Facile Fabrication of Cu2O Nanobelts in Ethanol on Nanoporous Cu and Their Photodegradation of Methyl Orange
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
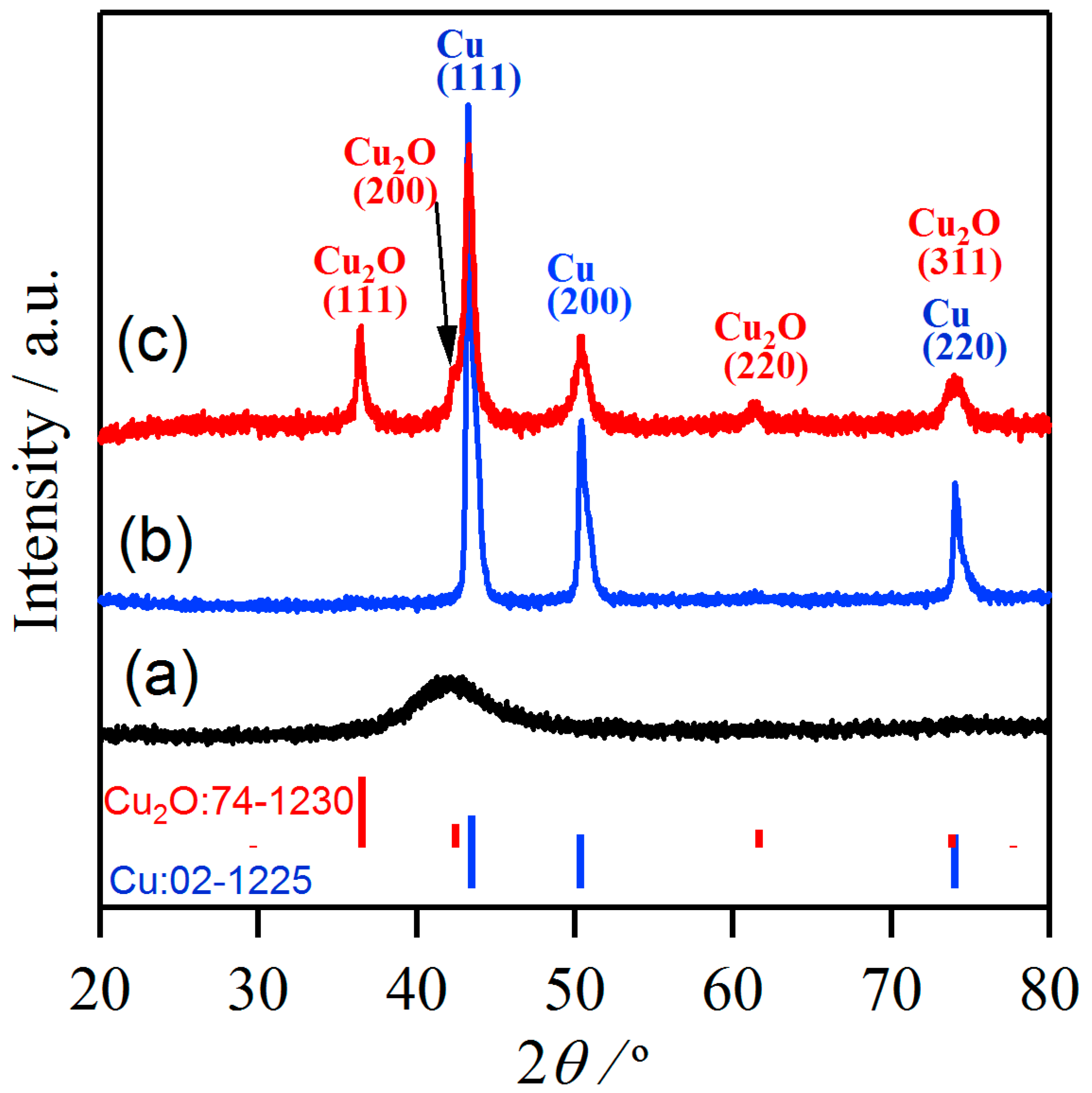
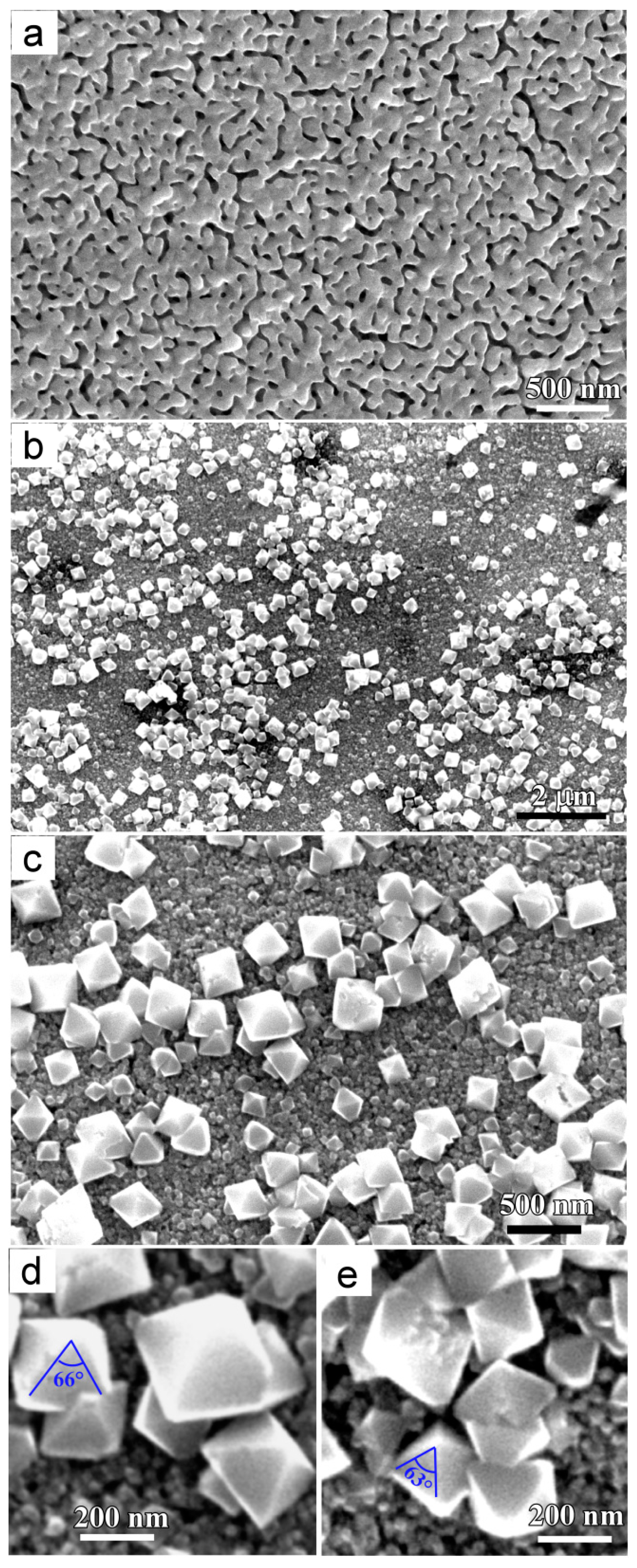
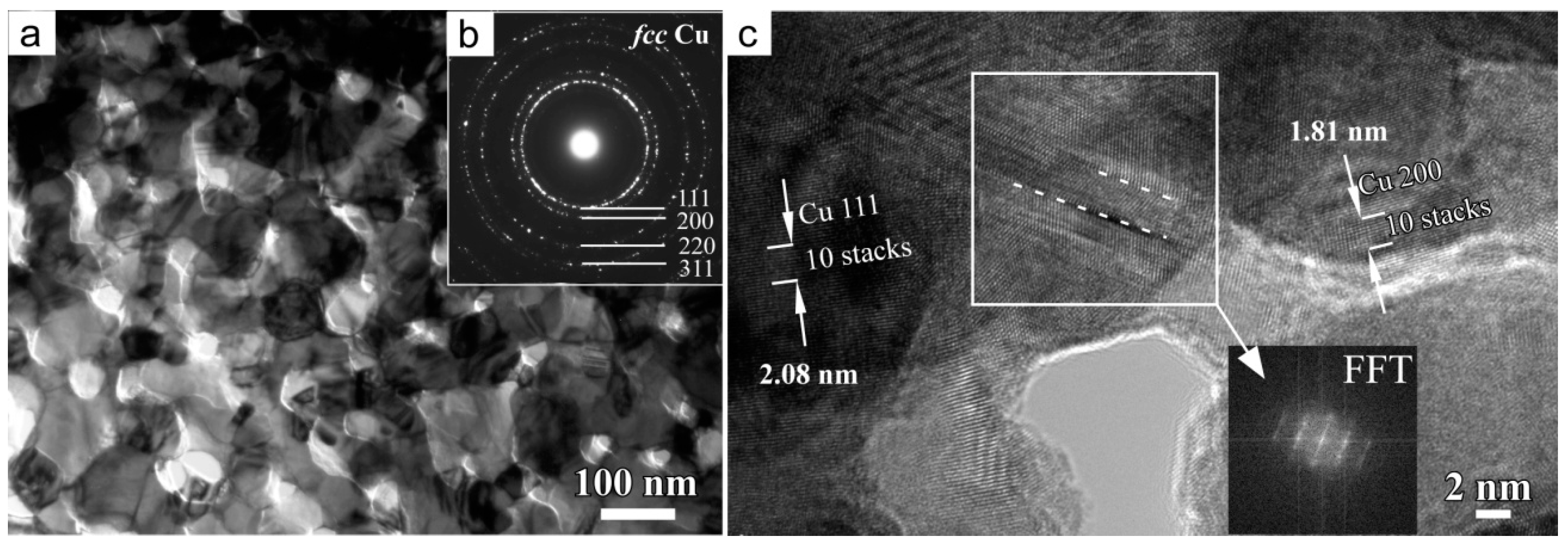
3.1. Characteristics of Cu2O Nanobelts
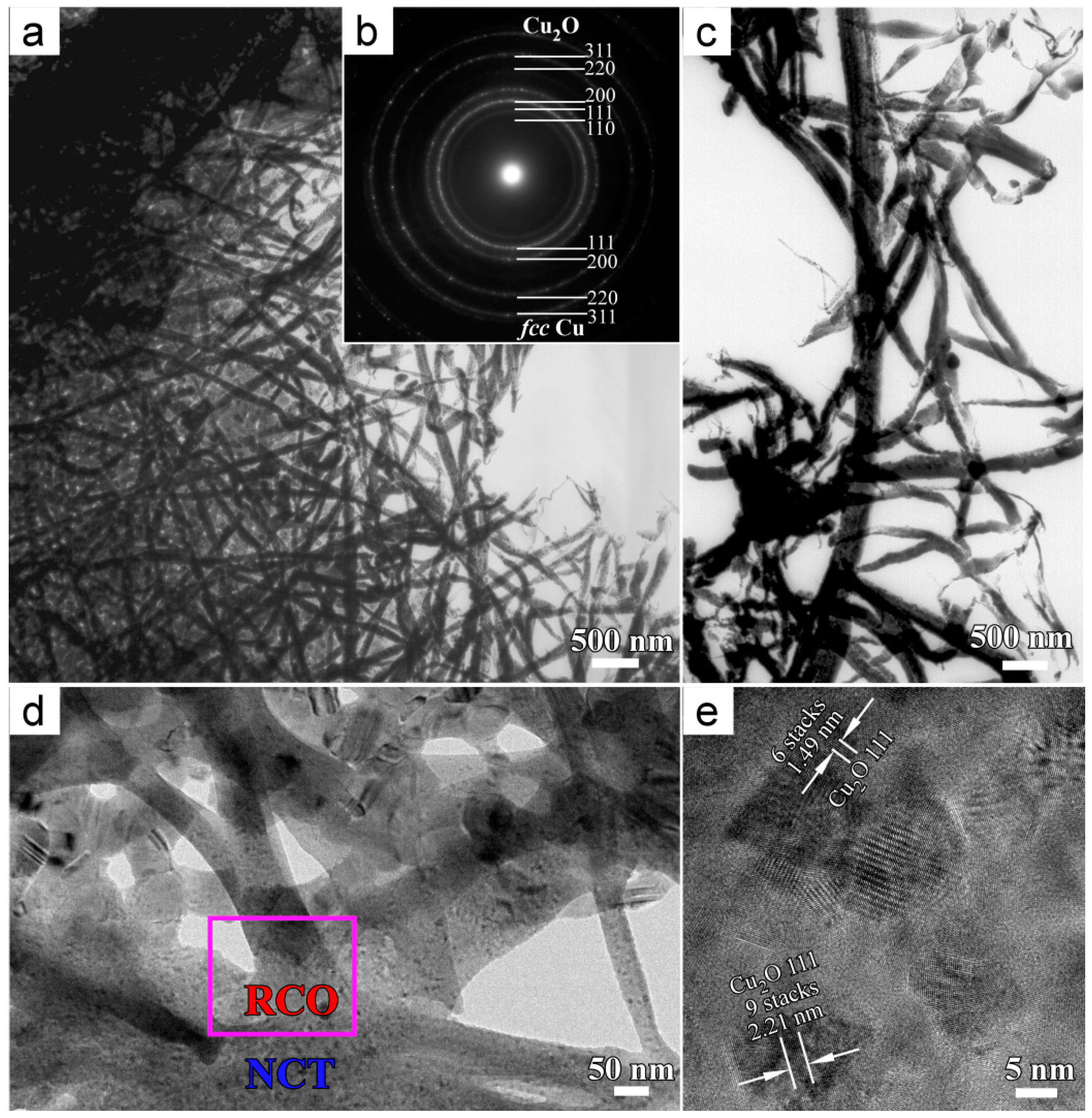
3.2. Formation Mechanism of Cu2O Nanobelts
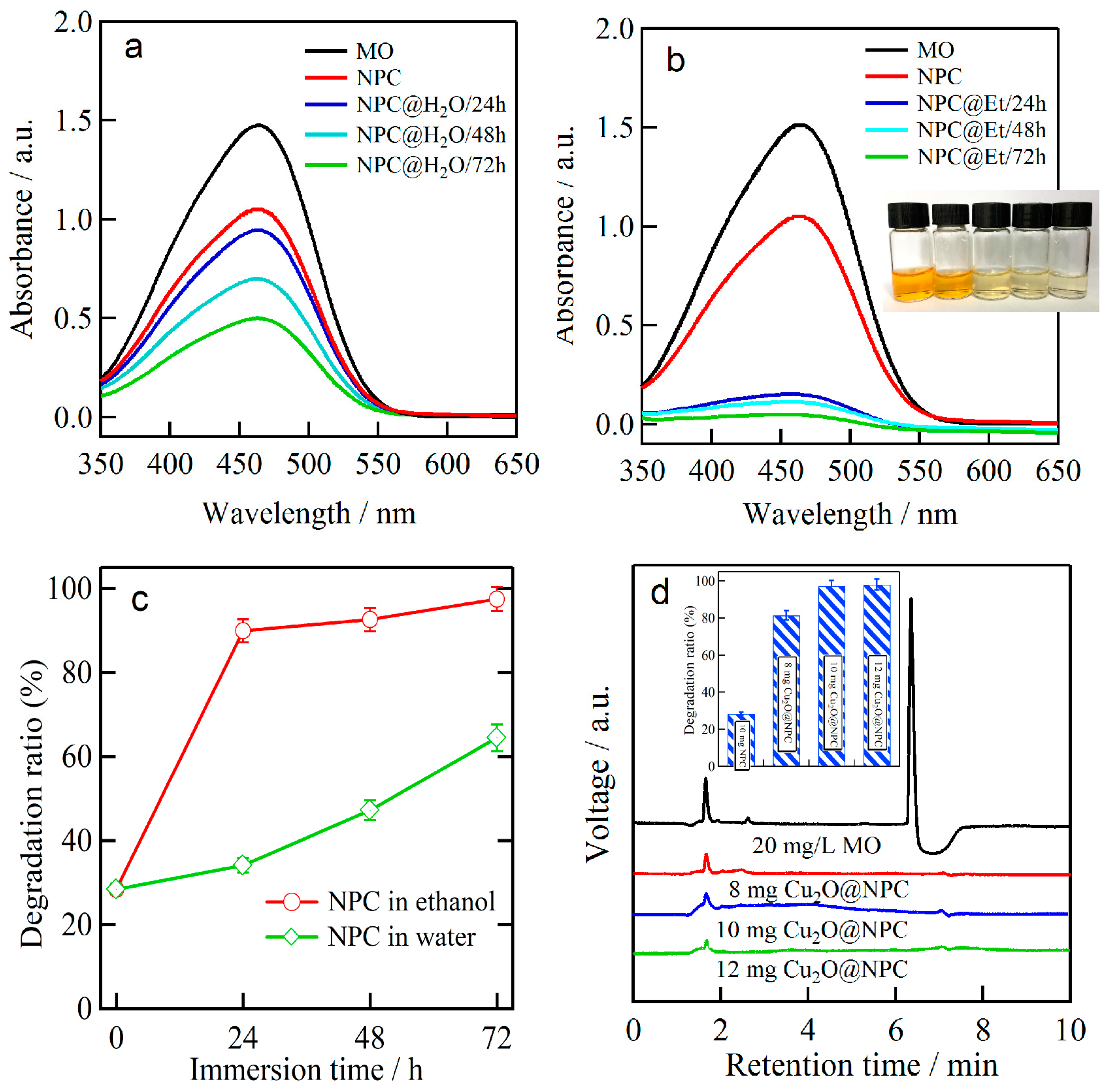
3.3. MO Photodegradation Performance of Cu2O Nanobelts
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zollinger, H. Colour Chemistry-Synthesis, Properties of Organic Dyes and Pigments; VCH Publishers: New York, NY, USA, 1987; pp. 92–100. [Google Scholar]
- Liu, W.B.; Chen, L.; Dong, X.; Yan, J.Z.; Li, N.; Shi, S.Q.; Zhang, S.C. A facile one-pot oxidation-assisted dealloying protocol to massively synthesize monolithic core-shell architectured nanoporous copper@cuprous oxide nanonetworks for photodegradation of methyl orange. Sci. Rep. 2016, 6, 36084. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wyatt, D.T., II; Bahorsky, M. Decolorization of dyes using UV/H2O2 photochemical oxidation. Text Chem. Color 1998, 30, 27–35. [Google Scholar]
- Briskman, R.N. A study of electrodeposited cuprous oxide photovoltaic cells. Sol. Energy Mater. Sol. Cells 1992, 27, 361–368. [Google Scholar] [CrossRef]
