Synthesis and Performance of Iron Oxide-Coated Ceramsite in a Biotrickling Filter for Nitric Oxide Removal under Thermophilic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbes
2.2. Growth Medium
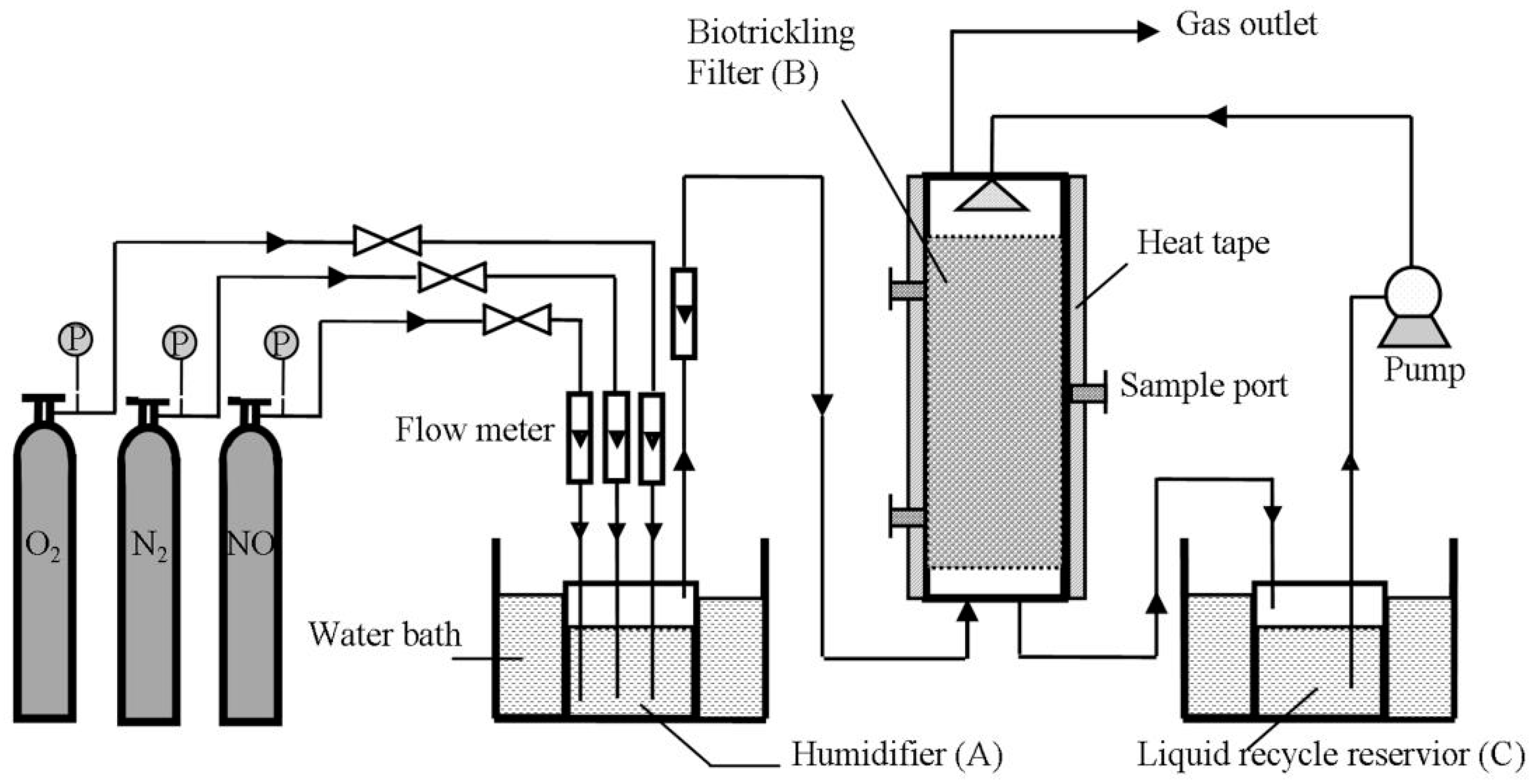
2.3. Biotrickling Filter Setup and Operation
2.4. Analytical Methods
2.5. Modified Ceramic
2.5.1. Ceramic Modification
2.5.2. Evaluation Parameters of the Modified Ceramic
Surface pH
Porosity
Isoelectric Point
Coating Content
3. Results and Discussion
3.1. Modification Conditions
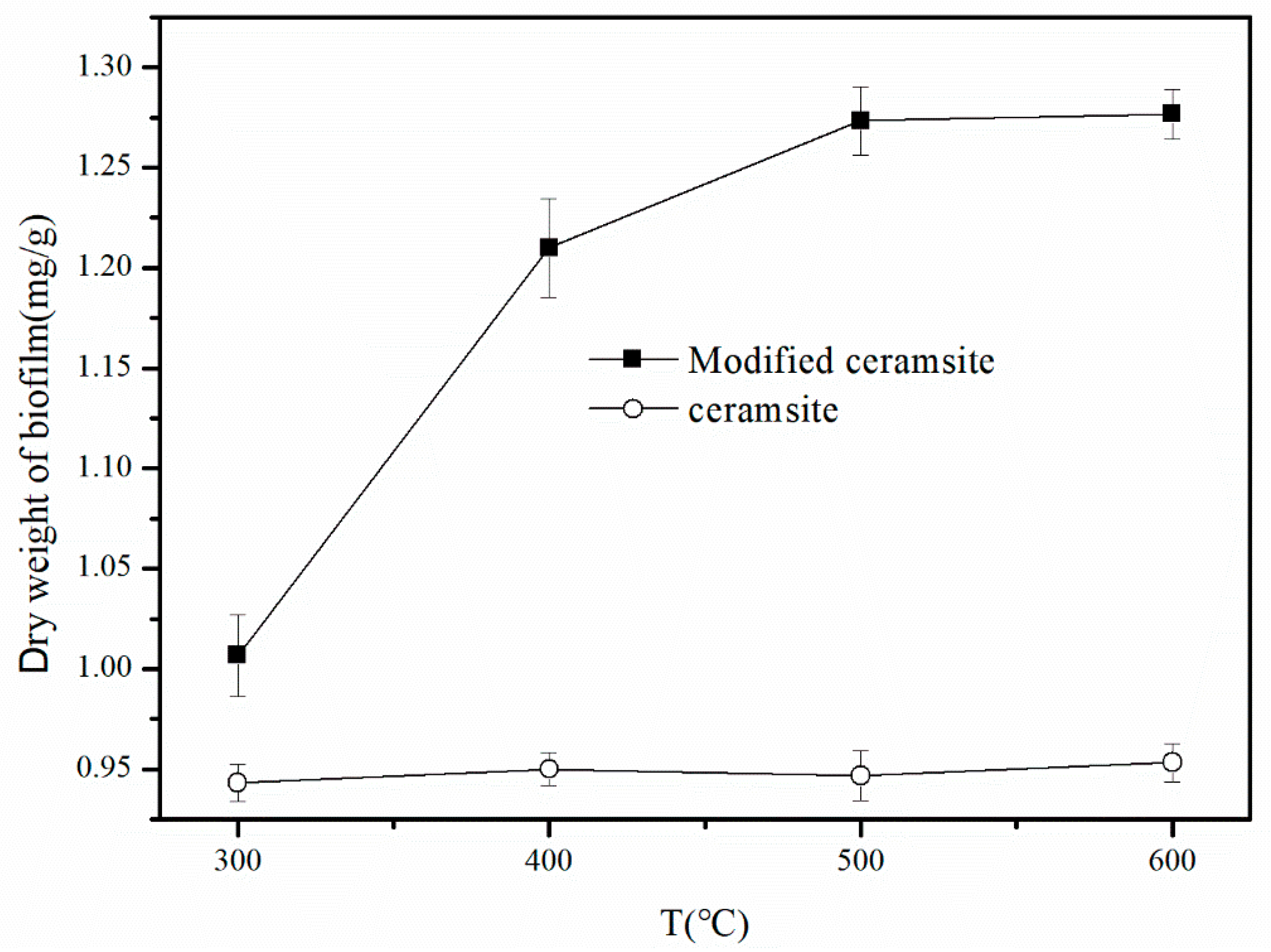
3.1.1. Calcination Temperature
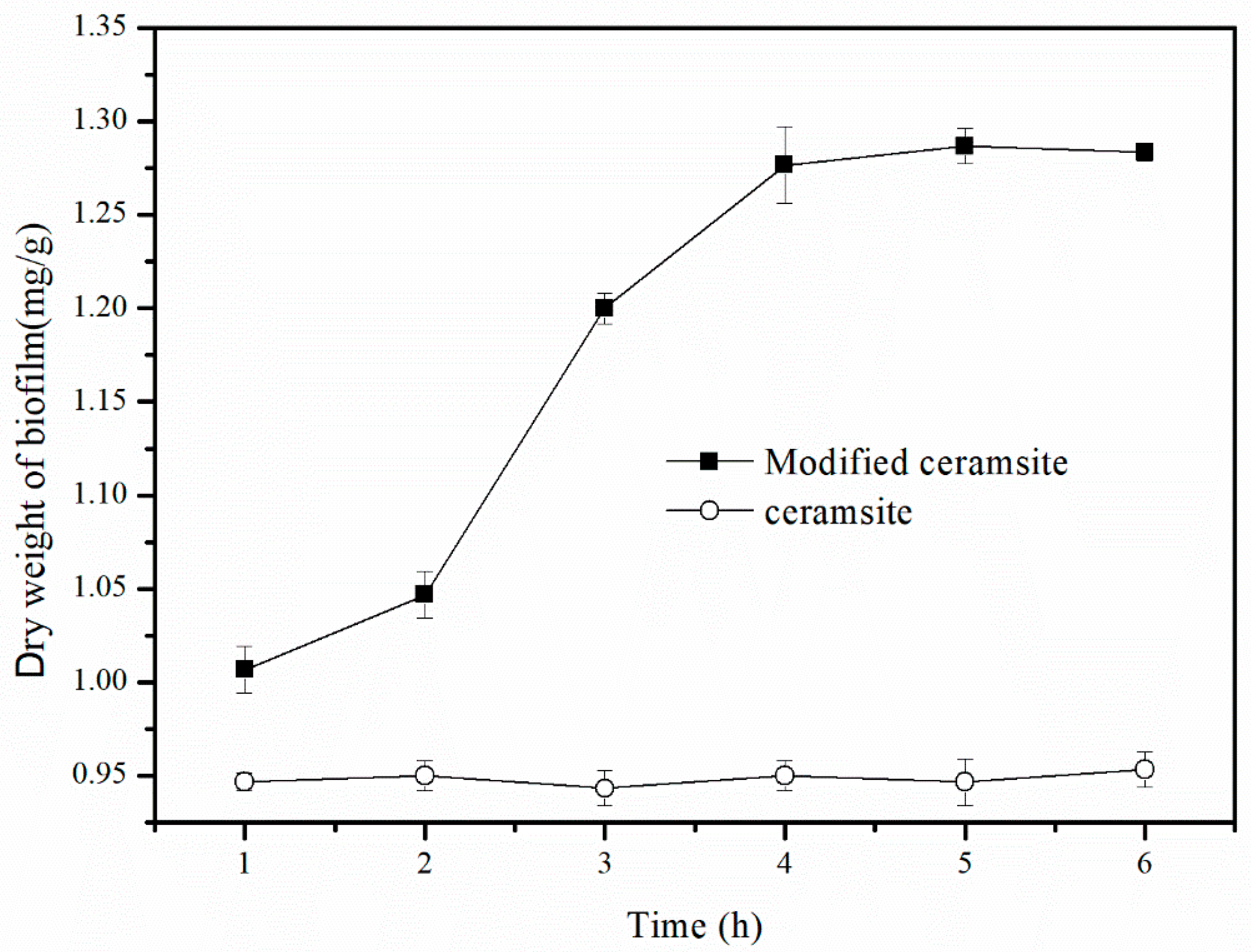
3.1.2. Calcination Time
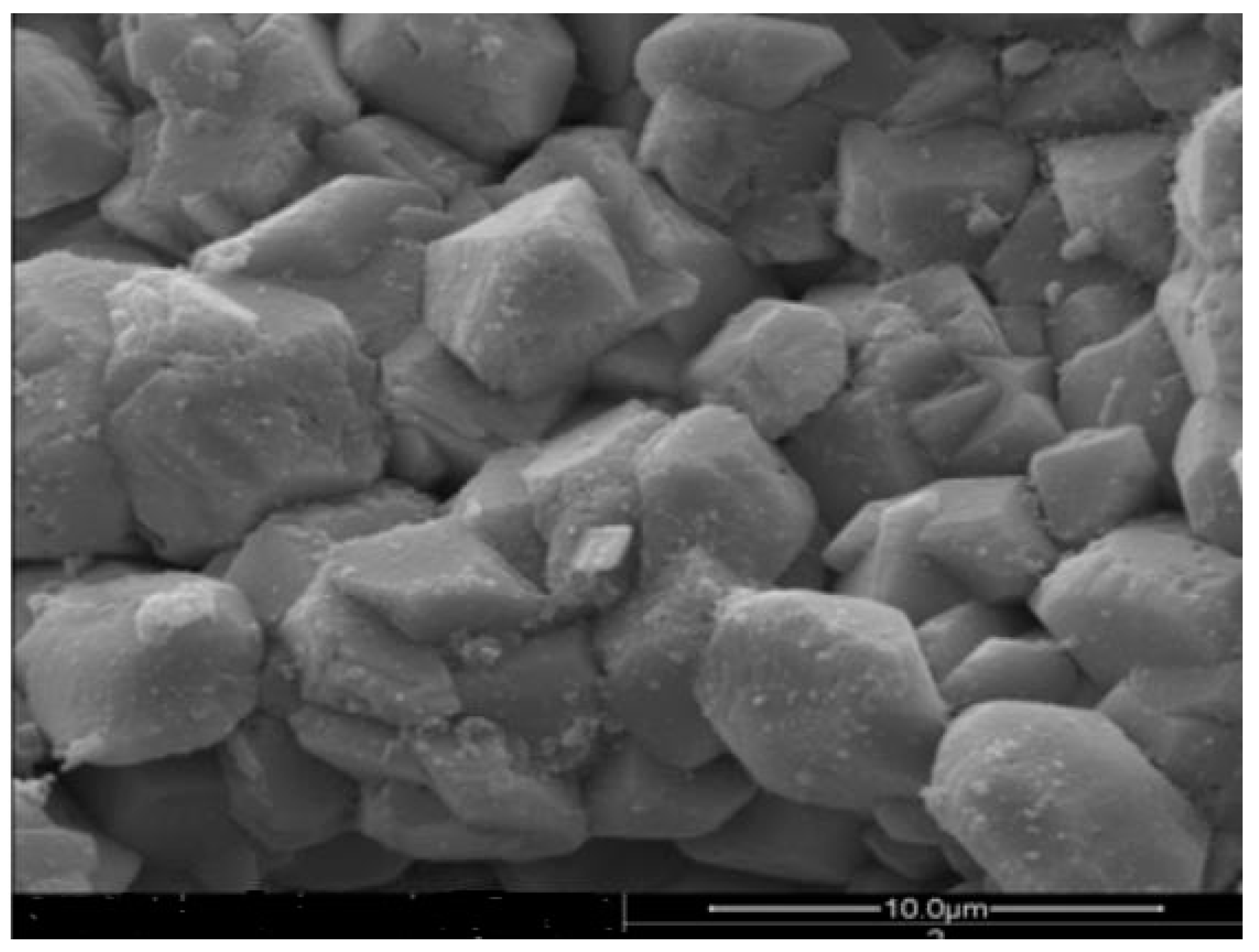
3.2. Properties of Modified Ceramics
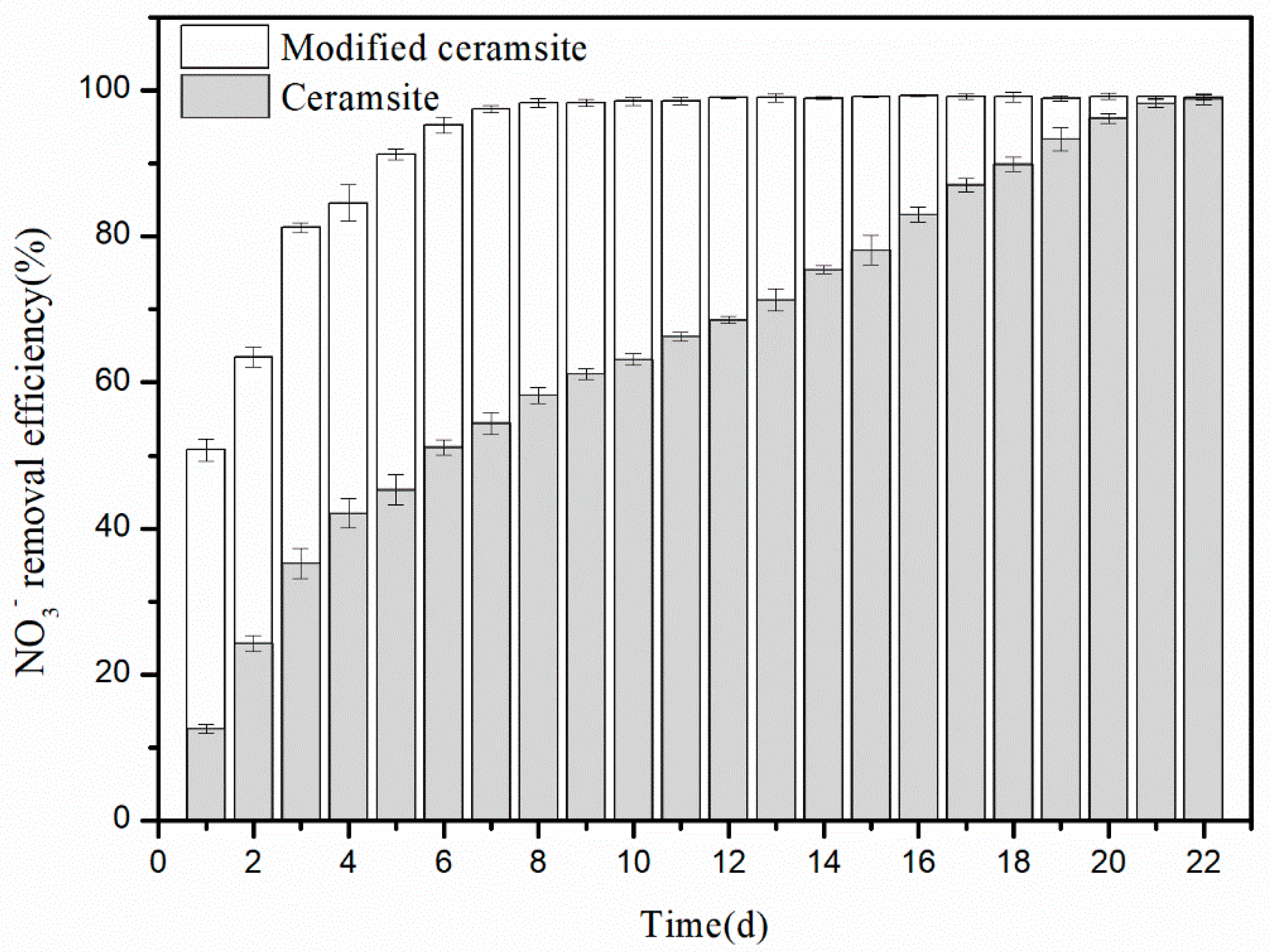
3.3. Start-up Performance of the Biotrickling Filter
3.4. Performance of the Biotrickling Filter
3.5. Analysis of Surface Stability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, Y.; Hao, R.; Yuan, B.; Jiang, J. Simultaneous removal of SO2, NO and Hg-0 through an integrative process utilizing a cost-effective complex oxidant. J. Hazard. Mater. 