Recent Advances in Discotic Liquid Crystal-Assisted Nanoparticles
Abstract
1. Introduction
1.1. Discotic Liquid Crystals
1.2. Nanomaterials
2. Nanoparticles in Discotic Liquid Crystals
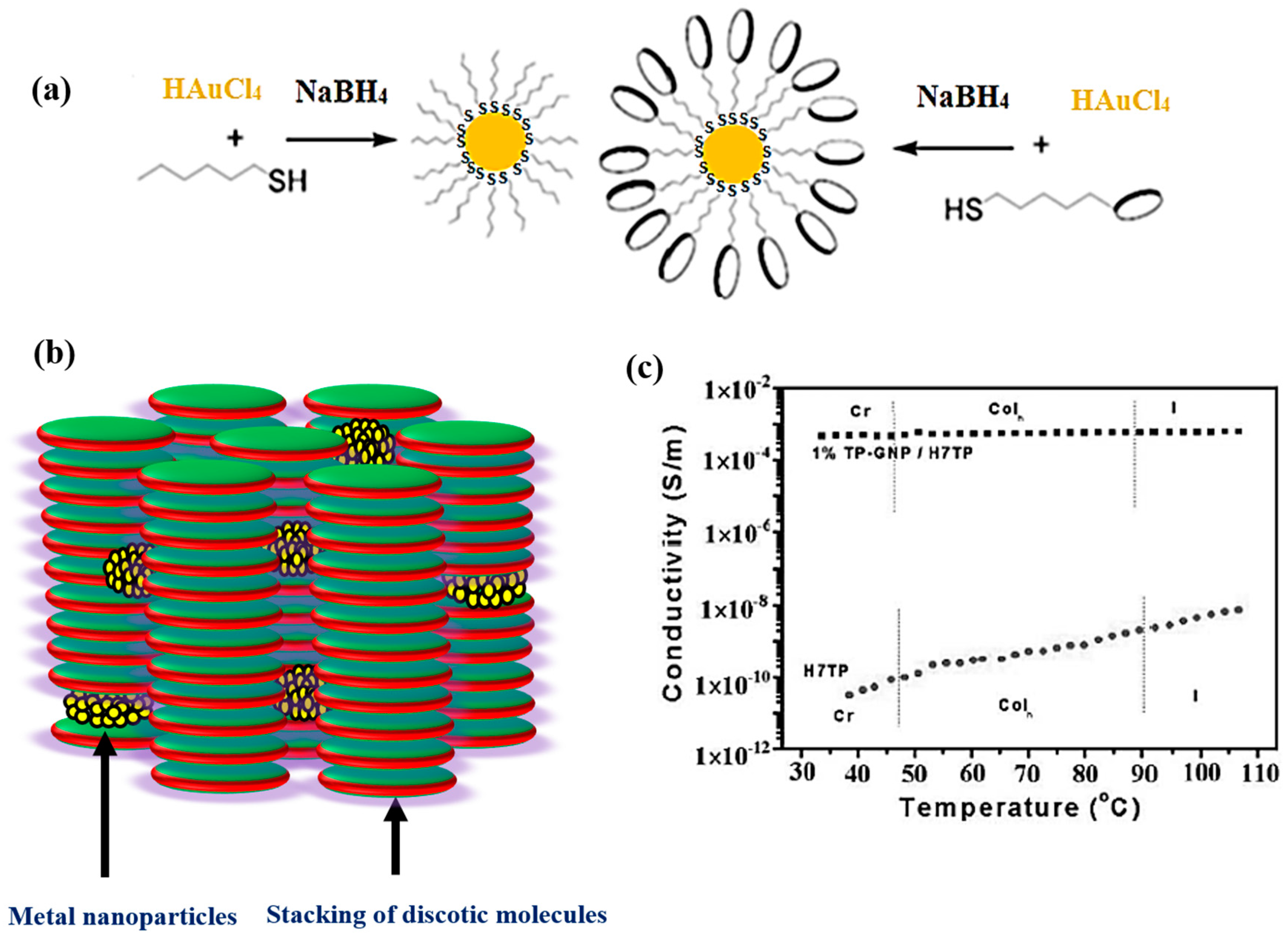
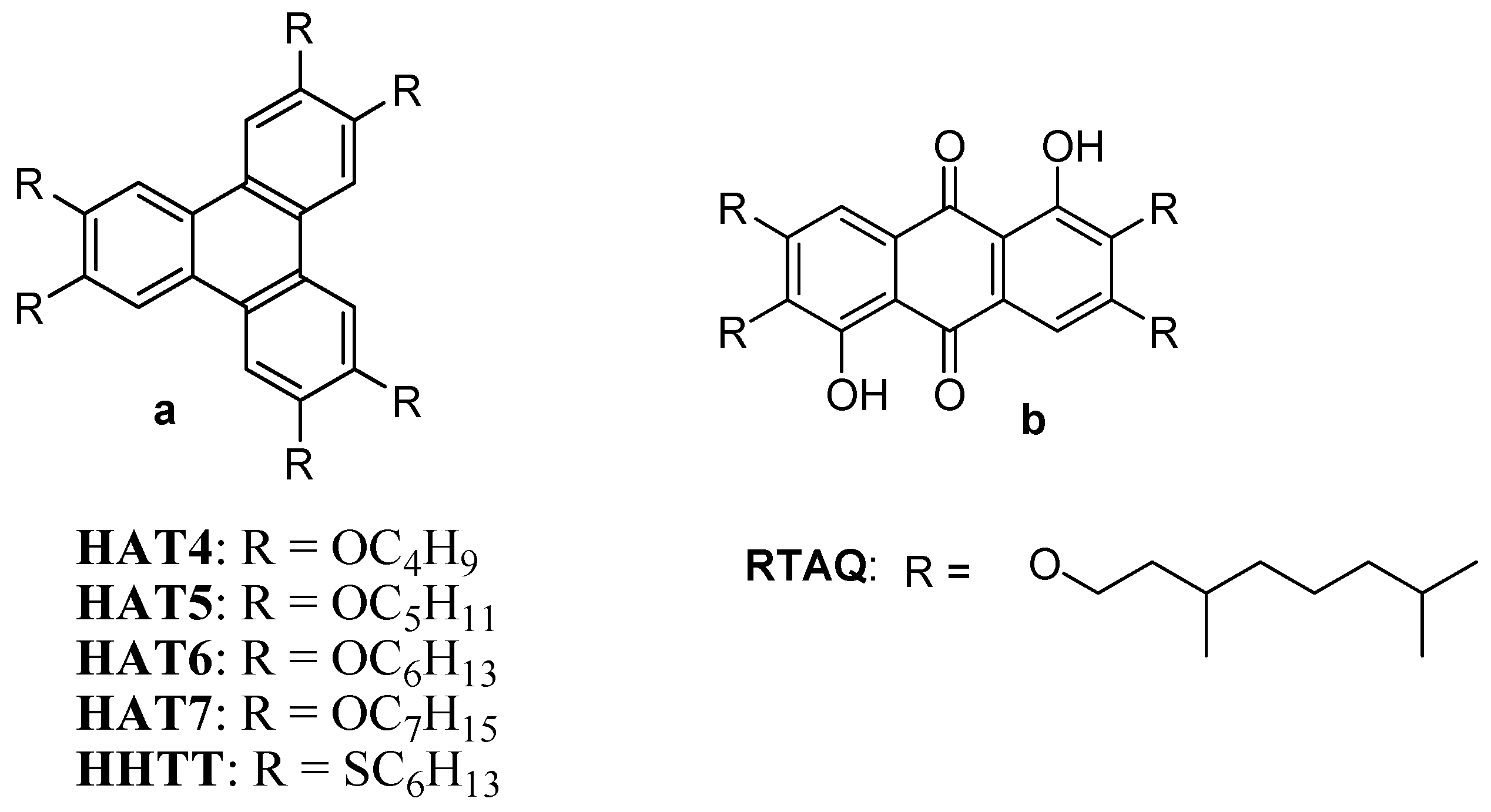
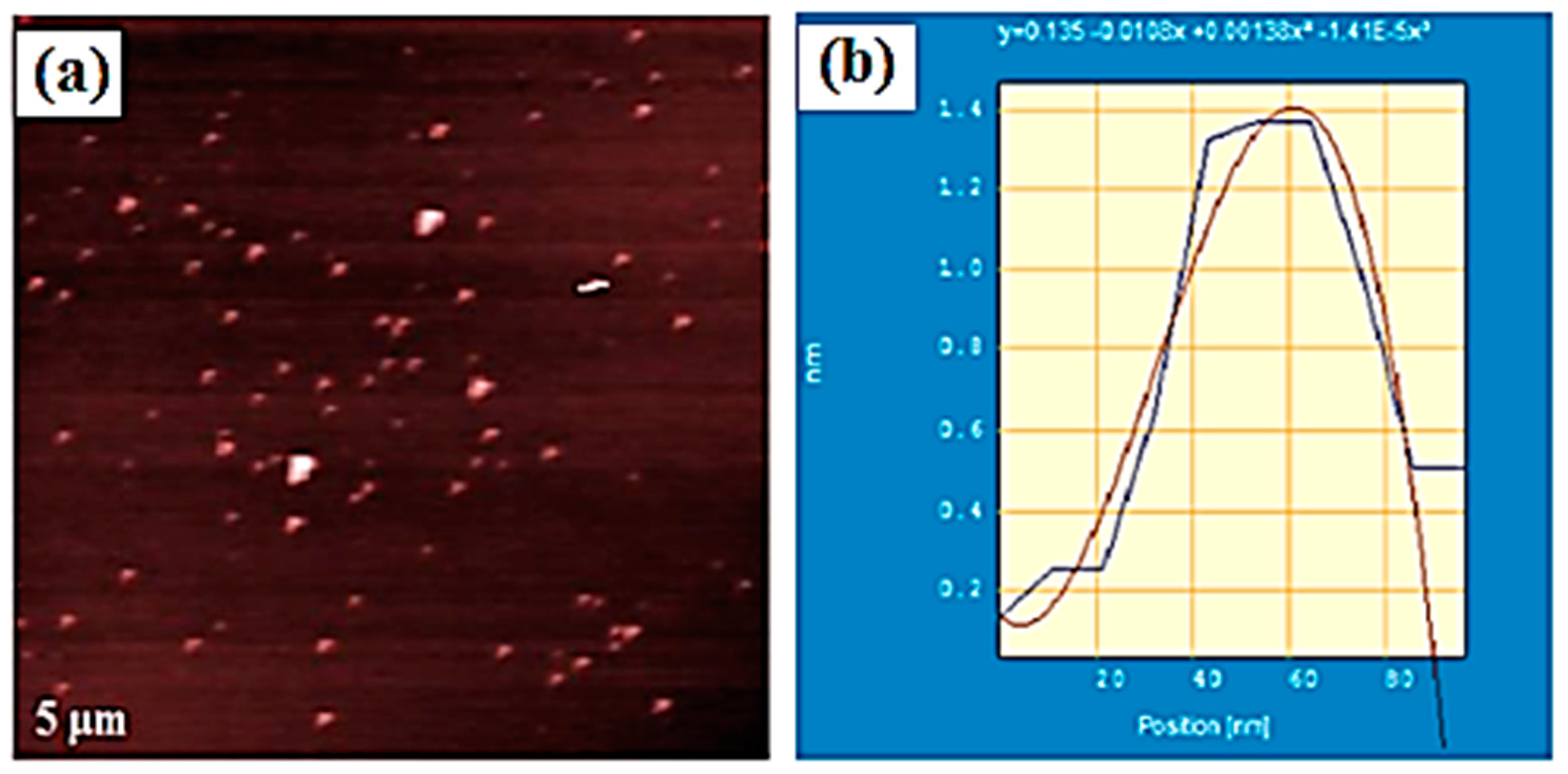
2.1. Spherical or Quasi-Spherical Metallic NPs in Discotic Liquid Crystals
2.2. Quantum Dots in Discotic Liquid Crystals
3. One-Dimensional Nanostructures in Discotic Liquid Crystals
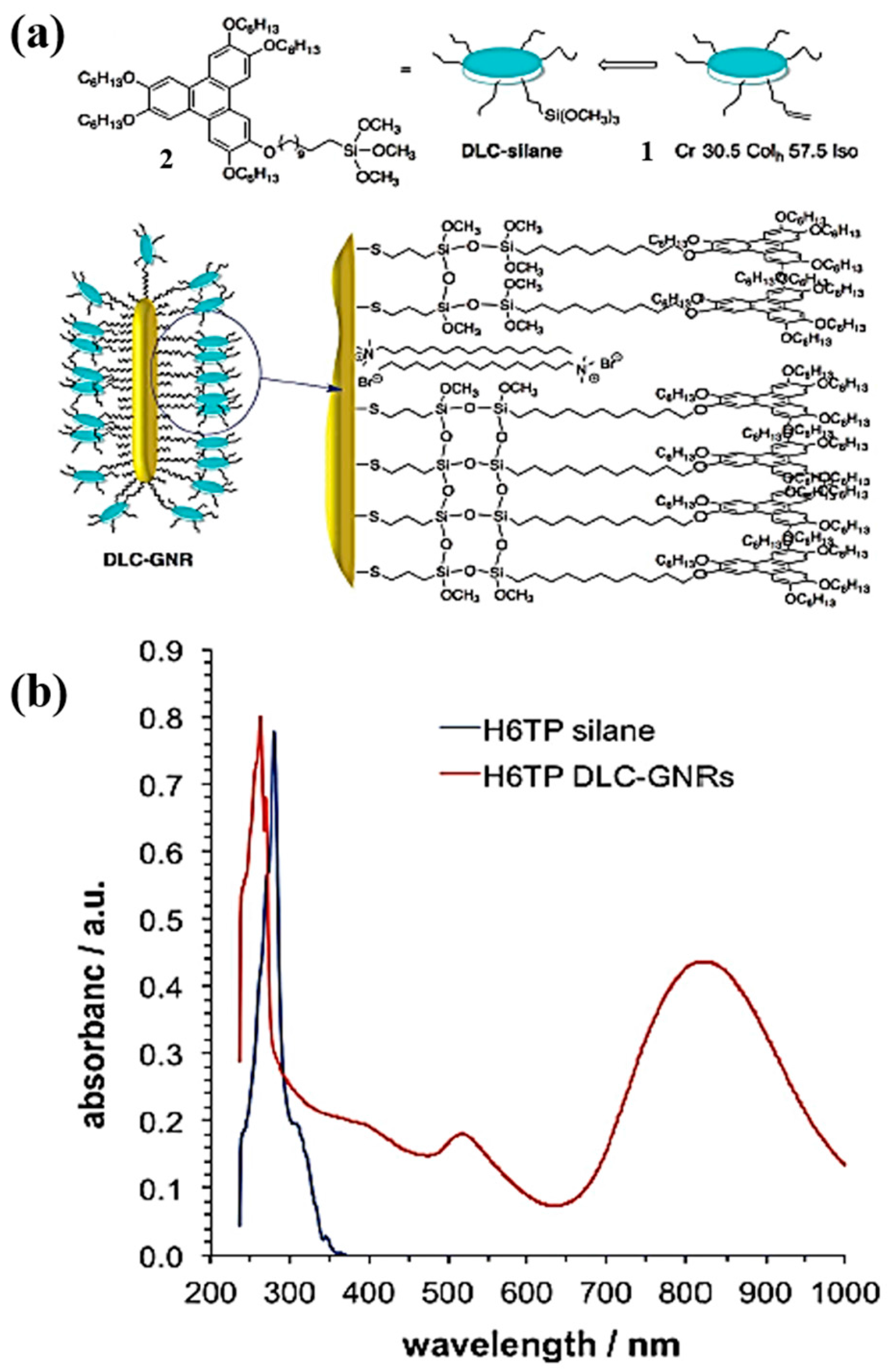
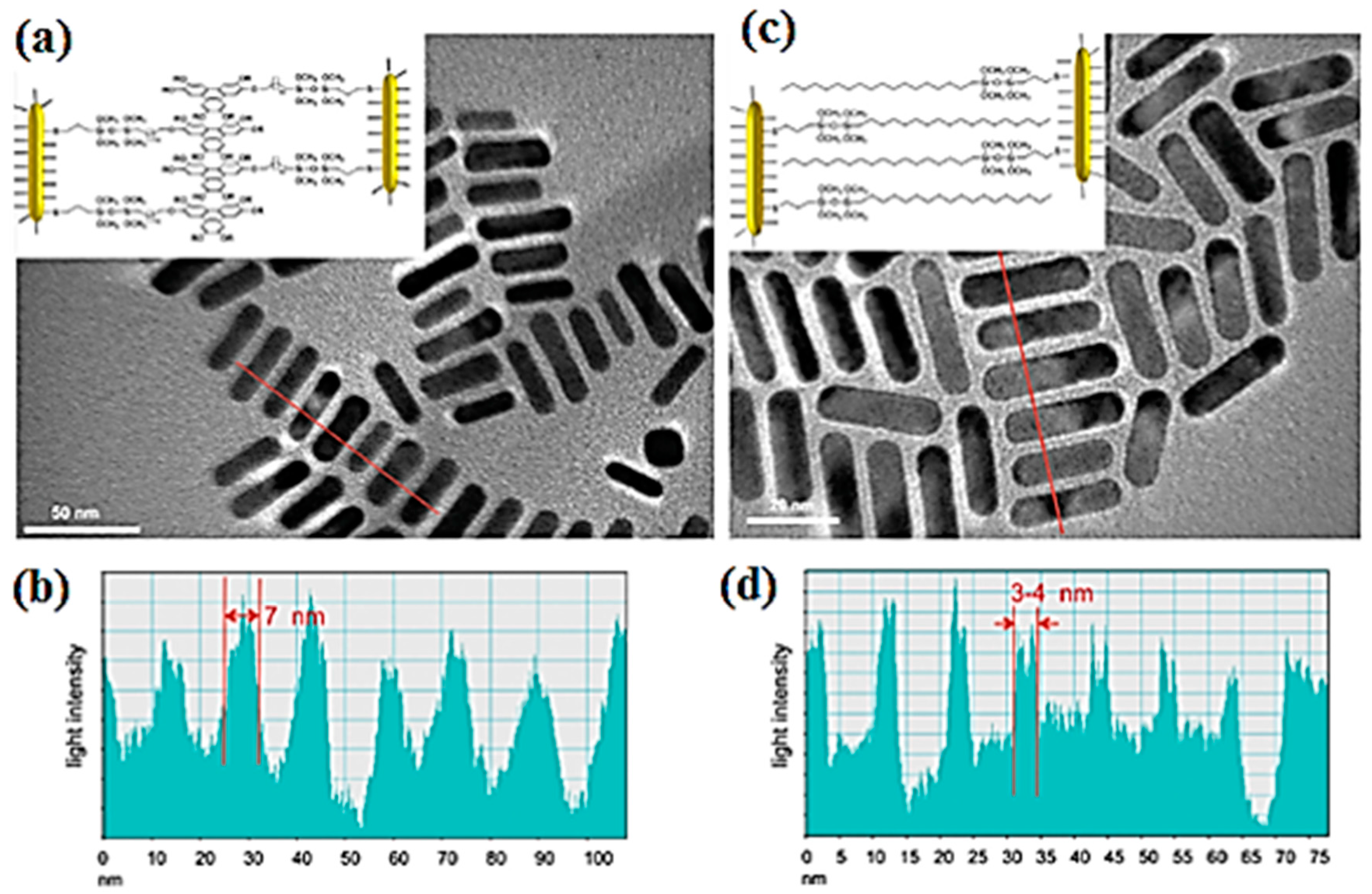
3.1. Gold Nanorods in DLCs
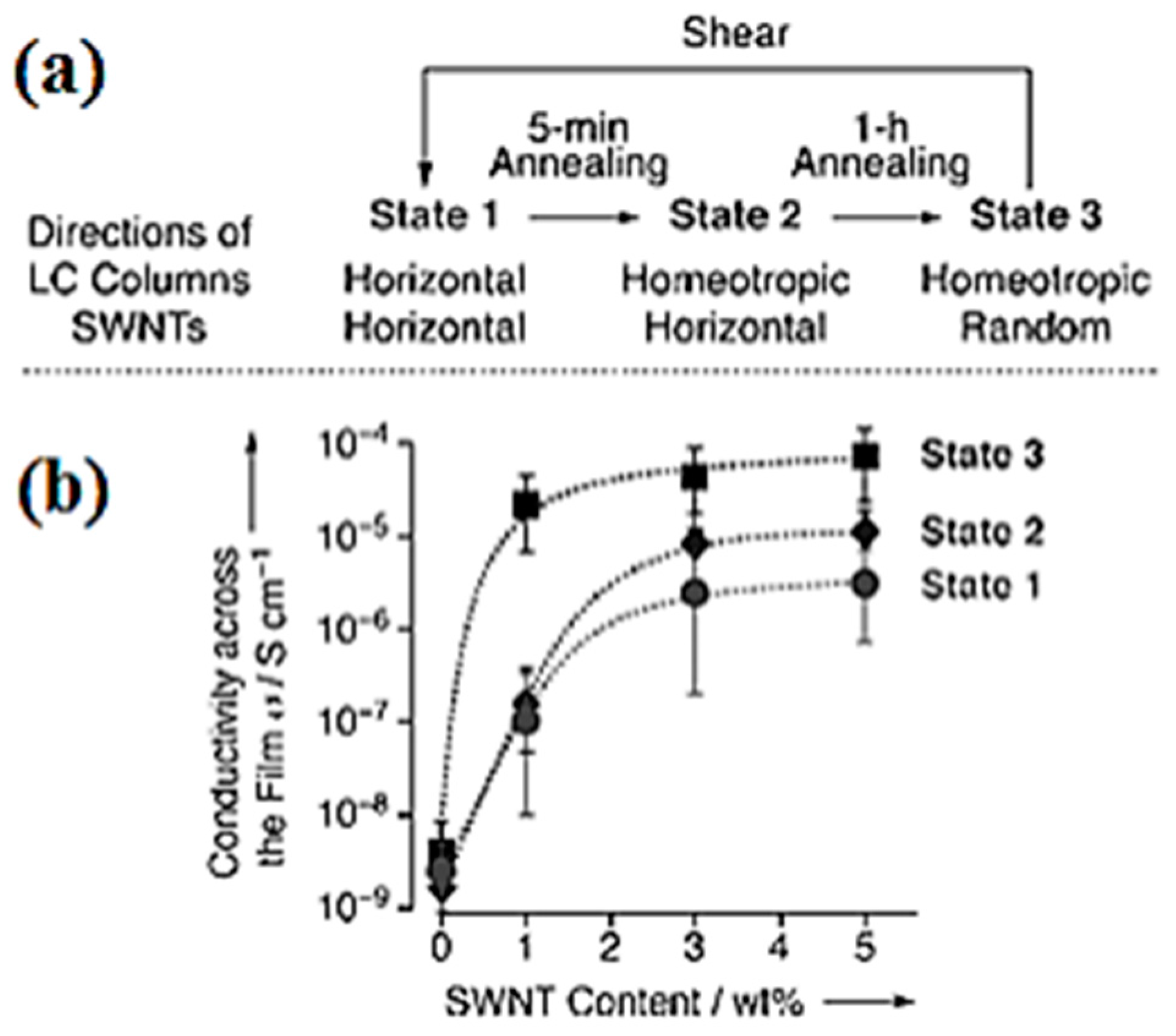
3.2. Carbon Nanotubes in DLCs
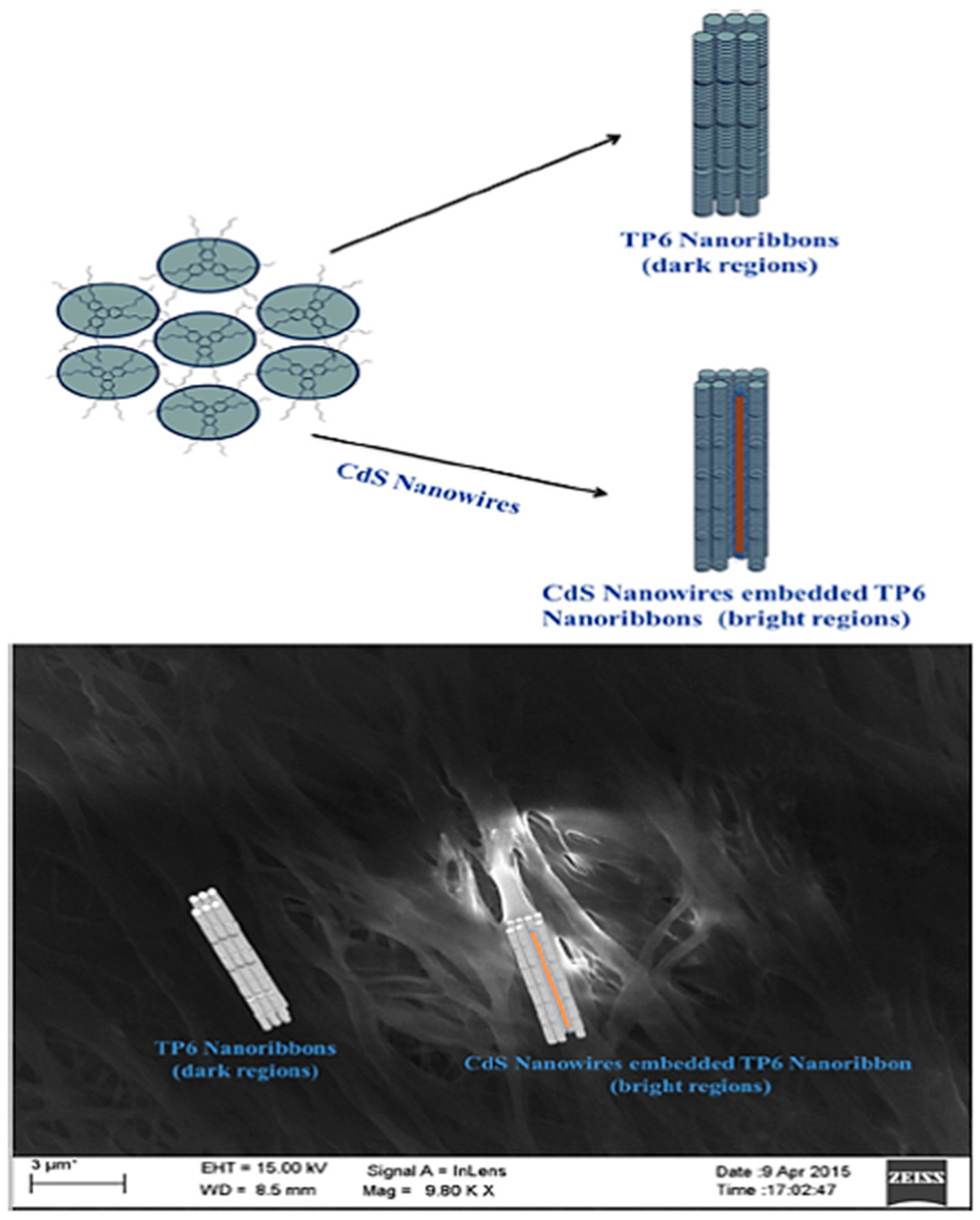
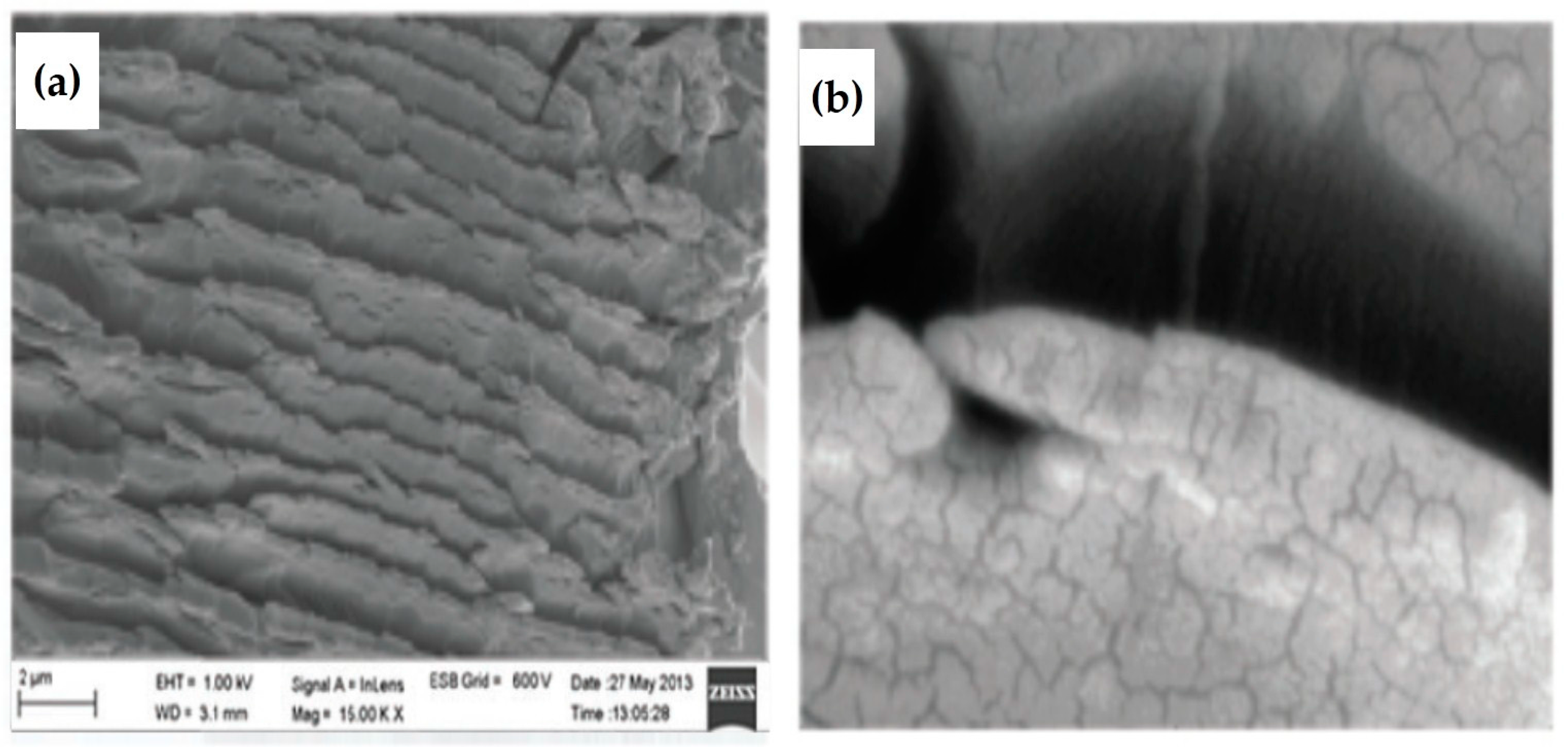
3.3. CdS Nanowires in DLCs
4. Two-Dimensional Nanostructures in Discotic Liquid Crystals
4.1. Graphene in Discotic Liquid Crystals
4.2. Metallic Nanodisks in Discotic Liquid Crystals
5. Conclusions and Outlook
Conflicts of Interest
References
- Goodby, J.W.; Collings, P.J.; Kato, T.; Tschierske, C.; Gleeson, H.; Raynes, P. Handbook of Liquid Crystals; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Kumar, S. Chemistry of Discotic Liquid Crystals: From Monomers to Polymers; CRC press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Reinitzer, F. Beiträge zur Kenntniss des Cholesterins. Monatsch. Chem. 1888, 9, 421–441. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Sadashiva, B.K.; Suresh, K.A. Liquid crystals of disc-like molecules. Pramana 1977, 7, 471–480. [Google Scholar] [CrossRef]
- Kawata, K. Orientation control and fixation of discotic liquid crystal. Chem. Rec. 2002, 2, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Bisoyi, H.K.; Kumar, S. Discotic nematic liquid crystals: Science and technology. Chem. Soc. Rev. 2010, 39, 264–285. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Varshney, S.K. A room-temperature discotic nematic liquid crystal. Angew. Chem. Int. Ed. 2000, 112, 3270–3272. [Google Scholar] [CrossRef]
- Kumar, S. Molecular engineering of discotic nematic liquid crystals. Pramana 2003, 61, 199–203. [Google Scholar] [CrossRef]
- Nair, G.G.; Rao, D.S.S.; Prasad, S.K.; Chandrasekhar, S.; Kumar, S. Electrooptic and viewing angle characteristics of a display device employing a discotic nematic liquid crystal. Mol. Cryst. Liq. Cryst. 2003, 397, 245–252. [Google Scholar] [CrossRef]
- Keulen, J.V.; Warmerdam, T.W.; Nolte, R.J.M.; Drenth, W. Electrical conductivity in hexaalkoxytriphenylenes. Recl. Trav. Chim. Pays-Bas 1987, 106, 534–536. [Google Scholar] [CrossRef]