- Singh, D.P.; Neti, N.R.; Sinha, A.S.K.; Srivastava, O.N. Growth of different nanostructures of Cu2O (Nanothreads, Nanowires, and Nanocubes) by simple electrolysis based oxidation of copper. J. Phys. Chem. C 2007, 111, 1638–1645. [Google Scholar] [CrossRef]
- Fujinaka, M.; Berezin, A.A. Cuprous oxide–indium–tin oxide thin film photovoltaic cells. J. Appl. Phys. 1983, 54, 3582–3588. [Google Scholar] [CrossRef]
- Olsen, L.C.; Addis, F.W.; Miller, W. Experimental and theoretical studies of Cu2O solar cells. Sol. Cells 1982, 7, 247–279. [Google Scholar] [CrossRef]
- Kumar, R.V.; Mastai, Y.; Diamant, Y.; Gedanken, A. Sonochemical synthesis of amorphous Cu and nanocrystalline Cu2O embedded in a polyaniline matrix. J. Mater. Chem. 2001, 11, 1209–1213. [Google Scholar] [CrossRef]
- Deki, S.; Akamatsu, K.; Yano, T.; Mizuhata, M.; Kajinami, A. Preparation and characterization of copper(I) oxide nanoparticles dispersed in a polymer matrix. J. Mater. Chem. 1998, 8, 1865–1868. [Google Scholar] [CrossRef]
- Wang, W.Z.; Wang, G.H.; Wang, X.S.; Zhan, Y.J.; Liu, Y.K.; Zhang, C.L. Synthesis and characterization of Cu2O nanowires by a novel reduction route. Adv. Mater. 2002, 14, 67–69. [Google Scholar] [CrossRef]
- Wang, W.Z.; Zhan, Y.J.; Wang, G.H. One-step, Solid-state reaction to the synthesis of copper oxide nanorods in the presence of a suitable surfactant. Chem. Commun. 2001, 8, 727–728. [Google Scholar] [CrossRef]
