Abstract
BiSnSbO6 with strong photocatalytic activity was first fabricated by a high-temperature, solid-state sintering method. The resulting BiSnSbO6 was characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), UV-vis diffuse reflectance spectroscopy (DRS) and X-ray photoelectron spectroscopy (XPS). The results showed that BiSnSbO6, with a pyrochlore structure and a cubic crystal system by a space group Fd3m, was well crystallized. The lattice parameter or the band gap of BiSnSbO6 was 10.234594 Å or 2.83 eV. Compared with N-doped TiO2, BiSnSbO6 showed higher photocatalytic activity in the degradation of benzotriazole and rhodamine B. The apparent first-order rate constant for BiSnSbO6 in the degradation of benzotriazole and rhodamine B was 0.0182 min−1 and 0.0147 min−1, respectively. On the basis of the scavenger experiment, during the photocatalytic process, the main active species were arranged in order of increasing photodegradation rate: •OH < •O2− < h+. The removal rate of benzotriazole or rhodamine B was approximately estimated to be 100% with BiSnSbO6 as a photocatalyst after 200 min visible-light irradiation. Plentiful CO2 produced by the experiment indicated that benzotriazole or rhodamine B was continuously mineralized during the photocatalytic process. Finally, the possible photodegradation pathways of benzotriazole and rhodamine B were deduced.
1. Introduction
Benzotriazole UV stabilizers (BZT-UVs) are one of most important synthetic compounds in plastic additives. Because of their large annual production, as well as their widespread use in building materials and paints, environmental and health risk assessments of BZT-UVs have gradually attracted increasing attention. In the current study, most of the target BZT-UVs have been included in the USEPA (United States Environmental Protection Agency) High Production Volume Challenge Program, and the HPV (High Production Volume) list for the Organization for Economic Co-operation and Development, and are each manufactured at over 450 tons and 1000 tons per year [1]. In addition, a substantial part of BZT-UVs have been measured in environmental samples—such as in surface water in rivers and lakes [2,3], soil [4,5], and fish [6,7]—which may result in the different degrees of bioaccumulation of BZT-UVs in an aquatic food web and finally in the human body. It is noteworthy that previous studies have shown the potential for endocrine disruption and liver toxicity by BZT-UVs [3,8]. Nowadays, the removal and mineralization of BZT-UVs have become more difficult, because of its high water solubility, low vapor pressure, low octanol–water distribution coefficients and fair resistance to biodegradation [9,10,11].
TiO2, an efficient photocatalyst, has drawn much attention due to its widespread use in self-cleaning construction materials, environmental protection technologies and other applications, but its catalytic activity is limited by ultraviolet (UV) light irradiation [12,13,14,15]. In recent years, there are only two studies advancing the photocatalytic activity of TiO2 particles on degrading BZT-UVs. Ding et al. [16] reported that the degradation removal of benzotriazole was significant, up to 89.8% under 180 min UV irradiation when a potential bias of +0.8 V was applied to the TiO2 thin film, indicating a synergistic reaction between photocatalysis and electrochemical oxidation. The TiO2 loaded on FeIIFe2IIIO4@C, synthesized by Jorfi et al. [17], effectively acted as an activator of peroxomonosulfate to oxidatively degrade benzotriazole. Another study reported by Xu et al. [11] also studied the impact of other efficient photocatalysts on degrading BZT-UVs. They found that BiOBr catalysts with different morphologies gradually removed nearly 90% of benzotriazole after visible light irradiation for 3 h, whereas P25 only removed about 50% of benzotriazole under the same conditions.
At present, in order to optimize visible light, which occupies 43% of sunlight, many efforts have been made to develop photocatalysis with high activity for visible light. Some new types of semiconductors with an obvious visible light response have been explored in recent years. In addition, elemental doping [18,19] and the formation of heterojunctions [20,21,22,23,24,25,26] have attracted researchers’ attention in order to improve the degradation efficiency of pollutants under visible light irradiation. Among these visible-light-driven photocatalysts, Bi-based photocatalysts with high photocatalytic activity, such as BiVO4 [27], BiOX (X = Br, I, Cl) [28,29] and Bi2MoO6 [30], have been widely used due to their wide range of visible light absorption and environmental friendliness. In addition, it has been found that the visible light absorption of photocatalysts can be enhanced via doping Sb [31]. Yang et al. [32] evaluated the photocatalytic activity of the reactions for CO2 reduction and gaseous iso-isopropanol oxidation using a novel Sb-doped SnO2/porous g-C3N4 photocatalyst. The obtained results showed that the prepared samples significantly improved the photocatalytic efficiency. Nasser et al. [33] reported that the energy change caused by Sb doping obviously led to the decrease of the band gap energy of ZnO. Moreover, previous works have shown that the photo-excitation of electrons in an O2p and Bi6s hybrid orbital may result in a charge transfer occurring to a d orbital of the other metal in the composite oxide [34,35,36]. Sn-Sb-Bi composite oxides may respond to visible light irradiation because Sn and Sb respectively occupy 5d orbits in the ground state. Based on above research and discussion, BiSnSbO6 was synthesized in this work to measure the photocatalytic degradation of BZT-UVs.
Rhodamine B (RhB), one of the most important elements of xanthene dyes, is resistant to biodegradation and direct photolysis, producing potentially carcinogenic aromatic amines after undergoing natural reductive anaerobic degradation [37,38,39,40]. However, it is widely used in the industrial field, especially as a photosensitizer, quantum counter and active medium in dye lasers [41,42,43]. In this report, therefore, RhB and benzotriazole (BZT) were used as the target contaminants to evaluate the photocatalytic activity of BiSnSbO6 with a xenon arc lamp as a visible light source.
The aim of this work was to prepare a new semiconductor catalyst BiSnSbO6 via a solid-state reaction method and evaluate the catalytic performance for the photodegradation of RhB and BZT. For comparison, we chose a conventional photocatalyst, nitrogen-doped titanium dioxide (N-doped TiO2), as a catalyst for the degradation of RhB and BZT under visible light irradiation. Additionally, the structure and photocatalytic properties of BiSnSbO6 have been researched via a variety of techniques, including XRD, SEM, TEM, XPS and UV–vis DRS. Finally, the possible degradation pathways of BZT and RhB have been proposed based on the identification of the reaction intermediates via liquid chromatograph-mass spectrometer (LC–MS) analysis.
2. Materials and Methods
2.1. Preparation of BiSnSbO6 and N-Doped TiO2 Photocatalysts
BiSnSbO6 was prepared by a high-temperature, solid-state sintering method. Bi2O3, SnO2 and Sb2O5 with a purity of 99.99% (Sinopharm Group Chemical Reagent Co., Ltd., Shanghai, China) were used as raw materials. Due to the volatile performance of Bi2O3 at high temperature, we ultimately made a resolve to increase the quantity of Bi2O3 up to 150%. These fully-mixed materials (n(Bi2O3):n(SnO2):n(Sb2O5) = 1.5:2:1) were ground in a ball mill until the powder particle size was 1–2 µm. All powders were dried at 200 °C for 4 h before synthesis. Then these powders were mixed in an aluminum oxide crucible (Shenyang Crucible Co., Ltd., Shenyang, China) in which above-mentioned materials were pressed into disks and were sintered in an electric furnace (KSL 1700X, Hefei Kejing Materials Technology CO., Ltd., Hefei, China) at 400 °C for 8 h. After crushing and pressing, the mixture was sintered again in an electric furnace at 900 °C for 25 h. Finally, pure BiSnSbO6 catalyst with a light yellow color was obtained after total grinding.
Nitrogen-doped titanium dioxide (N-doped TiO2) catalyst was synthesized by a sol-gel method and tetrabutyl titanate was selected as the titanium source. Firstly, 17 mL tetrabutyl titanate and 40 mL absolute ethyl alcohol were mixed as solution A. Then 10 mL glacial acetic acid and 5 mL double distilled water were sequentially dripped into solution A, and then the solution formed a transparent colloidal suspension named solution B after stirring for 0.5 h. Next, aqua ammonia with n(N)/n(Ti) of 8% was added into solution B with vigorous stirring for 1 h. After two days, a xerogel took shape. Eventually, after grinding the xerogel into powder and calcining at 500 °C for 2 h, N-doped TiO2 powder could be obtained by grinding these powders in agate mortar and screening by shaker.
2.2. Characterization
The particle morphology and chemical composition of BiSnSbO6 were obtained respectively by using a transmission electron microscope (TEM, Tecnal F20 S-Twin, FEI Corporation, Hillsboro, OR, USA) and scanning electron microscope-X-ray energy dispersion spectrum (SEM-EDS, LEO 1530VP, LEO Corporation, Dresden, Germany). The oxygen content, Bi3+ content, Sn4+ content and Sb5+ content of BiSnSbO6 were analyzed by X-ray photoelectron spectroscopy (XPS, ESCALABMK-2, VG Scientific Ltd., London, UK). In the depth profile of BiSnSbO6, its chemical composition was gauged by the argon ion denudation or XPS. The powder X-ray diffraction (XRD) spectra was recorded on a D/MAX-RB advance spectrometer (D/MAX-RB, Rigaku Corporation, Tokyo, Japan) with Cu Kα radiation (λ = 1.54056 Å), in which the operating temperature was 295 K and the scanning range was 2θ = 10°–95°. The Brunauer–Emmett–Teller (BET) surface area of BiSnSbO6 was determined via a ASAP 2020 physical adsorption apparatus (Micromeritics Corporation, Atlanta, GA, USA) with N2 adsorption at liquid nitrogen temperature. The optical properties of BiSnSbO6 were estimated by UV-visible spectrophotometer assembled with an integrating sphere (Shimadzu UV-2550 UV-Visible spectrometer, Kyoto, Japan). Room temperature photoluminescence (PL) measurement was performed by using a fluorescence spectrophotometer (Perkin Elmer, LS 55, Waltham, MA, USA).
