Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications
Abstract
:1. Introduction
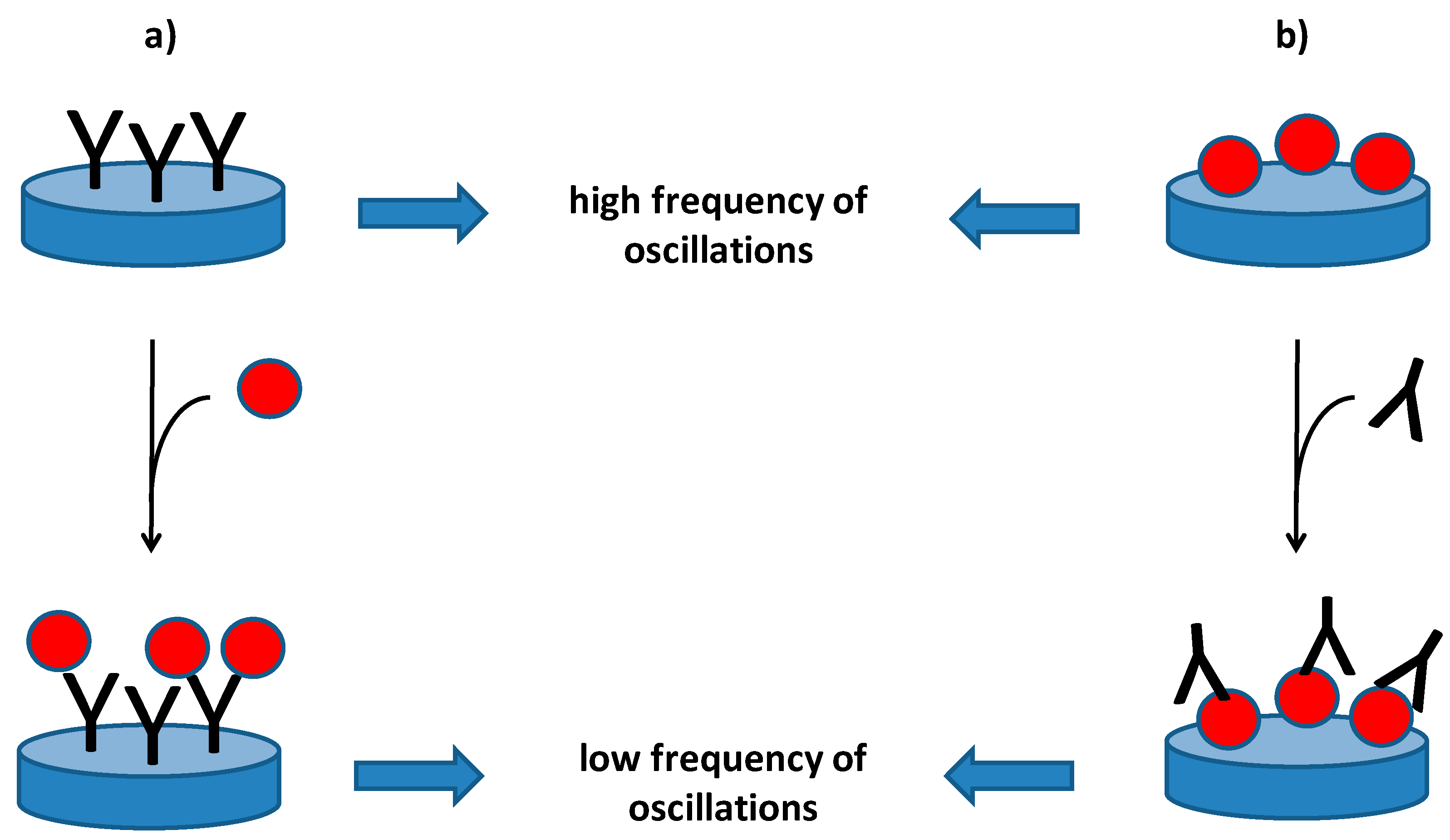
2. Piezoelectric Immunosensors
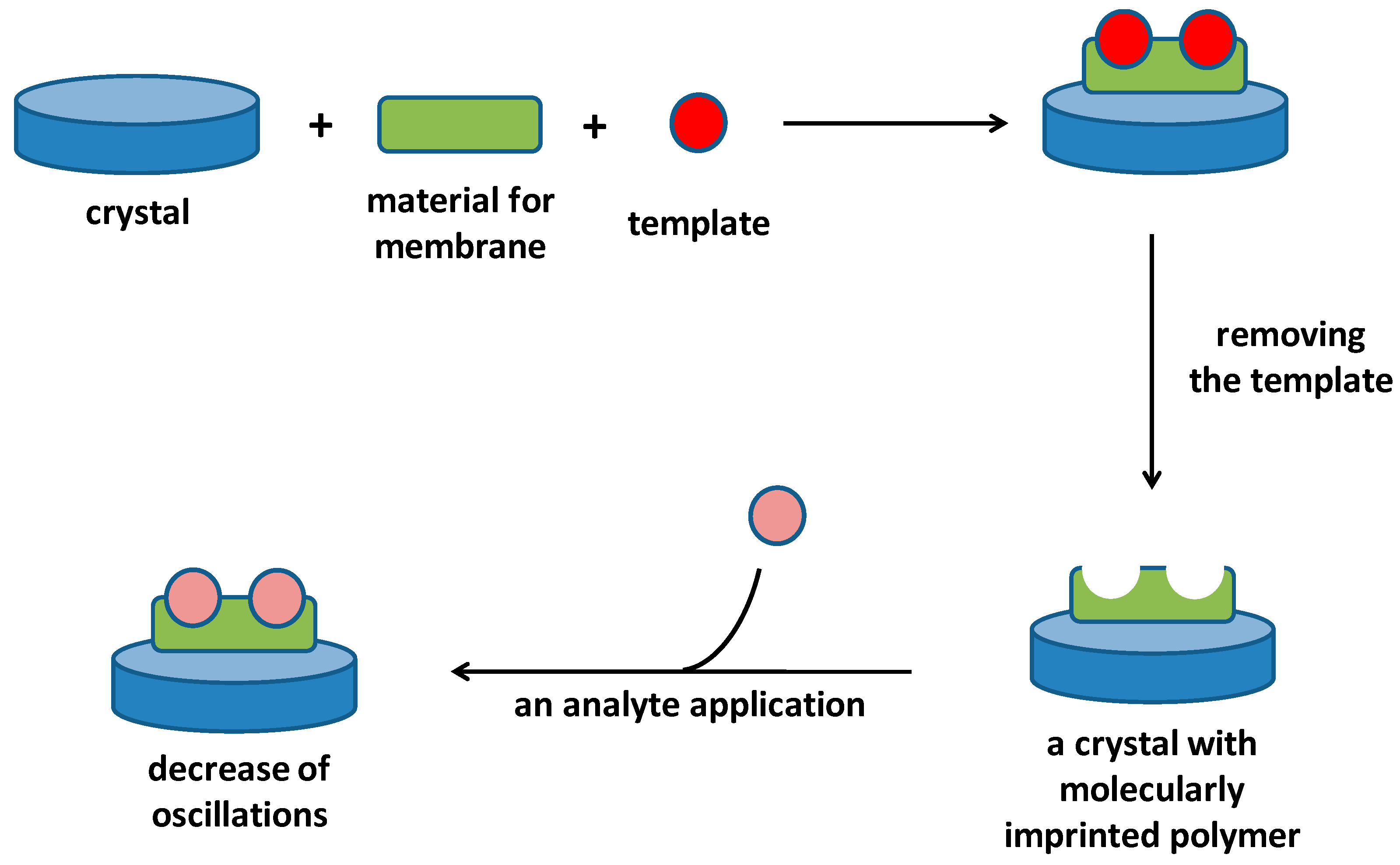
3. Molecularly Imprinted Polymers on Piezoelectric Platform
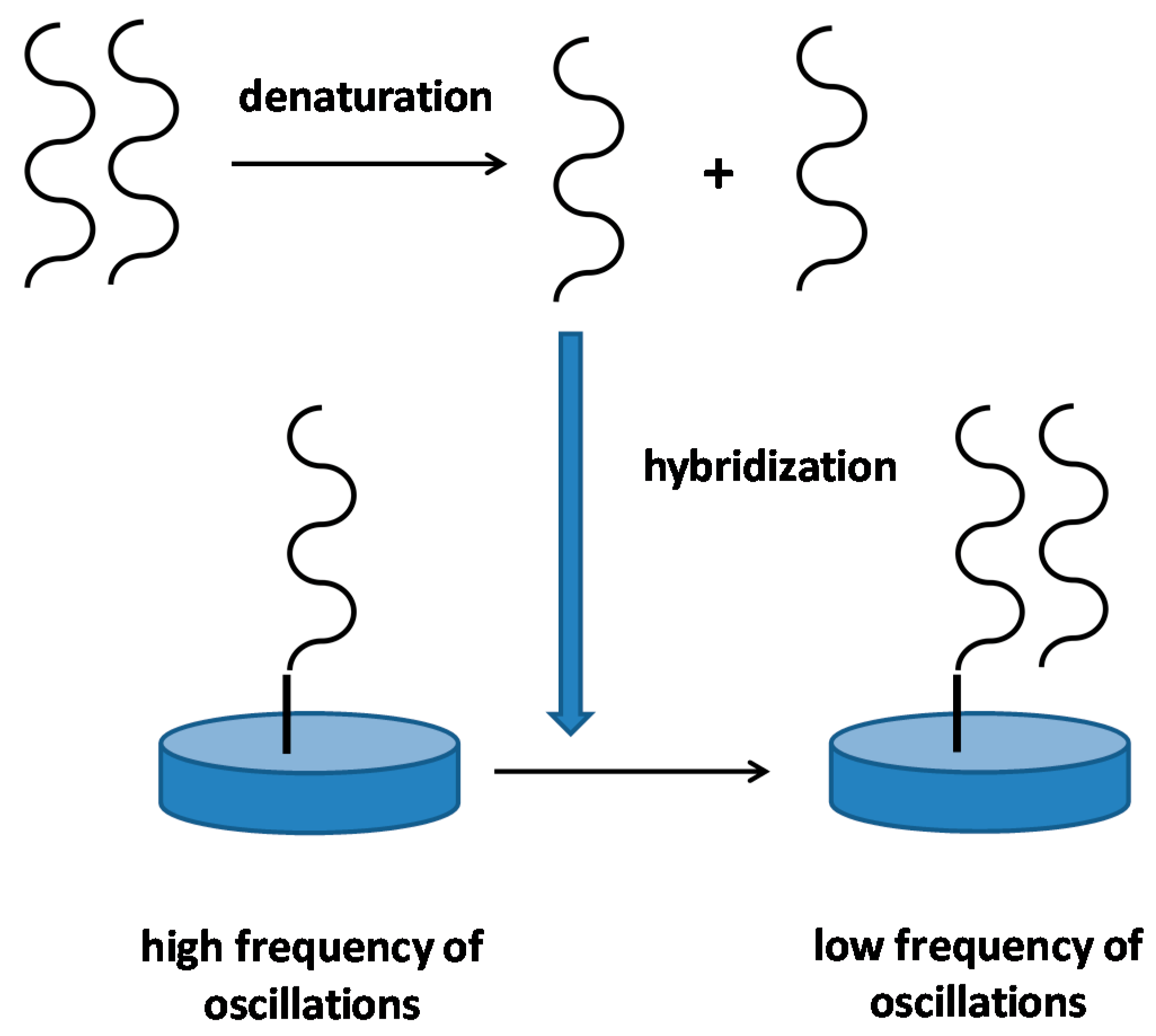
4. Genetic Information Using Piezoelectric Biosensors
5. Other Types of Piezoelectric Biosensors
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zu, H.; Wu, H.; Wang, Q.M. High-temperature piezoelectric crystals for acoustic wave sensor applications. IEEE 2016, 63, 486–505. [Google Scholar] [CrossRef] [PubMed]
- Hagood, N.W.; von Flotow, A.F. Damping of structural vibrations with piezoelectric materials and passive electrical networks. J. Sound Vib. 1991, 146, 243–268. [Google Scholar] [CrossRef]
- Hees, J.; Heidrich, N.; Pletschen, W.; Sah, R.E.; Wolfer, M.; Williams, O.A.; Lebedev, V.; Nebel, C.E.; Ambacher, O. Piezoelectric actuated micro-resonators based on the growth of diamond on aluminum nitride thin films. Nanotechnology 2013, 24, 025601. [Google Scholar] [CrossRef] [PubMed]
- Meyers, F.N.; Loh, K.J.; Dodds, J.S.; Baltazar, A. Active sensing and damage detection using piezoelectric zinc oxide-based nanocomposites. Nanotechnology 2013, 24, 185501. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.; Hou, R.Z.; Wu, A.; Willinger, M.G.; Vilarinho, P.M.; Mosa, J.; Laberty-Robert, C.; Boissiere, C.; Grosso, D.; Sanchez, C. Nanoporous piezo- and ferroelectric thin films. Langmuir 2012, 28, 2944–2949. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wereszczak, A.A. Effects of electric field and biaxial flexure on the failure of poled lead zirconate titanate. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008, 55, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Struth, B.; Decher, G.; Schmitt, J.; Hofmeister, W.; Neisendorfer, F.; Pietsch, U.; Brezesinski, G.; Mohwald, H. Chemical modification of topaz surfaces. Mater. Sci. Eng. C 1999, 10, 97–101. [Google Scholar] [CrossRef]
- Levitskii, R.R.; Zachek, I.R.; Verkholyak, T.M.; Moina, A.P. Dielectric, piezoelectric, and elastic properties of the rochelle salt NaKC4H4O6·4H2O: A theory. Phys. Rev. B 2003, 67, 174112. [Google Scholar] [CrossRef]
- Sawyer, C.B.; Tower, C.H. Rochelle salt as a dielectric. Phys. Rev. 1930, 35, 269. [Google Scholar] [CrossRef]