- Xiong, Y.J.; Li, Z.Q.; Zhang, R.; Xie, Y.; Yang, J.; Wu, C.Z. From complex chains to 1D metal oxides: A novel strategy to Cu2O nanowires. J. Phys. Chem. B 2003, 107, 3697–3702. [Google Scholar] [CrossRef]
- Yanagimoto, H.; Akamatsu, K.; Gotoh, K.; Deki, S. Synthesis and characterization of Cu2O nanoparticles dispersed in NH2-terminated poly(ethylene oxide). J. Mater. Chem. 2001, 11, 2387–2389. [Google Scholar] [CrossRef]
- Tench, D.; Warren, L.F. Electrodeposition of conducting transition metal oxide/hydroxide films from aqueous solution. J. Electrochem. Soc. 1983, 130, 869–872. [Google Scholar] [CrossRef]
- Rakhshani, A.E.; Varghese, J. Galvanostatic deposition of thin films of cuprous oxide. Solar Energy Mater. 1987, 15, 237–248. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.K.; Chakraborty, A.K.; Chatterjee, A.P.; Lahiri, S.K. Galvanostatic deposition and electrical characterization of cuprous oxide thin films. Thin. Solid. Films 1992, 209, 92–96. [Google Scholar] [CrossRef]
- Golden, T.D.; Shumsky, M.G.; Zhou, Y.; Vanderwerf, R.A.; Van Leeuwen, R.A.; Switzer, J. Electrochemical deposition of copper(I) oxide films. Chem. Mater. 1996, 8, 2499–2504. [Google Scholar] [CrossRef]
- Switzer, J.A.; Maune, B.M.; Raub, E.R.; Bohannan, W.E. Negative differential resistance in electrochemically self-Assembled layered nanostructures. J. Phys. Chem. B 1999, 103, 395–398. [Google Scholar] [CrossRef]