- Vaughan, G.B.M.; Heiney, P.A.; McCauley, J.P., Jr.; Smith, A.B., III. Conductivity and structure of a liquid-crystalline organic conductor. Phys. Rev. B 1992, 46, 2787–2791. [Google Scholar] [CrossRef]
- Boden, N.; Bushby, R.J.; Clements, J. Mechanism of quasi one dimensional electronic conductivity in discotic liquid crystals. J. Chem. Phys. 1993, 98, 5920–5931. [Google Scholar] [CrossRef]
- Kumar, P.S.; Kumar, S.; Lakshminarayanan, V. Electrical conductivity studies on discotic liquid crystal-ferrocenium donor-acceptor systems. J. Phys. Chem. B 2008, 112, 4865–4869. [Google Scholar] [CrossRef] [PubMed]
- Balagurusamy, V.S.K.; Krishnaprasad, S.; Chandrasekhar, S.; Kumar, S.; Manickam, M.; Yelamaggad, C.V. Quasi-one dimensional electrical conductivity and thermoelectric power studies on a discotic liquid crystal. Pramana 1999, 53, 3–11. [Google Scholar] [CrossRef]
- Pisula, W.; Feng, X.; Mullen, K. Charge-carrier transporting graphene-type molecules. Chem. Mater. 2011, 23, 554–567. [Google Scholar] [CrossRef]
- Sergeyev, S.; Pisula, W.; Geerts, Y.H. Discotic liquid crystals: a new generation of organic semiconductors. Chem. Soc. Rev. 2007, 36, 1902–1929. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Pisula, W.; Mullen, K. Graphenes as potential material for electronics. Chem. Rev. 2007, 107, 718–747. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Marcon, V.; Pisula, W.; Hansen, M.R.; Kirkpatrick, J.; Grozema, F.; Andrienko, D.; Kremer, Z.K.; Müllen, K. Towards high charge-carrier mobilities by rational design of the shape and periphery of discotics. Nat. Mater. 2009, 8, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional liquid-crystalline assemblies: selforganized soft materials. Angew. Chem. Int. Ed. 2006, 45, 38–68. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Hatsusaka, K.; Sugibayashi, M.; Ariyoshi, M.; Ban, K.; Maeda, F.; Naito, R.; Nishizawa, K.; van de Craats, A.M.; Warman, J.M. Discotic liquid crystalline semiconductors. Mol. Cryst. Liq. Cryst. 2003, 397, 25–45. [Google Scholar] [CrossRef]
- Hanna, J.-I. Liquid Crystalline Semiconductors; Bushby, R.J., Kelly, S.M., O’Neill, M., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 39–64. [Google Scholar]
- Holt, L.A.; Bushby, R.J.; Evans, S.D.; Burgess, A.; Seeley, G. A 106-fold enhancement in the conductivity of a discotic liquid crystal doped with only 1% (w/w) gold nanoparticles. J. Appl. Phys. 2008, 103, 063712. [Google Scholar] [CrossRef]
- Boden, N.; Bushby, R.J.; Cammidge, A.N.; Clements, J.; Luo, R.; Donovan, K.J. Transient Photoconductivity and Dark Conductivity in Discotic Liquid Crystals. Mol. Cryst. Liq. Cryst. 1995, 261, 251–257. [Google Scholar] [CrossRef]