- Fukada, E. History and recent progress in piezoelectric polymers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2000, 47, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Sinha, T.K.; Ghosh, S.K.; Maiti, R.; Jana, S.; Adhikari, B.; Mandal, D.; Ray, S.K. Graphene-silver-induced self-polarized PVDF-based flexible plasmonic nanogenerator toward the realization for new class of self powered optical sensor. ACS Appl. Mater. Interfaces 2016, 8, 14986–14993. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, G.; Bustabad, E.A.; Perrot, H.; Gabrielli, C.; Bucur, B.; Lazerges, M.; Rose, D.; Rodriguez-Pardo, L.; Farina, J.; Compere, C.; et al. Development of a mass sensitive quartz crystal microbalance (QCM)-based DNA biosensor using a 50 mHz electronic oscillator circuit. Sensors 2011, 11, 7656–7664. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. The piezoelectric biosensors: Principles and applications, a review. Int. J. Electrochem. Sci. 2017, 12, 496–506. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von schwingquarzen zur wägung dünner schichten und zur mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, N.; Yang, J.; Chen, W. Thickness-shear vibration of at-cut quartz plates carrying finite-size particles with rotational degree of freedom and rotatory inertia. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.K.; Gordon, J.G. Frequency of a quartz microbalance in contact with liquid. Anal. Chem. 1985, 57, 1770–1771. [Google Scholar] [CrossRef]
- Shana, Z.A.; Radtke, D.E.; Kelkar, U.R.; Josse, F.; Haworth, D.T. Theory and application of a quartz resonator as a sensor for viscous liquids. Anal. Chim. Acta 1990, 231, 317–320. [Google Scholar] [CrossRef]
- Muratsugu, M.; Ohta, F.; Miya, Y.; Hosokawa, T.; Kurosawa, S.; Kamo, N.; Ikeda, H. Quartz-crystal microbalance for the detection of microgram quantities of human serum-albumin—Relationship between the frequency change and the mass of protein adsorbed. Anal. Chem. 1993, 65, 2933–2937. [Google Scholar] [CrossRef]
- Deng, T.; Li, J.S.; Wang, H.; Shen, G.L.; Yu, R.Q. Piezoelectric immunoassay for complement c4 based on a nafion-modified interface for antibody immobilization. J. Immunol. Methods 2005, 299, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Funari, R.; Terracciano, I.; Della Ventura, B.; Ricci, S.; Cardi, T.; D’Agostino, N.; Velotta, R. Label-free detection of gliadin in food by quartz crystal microbalance-based immunosensor. J. Agric. Food Chem. 2017, 65, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Maraldo, D.; Mutharasan, R. 10-min assay for detecting Escherichia coli O157:H7 in ground beef samples using piezoelectric-excited millimeter-size cantilever sensors. J. Food Prot. 2007, 70, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Maraldo, D.; Mutharasan, R. Preparation-free method for detecting Escherichia coli O157:H7 in the presence of spinach, spring lettuce mix, and ground beef particulates. J. Food Prot. 2007, 70, 2651–2655. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.A.; Uknalis, J.; Tu, S.I.; Mutharasan, R. Detect of Escherichia coli O157:H7 in ground beef samples using piezoelectric excited millimeter-sized cantilever (PEMC) sensors. Biosens. Bioelectron. 2007, 22, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.V.; Sorokulova, I.B.; Petrenko, V.A.; Chen, I.H.; Brbaree, J.M.; Vodyanoy, V.J. Affinity-selected filamentous bacteriophage as a probe for acoustic wave biodetectors of salmonella typhimurium. Biosens. Bioelectron. 2006, 21, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Skladal, P. Piezoelectric immunosensor for the direct and rapid detection of francisella tularensis. Folia Microbiol. 2007, 52, 325–330. [Google Scholar] [CrossRef]
- Salam, F.; Uludag, Y.; Tothill, I.E. Real-time and sensitive detection of salmonella typhimurium using an automated quartz crystal microbalance (QCM) instrument with nanoparticles amplification. Talanta 2013, 115, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lin, C.S.; Chen, S.H.; Ye, R.; Wu, V.C. A piezoelectric immunosensor for specific capture and enrichment of viable pathogens by quartz crystal microbalance sensor, followed by detection with antibody-functionalized gold nanoparticles. Biosens. Bioelectron. 2012, 38, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Piezoelectric biosensor for the determination of tumor necrosis factor alpha. Talanta 2018, 178, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Kosslinger, C.; Drost, S.; Aberl, F.; Wolf, H. Quartz-crystal microbalance for immunosensing. Fresenius J. Anal. Chem. 1994, 349, 349–354. [Google Scholar] [CrossRef]
- Ramos-Jesus, J.; Carvalho, K.A.; Fonseca, R.A.; Oliveira, G.G.; Melo, S.M.; Alcantara-Neves, N.M.; Dutra, R.F. A piezoelectric immunosensor for leishmania chagasi antibodies in canine serum. Anal. Bioanal. Chem. 2011, 401, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Pavlis, O.; Skladal, P. Diagnosis of tularemia using piezoelectric biosensor technology. Talanta 2007, 71, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Crosson, C.; Rossi, C. Quartz crystal microbalance immunosensor for the quantification of immunoglobulin g in bovine milk. Biosens. Bioelectron. 2013, 42, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Naklua, W.; Suedee, R.; Lieberzeit, P.A. Dopaminergic receptor-ligand binding assays based on molecularly imprinted polymers on quartz crystal microbalance sensors. Biosens. Bioelectron. 2016, 81, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Kotova, K.; Lieberzeit, P.A. Molecularly imprinted polymer nanoparticles for formaldehyde sensing with QCM. Sensors 2016, 16, 1011. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhang, Y.; Pan, M.; Kong, L.; Wang, S. Development and application of quartz crystal microbalance sensor based on novel molecularly imprinted sol-gel polymer for rapid detection of histamine in foods. J. Agric. Food Chem. 2014, 62, 5269–5274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zhang, J.; Dai, C.M.; Zhou, X.F.; Liu, S.G. Sorption of carbamazepine from water by magnetic molecularly imprinted polymers based on chitosan-Fe(3)O(4). Carbohydr. Polym. 2013, 97, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, Y.A.; Zhu, Q.J.; Ye, C. Chitosan in molecularly-imprinted polymers: Current and future prospects. Int. J. Mol. Sci. 2015, 16, 18328–18347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, Z.; Zhang, H.; Qiu, Y. Selective removal of the genotoxic compound 2-aminopyridine in water using molecularly imprinted polymers based on magnetic chitosan and beta-cyclodextrin. Int. J. Environ. Res. Public Health 2017, 14, 991. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Hu, X.; Xu, Z. Beta-cyclodextrin molecularly imprinted solid-phase microextraction coatings for selective recognition of polychlorophenols in water samples. Anal. Bioanal. Chem. 2018, 410, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, J.; Meng, Q.; Tian, Y.; Xu, X.; Jin, Z. Photoirradiation surface molecularly imprinted polymers for the separation of 6-O-alpha-d-maltosyl-beta-cyclodextrin. J. Sep. Sci. 2017, 40, 4653–4660. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, T.; Yang, X.; She, Y.; Wang, M.; Wang, J.; Zhang, M.; Wang, S.; Jin, F.; Jin, M.; et al. Competitive fluorescence assay for specific recognition of atrazine by magnetic molecularly imprinted polymer based on fe3o4-chitosan. Carbohydr. Polym. 2016, 137, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Abdelbar, N.M.; Mohamed, A.A. Molecular imprinted chitosan-TiO2 nanocomposite for the selective removal of rose bengal from wastewater. Int. J. Biol. Macromol. 2018, 107, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singh, A.; Singh, M. QCM sensing of melphalan via electropolymerized molecularly imprinted polythiophene films. Biosens. Bioelectron. 2015, 74, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Shah, K.; Singh, M. An epitope-imprinted piezoelectric diagnostic tool for neisseria meningitidis detection. J. Mol. Recognit. 2016, 29, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, M. Electrochemical and piezoelectric monitoring of taurine via electropolymerized molecularly imprinted films. J. Mol. Recognit. 2017, 30, 13. [Google Scholar] [CrossRef] [PubMed]