2.3. Photocatalytic Properties Test
A total of 0.8 g photocatalyst (BiSnSbO6 or N-doped TiO2) powder was added into 300 mL 0.032 mM RhB solution (or 300 mL 0.032 mM BZT solution) in a pyrex glass cell (Jiangsu Yancheng Huaou Industry, Yancheng, China) with a magnetic rotor to form a suspension system. In order to guarantee the establishment of an adsorption/desorption equilibrium among the photocatalyst (BiSnSbO6 or N-doped TiO2), the RhB dye (or BZT) and dissolved oxygen, above suspension was magnetically stirred in the dark for 45 min before irradiation with simulated sunlight. The photodegradation of RhB (or BZT) (Tianjin Kermel Chemical Reagent Co., Ltd., Tianjin, China) was in progress. The photocatalytic reaction system (Nanjing JYZCPST CO., Ltd., Nanjing, China) that we used in this paper was made up of a 500 W xenon arc lamp of which the major emission wavelength was 436 nm, a cut-off filter with a cut-off wavelength of 400 nm (Jiangsu Nantong JSOL Corporation, Nantong, China), and a magnetic stirrer. The xenon arc lamp which was placed in the internal part of an optical quartz reactor vessel with a diameter of 5.8 cm and a length of 68 cm, was encircled by a quartz jacket, in which some pyrex glass cells with a homogeneous suspension solution of RhB (or BZT) and photocatalyst (BiSnSbO6 or N-doped TiO2) was circulated. Room temperature (25 °C) was maintained by an outer recycling water glass sleeve. The solution was continuously stirred and inflated. A total of 3 mL sample was sampled at different time intervals and also filtered by using a 0.22 μm filter membrane. The incident photon flux Io, which was gauged by a radiometer (Model FZ-A, Photoelectric Instrument Factory Beijing Normal University, Beijing, China), was measured to be 4.76 × 10−6 Einstein L−1·s−1 under visible light irradiation (wavelength coverage at 400–700 nm). The incident photon flux on the photoreactor was determined by the distance from the photoreactor to the xenon arc lamp. In addition, the initial pH value in this work was 7.0. The concentration of RhB was measured by an UV-Vis spectrophotometer (Shimadzu UV-2550 UV-Visible spectrometer, Kyoto, Japan) with the detecting wavelength at 553.5 nm. The concentration of BZT was evaluated by Agilent 1200 high performance liquid chromatography (Agilent Technologies, Palo Alto, CA, USA) with an UV detector and a Zorbax 300SB-C18 column (4.6 mm × 150 mm, 5 μm). A mixture of 50% CH3CN and 50% distilled deionized water was used as the mobile phase. The UV detection wavelengths were set at 254 nm. The injection volume of post-photodegradation BZT solution was 10 μL and the flow rate was 1 mL·min−1.
An ion chromatograph (DX-300, Dionex Corporation, Sunnyvale, CA, USA) was used to analyze the inorganic products generated in the process of RhB and BZT photodegradation. LC-MS (Thermo Quest LCQ Duo, Thermo Fisher Scientific Corporation, Silicon Valley, CA, USA, Beta Basic-C18 HPLC column: 150 mm × 2.1 mm, ID of 5 μm, Finnigan, Thermo Fisher Scientific Corporation, Silicon Valley, CA, USA) was applied to measure the appraisal of RhB or BZT and its photodegradation intermediate products. Here, the volume of post-photodegradation solution injected automatically into the LC-MS system was 20 μL. The mixture of 60% methanol and 40% water was served as eluent with a flow rate of 0.2 mL·min−1. MS conditions included an electrospray ionization interface, a capillary temperature of 300.15 K with a voltage of 19.00 V, a spray voltage of 5000 V and a constant sheath gas flow rate. Under the negative ion scan mode with the m/z range of 50–600, the spectrum could be acquired. The intersmatTM IGC120-MB gas chromatograph (Thermo Separation Products Corporation, Brussels, Belgium) assembled with a porapack Q column (length of 3 m and an inner diameter in 0.25 cm), which was linked to a catharometer detector, was used to analyze the CO2 production. A total organic carbon (TOC) analyzer (TOC-5000, Shimadzu Corporation, Kyoto, Japan) was used to measure the TOC concentration during the photodegradation of RhB or BZT. The photon efficiency of the photocatalyst (BiSnSbO6 or N-doped TiO2) was calculated on the basis of the following equation [44,45]:
where ϕ was the photon efficiency (%), R was the photodegradation rate of RhB (or BZT) (moL·L−1·s−1), and Io was the incident photon flux (Einstein·L−1·s−1).
ϕ = R/Io
2.4. Photoelectrochemical Properties Test
In order to explore the photoelectrochemical properties of BiSnSbO6, the modified electrodes were prepared as reported by Mao et al. [46] and the preparation technology was as following: firstly, 2 cm × 1 cm ITO glass was sequentially cleaned by methylbenzene, acetone, ethyl alcohol and deionized water before photoelectrochemical measurement. Secondly, 20 mg of BiSnSbO6 powder was mixed in 2 mL of dimethylformamide solution under sonication for 2 h to form the slurry. Thirdly, the slurry was uniformly daubed onto the conductive surface of the ITO substrate and dried at 120 °C for 10 min. The electrochemical measurements were then carried out on a CHI760E electrochemical workstation (Shanghai Chen Hua Instrument Co., Ltd., Shanghai, China) three-electrode photoelectrochemical cell in 0.2 M Na2SO4 aqueous. Here, the electrolyte with BiSnSbO6 coated on ITO glass was as the working electrode, and Ag/AgCl (3 M KCl) was as the counter electrode and a saturated calomel electrode was as the reference electrode. A 500 W xenon arc lamp was utilized as the light source.
3. Results and Discussion
3.1. Characterization
In order to obtain insight concerning the microstructure and morphology of BiSnSbO6, chemical analysis was carried out by using SEM and TEM. Figure 1 shows the SEM image of BiSnSbO6. As shown in Figure 1, the morphology of BiSnSbO6 was irregular and the diameter of the particles was about 0.5–1.5 µm. Figure 2a and Figure 2b show a high resolution TEM picture of BiSnSbO6 and the selected area electron diffraction pattern of BiSnSbO6. It was clear from Figure 2a that the particle size of BiSnSbO6 with irregularity was relatively uniform, and the particle size was about 200 nm to 300 nm. Some particles were agglomerated, and this result was similar to the SEM analysis. In Figure 2b, bright diffraction can be observed and the diffraction point is in a cycle arrangement, indicating the highly crystalline nature and single crystal of BiSnSbO6. Figure 2c shows the HRTEM image of BiSnSbO6 with clear lattice fringe spacing. The lattice fringe spacing shown in Figure 2c was estimated to be 0.304 nm and 0.256 nm, corresponding to the (222) and (400) lattice plane of BiSnSbO6, respectively.
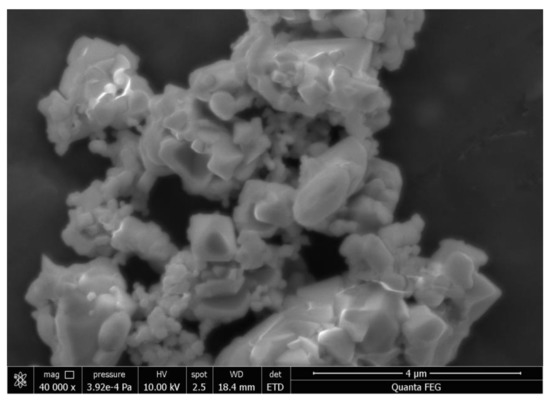
Figure 1.
SEM image of BiSnSbO6.
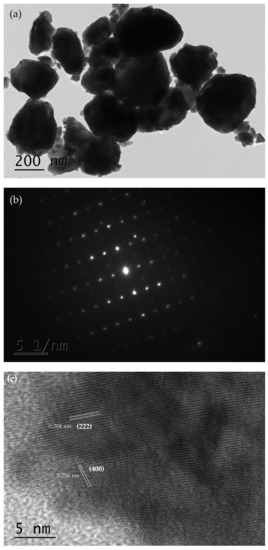
Figure 2.
The high resolution TEM picture of BiSnSbO6 (a), the selected area electron diffraction pattern of BiSnSbO6 (b) and the HRTEM image of BiSnSbO6 with clear lattice fringe spacing (c).
According to the Rietveld analysis, the comprehensive structure refinement of the data gathered from the RIETANTM [47] program and the XRD image of BiSnSbO6 are revealed in Figure 3a. The results indicated that BiSnSbO6, with a pyrochlore structure and a cubic system with a space group of Fd3m, was well crystallized. Meanwhile, it could be perceived from the XRD image that a good correlation was confirmed between the observed and calculational intensities of the structure of BiSnSbO6. The calculation results for BiSnSbO6 refinement received the unweighted R factors (RP = 15.43% < 20%), revealing good agreement with the experimental pattern in the whole 2θ range of 10°–95°. According to the high-purity precursors adopted in this work, we implied that the slightly refined structure of BiSnSbO6 could obtain slightly higher R factors. It should be noted that the defects or the disorder/order of a small portion of the atoms could lead to structural varieties, particularly for bond moment distributions, thermal displacement parameters and occupation factors for certain atoms [48]. Moreover, Figure 3b shows the XRD spectrum of N-doped TiO2. It could be seen from Figure 3b that the peaks for the as-prepared N-doped TiO2 sample corresponded to the anatase TiO2 phase according to the JCPDS No. 21-1272.