- Boden, N.; Movahar, B. Hand Book of Liquid Crystals; Demus, D., Goodby, J., Gray, G.W., Spiess, H.-W., Vill, V., Eds.; Wiley-VCH: Weinheim, Germany, 1998; Volume 2B, Chapter IX. [Google Scholar]
- Kaafarani, B.R. Discotic Liquid Crystals for Opto-Electronic Applications. Chem. Mater. 2011, 23, 378–396. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; Fechtenkotter, A.; Mullen, K.; Moons, E.; Friend, R.H.; MacKenzie, J.D. Self-Organized Discotic Liquid Crystals for High-Efficiency Organic Photovoltaics. Science 2001, 293, 1119–1122. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Nanoparticles in the supramolecular order of discotic liquid crystals. Liq. Cryst. 2014, 41, 353–367. [Google Scholar] [CrossRef]
- Kumar, S. Supramolecular Nanocomposites: Dispersion of Zero-, One- and Two-dimensional Nanoparticles in Discotic Liquid Crystals. J. Phys. Conf. Ser. 2016, 704, 012022. [Google Scholar] [CrossRef]
- Kumar, S. Discotic liquid crystal-nanoparticle hybrid systems. NPG Asia Mater. 2014, 6, e82. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Kumar, S. Liquid-crystal nanoscience: an emerging avenue of soft self-assembly. Chem. Soc. Rev. 2011, 40, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, S. Liquid crystals in photovoltaics: A new generation of organic photovoltaics. Polym. J. 2017, 49, 85–111. [Google Scholar] [CrossRef]
- Kumar, S. Functional discotic liquid crystals. Isr. J. Chem. 2012, 52, 820–829. [Google Scholar] [CrossRef]
- Kumar, S. Self-organization of disc-like molecules: Chemical aspects. Chem. Soc. Rev. 2006, 35, 83–109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Gowda, A.; Kumar, S. Discotic Liquid Crystals with Graphene: Supramolecular Self-assembly to Applications. Part. Part. Syst. Charact. 2017, 34, 1700003. [Google Scholar] [CrossRef]
- Gowda, A.; Kumar, M.; Kumar, S. Discotic liquid crystals derived from polycyclic aromatic cores: From the smallest benzene to the utmost graphene cores. Liq. Cryst. 2017, 44, 1990–2017. [Google Scholar] [CrossRef]
- Pal, S.K.; Setia, S.; Avinash, B.S.; Kumar, S. Triphenylene-based discotic liquid crystals: Recent advances. Liq. Cryst. 2013, 40, 1769–1816. [Google Scholar] [CrossRef]
- Kumar, S. Nanoparticles in discotic liquid crystals. In Liquid Crystals with Nano- and Microparticles; Lagerwall, J.P.F., Scalia, G., Eds.; World Scientific: Singapore, 2017; Volume I & II, pp. 461–496. [Google Scholar]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties and applications toward biology, catalysis and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G. Clusters and Colloids, From Theory to Applications; Wiley-VCH: Weinheim, Germany, 1994. [Google Scholar]