- Bohannan, E.W.; Huang, L.Y.; Miller, S.; Shumsky, M.G.; Switzer, J.A. In situ electrochemical quartz crystal microbalance study of potential oscillations during the electrodeposition of Cu/Cu2O layered nanostructures. Langmuir 1999, 15, 813–818. [Google Scholar] [CrossRef]
- Kou, T.Y.; Jin, C.H.; Zhang, C.; Sun, J.Z.; Zhang, Z.H. Nanoporous core–shell Cu@Cu2O nanocomposites with superior photocatalytic properties towards the degradation of methyl orange. RSC Adv. 2012, 2, 12636–12643. [Google Scholar] [CrossRef]
- Chen, L.Y.; Yu, J.S.; Fujita, T.; Chen, M.W. Nanoporous copper with tunable nanoporosity for SERS applications. Adv. Funct. Mater. 2009, 19, 1221–1226. [Google Scholar] [CrossRef]
- Xu, H.L.; Wang, W.Z.; Zhu, W. Shape evolution and size-controllable synthesis of Cu2O octahedra and their morphology-dependent photocatalytic properties. J. Phys. Chem. B 2006, 110, 13829–13834. [Google Scholar] [CrossRef] [PubMed]
- Dan, Z.H.; Lu, J.F.; Li, F.; Qin, F.X.; Chang, H. Ethanol-Mediated 2D growth of Cu2O nanoarchitectures on nanoporous Cu templates in anhydrous ethanol. Nanomaterials 2018, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Garrels, R.M.; Christ, C.L. Solutions, Minerals and Equilibria; Harper & Row: New York, NY, USA, 1965; pp. 216–222. [Google Scholar]
- Biesinger, M.C.; Hart, B.R.; Polack, R.; Kobe, B.A.; Smart, R.St.C. Analysis of mineral surface chemistry in flotation separation using imaging XPS. Miner. Eng. 2007, 20, 152–162. [Google Scholar] [CrossRef]
