Mechanical Properties of Optimized Diamond Lattice Structure for Bone Scaffolds Fabricated via Selective Laser Melting
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of Cellular Lattice Structures
2.2. Materials
2.3. Fabrication by SLM
2.4. Test Details
3. Results and Discussion
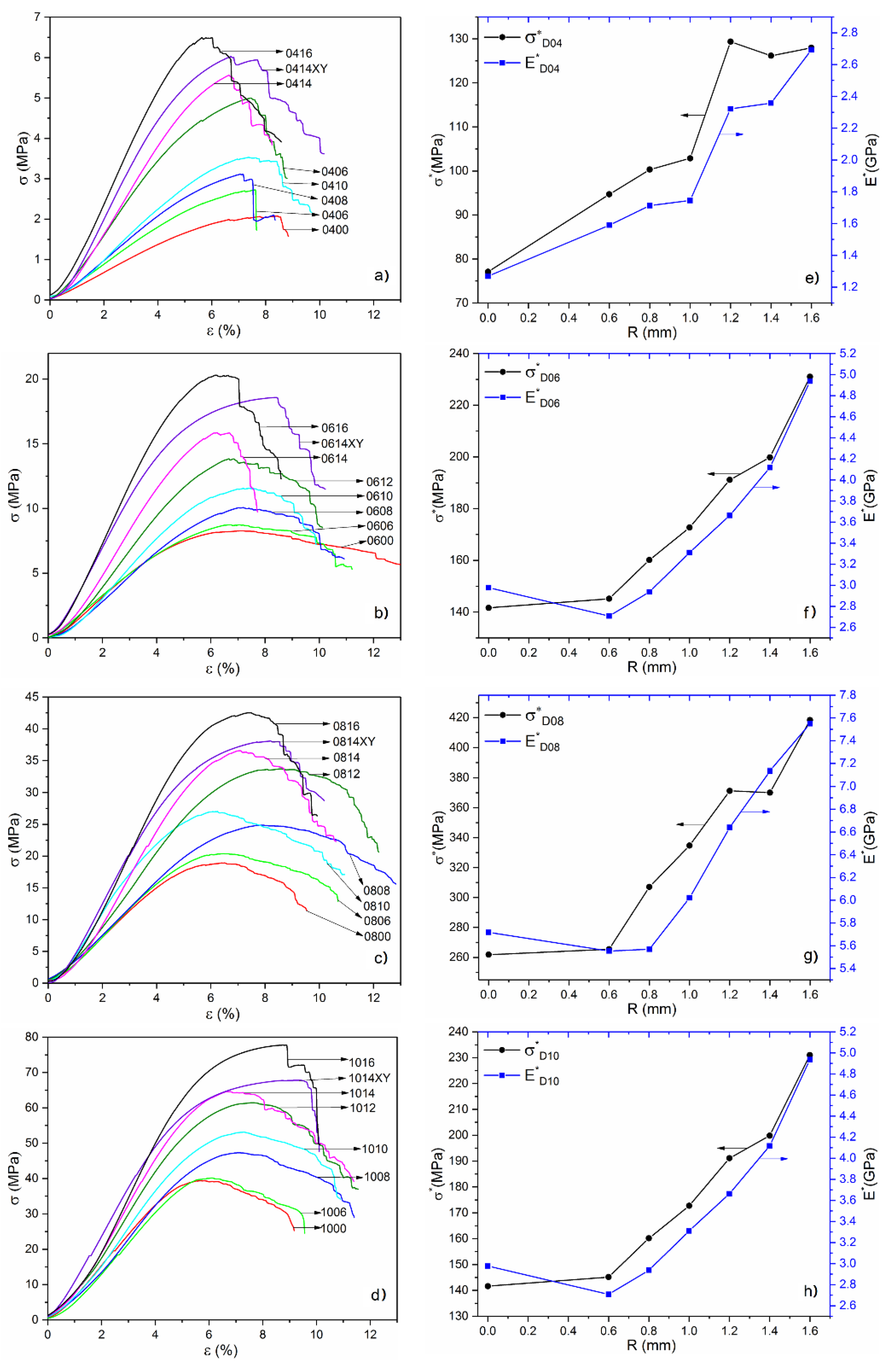
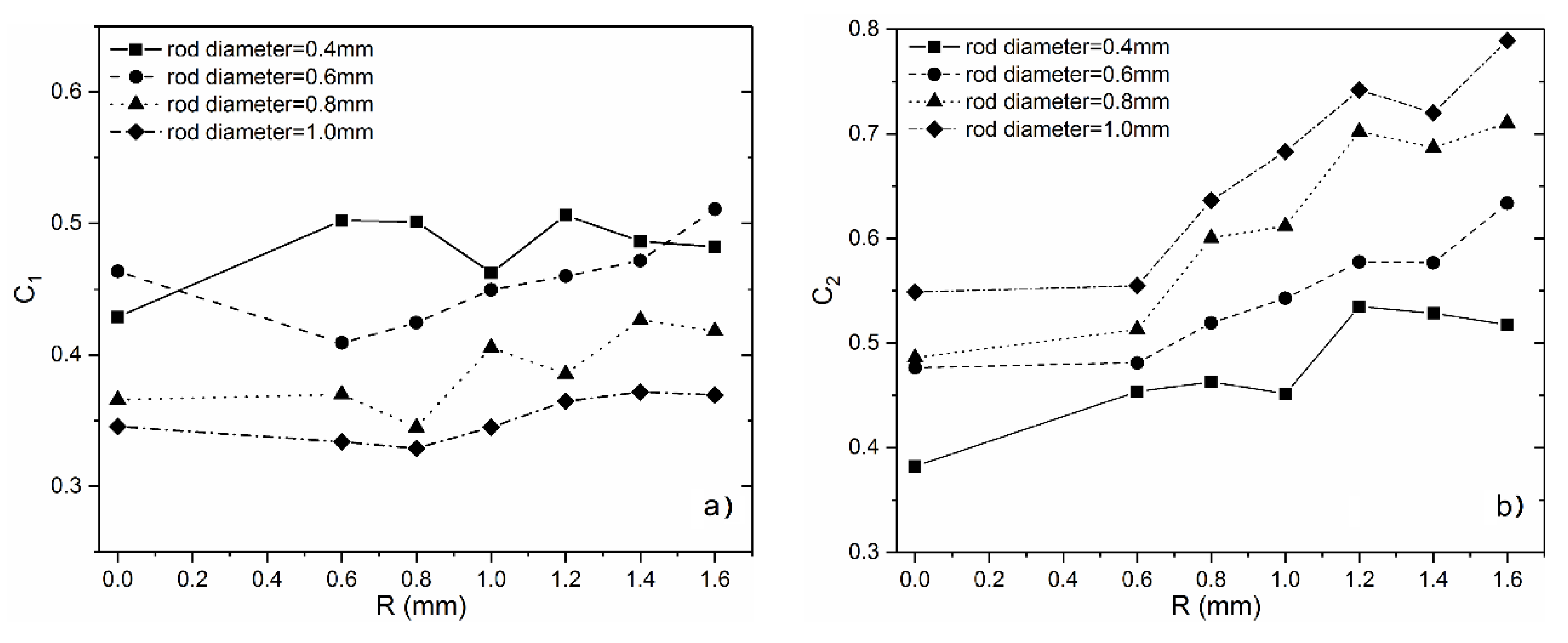
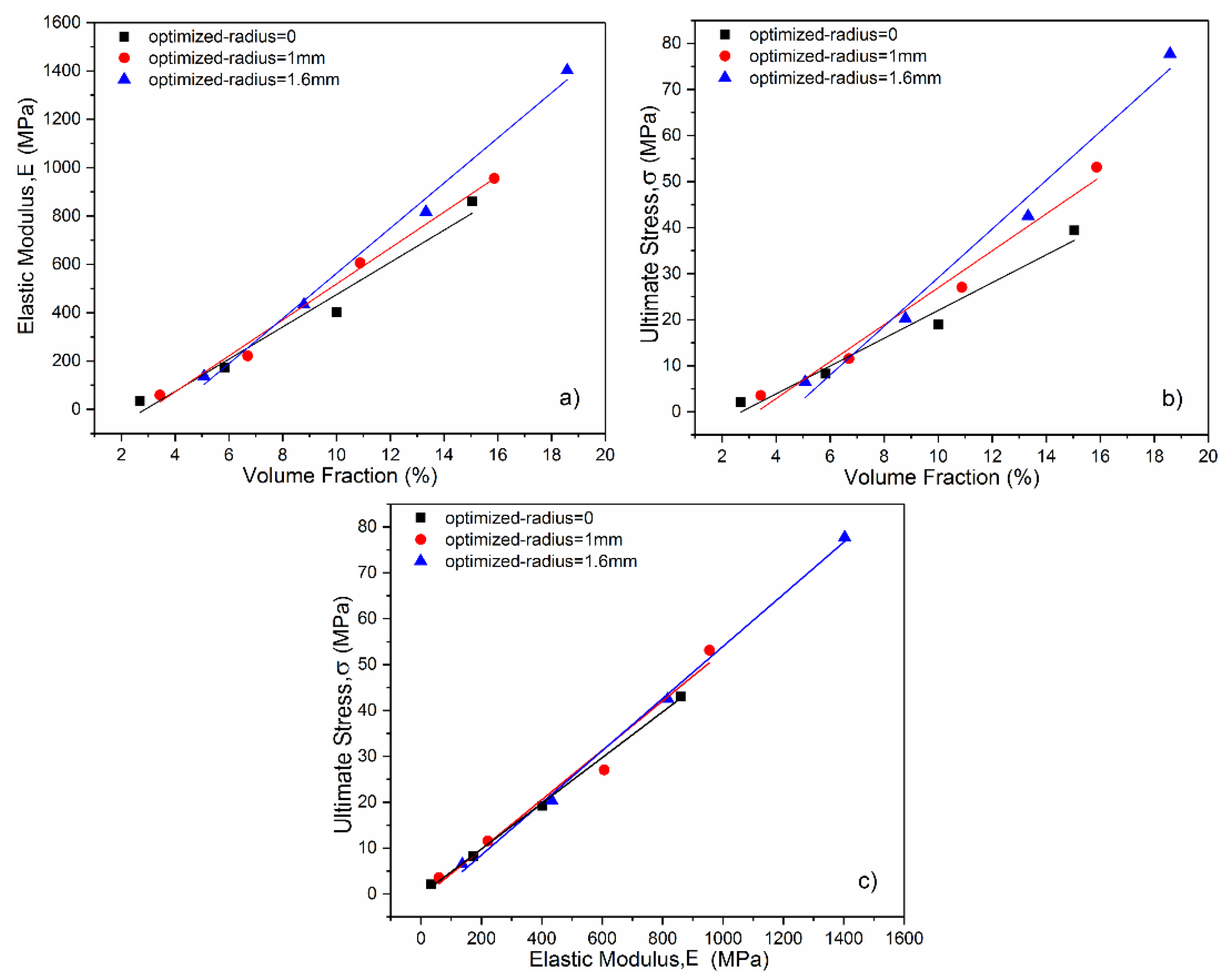
3.1. Analysis of Mechanical Properties
3.2. Bone Modulus Matching
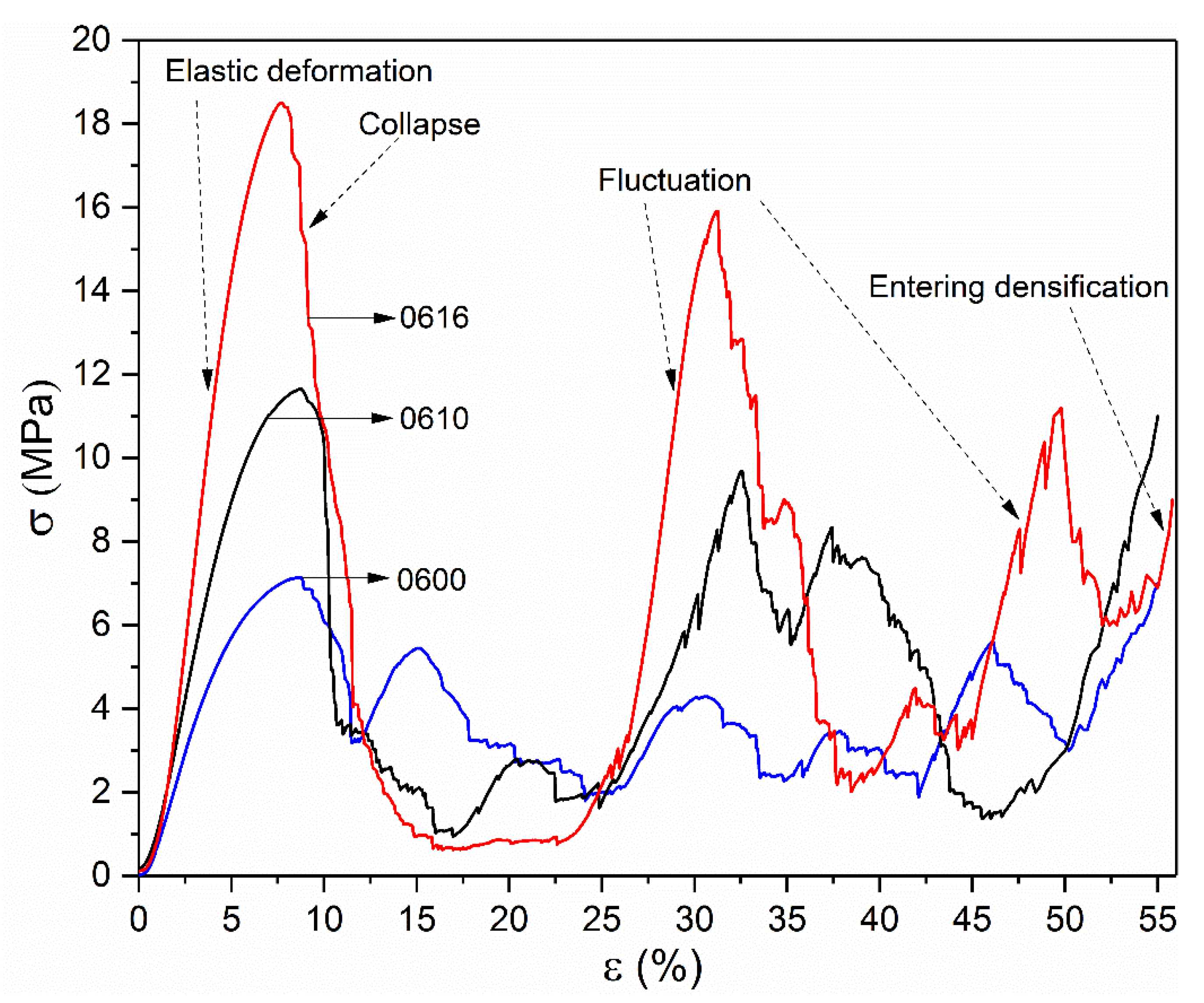
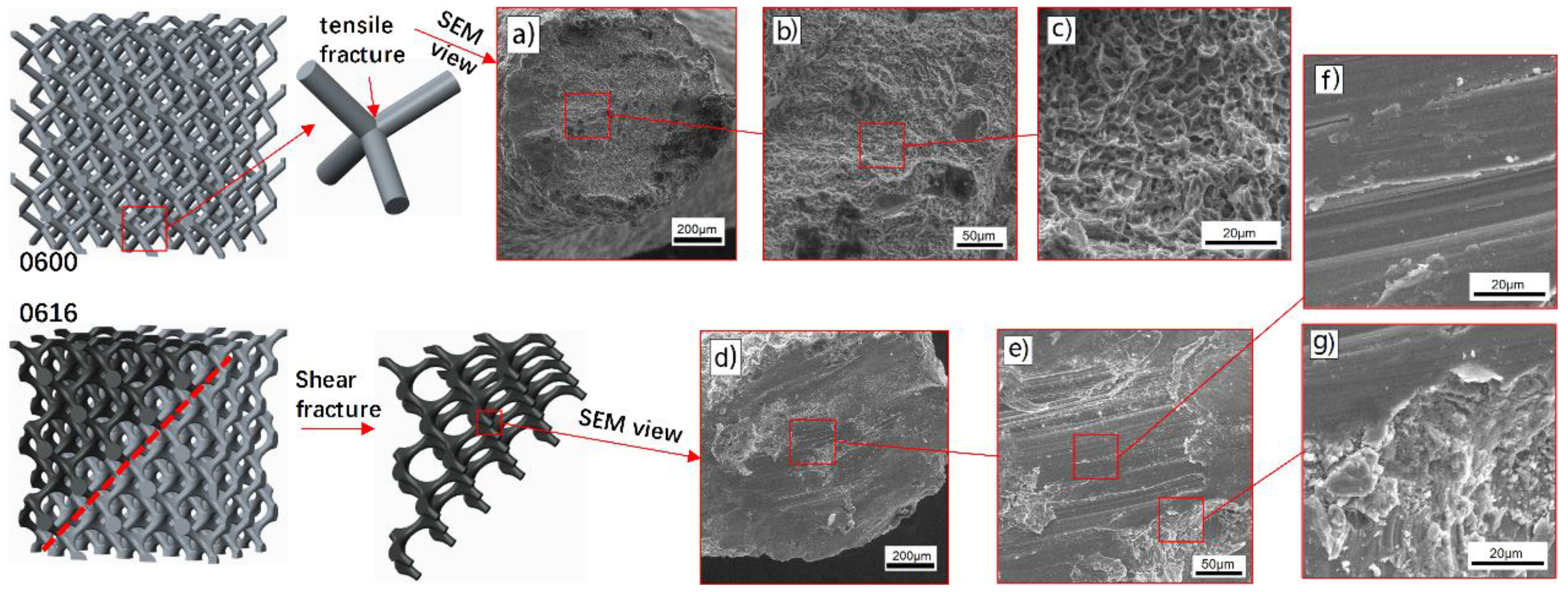
3.3. Deformation Behavior
4. Conclusions
- Mechanical properties including strength and modulus increase with the increase of optimized radius at a fixed volume fraction, indicating that the stress concentration at lattice nodes have been released by the introduction of surface optimization;
- A minitype database that correlates porosity levels to mechanical properties has been established. The compressive strength and elastic modulus of diamond lattice structures are in the range of 2–78 MPa and 34–1403 MPa, respectively, which are comparable with trabecular bone;
- By optimizing the nodes with different curvature in each unit cell, the relationship between compressive strength and elastic modulus can be regulated, enabling the cellular structures more suitable for bone implants implication;
- The change of stress distribution stemming from the optimized surface at nodes has a significant influence on the deformation behavior of these structures. With the analysis of in-situ images and SEM, it is revealed that the improvement of mechanical properties was mainly due to the transition of the deformation behavior from upward-layer collapse in non-optimization structures to diagonal (45°) shear band in optimized ones.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Evans, A.G.; Hutchinson, J.W.; Fleck, N.; Ashby, M.F.; Wadley, H.N.G. The topological design of multifunctional cellular metals. Prog. Mater. Sci. 2001, 46, 309–327. [Google Scholar] [CrossRef]