- Ebarvia, B.S.; Ubando, I.E.; Sevilla, F.B., III. Biomimetic piezoelectric quartz crystal sensor with chloramphenicol-imprinted polymer sensing layer. Talanta 2015, 144, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Ebarvia, B.S.; Binag, C.A.; Sevilla, F. Biomimetic piezoelectric quartz sensor for caffeine based on a molecularly imprinted polymer. Anal. Bioanal. Chem. 2004, 378, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Bartold, K.; Pietrzyk-Le, A.; Huynh, T.P.; Iskierko, Z.; Sosnowska, M.; Noworyta, K.; Lisowski, W.; Sannicolo, F.; Cauteruccio, S.; Licandro, E.; et al. Programmed transfer of sequence information into a molecularly imprinted polymer for hexakis(2,2′-bithien-5-yl) DNA analogue formation toward single-nucleotide-polymorphism detection. ACS Appl. Mater. Interfaces 2017, 9, 3948–3958. [Google Scholar] [CrossRef] [PubMed]
- Sivashankar, S.; Sapsanis, C.; Agambayev, S.; Buttner, U.; Salama, K.N. Label-free detection of sex determining region y (sry) via capacitive biosensor. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016, 10, 7591690. [Google Scholar]
- de las Heras, A.; Carreno, C.A.; de Lorenzo, V. Stable implantation of orthogonal sensor circuits in gram-negative bacteria for environmental release. Environ. Microbiol. 2008, 10, 3305–3316. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.N.; Nguyen, T.T.; Mai, C.T.; Bettarel, Y.; Hoang, T.Y.; Trinh, T.T.; Truong, N.H.; Chu, H.H.; Nguyen, V.T.; Nguyen, H.D.; et al. Procarcinogens—Determination and evaluation by yeast-based biosensor transformed with plasmids incorporating rad54 reporter construct and cytochrome p450 genes. PLoS ONE 2016, 11, e0168721. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhao, S.; Wu, S.; Wang, Z. Upconversion nanoparticles grafted molybdenum disulfide nanosheets platform for microcystin-lr sensing. Biosens. Bioelectron. 2017, 90, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Hayat, A.; Andreescu, S. Biomolecular detection at ssdna-conjugated nanoparticles by nano-impact electrochemistry. Biosens. Bioelectron. 2017, 87, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Kirimli, C.E.; Shih, W.H.; Shih, W.Y. DNA hybridization detection with 100 zm sensitivity using piezoelectric plate sensors with an improved noise-reduction algorithm. Analyst 2014, 139, 2754–2763. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Desai, D.; Kumar, A. Gene specific DNA sensors for diagnosis of pathogenic infections. Indian J. Microbiol. 