- Rao, C.N.R.; Muller, A.; Cheetham, A.K. The Chemistry of Nanomaterials: Synthesis, Properties and Applications; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Kumar, S.; Lakshminarayanan, V. Inclusion of gold nanoparticles into a discotic liquid crystalline matrix. Chem. Commun. 2004, 1600–1601. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pal, S.K.; Lakshminarayanan, V. Discoticdecorated gold nanoparticles. Mol. Cryst. Liq. Cryst. 2005, 434, 251–258. [Google Scholar] [CrossRef]
- Kumar, P.S.; Pal, S.K.; Kumar, S.; Lakshminarayanan, V. Dispersion of thiol stabilized gold nanoparticles in lyotropic liquid crystalline systems. Langmuir 2007, 23, 3445–3449. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pal, S.K.; Kumar, P.S.; Lakshminarayanan, V. Novel conducting nanocomposites: synthesis of triphenylene-covered gold nanoparticles and their insertion into a columnar matrix. Soft Matter 2007, 3, 896–900. [Google Scholar] [CrossRef]
- Kumar, S.; Bisoyi, H.K. Aligned carbon nanotubes in the supramolecular order of discotic liquid crystals. Angew. Chem. Int. Ed. 2007, 46, 1501–1503. [Google Scholar] [CrossRef] [PubMed]
- Bisoyi, H.K.; Kumar, S. Carbon nanotubes in triphenylene and rufigallol-based room temperature monomeric and polymeric discotic liquid crystals. J. Mater. Chem. 2008, 18, 3032–3039. [Google Scholar] [CrossRef]
- Gupta, R.K.; Suresh, K.A.; Kumar, S. Monolayer of amphiphilic functionalized gold nanoparticles at an air-water interface. Phys. Rev. E 2008, 78, 032601. [Google Scholar] [CrossRef] [PubMed]
- Dhar, R.; Pandey, A.S.; Pandey, M.B.; Kumar, S.; Dabrowski, R. Optimization of the display parameters of a room temperature twisted nematic display material (6CHBT) by doping single-wall carbon nanotubes. Appl. Phys. Express 2008, 1, 121501. [Google Scholar] [CrossRef]
- Vijayaraghavan, D.; Kumar, S. Self-assembled superlattices of gold nanoparticles in a discotic liquid crystal. Mol. Cryst. Liq. Cryst. 2009, 508, 101–114. [Google Scholar] [CrossRef]
- Kumar, S.; Sagar, L.K. CdSe quantum dots in a columnar matrix. Chem. Commun. 2011, 47, 12182–12184. [Google Scholar] [CrossRef] [PubMed]
- Manjuladevi, V.; Gupta, R.K.; Kumar, S. Effect of functionalized carbon nanotube on electro-optic and dielectric properties of a liquid crystal. J. Mol. Liq. 2012, 171, 60–63. [Google Scholar] [CrossRef]