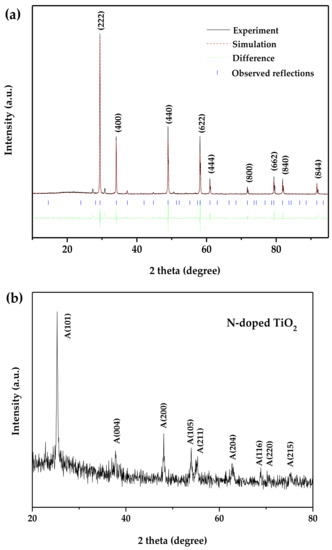
Figure 3.
(a) The Pawley refinement outcomes of XRD data for BiSnSbO6; (b) XRD spectrum of N-doped TiO2.
In addition, BiSnSbO6 was a single phase and the lattice parameter α for BiSnSbO6 was 10.234594 Å according to above refinement results of the XRD data. The Bragg equation (2dsinθ = nλ, here, n = 1, λ = 1.54056 Å) and the calculation formula for the cubic crystal face spacing equation (d = α(h2 + k2 + l2)−1/2) were combined to calculate the lattice parameter α for BiSnSbO6. Here, d was the face spacing, and θ was the diffraction angle, and h, k and l were the indices of crystallographic plane. On the basis of the lattice fringe spacing shown in Figure 2c, the lattice parameter α for BiSnSbO6 was found to be 10.530869 Å or 10.240000 Å, which was basically accordant to above refined lattice parameter result. According to the calculation of the lattice parameter for every diffraction peak of the XRD data and the refinement results of BiSnSbO6, an optimal lattice parameter for BiSnSbO6 could be clearly received.
On the basis of the lattice parameter α for BiSnSbO6 with a cubic system, the unit cell bulk V value of BiSnSbO6 was found to be 1072.04(2) Å3, which was 2.2 times than that of Bi2WO6 (V = 487.30(5) Å3) [49], and 7.8 times than that of N-doped TiO2 (V =136.57 Å3) [50]. Compared with N-doped TiO2, the size expansion of BiSnSbO6 might result in an increase of the bond lengths. Previous work had found that the closer the M-O-M bond angle was to 180°, the more delocalized the excited state was, which could lead to the easier movement of charge carriers in the matrix [49,50,51]. The increase of the photon efficiency of BiSnSbO6 was owing to high diffusivity, which contributed to more excited photogenerated electrons and holes on the surface of a photocatalyst. In addition, the O-Bi-O bond angle of BiSnSbO6 was 90°, while the O-Bi-O bond angle of Bi2WO6 only ranged from 65.5(7)° to 87.3(6)°. As for BiSnSbO6, the angle of the Sn-O-Bi bond or Bi-Sn-Sn bond or Bi-O-Bi bond was 116.5(6)° or 135° or 126.8(7)°, which was close to 180°. Moreover, the angle of the Bi-O-Sn bond or Sn-O-Sn bond or O-Sn-O bond was 180°. Therefore, the photocatalytic activity of BiSnSbO6 might be accordingly higher.
The absorption spectra of BiSnSbO6 and N-doped TiO2 are shown in Figure 4. In comparison with the N-doped TiO2, of which the photoresponse wavelength was less than 410 nm, the photoresponse wavelength of newly synthesized BiSnSbO6 was invented at 440 nm, indicating that BiSnSbO6 photocatalyst could response to visible light and rise in the range of the whole absorption spectrum.
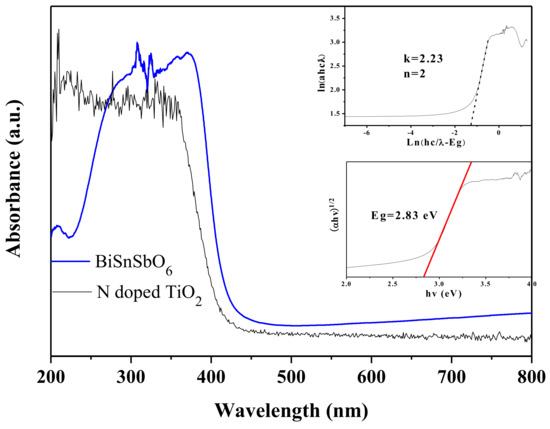
Figure 4.
UV-Vis diffuse reflectance spectra of BiSnSbO6 and N-doped TiO2 (inset was plot of ln(αhν) versus ln(hν − Eg) or plot of (αhν)1/2 versus hν for BiSnSbO6).
The light absorption near the band edge for a crystalline semiconductor was found according to the following equation [52,53]: αhν = A(hν − Eg)n, where A, α, Eg and ν were proportional constant, absorption coefficient, band gap and light frequency, respectively. Within this equation, the peculiarity of the interim in a crystalline semiconductor was decided by the superscript n. The following steps were to count Eg and n: (i) assuming an approximate value of Eg through the equation Eg = 1240/λ; (ii) drawing plot ln(αhν) versus ln(hν − Eg) spectra to obtain the value of n; (iii) drawing plot (αhν)1/n versus hν to refine the value of Eg. Additionally, hν was equal to ch/λ, where c, h and λ were light velocity, Planck constant and wavelength of incident light. The Eg of BiSnSbO6 was found to be 2.83 eV in this way. The n of BiSnSbO6 was found to be 2, indicating the indirect light transition for BiSnSbO6.
The XPS full spectrum of BiSnSbO6 and the characteristic peak XPS spectra of their elements are shown in Figure 5. From Figure 5a, it could be seen that the BiSnSbO6 catalyst contained only the elements bismuth, stannum, antimony, oxygen and carbon, indicting the absence of any other impurity elements. Here, the carbon element was due to the addition of hydrocarbons which were used to facilitate the testing and calibration of the elements. Hence, it could be easily found that the prepared substance BiSnSbO6 was of high purity. Moreover, the XPS peaks of BiSnSbO6 showed side-by-side peaks between Sb and O. In order to further analyze the surface chemical states of BiSnSbO6, the binding energy of the characteristic peaks of each element in BiSnSbO6 are set out in Table 1. The binding energies obtained through the XPS analysis were corrected by referencing the C 1 s line to 285.0 eV. The binding energies of Bi4f7/2, Sn3d5/2, Sb3d5/2 and O1s were 159.80, 486.90, 529.70 and 530.70 eV respectively after correcting C. By comparing the XPS standard binding energy data table and the chemical shifts of each element, the valence state of each element in BiSnSbO6 was determined and the oxidation state of Bi, Sn, Sb and O ions from BiSnSbO6 were +3, +4, +5 and −2, respectively. In addition, the mean atomic ratio of Bi: Sn: Sb: O measured by the XPS data was 1.00: 0.98: 1.02: 5.98, which was near the theoretical value for BiSnSbO6.
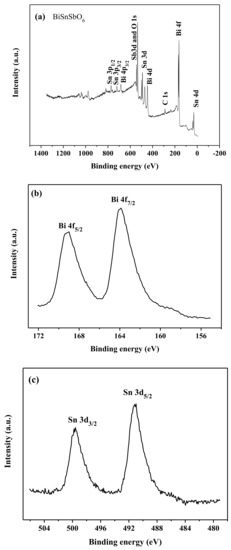
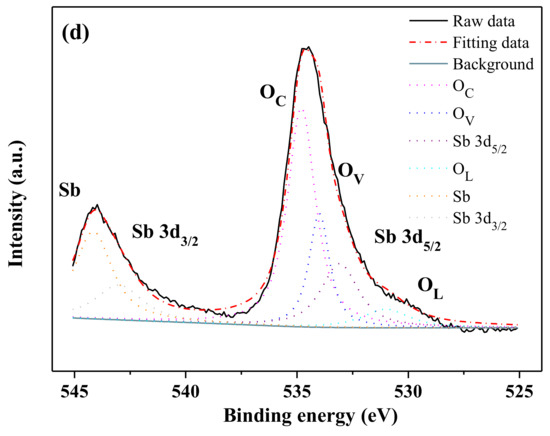
Figure 5.
The XPS spectra of BiSnSbO6 and every element in BiSnSbO6. The full spectrum of BiSnSbO6 (a); Bi 4f (b); Sn 3d (c); Sb 3d and O1s (d).

Table 1.
The binding energies of the element characteristic peaks in the BiSnSbO6 photocatalyst.
3.2. Photocatalytic Activity
The photodegradation curves of RhB and BZT over different photocatalysts are shown in Figure 6. From Figure 6, the initial rate and the initial photon efficiency of the RhB and BZT degradation under visible light irradiation with BiSnSbO6 as catalyst were measured, respectively. The surveys were in progress under oxygen saturation conditions ([O2]sat = 1.02 × 10−3 M). After stirring in the darkness for 45 min, the suspension had already achieved adsorption/desorption equilibrium. With the absence of the photocatalysts, the RhB and BZT were scarcely reduced under simulated solar irradiation. Under visible light irradiation for 200 min, the removal rate of RhB was estimated to be 72.72% with N-doped TiO2 as catalyst, while BiSnSbO6 could attain a photodegradation efficiency of 100%. Here, the initial photodegradation rate of RhB was 2.398 × 10−9 moL·L−1·s−1 and the initial photon efficiency was found to be 0.0501% (λ = 420 nm) for BiSnSbO6. In contrast, the photocatalytic degradation ratio with BiSnSbO6 as catalyst was better than that with N-doped TiO2 as catalyst. Under the same reaction conditions for 200 min, the initial photodegradation rate of RhB was 1.417 × 10−9 moL·L−1·s−1 and the initial photon efficiency was 0.0298% for N-doped TiO2. Meanwhile, as shown in Figure 6d, under visible light irradiation for 200 min over N-doped TiO2, the initial photodegradation rate of BZT and the initial photon efficiency were respectively found to be 1.908 × 10−9 moL·L−1·s−1 and 0.0401%. However, the initial photodegradation rate of BZT with BiSnSbO6 as a photocatalyst was about 2.568 × 10−9 moL·L−1·s−1, which was 1.35 times greater than that with N-doped TiO2 as catalyst, and the initial photon efficiency was found to be 0.0539% (λ = 420 nm). Therefore, it could be obtained from above results that BiSnSbO6 had better photocatalytic activity than N-doped TiO2.