2017, 57, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; He, F.; Wang, H.; Tong, F. A new aptamer/graphene interdigitated gold electrode piezoelectric sensor for rapid and specific detection of staphylococcus aureus. Biosens. Bioelectron. 2015, 65, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Rijal, K.; Mutharasan, R. A method for DNA-based detection of E. coli O157:H7 in a proteinous background using piezoelectric-excited cantilever sensors. Analyst 2013, 138, 2943–2950. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Chuang, Y.C.; Lu, Y.C.; Lin, H.C.; Yang, Y.L.; Lin, C.S. A method of layer-by-layer gold nanoparticle hybridization in a quartz crystal microbalance DNA sensing system used to detect dengue virus. Nanotechnology 2009, 20, 215501. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Li, J.; Jiang, J.; Shen, G.; Yu, R. DNA point mutation detection based on DNA ligase reaction and nano-au amplification: A piezoelectric approach. Anal. Biochem. 2006, 358, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, Y.; Li, H.; Tan, P.; Tang, H.; Yao, S. A novel method for the detection of point mutation in DNA using single-base-coded cds nanoprobes. Biosens. Bioelectron. 2009, 24, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- Gabius, H.J.; Siebert, H.C.; Andre, S.; Jimenez-Barbero, J.; Rudiger, H. Chemical biology of the sugar code. ChemBioChem 2004, 5, 740–764. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.Q.; Qu, K.; Rehman, A. Glycosylated conductive polymer: A multimodal biointerface for studying carbohydrate-protein interactions. Acc. Chem. Res. 2016, 49, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Pesquero, N.C.; Carvalho, F.C.; Faria, R.C.; Roque-Barreira, M.C.; Bueno, P.R. Artinm binding effinities and kinetic interaction with leukemia cells: A quartz crystal microbalance bioelectroanalysis on the cytotoxic effect. Electroanalysis 2017, 29, 1554–1558. [Google Scholar] [CrossRef]
- Pizzoni, D.; Mascini, M.; Lanzone, V.; Del Carlo, M.; Di Natale, C.; Compagnone, D. Selection of peptide ligands for piezoelectric peptide based gas sensors arrays using a virtual screening approach. Biosens. Bioelectron. 2014, 52, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Mascini, M.; Pizzoni, D.; Perez, G.; Chiarappa, E.; Di Natale, C.; Pittia, P.; Compagnone, D. Tailoring gas sensor arrays via the design of short peptides sequences as binding elements. Biosens. Bioelectron. 2017, 93, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Compagnone, D.; Fusella, G.C.; Del Carlo, M.; Pittia, P.; Martinelli, E.; Tortora, L.; Paolesse, R.; Di Natale, C. Gold nanoparticles-peptide based gas sensor arrays for the detection of food aromas. Biosens. Bioelectron. 2013, 42, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, X.; Zhang, F.Y.; He, X.X.; Fang, G.Z.; Liu, J.F.; Wang, S. Design of cyclic peptide based glucose receptors and their application in glucose sensing. Anal. Chem. 2017, 89, 10431–10438. [Google Scholar] [CrossRef] [PubMed]