- Supreet, K.S.; Raina, K.K.; Pratibha, R. Enhanced stability of the columnar matrix in a discotic liquid crystal by insertion of ZnO nanoparticles. Liq. Cryst. 2013, 40, 228–236. [Google Scholar] [CrossRef][Green Version]
- Avinash, B.S.; Lakshminarayanan, V.; Kumar, S.; Vij, J.K. Gold nanorods embedded discotic nanoribbons. Chem. Commun. 2013, 49, 978–980. [Google Scholar] [CrossRef] [PubMed]
- Supreet; Pratibha, R.; Kumar, S.; Raina, K.K. Effect of Dispersion of Gold Nanoparticles on the Optical and Electrical Properties of Discotic Liquid Crystal. Liq. Cryst. 2014, 41, 933–939. [Google Scholar] [CrossRef]
- Mishra, M.; Kumar, S.; Dhar, R. Effect of the dispersed colloidal gold nano particles on the electrical properties of a columnar discotic liquid crystal. RSC Adv. 2014, 4, 62404–62412. [Google Scholar] [CrossRef]
- Shivanandareddy, A.B.; Krishnamurthy, V.; Lakshminarayanan, V.; Kumar, S. Mutually ordered self-assembly of discotic liquid crystal-graphene nanocomposites. Chem. Commun. 2014, 50, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, S. Luminescent CdTe quantum dots incarcerated in supramolecular columnar matrix for optoelectronic applications. RSC Adv. 2015, 5, 1262–1267. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, S. Stacking of ultra-thin reduced graphene oxide nanoparticles in supramolecular structures for optoelectronic applications. RSC Adv. 2015, 5, 14871–14878. [Google Scholar] [CrossRef]
- Yaduvanshi, P.; Kumar, S.; Dhar, R. Effects of copper nanoparticles on the thermodynamic, electrical and optical properties of a disc-shaped liquid crystalline material showing columnar phase. Phase Transit. 2015, 88, 489–502. [Google Scholar] [CrossRef]
- Yaduvanshi, P.; Mishra, A.; Kumar, S.; Dhar, R. Enhancement of the thermodynamic, electrical and optical properties of hexabutyloxytriphenylene due to copper nanoparticles. J. Mol. Liq. 2015, 208, 160–164. [Google Scholar] [CrossRef]
- Shivanandareddy, A.V.; Kumar, M.; Lakshminarayanan, V.; Kumar, S. Self-assembly of thiolated graphene oxide onto gold surface and in the supramolecular order of discotic liquid crystals. RSC Adv. 2015, 5, 47692–47700. [Google Scholar] [CrossRef]
- Yaduvanshi, P.; Mishra, A.; Kumar, S.; Dhar, R. Effect of silver nano particles on frequency and temperature dependent electrical parameters of a discotic liquid crystalline material. Liq. Cryst. 2015, 42, 1478–1489. [Google Scholar] [CrossRef]
- Yadav, N.; Kumar, S.; Dhar, R. Cadmium selenide quantum dots to ameliorate the properties of a room temperature discotic liquid crystalline material. RSC Adv. 2015, 5, 78823–78832. [Google Scholar] [CrossRef]