- Olivares, A.L.; Marsal, È.; Planell, J.A.; Lacroix, D. Finite element study of scaffold architecture design and culture conditions for tissue engineering. Biomaterials 2009, 30, 6142–6149. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.M.; Campoli, G.; Amin Yavari, S.; Sajadi, B.; Wauthle, R.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Mechanical behavior of regular open-cell porous biomaterials made of diamond lattice unit cells. J. Mech. Behav. Biomed. 2014, 34, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Kadkhodapour, J.; Montazerian, H.; Darabi, A.C.; Anaraki, A.P.; Ahmadi, S.M.; Zadpoor, A.A.; Schmauder, S. Failure mechanisms of additively manufactured porous biomaterials: Effects of porosity and type of unit cell. J. Mech. Behav. Biomed. 2015, 50, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Cansizoglu, O.; Harrysson, O.; Cormier, D.; West, H.; Mahale, T. Properties of ti–6al–4v non-stochastic lattice structures fabricated via electron beam melting. Mater. Sci. Eng. A-Struct. 2008, 492, 468–474. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Li, S.J.; Murr, L.E.; Zhang, Z.B.; Hao, Y.L.; Yang, R.; Medina, F.; Wicker, R.B. Compression deformation behavior of ti-6al-4v alloy with cellular structures fabricated by electron beam melting. J. Mech. Behav. Biomed. 2012, 16, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kapfer, S.C.; Hyde, S.T.; Mecke, K.; Arns, C.H.; Schröder-Turk, G.E. Minimal surface scaffold designs for tissue engineering. Biomaterials 2011, 32, 6875–6882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jones, D.; Yue, S.; Lee, P.D.; Jones, J.R.; Sutcliffe, C.J.; Jones, E. Hierarchical tailoring of strut architecture to control permeability of additive manufactured titanium implants. Mater. Sci. Eng. C-Mater. 