Evaluation of Medical Devices—Part 5: Tests for Cytotoxicity: In Vitro Methods; American National Standards Institute: Arlington, VA, USA, 1999. [Google Scholar]
- Samal, C.P.; Parihar, J.S.; Chaira, D. The effect of milling and sintering techniques on mechanical properties of Cu-graphite metal matrix composite prepared by powder metallurgy route. J. Alloys Compd. 2013, 569, 95–110. [Google Scholar] [CrossRef]
- Wen, M.; Wen, C.E.; Hodgson, P.; Li, Y.C. Fabrication of Ti-Nb-Ag alloy via powder metallurgy for biomedical applications. Mater. Des. 2014, 56, 629–634. [Google Scholar] [CrossRef]
- Li, Y.; Wen, C.W.; Mushahary, D.; Sravanthi, R.; Harishankar, N.; Pande, G.; Hodgson, P. Mg-Zr-Sr alloys as biodegradable implant materials. Acta Biomater. 2012, 8, 3177–3188. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Li, L.; Zheng, Y.F. In vitro cytotoxicity and hemocompatibility studies of Ti-Nb, Ti-Nb-Zr and Ti-Nb-Hf biomedical shape memory alloys. Biomed. Mater. 2010, 5, 044102. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.; Carvalho, O.A.S.; Schneider, S.G.; Schneider, S. Corrosion behavior of Ti-xNb-13Zr alloys in Ringer’s solution. Mater. Corros. 2015, 59, 929–933. [Google Scholar] [CrossRef]
- Robin, A.; Carvalho, O.A.S. Influence of pH and Fluoride Species on the Corrosion Behavior of Ti-xNb-13Zr Alloys in Ringer’s Solution. Adv. Mater. Sci. Eng. 2013, 1, 1–10. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Li, M.; Wen, C.; Lv, S.; Liu, C.; Lu, X.; Qu, X. The Mechanical Properties and In Vitro Biocompatibility of PM-Fabricated Ti-28Nb-35.4Zr Alloy for Orthopedic Implant Applications. Materials 2018, 11, 531. https://doi.org/10.3390/ma11040531
Xu W, Li M, Wen C, Lv S, Liu C, Lu X, Qu X. The Mechanical Properties and In Vitro Biocompatibility of PM-Fabricated Ti-28Nb-35.4Zr Alloy for Orthopedic Implant Applications. Materials. 2018; 11(4):531. https://doi.org/10.3390/ma11040531
Chicago/Turabian StyleXu, Wei, Ming Li, Cuie Wen, Shaomin Lv, Chengcheng Liu, Xin Lu, and Xuanhui Qu. 2018. "The Mechanical Properties and In Vitro Biocompatibility of PM-Fabricated Ti-28Nb-35.4Zr Alloy for Orthopedic Implant Applications" Materials 11, no. 4: 531. https://doi.org/10.3390/ma11040531
APA StyleXu, W., Li, M., Wen, C., Lv, S., Liu, C., Lu, X., & Qu, X. (2018). The Mechanical Properties and In Vitro Biocompatibility of PM-Fabricated Ti-28Nb-35.4Zr Alloy for Orthopedic Implant Applications. Materials, 11(4), 531. https://doi.org/10.3390/ma11040531