- Mishra, M.; Kumar, S.; Dhar, R. Gold nanoparticles in plastic columnar discotic liquid crystalline Material. Thermochim. Acta 2016, 631, 59–70. [Google Scholar] [CrossRef]
- Gowda, A.N.; Kumar, S.; Thomas, A.R.; Philip, R.; Kumar, S. Self-Assembly of Silver and Gold Nanoparticles in a Metal-Free Phthalocyanine Liquid Crystalline Matrix: Structural, Thermal, Electrical and Nonlinear Optical Characterization. Chem. Sel. 2016, 1, 1361–1370. [Google Scholar] [CrossRef]
- Mishra, M.; Kumar, S.; Dhar, R. Effect of high concentration of colloidal gold nanoparticles on the thermodynamic, optical and electrical properties of 2, 3, 6, 7, 10, 11-hexabutyloxytryphenylene discotic liquid crystalline material. Soft Matter 2017, 15, 34–44. [Google Scholar] [CrossRef]
- Varshney, S.; Kumar, M.; Gowda, A.; Kumar, S. Soft discotic matrix with 0-D silver nanoparticles: Impact on molecular ordering and conductivity. J. Mol. Liq. 2017, 238, 290–295. [Google Scholar] [CrossRef]
- Tripathi, P.; Mishra, M.; Kumar, S.; Dhar, R. Thermodynamic study of a plastic columnar discotic material 2, 3, 6, 7, 10, 11 hexabutyloxytriphenylene dispersed with gold nanoparticles under elevated pressure. J. Therm. Anal. Calorim. 2017, 129, 315–322. [Google Scholar] [CrossRef]
- Kumar, M.; Varshney, S.; Gowda, A.; Kumar, S. Silver Nanodisks in Soft Discotic Matrix: Impact on Self-assembly, Conductivity and Molecular Packing. J. Mol. Liq. 2017, 241, 666–674. [Google Scholar] [CrossRef]
- Shivanandareddy, A.B.; Kumar, M.; Gowda, A.; Kumar, S. Trapping of inorganic nanowires in supramolecular organic nanoribbons. J. Mol. Liq. 2017, 244, 1–6. [Google Scholar] [CrossRef]
- Shen, Z.; Yamada, M.; Miyake, M. Control of Stripelike and Hexagonal Self-Assembly of Gold Nanoparticles by the Tuning of Interactions between Triphenylene Ligands. J. Am. Chem. Soc. 2007, 129, 14271–14280. [Google Scholar] [CrossRef] [PubMed]
- Basova, T.V.; Parkhomenko, R.G.; Igumenov, I.K.; Hassan, A.; Durmu, M.; Gurek, A.G.; Ahsen, V. Composites of liquid crystalline nickel phthalocyanine with gold nanoparticles: Liquid crystalline behaviour and optical properties. Dyes Pigments 2014, 111, 58–63. [Google Scholar] [CrossRef]
- Thuy, U.T.D.; Toan, P.S.; Chi, T.T.K.; Khang, D.D.; Liem, N.Q. CdTe quantum dots for an application in the life sciences. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 045009. [Google Scholar] [CrossRef]
- Chen, W.C.W.; Nie, S. Quantum Dot Bioconjugates for Ultrasensitive Nonisotopic Detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Ung, T.D.T.; Vu, T.H.; Tran, T.K.C.; Dong, V.Q.; Dinh, D.K.; Nguyen, Q.L. Fluorescence biosensor based on CdTe quantum dots for specific detection of H5N1 avian influenza virus. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 035014. [Google Scholar] [CrossRef]