2013, 33, 4055–4062. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Hao, L.; Hussein, A.; Young, P. Ti–6al–4v triply periodic minimal surface structures for bone implants fabricated via selective laser melting. J. Mech. Behav. Biomed. 2015, 51, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Barradas, A.M.C.; van Blitterswijk, C.A.; de Boer, J.; Feijen, J.; Grijpma, D.W. Effects of the architecture of tissue engineering scaffolds on cell seeding and culturing. Acta Biomater. 2010, 6, 4208–4217. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Roden, L.C.; Renton, L.F. Osteoinductive hydroxyapatite-coated titanium implants. Biomaterials 2012, 33, 3813–3823. [Google Scholar] [CrossRef] [PubMed]
- Heinl, P.; Müller, L.; Körner, C.; Singer, R.F.; Müller, F.A. Cellular ti-6al-4v structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008, 4, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Xu, Q.S.; Wang, Z.; Hou, W.T.; Hao, Y.L.; Yang, R.; Murr, L.E. Influence of cell shape on mechanical properties of ti-6al-4v meshes fabricated by electron beam melting method. Acta Biomater. 2014, 10, 4537–4547. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Song, W.; Wang, C.; Liu, H.; Tang, H.; Wang, J. Mechanical behavior of open-cell rhombic dodecahedron ti–6al–4v lattice structure. Mater. Sci. Eng. A-Struct. 2015, 640, 375–384. [Google Scholar] [CrossRef]
- Babaee, S.; Jahromi, B.H.; Ajdari, A.; Nayeb-Hashemi, H.; Vaziri, A. Mechanical properties of open-cell rhombic dodecahedron cellular structures. Acta Mater. 2012, 60, 2873–2885. [Google Scholar] [CrossRef]
- Knorr, T.; Heinl, P.; Schwerdtfeger, J.; Körner, C.; Singer, R.F.; Etzold, B.J.M. Process specific catalyst supports—selective electron beam melted cellular metal structures coated with microporous carbon. Chem. Eng. J. 2012, 181, 725–733. [Google Scholar] [CrossRef]