2016, 301, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Li, C.M.; Yu, C.; Tran, T.; Guo, F.; Yang, Y.Q.; Yu, J.; Xu, G.W. Synthesis, characterization and activity evaluation of Cu-based catalysts derived from layered double hydroxides (LDHs) for DeNO(x) reaction. Chem. Eng. J. 2017, 330, 1082–1090. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wang, Y.; Liu, Z.Y.; Wang, Q. Oxidation Removal of Nitric Oxide from Flue Gas Using UV Photolysis of Aqueous Hypochlorite. Environ. Sci. Technol. 2017, 51, 11950–11959. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pan, D.P.; Zhang, R.; Yang, L.J.; Peng, Z.M.; Yang, B. Reducing fine particle emissions by heterogeneous vapor condensation after wet desulfurization process. J. Chem. Technol. Biotechnol. 2017, 92, 2342–2350. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chou, M.S. Reduction of nitrogen dioxide from etching vent gases by scrubbing with caustic sodium sulfide solution. J. Chem. Technol. Biotechnol. 2014, 89, 1850–1858. [Google Scholar] [CrossRef]
- Yang, S.L.; Pan, X.X.; Han, Z.T.; Zhao, D.S.; Liu, B.J.; Zheng, D.K.; Yan, Z.J. Removal of NOx and SO2 from simulated ship emissions using wet scrubbing based on seawater electrolysis technology. Chem. Eng. J. 2018, 331, 8–15. [Google Scholar] [CrossRef]
- Zouzelka, R.; Rathousky, J. Photocatalytic abatement of NOx pollutants in the air using commercial functional coating with porous morphology. Appl. Catal. B-Environ. 2017, 217, 466–476. [Google Scholar] [CrossRef]
- Angelo, J.; Andrade, L.; Madeira, L.M.; Mendes, A. An overview of photocatalysis phenomena applied to NOx abatement. J. Environ. Manag. 2013, 129, 522–539. [Google Scholar] [CrossRef] [PubMed]
- Boyjoo, Y.; Su, H.Q.; Liu, J.; Pareek, V.K.; Wang, S.B. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Jablonska, M.; Palkovits, R. Nitrogen oxide removal over hydrotalcite-derived mixed metal oxides. Catal. Sci. Technol. 2016, 6, 49–72. [Google Scholar] [CrossRef]