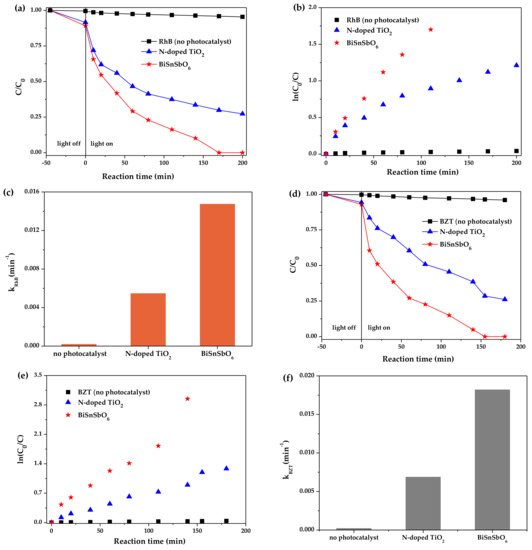
Figure 6.
(a) Degradation curves of RhB over different samples; (b) Linear relation plots of ln(C0/C) vs. reaction time; (c) The photodegradation rate constants (k) calculated from (b); (d) Degradation curves of BZT over different samples; (e) Linear relation plots of ln(C0/C) vs. reaction time; (f) The photodegradation rate constants (k) calculated from (e).
The kinetic results of RhB and BZT degradation under visible light irradiation are inferred and are shown in Figure 6b,c,e,f, which show the kinetic results with BiSnSbO6, N-doped TiO2 and no photocatalyst. Here, C and C0 stood for the RhB (or BZT) concentration at time t and t0. The relationship between ln(C/C0) and the irradiation time was linear correlation. Assuming that the RhB photodegradation process adhered to pseudo first-order decay kinetics, the calculational k value for BiSnSbO6 was 0.0147 min−1, which was 2.7 times greater than the k value of 0.0055 min−1 for N-doped TiO2. As shown in Figure 6f, the first-order rate constant k for BiSnSbO6 on degrading BZT was found to be 0.0182 min−1, while that of N-doped TiO2 was 0.0069 min−1. It was clear that BiSnSbO6 had better activity than N-doped TiO2 for both RhB and BZT photodegradation under visible light irradiation.
In order to exclude the photosensitization effect under light irradiation, the photocatalytic properties of BiSnSbO6 and N-doped TiO2 toward the colorless phenol (0.032 mM and 300 mL) are evaluated and the results can be seen in Figure 7. Under visible light irradiation for 230 min, the removal rate of phenol over BiSnSbO6 was up to 100%, while that over N-doped TiO2 was only 33.16%. As shown in Figure 7b,c, the calculational k value of BiSnSbO6 for the phenol photodegradation was higher than that of N-doped TiO2. Above results demonstrated that the photosensitive effect was not a main factor in the photodegradation process of RhB with BiSnSbO6 as a photocatalyst and BiSnSbO6 itself had photocatalytic activity [54,55].
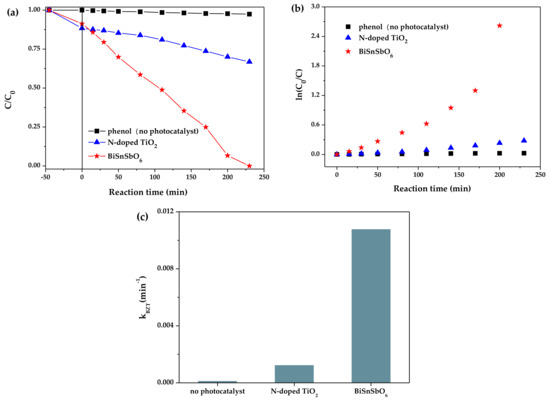
Figure 7.
(a) Degradation curves of phenol over different samples; (b) Linear relation plots of ln(C0/C) vs. reaction time; (c) The photodegradation rate constants (k) calculated from (b).
In order to compare the photocatalytic activity results of the current system with the data from the literature, the catalytic activity of the photocatalyst was evaluated by using kBZT/SBET (i.e., degradation rate constant per unit area). Here, kBZT was the first-order rate constant k for BZT photodegradation, and SBET was the BET surface of the photocatalyst. Table 2 shows the photocatalytic activity results from the literature data and our work for BZT degradation. As shown in Table 2, we could observe that BiSnSbO6 showed a slightly higher kBZT/SBET value compared with BiOBr photocatalyst, indicating that BiSnSbO6 is considered a potential photocatalyst to remove BZT contaminant.

Table 2.
The photocatalytic activity results from the literature data on BZT degradation compared with this work.
Figure 8a,c describe the removal rate of the total organic carbon in solutions containing BiSnSbO6 or N-doped TiO2 within 200 min. Figure 8b,d,e show the effect of the irradiation time on the ln(TOC0/TOC) and the removal rate constants of TOC. The results showed that the TOC removal rate of RhB reached 99.59% with BiSnSbO6 as catalyst, while the TOC removal rate of RhB was only 74.27% with N-doped TiO2 as catalyst, after simulated sunlight irradiation for 200 min. Meanwhile, under simulated sunlight irradiation for 180 min, the TOC removal rate of BZT was up to 100% with BiSnSbO6 as catalyst, while the TOC removal rate of BZT was only 71.20% with N-doped TiO2 as catalyst. Above results showed that the target contaminants (RhB and BZT) were almost completely mineralized when BiSnSbO6 was used as a photocatalyst under visible light irradiation. In addition, the intermediate products might be produced during the photodegradation of RhB and BZT. The relationship between the ln(TOC0/TOC) and the irradiation time of RhB and BZT photodegradation by using BiSnSbO6 or N-doped TiO2 could be easily obtained from Figure 8b,d. It was noteworthy that the relationship between ln(TOC0/TOC) and irradiation time was linear. In the process of RhB photodegradation, the apparent first-order rate constant k for BiSnSbO6 was calculated to be 0.0152 min−1, which was 2.9 times greater than that of 0.0053 min−1 for N-doped TiO2. For the BZT photodegradation process, the pseudo first-order rate constant k for BiSnSbO6 was estimated to be 0.0209 min−1, which was 3.1 times greater than that of 0.0067 min−1 for N-doped TiO2. Above results of photocatalytic degradation indicated that BiSnSbO6 could be more effectively excited by visible light than N-doped TiO2.
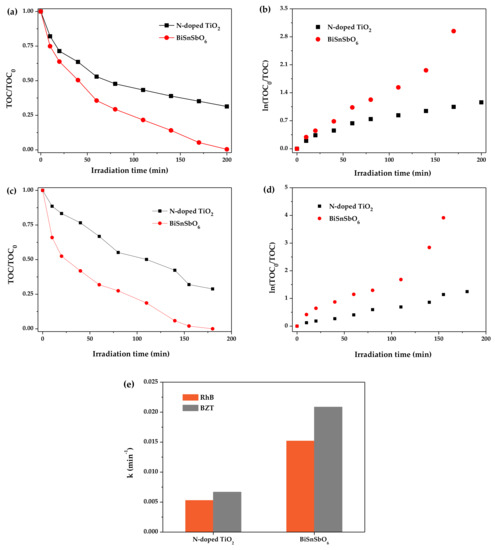
Figure 8.
(a) Total organic carbon curves of RhB over different samples; (b) Linear plots of ln(TOC0/TOC) vs. reaction time; (c) Total organic carbon curves of BZT over different samples; (d) Linear plots of ln(TOC0/TOC) vs. reaction time; (e) The removal rate constants (k) of total organic carbon calculated from (b,d).
In order to further investigate the mineralization of RhB and BZT during the photocatalytic degradation process over BiSnSbO6 or N-doped TiO2, the amount of CO2 generation was measured. Figure 9 shows CO2 generation during the photodegradation of RhB or BZT with BiSnSbO6 or N-doped TiO2 as catalyst. The results showed that the amount of CO2 was gradually increased with the gradual extension of the reaction time. As shown in Figure 9, the rate of CO2 production for RhB or BZT photocatalytic degradation with BiSnSbO6 as catalyst was faster than that with N-doped TiO2 as catalyst. For example, after exposing BiSnSbO6, N-doped TiO2, RhB and BZT to simulated sunlight for 200 min, the amount of CO2 produced from degrading RhB was 0.2441 mmol with BiSnSbO6 as catalyst, which was 1.5 times greater than that of 0.1667 mmol with N-doped TiO2 as catalyst. The turnover number which was the ratio between total amount of gas produced and photocatalyst in RhB photodegradation, was found to be more than 0.30 for BiSnSbO6 and 0.20 for N-doped TiO2. Furthermore, after photocatalytic reaction for 180 min under visible light irradiation, the CO2 generated during the degradation of BZT was 0.0369 mmol over N-doped TiO2, which was 0.7 times greater than that of 0.0523 mmol with BiSnSbO6 as catalyst. The turnover number for BZT photodegradation was found to be more than 0.06 with BiSnSbO6 as catalyst and 0.04 with N-doped TiO2 as catalyst. These turnover numbers fully confirmed the occurrence of catalytic reactions.
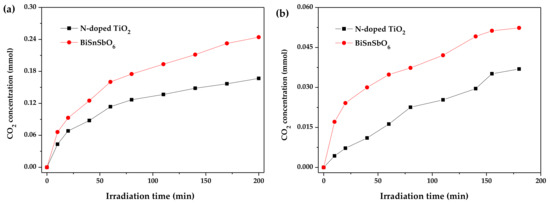
Figure 9.