- Hussein, A.; Hao, L.; Yan, C.; Everson, R.; Young, P. Advanced lattice support structures for metal additive manufacturing. J. Mater. Process. Technol. 2013, 213, 1019–1026. [Google Scholar] [CrossRef]
- Yan, C.; Hao, L.; Hussein, A.; Bubb, S.L.; Young, P.; Raymont, D. Evaluation of light-weight alsi10mg periodic cellular lattice structures fabricated via direct metal laser sintering. J. Mater. Process. Technol. 2014, 214, 856–864. [Google Scholar] [CrossRef]
- Yan, C.; Hao, L.; Hussein, A.; Young, P.; Huang, J.; Zhu, W. Microstructure and mechanical properties of aluminium alloy cellular lattice structures manufactured by direct metal laser sintering. Mater. Sci. Eng. A-Struct. 2015, 628, 238–246. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Robb, R.A. Schwarz meets schwann: Design and fabrication of biomorphic and durataxic tissue engineering scaffolds. Med. Image Anal. 2006, 10, 693–712. [Google Scholar] [CrossRef] [PubMed]
- Brenne, F.; Niendorf, T.; Maier, H.J. Additively manufactured cellular structures: Impact of microstructure and local strains on the monotonic and cyclic behavior under uniaxial and bending load. J. Mater. Process. Technol. 2013, 213, 1558–1564. [Google Scholar] [CrossRef]
- Gümrük, R.; Mines, R.A.W.; Karadeniz, S. Static mechanical behaviours of stainless steel micro-lattice structures under different loading conditions. Mater. Sci. Eng. A-Struct. 2013, 586, 392–406. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Bertoldi, K.; Gabbrielli, R.; Velders, A.H.; Feijen, J.; Grijpma, D.W. Mathematically defined tissue engineering scaffold architectures prepared by stereolithography. Biomaterials 2010, 31, 6909–6916. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Tian, Y.; Zhang, D. Novel real function based method to construct heterogeneous porous scaffolds and additive manufacturing for use in medical engineering. Med. Eng. Phys. 2015, 37, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Du, C.; Wang, S.; Yang, Y.; Zhang, C. Mathematically defined gradient porous materials. Mater. Lett. 2016, 173, 136–140. [Google Scholar] [CrossRef]