- Capobianco, J.A.; Shih, W.Y.; Adams, G.P.; Shih, W.H. Label-free growth receptor-2 detection and dissociation constant assessment in diluted human serum using a longitudinal extension mode of a piezoelectric microcantilever sensor. Sens. Actuators B Chem. 2011, 160, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Ben Yahia, M.; Hsan, L.B.H.; Knani, S.; Nasri, H.; Ben Lamine, A. Modeling of adsorption isotherms of zinc nitrate on a thin layer of porphyrin. J. Mol. Liq. 2016, 222, 576–585. [Google Scholar] [CrossRef]


| Recognition Part | Piezoelectric Part | Analyte | Limit of Detection | References |
|---|---|---|---|---|
| Antibody | QCM 1 | Albumin in urea (albuminuria) | 0.1 µg/mL | [18] |
| Antibody | QCM | Francisella tularensis | 105 CFU/mL | [25] |
| Antibody on the sensor surface and gold nanoparticles covered by antibodies | QCM | Escherichia coli O157:H7 | 10 CFU/mL | [27] |
| Molecularly Imprinted Polymer from electropolymerized 3-thiophene acetic acid | QCM | Drug melphalan | 5.40 ng/mL | [43] |
| Electrochemically polymerized I-methionine with molecularly imprinted taurine | QCM | Taurine | 0.12 µmol/L | [45] |
| DNA probe | 8 µm thick lead magnesium niobite-lead titanate piezoelectric plates | DNA | 10−19 mol/L in urine samples | [54] |
| DNA specific to stx2 gene from Escherichia coli O157:H7 | Piezoelectric-excited cantilever sensor | Escherichia coli O157:H7 | 700 cells/mL | [57] |
| Oligonucleotide functionalized gold nanoparticles | QCM | Denque virus | 2 PFU/mL | [58] |
| Antibody | Lead zirconate-lead titanate glass piezoelectric microcantilever sensor | Marker of cancer Her2 | 0.06 nmol/L | [68] |
| Various short peptides | QCM | volatile compounds | - | [64] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pohanka, M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials 2018, 11, 448. https://doi.org/10.3390/ma11030448
Pohanka M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials. 2018; 11(3):448. https://doi.org/10.3390/ma11030448
Chicago/Turabian StylePohanka, Miroslav. 2018. "Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications" Materials 11, no. 3: 448. https://doi.org/10.3390/ma11030448
APA StylePohanka, M. (2018). Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials, 11(3), 448. https://doi.org/10.3390/ma11030448