CO2 generation during the RhB photodegradation (a) and the BZT photodegradation (b) over BiSnSbO6 or N-doped TiO2.
Figure 10 shows the photoluminescence spectra and transient photocurrent response of BiSnSbO6 and N-doped TiO2. The photoluminescence spectra patterns of BiSnSbO6 and N-doped TiO2 in Figure 10a showed a higher photoluminescence spectra intensity of N-doped TiO2 than that of BiSnSbO6. In general, the higher photoluminescence spectra intensity indicated a higher recombination rate of photogenerated electrons and holes, which had an important effect on the photocatalytic activity [56,57]. However, in this work, BiSnSbO6 which owned higher photoluminescence spectra intensity exhibited higher photocatalytic activity than N-doped TiO2. Above-mentioned discrepancy might be caused by oxygen vacancies and crystalline defects [58,59,60,61] on the surface of BiSnSbO6. During the preparation of BiSnSbO6, the volatilization of Bi2O3 and oxygen vacancies might appear on the surface of the BiSnSbO6 samples when the calcination temperature was higher than 820 °C [62]. The photocurrent analysis was also performed to study the interface charge transfer kinetics. As shown in Figure 10b, the transient photocurrent response produced by BiSnSbO6 was more clearly observed than that produced by N-doped TiO2. Since the high photocurrent intensity indicated highly separable electron–hole pairs and a favourable photocatalytic performance [63], it could be concluded that BiSnSbO6 had a more powerful charge separation capacity compared with N-doped TiO2. Moreover, the photocurrent intensity rapidly increased when the light was turned on, indicating the apparent photoresponse of BiSnSbO6 and N-doped TiO2.
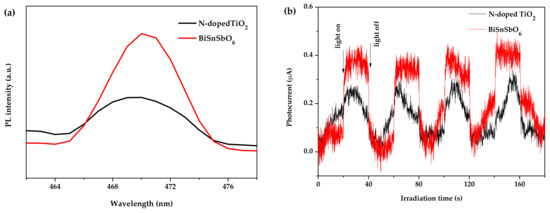
Figure 10.
(a) The photoluminescence spectra (PL) and (b) transient photocurrent response of BiSnSbO6 and N-doped TiO2.
Figure 11a shows the degradation of BZT with BiSnSbO6 as catalyst in the presence of different scavengers. Figure 11b shows the linear relation curves between ln(C0/C) and reaction time. Figure 11c shows the photodegradation rate constants by using different scavengers. As shown in Figure 11, ethylene diamine tetraacetic acid (EDTA), tert-butanol (TBA) and superoxide dismutase (SOD) were used as scavengers to remove h+, •OH and •O2− [64,65,66] respectively and the main active substances could be further obtained and verified during the photodegradation of BZT with BiSnSbO6 as catalyst. For the TBA and SOD photocatalytic systems, the photodegradation efficiency of BZT over BiSnSbO6 remained almost the same. Comparing the apparent first-order rate constant k calculated from the TBA and SOD photocatalysis system, the kSOD of 0.0103 min−1 was smaller than kTBA of 0.0131 min−1, which confirmed that •O2− was another main active species. Above active species could be ranked to increase photodegradation rate: •OH < •O2− < h+.
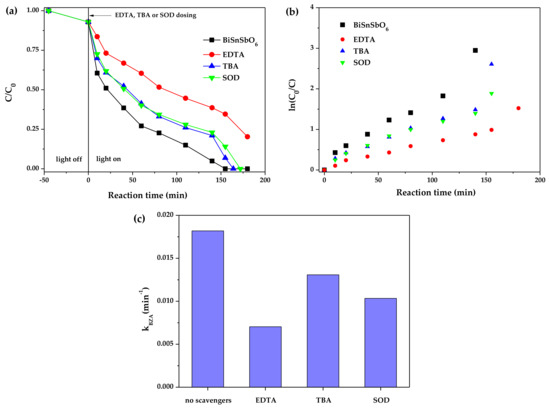
Figure 11.
(a) Degradation of BZT over BiSnSbO6 in the presence of different scavengers (1.5 mM, 2.5 vol% of reaction solution); (b) Linear relation plots of ln(C0/C) vs. reaction time; (c) The photodegradation rate constants (k) calculated from (b).
The intermediates of RhB photodegradation in this work were measured as N-ethyl-N’-ethylrhodamine (m/z: 387), N-ethylrhodamine (m/z: 359), rhodamine (m/z: 331), benzoic acid (m/z: 122), 3-nitrobenzoic acid, 1,2-benzenedicarboxylic acid, terephthalic acid (m/z: 166), adipic acid (m/z: 146), 3-hydroxybenzoic acid (m/z: 138), 2-hydroxypentanedioic acid, pentanedioic acid (m/z: 132), maleic acid (m/z: 116), succinic acid (m/z: 118), malonic acid (m/z: 104) and oxalic acid (m/z: 90). Based on these photocatalytic intermediates, a probable photodegradation pathway of RhB is shown in Figure 12. This pathway was similar to, but not the same as the pathway presented by Horikoshi et al. [67] or our previous work [48]. In the research which came from Li et al. [68], the photocatalytic degradation of RhB included two major complex processes: the N-demethylation process and the destruction of the conjugate structure. In this work, the photodegradation process of RhB was mainly controlled by chromophore decomposition, ring opening and mineralization. In addition, according to the detected photodegradative intermediates of BZT, a possible photodegradation pathway of BZT is also deduced, as shown in Figure 13. When BiSnSbO6 was irradiated by photons whose energy was higher than the band gap energy of BiSnSbO6, photogenerated electron–hole pairs were formed on the surface of BiSnSbO6 [11]. The oxidation of BZT might be triggered by the combined oxidation of h+ and •O2−, and the opening process of triazole ring was similar to the results reported by Ding et al. [16] and Xu et al. [11]. In summary, RhB and BZT were first converted into small-molecule organic compounds which were eventually combined with other organic active groups to be turned into CO2 and water.
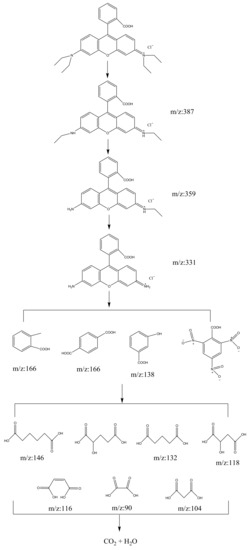
Figure 12.
Conceivable photodegradation pathway of RhB under simulated sunlight irradiation with BiSnSbO6 as catalyst.
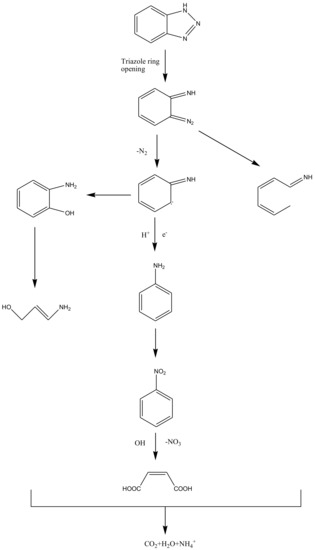
Figure 13.
Conceivable photodegradation pathway of BZT under simulated sunlight irradiation with BiSnSbO6 as catalyst.
As shown in Figure 6a, a slight decrease in RhB degradation was observed under visible light irradiation in the absence of photocatalysts. After reacting for 200 min under visible light irradiation, the initial photodegradation rate of RhB in the absence of photocatalysts was 9.467 × 10−11 moL·L−1·s−1 and the photon efficiency was found to be 0.0020% (λ = 420 nm), which was 0.07 times greater than N-doped TiO2, and 0.04 times greater than BiSnSbO6. The main reason for the photodegradation of RhB without photocatalysts might be due to the direct photosensitization of dye, which was similar to the report from Liu et al. [69]. In addition, under visible light irradiation for 180 min, the initial photodegradation rate of BZT in the absence of photocatalysts was 1.058 × 10−10 moL·L−1·s−1 and the photon efficiency was found to be 0.0022%.
As shown in Figure 4, the apparent photon efficiency at wavelengths which ranged from 440 nm to 800 nm corresponding to sub-Eg energy of BiSnSbO6 could be easily observed. The photon efficiency without photon assimilation occurred especially between the spectrum in the low energy region and the absorption spectrum of RhB or BZT. Scheme 1 shows the photosensitization effect on RhB and BZT. The result clearly demonstrated that the photocatalytic degradation of RhB or BZT with BiSnSbO6 as catalyst at an absorption wavelength which was greater than 440 nm should be attributed to the photosensitization of RhB or BZT itself.
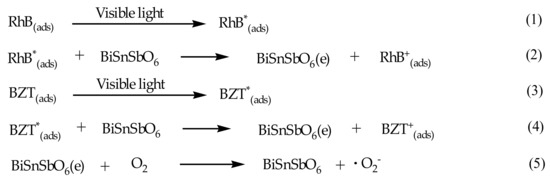
Scheme 1.
The photosensitization effect on RhB and BZT.
Based on above mechanism, RhB or BZT which was absorbed on the surface of BiSnSbO6 was driven by visible light irradiation. Electrons skipped from the excited state of the RhB surface or BZT surface to the conduction band of BiSnSbO6. The molecular oxygen and electrons combined to produce superoxide anion. Scheme 1 could explain the results which were gained under visible light irradiation with BiSnSbO6 as catalyst, where BiSnSbO6 might reduce a recombination of the photogenerated electrons and photogenerated holes via scavenging of the electrons [70].