- Yoo, D.J. Porous scaffold design using the distance field and triply periodic minimal surface models. Biomaterials 2011, 32, 7741–7754. [Google Scholar] [CrossRef] [PubMed]
- Arabnejad, S.; Burnett Johnston, R.; Pura, J.A.; Singh, B.; Tanzer, M.; Pasini, D. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints. Acta Biomater. 2016, 30, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Egan, P.F.; Gonella, V.C.; Engensperger, M.; Ferguson, S.J.; Shea, K. Computationally designed lattices with tuned properties for tissue engineering using 3d printing. PLoS ONE 2017, 12, e0182902. [Google Scholar] [CrossRef] [PubMed]
- Giannitelli, S.M.; Accoto, D.; Trombetta, M.; Rainer, A. Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomater. 2014, 10, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Fang, G.; Zhou, J. Additively manufactured scaffolds for bone tissue engineering and the prediction of their mechanical behavior: A review. Materials 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Humbeeck, J.V.; Kruth, J.-P. A study of the micro structural evolution during selective laser melting of ti-6al-4v. Acta Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Smith, M.; Guan, Z.; Cantwell, W.J. Finite element modelling of the compressive response of lattice structures manufactured using the selective laser melting technique. Int. J. Mech. Sci. 2013, 67, 28–41. [Google Scholar] [CrossRef]
- Caiazzo, F.; Campanelli, S.L.; Cardaropoli, F.; Contuzzi, N.; Sergi, V.; Ludovico, A.D. Manufacturing and characterization of similar to foam steel components processed through selective laser melting. Int. J. Adv. Manuf. Technol. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Guan, K.; Wang, Z.; Gao, M.; Li, X.; Zeng, X. Effects of processing parameters on tensile properties of selective laser melted 304 stainless steel. Mater. Des. 2013, 50, 581–586. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: New York, NY, USA, 1997. [Google Scholar]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, K.; Nakajima, H. Metallic scaffolds for bone regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Rohlmann, A.; Zilch, H.; Bergmann, G.; Kölbel, R. Material properties of femoral cancellous bone in axial loading-part i: Time independent properties. Arch. Orthop. Traum. Surg. 1980, 97, 95–102. [Google Scholar] [CrossRef]
- Goldstein, S.A.; Wilson, D.L.; Sonstegard, D.A.; Matthews, L.S. The mechanical properties of human tibial trabecular bone as a function of metaphyseal location. J. Biomech. 1983, 16, 965–969. [Google Scholar] [CrossRef]
- Goldstein, S.A. The mechanical properties of trabecular bone: Dependence on anatomic location and function. J. Biomech. 1987, 20, 1055–1061. [Google Scholar] [CrossRef]
- Maskery, I.; Aboulkhair, N.T.; Aremu, A.O.; Tuck, C.J.; Ashcroft, I.A.; Wildman, R.D.; Hague, R.J.M. A mechanical property evaluation of graded density al-si10-mg lattice structures manufactured by selective laser melting. Mater. Sci. Eng. A-Struct. 2016, 670, 264–274. [Google Scholar] [CrossRef]
- Amirkhani, S.; Bagheri, R.; Yazdi, A.Z. Effect of pore geometry and loading direction on deformation mechanism of rapid prototyped scaffolds. Acta Mater. 2012, 60, 2778–2789. [Google Scholar] [CrossRef]
- Deshpande, V.S.; Ashby, M.F.; Fleck, N.A. Foam topology: Bending versus stretching dominated architectures. Acta Mater. 2001, 49, 1035–1040. [Google Scholar] [CrossRef]

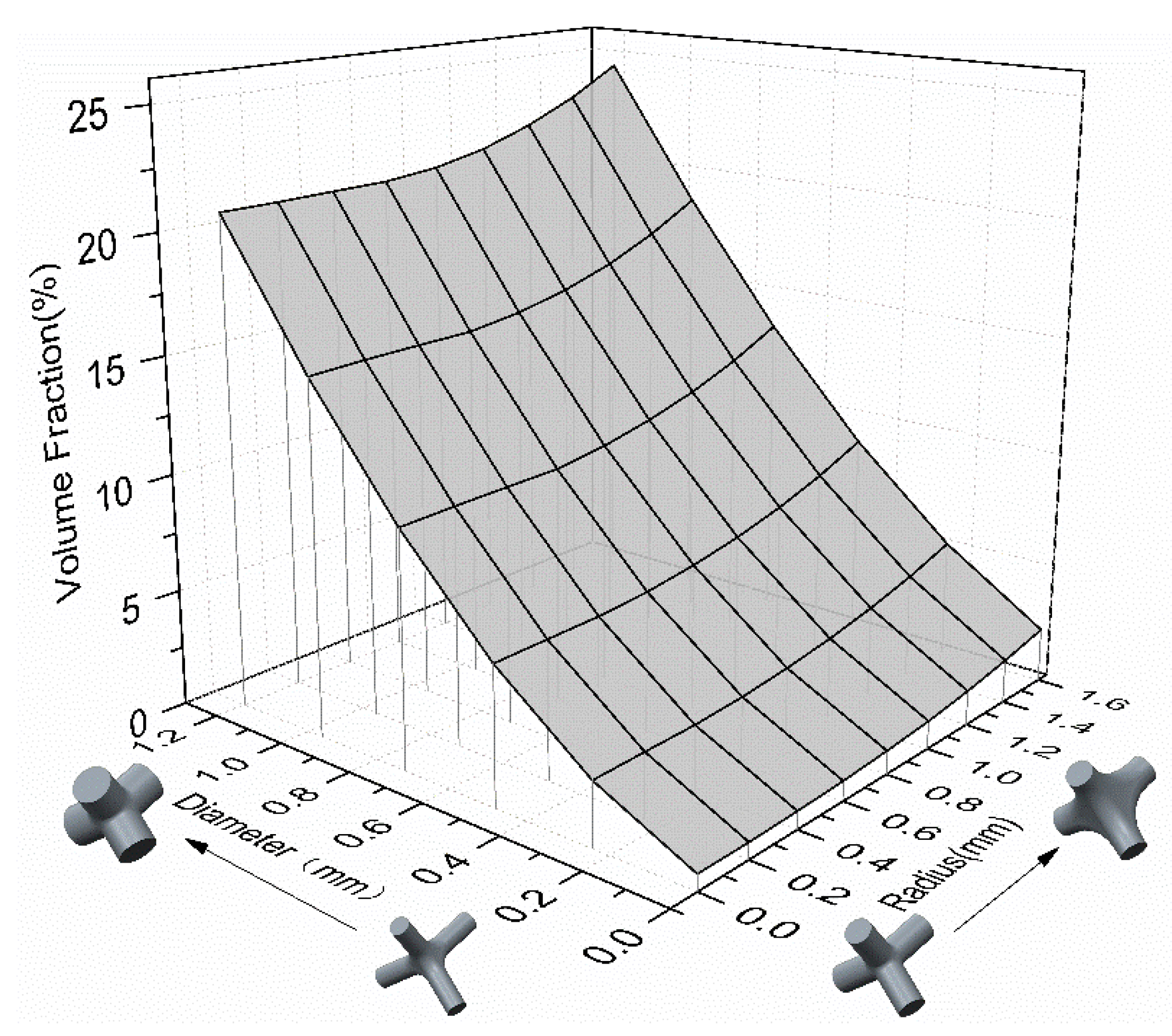

| Parameters | C (mm) | D (mm) | R (mm) | L (mm) | (mm) |
|---|---|---|---|---|---|
| CAD size | 5.5 | 0.2–1.0 (interval 0.2) | 0–1.6 (interval 0.2) |
| Ti6Al4V | Ti | Al | V | O | N | C | H | Fe |