- Kershaw, S.V.; Harrison, M.; Rogach, A.L.; Kornowski, A. Development of IR-emitting colloidal II-VI quantum-dot materials. IEEE J. Sel. Top. Quantum Electron. 2000, 6, 534–543. [Google Scholar] [CrossRef]
- Cushing, B.L.; Kolesnichenko, V.L.; O’Connor, C.J. Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem. Rev. 2004, 104, 3893–3946. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Vivekchand, S.R.C.; Biswas, K.; Govindaraj, A. Synthesis of inorganic nanomaterials. Dalton Trans. 2007, 3728–3749. [Google Scholar] [CrossRef] [PubMed]
- Debasis, B.; Lei, Q.; Teng-Kuan, T.; Paul, H. Quantum dots and their multimodal applications: A review. Materials 2010, 3, 2260–2345. [Google Scholar]
- Karanikolos, G.N.; Alexandridis, P.; Mountziaris, T.J. Block copolymer-templated synthesis and organization of semiconductor nanocrystals. Macromol. Symp. 2010, 289, 43–51. [Google Scholar] [CrossRef]
- Mirzaei, J.; Reznikov, M.; Hegmann, T. Quantum dots as liquid crystal dopants. J. Mater. Chem. 2012, 22, 22350–22365. [Google Scholar] [CrossRef]
- Zlateva, G.; Zhelev, Z.; Bakalova, R.; Kanno, I. Precise Size Control and Synchronized Synthesis of Six Colors of CdSe Quantum Dots in a Slow-Increasing Temperature Gradient. Inorg. Chem. 2007, 46, 6212–6214. [Google Scholar] [CrossRef] [PubMed]
- Wuister, S.F.; Swart, I.; Driel, F.V.; Hickey, S.G.; Donega, C.D.M. Highly Luminescent Water-Soluble CdTe Quantum Dots. Nano Lett. 2003, 3, 503–507. [Google Scholar] [CrossRef]
- Nealon, G.L.; Greget, R.; Dominguez, C.; Nagy, Z.T.; Guillon, D.; Gallani, J.-L.; Donnio, B. Liquid-crystalline nanoparticles: Hybrid design and mesophase structures. Beilstein. J. Org. Chem. 2012, 8, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Stamatoiu, O.; Mirzaei, J.; Feng, X.; Hegmann, T. Nanoparticles in liquid crystals and liquid crystalline nanoparticles. Top. Curr. Chem. 2012, 318, 331–393. [Google Scholar] [PubMed]
- Umadevi, S.; Ganesh, V.; Hegmann, T. Nanoparticles: Additives and building blocks for liquid crystal phases. In Handbook of Liquid Crystals, 2nd ed.; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume VI, pp. 27–76. [Google Scholar]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seed-Mediated Growth Approach for Shape-Controlled Synthesis of Spheroidal and Rod-like Gold Nanoparticles Using a Surfactant Template. Adv. Mater. 2001, 13, 1389. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Xiao, S.; Tang, J.; Beetz, T.; Guo, X.; Tremblay, N.; Siegrist, T.; Zhu, Y.; Steigerwald, M.; Nuckolls, C. Transferring Self-Assembled, Nanoscale Cables into Electrical Devices. J. Am. Chem. Soc. 2006, 128, 10700–10701. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Sosa-Vargas, L.; Umadevi, S.; Mori, T.; Shimizu, Y.; Hegmann, T. Discotic Liquid Crystal-Functionalized Gold Nanorods: 2- and 3D Self-Assembly and Macroscopic Alignment as well as Increased Charge Carrier Mobility in Hexagonal Columnar Liquid Crystal Hosts Affected by Molecular Packing and π–π Interactions. Adv. Funct. Mater. 2015, 25, 1180–1192. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Volder, M.F.L.D.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Vosguerichian, M.; Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef] [PubMed]
- Scarselli, M.; Castrucci, P.; Crescenzi, M.D. Electronic and optoelectronic nano-devices based on carbon nanotubes. J. Phys. Condens. Matter 2012, 24, 313202. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Chang, D.W.; Baek, J.-B.; Lu, W. Carbon Nanomaterials for Advanced Energy Conversion and Storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef] [PubMed]
- Endo, M. Carbon Nanotube Research: Past and Future. Jpn. J. Appl. Phys. 2012, 51, 040001. [Google Scholar] [CrossRef]
- Lee, J.J.; Yamaguchi, A.; Alam, M.A.; Yamamoto, Y.; Fukushima, T.; Kato, K.; Takata, M.; Fujita, N.; Aida, T. Discotic Ionic Liquid Crystals of Triphenylene as Dispersants for Orienting Single-Walled Carbon Nanotubes. Angew. Chem. Int. Ed. 2012, 51, 8490–8494. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Hummers, S.W.; Offeman, E.R. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Liu, J.; Tang, J.; Gooding, J.J.J. Strategies for chemical modification of graphene and applications of chemically modified graphene. J. Mater. Chem. 2012, 22, 12435–12452. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gowda, A.; Kumar, S. Recent Advances in Discotic Liquid Crystal-Assisted Nanoparticles. Materials 2018, 11, 382. https://doi.org/10.3390/ma11030382
Gowda A, Kumar S. Recent Advances in Discotic Liquid Crystal-Assisted Nanoparticles. Materials. 2018; 11(3):382. https://doi.org/10.3390/ma11030382
Chicago/Turabian StyleGowda, Ashwathanarayana, and Sandeep Kumar. 2018. "Recent Advances in Discotic Liquid Crystal-Assisted Nanoparticles" Materials 11, no. 3: 382. https://doi.org/10.3390/ma11030382
APA StyleGowda, A., & Kumar, S. (2018). Recent Advances in Discotic Liquid Crystal-Assisted Nanoparticles. Materials, 11(3), 382. https://doi.org/10.3390/ma11030382