The situation was not the same at absorption wavelength which was below 440 nm. Once the wavelength was below 440 nm, the photon efficiency would correlate intimately with the absorption spectrum of BiSnSbO6. In such a case, it was obvious that the band gap excitation of BiSnSbO6 was the main mechanism for RhB photodegradation or BZT photodegradation. Although detailed examination of the effects of oxygen and water during the photodegradation of RhB or BZT was not carried out, it was reasonable to conclude that this mechanism was similar to the mechanism which was measured under supra-band gap irradiation. Scheme 2 shows the generation of oxidative radicals with BiSnSbO6 as catalyst.
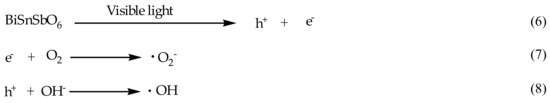
Scheme 2.
The generation scheme of oxidative radicals with BiSnSbO6 as catalyst.
Figure 14 shows the proposed band structure of BiSnSbO6. The electronic structures of InMO4 (M = V, Nb or Ta) and BiVO4 were calculated by using the first principles in the work of Oshikiri et al. [71]. This report showed that the conduction band of InMO4 (M = V, Nb or Ta) consisted mainly of V 3d orbital component, Nb 4d orbital component and Ta 5d orbital component. The valence band of BiVO4 was composed of a partial Bi 6s orbital constituent and a prevailing O 2p orbital constituent. Similarly, the band structure of BiSnSbO6 should be similar to that of InMO4 (M = V, Nb or Ta) and BiVO4. Therefore, the conduction band of BiSnSbO6 was constitutive of Bi 6p orbital component, Sn 5p orbital component and Sb 5p orbital component. The valence band of BiSnSbO6 consisted of a partial Bi 6s orbital constituent and a prevailing O 2p orbital constituent. Electron–hole pairs were generated in the photocatalyst after BiSnSbO6 absorbed photons, indicating that plenitudinous energy which was higher than the band gap energy of BiSnSbO6 was essential for the photodecomposition of RhB and BZT.
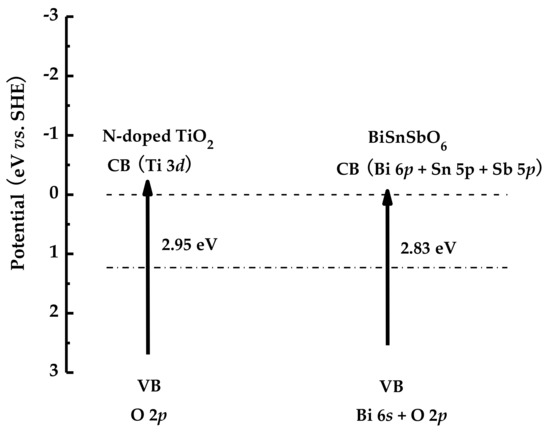
Figure 14.
Proposed band structures of BiSnSbO6 and N-doped TiO2.
The stability and recyclability of the photocatalysts are important factors for the practical application of photocatalytic materials. Recycling experiments, XRD analysis and XPS analysis were performed to evaluate the stability and recyclability of BiSnSbO6. Figure 15a shows the photocatalytic efficiency of RhB degradation or BZT degradation at different recycling time with BiSnSbO6 as catalyst. It could be seen from Figure 15a that the photocatalytic efficiencies of RhB over BiSnSbO6 under the condition of four cycles were respectively 100% (first cycle), 98.53% (second cycle), 94.84% (third cycle) and 87.88% (fourth cycle). The photocatalytic degradation rates of BZT over BiSnSbO6 under the condition of four cycles were respectively 100% (first cycle), 97.63% (second cycle), 91.94% (third cycle) and 84.63% (fourth cycle). Figure 15b shows the XRD spectra of BiSnSbO6 after four recycles of RhB photodegradation or BZT photodegradation. The XRD spectra patterns of BiSnSbO6 in Figure 15b showed the unchanged phase and structure of BiSnSbO6. Figure 15c shows the XPS spectra of BiSnSbO6 after four recycles of RhB photodegradation or BZT photodegradation. It could be seen from Figure 15c that the XPS spectra of BiSnSbO6 which was gained before and after four recycling reactions revealed that BiSnSbO6 had excellent stability and high performance under the condition of photodegradation.
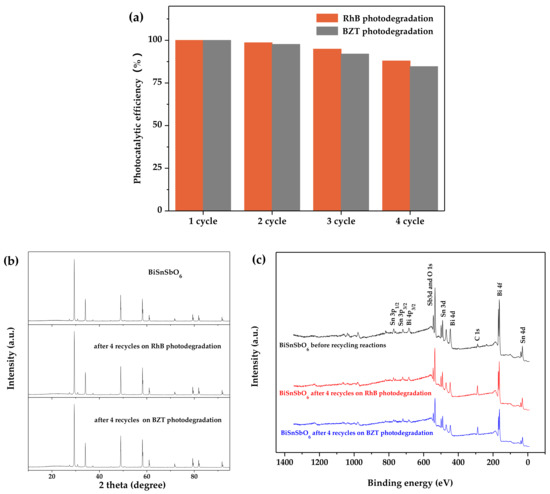
Figure 15.
(a) The photocatalytic efficiency of RhB degradation or BZT degradation at different recycling time with BiSnSbO6 as catalyst.; (b) XRD spectra of BiSnSbO6 after four recycles of RhB photodegradation or BZT photodegradation; (c) XPS spectra of BiSnSbO6 after four recycles of RhB photodegradation or BZT photodegradation.
Above results showed that a BiSnSbO6/(visible light) photocatalytic system was an effective method to deal with diluted colored wastewater or surface water polluted by BZT-UVs. The BiSnSbO6/(visible light) photocatalytic system in this work could be widely used in decolorization, detoxification and purification in the textile, printing and dyeing industries because above system did not demand heating, high pression of oxygen and chemical reagents.
4. Conclusions
BiSnSbO6 with high photocatalytic activity was first prepared by a high-temperature, solid-state sintering method. The photophysical properties and the photocatalytic properties were investigated via SEM, TEM, XRD, UV-vis and XPS. The results showed that BiSnSbO6, with a pyrochlore structure and a cubic crystal system by the space group Fd3m, was well crystallized and was a single phase. The lattice parameter and the band gap of BiSnSbO6 were 10.234594 Å and 2.83 eV, respectively. Furthermore, BiSnSbO6 showed significant optical absorption in the visible light region (λ > 400 nm). BiSnSbO6 had a higher photocatalytic activity for degrading BZT and RhB than N-doped TiO2 under visible light irradiation. The complete removal of organic carbon could be achieved by measuring the total organic carbon in the presence of BiSnSbO6. According to scavenger experiments, during the photocatalytic process the main active species were arranged in order of increasing photodegradation rate: •OH < •O2− < h+. Thence, it could be concluded that a BiSnSbO6/(visible light) photocatalytic system might be a potent method for treating colored wastewater or surface water polluted by BZT-UVs. Finally, the possible photodegradation pathways for RhB and BZT were speculated.
Acknowledgments
This work was supported by a grant from China-Israel Joint Research Program in Water Technology and Renewable Energy (No. 5).