- Yen, H.W.; Ho, S.H.; Chen, C.Y.; Chang, J.S. CO2, NOx and SOx removal from flue gas via microalgae cultivation: A critical review. Biotechnol. J. 2015, 10, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Yang, K.; Zhu, L.; Jiang, Y.; Wang, H.Y.; Zhou, J.; Zhang, H.N. Aerobic denitrification: A review of important advances of the last 30 years. Biotechnol. Bioprocess. Eng. 2015, 20, 643–651. [Google Scholar] [CrossRef]
- Yang, L.; Ren, Y.X.; Zhao, S.Q.; Liang, X.; Wang, J.P. Isolation and characterization of three heterotrophic nitrifying-aerobic denitrifying bacteria from a sequencing batch reactor. Ann. Microbiol. 2016, 66, 737–747. [Google Scholar] [CrossRef]
- De Morais, M.G.; Vieira Costa, J.A. Bioprocesses for removal of carbon dioxide and nitrogen oxide by microalgae for the utilization of gas generated during coal burning. Quim. Nova 2008, 31, 1038–1042. [Google Scholar]
- Li, H.; Huang, S.B.; Wei, Z.D.; Chen, P.F.; Zhang, Y.Q. Performance of a new suspended filler biofilter for removal of nitrogen oxides under thermophilic conditions and microbial community analysis. Sci. Total Environ. 2016, 562, 533–541. [Google Scholar]