- Kenane, S.; Piraux, L. Electrochemical self-assembly of Cu/Cu2O nanowires. J. Mater. Res. 2002, 17, 401–406. [Google Scholar] [CrossRef]
- Luo, Y.S.; Li, S.Q.; Ren, Q.F.; Liu, J.P.; Xing, L.L.; Wang, Y.; Yu, Y.; Jia, Z.J.; Li, J.L. Facile synthesis of flowerlike Cu2O nanoarchitectures by a solution phase route. Cryst. Growth Des. 2007, 7, 87–92. [Google Scholar] [CrossRef]
- Ko, E.S.; Choi, J.; Okamoto, K.; Tak, Y.S.; Lee, J.Y. Cu2O nanowires in an alumina template: Electrochemical conditions for the synthesis and photoluminescence characteristics. ChemPhysChem 2006, 7, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; McCann, J.T.; Gratt, M.; Xia, Y.N. Photocatalytic deposition of gold nanoparticles on electrospun nanofibers of titania. Chem Phys Lett 2004, 394, 387–391. [Google Scholar] [CrossRef]
- Leff, D.V.; Ohara, P.C.; Heath, J.R.; Gelbart, W.M. Thermodynamic Control of Gold Nanocrystal Size: Experiment and Theory. J. Phys. Chem. 1995, 99, 7036–7041. [Google Scholar] [CrossRef]
- Wiley, B.J.; Xiong, Y.J.; Li, Z.Y.; Yin, Y.D.; Xia, Y.N. Right bipyramids of silver: A new shape derived from single twinned seeds. Nano Lett. 2006, 6, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, C.; Brussino, M.C.; Pramauro, E.; Prevot, A.B.; Palmisano, L.; Marcì, G. Characterization of methyl orange and its photocatalytic degradation products by HPLC/UV–VIS diode array and atmospheric pressure ionization quadrupole ion trap mass spectrometry. Int. J. Mass Spectr. 2002, 214, 247–256. [Google Scholar] [CrossRef]