Author Contributions
Jingfei Luan were involved with all aspects of the work including visualizing, planning, and data explication. Panqi Huang carried out the experiments, analyzed the data and wrote the paper. All authors read and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhe, L.; Shirley, A.S.; Thomas, E.P.; Amila, O.D.S. Occurrence and fate of substituted diphenylamine antioxidants and benzotriazole UV stabilizers in various Canadian wastewater treatment processes. Water Res. 2017, 124, 158–166. [Google Scholar]
- Voutsa, D.; Hartmann, P.; Schaffner, C.; Giger, W. Benzotriazoles, alkylphenols and bisphenol A in bunicipal wastewaters and in the Glatt River, Switzerland. Environ. Sci. Pollut. Res. 2006, 13, 333–341. [Google Scholar] [CrossRef]
- Liu, R.; Ruan, T.; Wang, T.; Song, S.; Guo, F.; Jiang, G. Determination of nine benzotriazole UV stabilizers in environmental water samples by automated on-line solid phase extraction coupled with high-performance liquid chromatography−tandem mass spectrometry. Talanta 2014, 120, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Ying, G.; Ma, Y.; Chen, Z.; Chen, F.; Liu, Y. Occurrence and dissipation of benzotriazoles and benzotriazole ultraviolet stabilizers in biosolid-amended soils. Environ. Toxicol. Chem. 2014, 33, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Bakken, L.R.; Breedveld, G.D.; Aagaard, P.; Frostegård, Å. Organic compounds that reach subsoil may threaten groundwater quality: Effect of benzotriazole on degradation kinetics and microbial community composition. Soil Biol. Biochem. 2006, 38, 2543–2556. [Google Scholar] [CrossRef]
- Nakata, H.; Murata, S.; Filatreau, J. Occurrence and concentrations of benzotriazole UV stabilizers in marine organisms and sediments from the Ariake Sea, Japan. Environ. Sci. Technol. 2009, 43, 6920–6926. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ramaswamy, B.R.; Chang, K.; Isobe, T.; Tanabe, S. Multiresidue analytical method for the determination of antimicrobials, preservatives, benzotriazole UV stabilizers, flame retardants and plasticizers in fish using ultra high performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3511–3520. [Google Scholar] [CrossRef] [PubMed]
- Zhe, L.; Amila, O.D.S.; Thomas, E.P.; Cyril, J.C.; Gerald, R.T.; Mark, R.S.; Derek, C.G.M. Distribution, partitioning and bioaccumulation of substituted diphenylamine antioxidants and benzotriazole UV stabilizers in an Urban Creek in Canada. Environ. Sci. Technol. 2016, 50, 9089–9097. [Google Scholar]
- Hem, L.J.; Hartnik, T.; Roseth, R.; Breedveld, G.D. Photochemical degradation of benzotriazole. J. Environ. Sci. Health A 2003, 38, 471–481. [Google Scholar] [CrossRef]
- Hollingsworth, J.; Sierra-Alvarez, R.; Zhou, M.; Ogden, K.L.; Field, J.A. Anaerobic biodegradability and methanogenic toxicity of key constituents in copper chemical mechanical planarization effluents of the semiconductor industry. Chemosphere 2005, 59, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, L.; Guo, C.S.; zhang, Y.; Wang, S.F. Removal of benzotriazole from solution by BiOBr photocatalysis under simulated solar irradiation. Chem. Eng. J. 2013, 221, 230–237. [Google Scholar] [CrossRef]
- Xu, X.J.; Fang, X.S.; Zhai, T.Y.; Zeng, H.B.; Liu, B.D.; Hu, X.Y.; Bando, Y.; Golberg, D. Tube-in-tube TiO2 nanotubes with porous walls: Fabrication, formation mechanism, and photocatalytic properties. Small 2011, 7, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Mahata, A. Demineralization of organic pollutants on the dye modified TiO2 semiconductor particulate system using visible light. Appl. Catal. B 2001, 33, 119–125. [Google Scholar] [CrossRef]
- Katoh, R.; Murai, M.; Furube, A. Electron-hole recombination in the bulk of a rutile TiO(2) single crystal studied by sub-nanosecond transient absorption spectroscopy. Chem. Phys. Lett. 2008, 461, 238–248. [Google Scholar] [CrossRef]
- Wang, W.; Dong, L.; Wang, J.P.; Shi, X.M.; Han, S.Y. Characterization and photocatalytic activity of mesoporous TiO2 prepared from an ethanol-diethyl ether binary solvent system. Chem. Phys. Lett. 2014, 616, 1–5. [Google Scholar] [CrossRef]
- Ding, Y.B.; Yang, C.Z.; Zhu, L.H.; Zhang, J.D. Photoelectrochemical activity of liquid phase deposited TiO2 film for degradation of benzotriazole. J. Hazard. Mater. 2010, 175, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Jorfiab, S.; Kakavandicd, B.; Motlaghab, H.R.; Ahmadiab, M.; Jaafarzadeh, N. A novel combination of oxidative degradation for benzotriazole removal using TiO2 loaded on FeIIFe2IIIO4@C as an efficient activator of peroxymonosulfate. Appl. Catal. B 2017, 219, 216–230. [Google Scholar] [CrossRef]
- Reddy, D.A.; Choi, J.; Lee, S.; Kim, T.K. Controlled synthesis of heterostructured Ag@AgI/ZnS microspheres with enhanced photocatalytic activity and selective separation of methylene blue from mixture dyes. J. Taiwan Inst. Chem. Eng. 2016, 66, 200–209. [Google Scholar] [CrossRef]
- Liu, L.Q.; Dao, T.D.; Kodiyath, R.; Kang, Q.; Abe, H.; Nagao, T.; Ye, J.H. Plasmonic janus-composite photocatalyst comprising Au and C-TiO2 for enhanced aerobic oxidation over a broad visible-light range. Adv. Funct. Mater. 2014, 24, 7754–7762. [Google Scholar] [CrossRef]
- Lee, S.; Reddy, D.A.; Kim, T.K. Well-wrapped reduced graphene oxide nanosheets on Nb3O7(OH) nanostructures as good electron collectors and transporters for efficient photocatalytic degradation of rhodamine B and phenol. RSC Adv. 2016, 6, 37180–37188. [Google Scholar] [CrossRef]
- Reddy, D.A.; Lee, S.; Choi, J.; Park, S.; Ma, R.; Yang, H.; Kim, T.K. Green synthesis of AgI-reduced graphene oxide nanocomposites: Toward enhanced visible-light photocatalytic activity for organic dye removal. Appl. Surf. Sci. 2015, 341, 175–184. [Google Scholar] [CrossRef]
- Reddy, D.A.; Ma, R.; Kim, T.K. Efficient photocatalytic degradation of methylene blue by heterostructured ZnO-RGO/RuO2 nanocomposite under the simulated sunlight irradiation. Ceram. Int. 2015, 41, 6999–7009. [Google Scholar] [CrossRef]
- Reddy, D.A.; Choi, J.; Lee, S.; Ma, R.; Kim, T.K. Self-assembled macro porous ZnS-graphene aerogels for photocatalytic degradation of contaminants in water. RSC Adv. 2015, 5, 18342–18351. [Google Scholar] [CrossRef]
- Zhang, A.Y.; Wang, W.K.; Pei, D.N.; Yu, H.Q. Degradation of refractory pollutants under solar light irradiation by a robust and self-protected ZnO/CdS/TiO2 hybrid photocatalyst. Water Res. 2016, 92, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.A.; Ma, R.; Choi, M.Y.; Kim, T.K. Reduced graphene oxide wrapped ZnS-Ag2S ternary composites synthesized via hydrothermal method: Applications in photocatalyst degradation of organic pollutants. Appl. Surf. Sci. 2015, 324, 725–735. [Google Scholar] [CrossRef]
- Reddy, D.A.; Choi, J.; Lee, S.; Ma, R.; Kim, T.K. Green synthesis of AgI nanoparticle-functionalized reduced graphene oxide aerogels with enhanced catalytic performance and facile recycling. RSC Adv. 2015, 5, 67394–67404. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, X.H. Synthesis of novel visible light driven BiVO4 photocatalysts via microemulsion process and its photocatalytic performance. J. Inorg. Mater. 2009, 24, 453–456. [Google Scholar] [CrossRef]
- Islam, M.J.; Kim, H.K.; Reddy, D.A.; Kim, Y.; Ma, R.; Baek, H.; Kim, J.; Kim, T.K. Hierarchical BiOI nanostructures supported on a metal organic framework as efficient photocatalysts for degradation of organic pollutants in water. Dalton Trans. 2017, 46, 6013–6023. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Q.; Xiong, X.Y.; Ding, S.P.; Liu, X.F.; Jiang, Q.Q.; Hu, J.C. In-situ topotactic synthesis and photocatalytic activity of plate-like BiOCl/2D networks Bi2S3 heterostructures. Appl. Catal. B 2018, 220, 570–580. [Google Scholar] [CrossRef]
- Meng, Q.Q.; Zhou, Y.S.; Chen, G.; Hu, Y.D.; Lv, C.D.; Qiang, L.S.; Xing, W.N. Integrating both homojunction and heterojunction in QDs self-decorated Bi2MoO6/BCN composites to achieve an efficient photocatalyst for Cr(VI) reduction. Chem. Eng. J. 2018, 334, 334–343. [Google Scholar] [CrossRef]
- Yang, L.Q.; Huang, J.F.; Shi, L.; Cao, L.Y.; Zhou, W.; Chang, K.; Meng, X.G.; Liu, G.G.; Jie, Y.N.; Ye, J.H. Efficient hydrogen evolution over Sb doped SnO2 photocatalyst sensitized by Eosin Y under visible light irradiation. Nano Energy 2017, 36, 331–340. [Google Scholar] [CrossRef]
- Yang, L.Q.; Huang, J.F.; Shi, L.; Cao, L.Y.; Liu, H.M.; Liu, Y.Y.; Li, Y.X.; Song, H.; Jie, Y.N.; Ye, J.H. Sb doped SnO2-decorated porous g-C3N4 nanosheet heterostructures with enhanced photocatalytic activities under visible light irradiation. Appl. Catal. B 2018, 221, 670–680. [Google Scholar] [CrossRef]
- Nasser, R.; Othmen, W.B.; Elhouichet, H.; Ferid, M. Preparation, characterization of Sb-doped ZnO nanocrystals and their excellent solar light driven photocatalytic activity. Appl. Catal. B 2017, 393, 486–495. [Google Scholar] [CrossRef]
- Shimodaira, Y.; Kato, H.; Kobayashi, H.; Kudo, A. Photophysical properties and photocatalytic activities of bismuth molybdates under visible light irradiation. J. Phys. Chem. B 2006, 110, 17790–17797. [Google Scholar] [CrossRef] [PubMed]
- He, G.P.; Xing, C.L.; Xiao, X.; Hu, R.P.; Zuo, X.X.; Nan, J.M. Facile synthesis of flower-like Bi12O17Cl2/β-Bi2O3 composites with enhanced visible light photocatalytic performance for the degradation of 4-tert-butylphenol. Appl. Catal. B 2015, 170, 1–9. [Google Scholar] [CrossRef]