|---|---|---|---|---|---|---|---|---|
| Wt.% | (balance) | 5.5–6.75 | 3.5–4.5 | <0.2 | <0.05 | <0.08 | <0.015 | <3 |
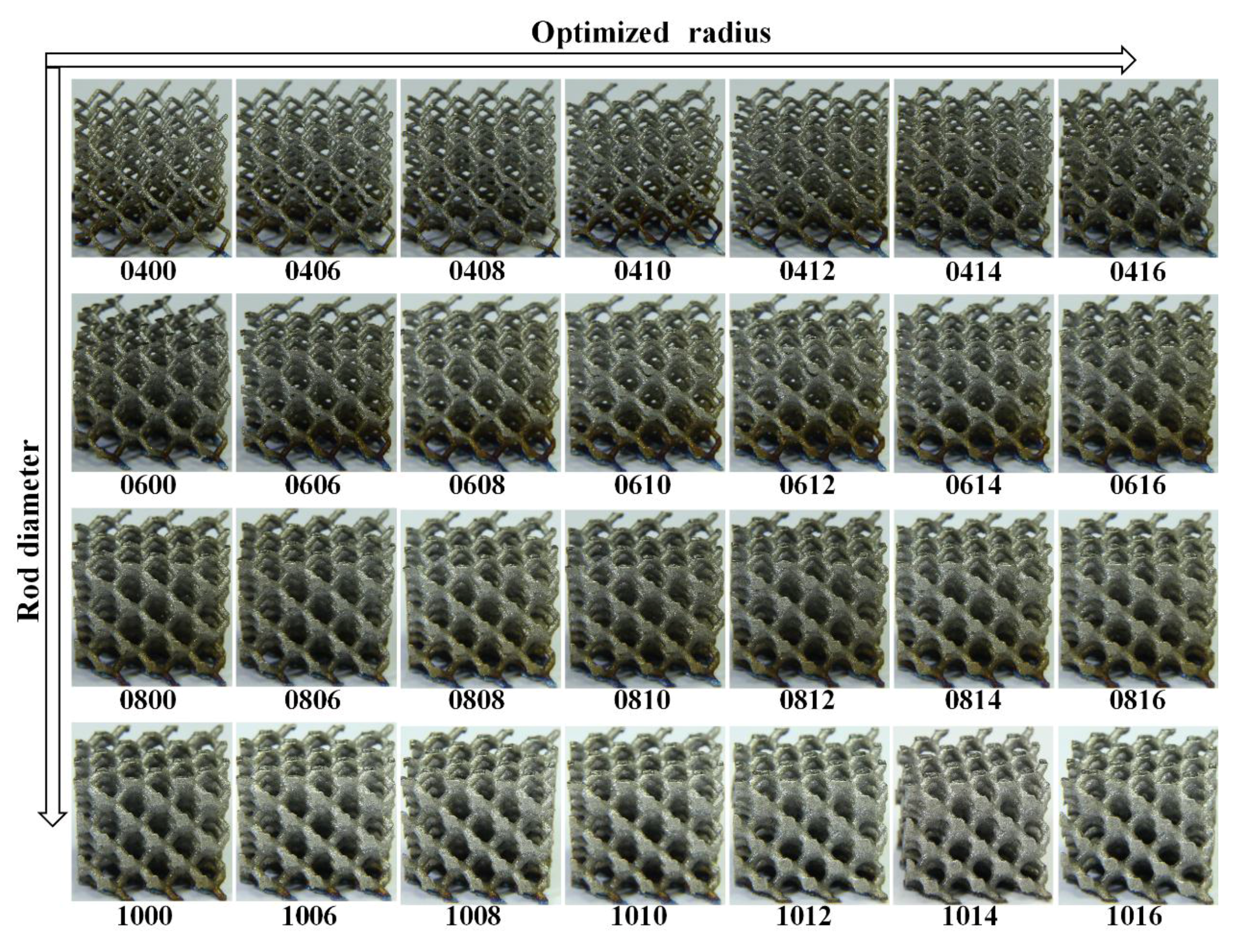
| Samples | Rod Diameter, D (mm) | Optimized-Radius, R (mm) | Volume Fraction, Vf (%) | Modulus, (MPa) | Ultimate Stress, (MPa) |
|---|---|---|---|---|---|
| 0200 | 0.2 | 0 | 1.28 | -- | -- |
| 0400 | 0.4 | 0 | 2.69 | 34.12 | 2.07 |
| 0406 | 0.6 | 2.88 | 45.75 | 2.72 | |
| 0408 | 0.8 | 3.11 | 53.17 | 3.12 | |
| 0410 | 1 | 3.43 | 59.86 | 3.53 | |
| 0412 | 1.2 | 3.86 | 89.68 | 5.00 | |
| 0414 | 1.4 | 4.41 | 103.97 | 5.56 | |
| 0416XY | 1.4 | 4.41 | 122.34 | 6.03 | |
| 0416 | 1.6 | 5.08 | 136.66 | 6.50 | |
| 0600 | 0.6 | 0 | 5.84 | 173.80 | 8.27 |
| 0606 | 0.6 | 6.02 | 162.97 | 8.74 | |
| 0608 | 0.8 | 6.29 | 184.73 | 10.07 | |
| 0610 | 1 | 6.7 | 221.64 | 11.56 | |
| 0612 | 1.2 | 7.24 | 265.25 | 13.84 | |
| 0614 | 1.4 | 7.94 | 326.79 | 15.86 | |
| 0614XY | 1.4 | 7.94 | 370.69 | 18.58 | |
| 0616 | 1.6 | 8.79 | 433.83 | 20.30 | |
| 0800 | 0.8 | 0 | 10 | 402.75 | 18.92 |
| 0806 | 0.6 | 10.14 | 418.52 | 20.39 | |
| 0808 | 0.8 | 10.43 | 412.53 | 24.87 | |
| 0810 | 1 | 10.88 | 606.42 | 27.02 | |
| 0812 | 1.2 | 11.51 | 561.95 | 33.73 | |
| 0814 | 1.4 | 12.33 | 712.83 | 36.56 | |
| 0814XY | 1.4 | 12.33 | 748.58 | 38.07 | |
| 0816 | 1.6 | 13.33 | 817.10 | 42.52 | |
| 1000 | 1.0 | 0 | 15.04 | 860.01 | 39.38 |
| 1006 | 0.6 | 15.13 | 840.26 | 40.16 | |
| 1008 | 0.8 | 15.4 | 857.61 | 47.29 | |
| 1010 | 1 | 15.87 | 955.56 | 53.12 | |
| 1012 | 1.2 | 16.55 | 1099.06 | 61.45 | |
| 1014 | 1.4 | 17.45 | 1245.33 | 64.59 | |
| 1014XY | 1.4 | 17.45 | 1260.84 | 67.79 | |
| 1016 | 1.6 | 18.58 | 1402.69 | 77.73 |
| Material | E (MPa) | Direction and Type of Load | (MPa) | Reference | |
|---|---|---|---|---|---|
| Cortical bone | Mid-femoral | Mean:17(×103) | Longitudinal | Mean: 193 | [37] |
| Mean:11.5(×103) | Transverse | Mean: 33 | |||