- He, Y.H.; Grieser, F.; Ashokkumar, M. The mechanism of sonophotocatalytic degradation of methyl orange and its products in aqueous solutions. Ultrason. Sonochem. 2011, 18, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Huang, B.; Wang, Z.; Guo, M.; Qin, X.; Zhang, X.; Wang, P.; Dai, Y. Crystal faces of Cu2O and their stabilities in photocatalytic reactions. J. Phys. Chem. C 2009, 113, 14448–14453. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.; Kamar, P.V. Semiconductor−Metal composite nanostructures. To what extent do metal nanoparticles improve the photocatalytic activity of TiO2 films? J. Phys. Chem. B 2001, 105, 11439–11446. [Google Scholar] [CrossRef]
- Louis, M.; Petr, Z. CRC Handbook Series in Organic Electrochemistry; CRC Press: Boca Raton, FL, USA, 1977; Volume 1. [Google Scholar]
- Huang, L.; Peng, F.; Yu, H.; Wang, H. Preparation of cuprous oxides with different sizes and their behaviors of adsorption, visible-light driven photocatalysis and photocorrosion. Solid State Sci. 2009, 11, 129–138. [Google Scholar] [CrossRef]
- Batista, A.P.L.; Carvalho, H.W.P.; Luz, G.H.P.; Martins, P.F.Q.; Goncalves, M.; Oliveira, L.C.A. Preparation of CuO/SiO2 and photocatalytic activity by degradation of methylene blue. Environ. Chem. Lett. 2010, 8, 63–67. [Google Scholar] [CrossRef]