- Luan, J.F.; Shen, Y.; Zhang, L.Y.; Guo, N.B. Property characterization and photocatalytic activity evaluation of BiGdO3 nanoparticles under visible light irradiation. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.B.; Pan, C.S.; Yao, W.Q.; Zhu, Y.F. Visible-light-induced degradation of rhodamine B by nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Zhang, T.W. Photodegradation of rhodamine B in water assisted by titania films prepared through a novel procedure. J. Photochem. Photobiol. A Chem. 2004, 162, 171–177. [Google Scholar] [CrossRef]
- Guo, Y.P.; Zhao, J.Z.; Zhang, H.; Yang, S.F.; Qi, J.R.; Wang, Z.C.; Xu, H.D. Use of rice husk-based porous carbon for adsorption of Rhodamine B from aqueous solutions. Dyes Pigments 2005, 66, 123–128. [Google Scholar] [CrossRef]
- Horikoshi, S.; Hojo, F.; Hikaka, H.; Serpone, N. Environmental remediation by an integrated microwave/UV illumination technique. 8. Fate of carboxylic acids, aldehydes, alkoxycarbonyl and phenolic substrates in a microwave radiation field in the presence of TiO2 particles under UV irradiation. Environ. Sci. Technol. 2004, 38, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Suhas; Ali, I.; Saini, V.K. Removal of rhodamine B, fast green, and methylene blue from wastewater using red mud, an aluminum industry waste. Ind. Eng. Chem. Res. 2004, 43, 1740–1747. [Google Scholar] [CrossRef]
- Fujii, T.; Nishikiori, H.; Tamura, T. Absorpyion-spectra of rhodamine-B dimers in dip-coated thin-films prepared by the sol-gel method. Chem. Phys. Lett. 1995, 233, 424–429. [Google Scholar] [CrossRef]
- Valdes-Aguilera, O.; Neckers, D.C. Aggregation phenomena in xanthene dyes. Accounts Chem. Res. 1989, 22, 171–177. [Google Scholar] [CrossRef]
- Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Bahnemann, D.W.; Murugesan, V. Enhancement of photocatalytic activity by metal deposition: Characterisation and photonic efficiency of Pt, Au and Pd deposited on TiO2 catalyst. Water Res. 2004, 38, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Marugan, J.; Hufschmidt, D.; Sagawe, G.; Selzer, V.; Bahnemann, D. Optical density and photonic efficiency of silica-supported TiO2 photocatalysts. Water Res. 2006, 40, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.J.; Ding, S.S.; Meng, L.J.; Dai, Y.X.; Sun, C.; Yang, S.G. One-pot microemulsion-mediated synthesis of Bi-rich Bi4O5Br2 with controllable morphologies and excellent visible-light photocatalytic removal of pollutants. Appl. Catal. B 2017, 207, 153–165. [Google Scholar] [CrossRef]
- Izumi, F.; Murata, H.; Murata, H.; Watanabe, N. Rietveld analysis of powder patterns obtained by tof neutron-diffraction using gold neutron sources. J. Appl. Crystallogr. 1987, 20, 411–418. [Google Scholar] [CrossRef]
- Luan, J.F.; Ma, K.; Pan, B.C.; Li, Y.M.; Wu, X.S.; Zou, Z.G. Synthesis and catalytic activity of new Gd2BiSbO7 and Gd2YSbO7 nanocatalysts. J. Mol. Catal. A Chem. 2010, 321, 1–9. [Google Scholar] [CrossRef]
- Nithya, V.D.; Selvan, R.K.; Kalpana, D.; Vasylechko, L.; Sanjeeviraja, C. Synthesis of Bi2WO6 nanoparticles and its electrochemical properties in different electrolytes for pseudocapacitor electrodes. Electrochim. Acta 2013, 109, 720–731. [Google Scholar] [CrossRef]
- Le, N.T.H.; Thanh, T.D.; Pham, V.T.; Phan, T.L.; Lam, V.D.; Manh, D.H.; Anh, T.X.; Le, T.K.C.; Thammajak, N.; Hong, L.V.; et al. Structure and high photocatalytic activity of (N, Ta)-doped TiO2 nanoparticles. J. Appl. Phys. 2016, 120, 142110. [Google Scholar] [CrossRef]
- Wiegel, M.; Middel, W.; Blasse, G. Influence of ns2 ions on the luminescence of niobates and tantalates. J. Mater. Chem. 1995, 5, 981–983. [Google Scholar] [CrossRef]
- Nagatani, H.; Suzuki, I.; Kita, M.; Tanaka, M.; Katsuya, Y.; Sakata, O.; Omata, T. Structure of beta-AgGaO2; ternary I-III-VI2 oxide semiconductor with a wurtzite-derived structure. J. Solid State Chem. 2015, 222, 66–70. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis and physical-properties of semiconducting electrode WO3. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Calza, P.; Rigo, L.; Sangermano, M. Investigations of photocatalytic activities of photosensitive semiconductors dispersed into epoxy matrix. Appl. Catal. B Environ. 2011, 106, 657–663. [Google Scholar] [CrossRef]
- Luan, J.F.; Shen, Y.; Li, Y.Y.; Paz, Y. The structural, photocatalytic property characterization and enhanced photocatalytic activities of novel photocatalysts Bi2GaSbO7 and Bi2InSbO7 during visible light irradiation. Material 2016, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Reddy, D.A.; Choi, J.; Kim, T.K. Surface oxygen vacancy assisted electron transfer and shuttling for enhanced photocatalytic activity of a Z-scheme CeO2-Agl nanocomposite. RSC Adv. 2016, 6, 19341–19350. [Google Scholar] [CrossRef]
- Yu, C.L.; Wu, Z.; Liu, R.Y.; Dionysiou, D.D.; Yang, K.; Wang, C.Y.; Liu, H. Novel fluorinated Bi2MoO6 nanocrystals for efficient photocatalytic removal of water organic pollutants under different light source illumination. Appl. Catal. B 2017, 209, 1–11. [Google Scholar] [CrossRef]
- Choi, J.; Reddy, D.A.; Kim, T.K. Enhanced photocatalytic activity and anti-photocorrosion of AgI nanostructures by coupling with graphene-analogue boron nitride nanosheets. Ceram. Int. 2015, 41, 13793–13803. [Google Scholar] [CrossRef]
- Choi, J.; Reddya, D.A.; Islam, M.J.; Seo, B.; Joo, S.H.; Kim, T.K. Green synthesis of the reduced graphene oxide-CuI quasi-shell-core nanocomposite: A highly efficient and stable solar-light-induced catalyst for organic dye degradation in water. Appl. Surf. Sci. 2015, 358, 159–167. [Google Scholar] [CrossRef]
- Choi, J.; Reddy, D.A.; Islam, M.J.; Ma, R.; Kim, T.K. Self-assembly of CeO2 nanostructures/reduced graphene oxide composite aerogels for efficient photocatalytic degradation of organic pollutants in water. J. Alloy. Compd. 2016, 688, 527–536. [Google Scholar] [CrossRef]
- Yang, C.M.; Gao, G.M.; Zhang, J.J.; Liu, R.P.; Fan, R.C.; Zhao, M.; Wang, Y.W.; Gan, S.C. Surface oxygen vacancy induced solar light activity enhancement of a CdWO4/Bi2O2CO3 core-shell heterostructure photocatalyst. Phys. Chem. Chem. Phys. 2017, 19, 14431–14441. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Wu, J.; Li, Q.F.; Liu, Q.Z.; Qi, Y.F.; You, L.; Ji, Z.; He, P.; Sheng, P.F.; Ren, J.X. Fabrication of BiOIO3 with induced oxygen vacancies for efficient separation of the electron-hole pairs. Appl. Catal. B 2017, 218, 80–90. [Google Scholar] [CrossRef]
- Islam, M.J.; Reddy, D.A.; Han, N.S.; Choi, J.; Song, J.K.; Kim, T.K. An oxygen-vacancy rich 3D novel hierarchical MoS2/BiOI/AgI ternary nanocomposite: Enhanced photocatalytic activity through photogenerated electron shuttling in a Z-scheme manner. Phys. Chem. Chem. Phys. 2016, 18, 24984–24993. [Google Scholar] [CrossRef] [PubMed]
- Song, C.D.; Wang, X.; Zhang, J.; Chen, X.B.; Li, C. Enhanced performance of direct Z-scheme CuS-WO3 system towards photocatalytic decomposition of organic pollutants under visible light. Appl. Surf. Sci. 2017, 425, 788–795. [Google Scholar] [CrossRef]
- Ye, Y.; Feng, Y.; Bruning, H.; Yntem, D.; Rijnaarts, H.H.M. Photocatalytic degradation of metoprolol by TiO2 nanotube arrays and UV-LED: Effects of catalyst properties, operational parameters, commonly present water constituents, and photo-induced reactive species. Appl. Catal. B 2018, 220, 171–181. [Google Scholar] [CrossRef]
- Fotiou, T.; Triantis, T.M.; Kaloudis, T.; O’Shea, K.E.; Dionysiou, D.D.; Hiskia, A. Assessment of the roles of reactive oxygen species in the UV and visible light photocatalytic degradation of cyanotoxins and water taste and odor compounds using C-TiO2. Water Res. 2016, 90, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Saitou, A.; Hidaka, H.; Serpone, N. Environmental remediation by an integrated microwave/UV illumination method. V. Thermal and nonthermal effects of microwave radiation on the photocatalyst and on the photodegradation of rhodamine-b under UV/Vis radiation. Environ. Sci. Technol. 2003, 37, 5813–5822. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Zhang, X.; Ai, Z.H.; Jia, F.L.; Zhang, L.Z.; Lin, J. Efficient visible light degradation of rhodamine B by a photo-electrochemical process based on a Bi2WO6 nanoplate film electrode. J. Phys. Chem. C 2007, 111, 6832–6836. [Google Scholar] [CrossRef]
- Liu, G.; Wu, T.; Zhao, J.; Hidaka, H.; Serpone, N. Photoassisted degradation of dye pollutants. 8. Irreversible degradation of alizarin red under visible light radiation in air-equilibrated aqueous TiO2 dispersions. Environ. Sci. Technol. 1999, 33, 2081–2087. [Google Scholar] [CrossRef]
- Nasr, C.; Vinodgopal, K.; Fisher, L.; Hotchandani, S.; Chattopadhyay, A.K.; Kamat, P.V. Environmental photochemistry on semiconductor surfaces. Visible light induced degradation of a textile diazo dye, naphthol blue black, on TiO2 nanoparticles. J. Phys. Chem. 1996, 100, 8436–8442. [Google Scholar] [CrossRef]
- Oshikiri, M.; Boero, M.; Ye, J.H.; Zou, Z.G.; Kido, G. Electronic structures of promising photocatalysts InMO4 (M = V, Nb, Ta) and BiVO4 for water decomposition in the visible wavelength region. J. Chem. Phys. 2002, 117, 7313–7318. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).