| -- | 14.1–27.6(×103) | Longitudinal | 219 ± 26 | [38] | |
| Transverse | 153 ± 20 | ||||
| Trabecular bone | Proximal femoral | Mean:441 | Longitudinal | Mean: 6.8 | [37] |
| -- | 100–400 | -- | 1.5–9.3 | [38] | |
| Femoral bone | 65.7–873 (mean: 315) | -- | 0.8–12.7 (mean: 6) | [39] | |
| Proximal tibia | 4–430 | Longitudinal | 1–13 | [40] | |
| Num | (%) | |||||
|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 30 | 40 | |
| 0600 |  |  |  |  |  |  |
| 0610 |  |  |  |  |  |  |
| 0616 |  |  |  |  |  |  |
| Structures | Optimization | Volume Fraction, (%) | Deformation Modes | Deformation Pattern | |
|---|---|---|---|---|---|
 | Non | 5.84 |  | Layer-by-layer (cell row) | stretch dominated |
 | Non | 22 | 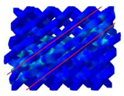 | 45° shear | bending dominated |
 | Surface optimization at nodes | 8.79 |  | 45° shear | bending dominated |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Zhang, D.Z.; Zhang, P.; Zhao, M.; Jafar, S. Mechanical Properties of Optimized Diamond Lattice Structure for Bone Scaffolds Fabricated via Selective Laser Melting. Materials 2018, 11, 374. https://doi.org/10.3390/ma11030374
Liu F, Zhang DZ, Zhang P, Zhao M, Jafar S. Mechanical Properties of Optimized Diamond Lattice Structure for Bone Scaffolds Fabricated via Selective Laser Melting. Materials. 2018; 11(3):374. https://doi.org/10.3390/ma11030374
Chicago/Turabian StyleLiu, Fei, David Z. Zhang, Peng Zhang, Miao Zhao, and Salman Jafar. 2018. "Mechanical Properties of Optimized Diamond Lattice Structure for Bone Scaffolds Fabricated via Selective Laser Melting" Materials 11, no. 3: 374. https://doi.org/10.3390/ma11030374
APA StyleLiu, F., Zhang, D. Z., Zhang, P., Zhao, M., & Jafar, S. (2018). Mechanical Properties of Optimized Diamond Lattice Structure for Bone Scaffolds Fabricated via Selective Laser Melting. Materials, 11(3), 374. https://doi.org/10.3390/ma11030374