- Sun, W.; Sun, W.D.; Zhuo, Y.J.; Chu, Y. Facile synthesis of Cu2O nanocube/polycarbazole composites and their high visible-light photocatalytic properties. J. Solid. State Chem. 2011, 184, 1638–1643. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, Z.; Wang, H.; Yang, Y.; Su, W. Experimental Study on Photocatalytic Activity of Cu2O/Cu Nanocomposites Under Visible Light. Catal. Lett. 2009, 132, 75–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, B.; Zhang, T.R.; Gao, D.M.; Xu, A.W. Shape effects of Cu2O polyhedral microcrystals on photocatalytic activity. J. Phys. Chem. C 2010, 114, 5073–5079. [Google Scholar] [CrossRef]
- Huang, W.C.; Lyu, L.M.; Yang, Y.C.; Huang, H. Synthesis of Cu2O nanocrystals from cubic to rhombic dodecahedral structures and their comparative photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.J.; Wise, H.; Yolles, R.S. Selectivity and stoichiometry of copper oxide in propylene oxidation. J. Catal. 1969, 15, 355–362. [Google Scholar] [CrossRef]
- Kansal, S.K.; Singh, M.; Sud, D. Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts. J. Hazard. Mater. 2007, 141, 581–590. [Google Scholar] [CrossRef] [PubMed]


| Photocatalyst | Additive Amount | MO Concentration | Degradation Ratio | Irradiation Time | Degradation Rate | Light Source |
|---|---|---|---|---|---|---|
| mg | mg L−1 | % | min | mg min−1 gcat−1 | ||
| Cu2O nanobelts@NPC (This work) | 8 | 20 | 81.5 | 150 | 0.68 | 40 W daylight lamp (460–610 nm) |
| 10 | 20 | 97.4 | 150 | 0.65 | ||
| 12 | 20 | 98.1 | 150 | 0.55 | ||
| NPC (This work) | 10 | 20 | 28.4 | 150 | 0.19 | |
| Core-shell Cu@Cu2O [20] | 4 | 20 | 90 | 100 | 2.25 | Sun light (300–2500 nm) |
| Cu2O nanocube [39] | 8 | 20 | 83.6 | 120 | 0.70 | 300 W xenon lamp (190–1100 nm) |
| Cu2O@Cu [40] | 30 | 10 | 90 | 120 | 0.14 | 40 W tungsten lamp (350–2500 nm) |
| Cu2O polyhedral [41] | 50 | 15 | 96 | 180 | 0.06 | 500 W xenon lamp (≥400 nm) |
| Cu2O octahedra [41] | 50 | 15 | 80 | 180 | 0.05 | 500 W xenon lamp (≥400 nm) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dan, Z.; Yang, Y.; Qin, F.; Wang, H.; Chang, H. Facile Fabrication of Cu2O Nanobelts in Ethanol on Nanoporous Cu and Their Photodegradation of Methyl Orange. Materials 2018, 11, 446. https://doi.org/10.3390/ma11030446
Dan Z, Yang Y, Qin F, Wang H, Chang H. Facile Fabrication of Cu2O Nanobelts in Ethanol on Nanoporous Cu and Their Photodegradation of Methyl Orange. Materials. 2018; 11(3):446. https://doi.org/10.3390/ma11030446
Chicago/Turabian StyleDan, Zhenhua, Yulin Yang, Fengxiang Qin, Hao Wang, and Hui Chang. 2018. "Facile Fabrication of Cu2O Nanobelts in Ethanol on Nanoporous Cu and Their Photodegradation of Methyl Orange" Materials 11, no. 3: 446. https://doi.org/10.3390/ma11030446
APA StyleDan, Z., Yang, Y., Qin, F., Wang, H., & Chang, H. (2018). Facile Fabrication of Cu2O Nanobelts in Ethanol on Nanoporous Cu and Their Photodegradation of Methyl Orange. Materials, 11(3), 446. https://doi.org/10.3390/ma11030446