- Wang, H.L.; Cheng, L. Research on the Oxygenation Capacity and Energy Efficiency of Aeration Tank Impacted by Cellular Standpipe Filler; Beijing Institute of Technology: Beijing, China, 2007; pp. 1011–1014. [Google Scholar]
- Zhao, B.; Zhang, J.; Jiang, Y. Presence of bacteria in aqueous solution influences virus adsorption on nanoparticles. Environ. Sci. Pollut. Res. 2013, 20, 8245–8254. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Peng, L.; Guo, R.; Miao, D.; Shang, S.; Xu, J.; Li, A. Preparation and characterization of Fe2O3 coating on quartz fabric by electron beam evaporation. Ceram. Int. 2016, 42, 19386–19392. [Google Scholar] [CrossRef]
- Lu, M.; Xia, G.-H.; Liao, R.-H.; Zhao, X.-D. Preparation and modification of porous lightweight ceramsite and its performance investigation. Desalination Water. Treat. 2013, 51, 4651–4657. [Google Scholar] [CrossRef]
- Chen, J.N.; Truesdail, S.; Lu, F.H.; Zhan, G.G.; Belvin, C.; Koopman, B.; Farrah, S.; Shah, D. Long-term evaluation of aluminum hydroxide-coated sand for removal of bacteria from wastewater. Water Res. 1998, 32, 2171–2179. [Google Scholar] [CrossRef]
- Cho, E.; Galera, M.M.; Lorenzana, A.; Chung, W.-J. Ethylbenzene, o-Xylene, and BTEX Removal by Sphingomonas sp. D3K1 in Rock Wool-Compost Biofilters. Environ. Eng. Sci. 2009, 26, 45–52. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, S.; Wang, G.; Du, M.A. Performances and nitrification properties of biological aerated filters with zeolite, ceramic particle and carbonate media. Bioresour. Technol. 2010, 101, 7245–7251. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Wang, H.; Yang, K. Nitrate and COD removal in an upflow biofilter under an aerobic atmosphere. Bioresour. Technol. 2014, 158, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, N.; Luo, R.; Zhang, Y.; Zhou, Q.; Chen, X. Experimental study on thermal effect on infiltration mechanisms of glycerol into ZSM-5 zeolite under cyclic loadings. J. Phys. D-Appl. Phys. 2016, 49. [Google Scholar] [CrossRef]
- Qiu, H.; Liang, C.; Yu, J.; Zhang, Q.; Song, M.; Chen, F. Preferable phosphate sequestration by nano-La(III) (hydr)oxides modified wheat straw with excellent properties in regeneration. Chem. Eng. J. 2017, 315, 345–354. [Google Scholar] [CrossRef]
- Guzek, Z. The effect of modifying agents on the structure and electromechanical properties of steatite materials. Szklo I Ceram. 1972, 23, 137–144. [Google Scholar]
- Zhou, H.; Feng, S.; Dongdong, C.; Chun, Z.; Xuehong, G.; Nanping, X. Improvement of hydrogen-separating performance by on-stream catalytic cracking of silane over hollow fiber MFI zeolite membrane. Int. J. Hydrogen Energy 2013, 38, 8409–8414. [Google Scholar]
- Bao, T.; Chen, T.H.; Tan, J.; Wille, M.L.; Zhu, D.; Chen, D.; Xi, Y.F. Synthesis and performance of iron oxide-based porous ceramsite in a biological aerated filter for the simultaneous removal of nitrogen and phosphorus from domestic wastewater. Sep. Purif. Technol. 2016, 167, 154–162. [Google Scholar] [CrossRef]
- Bao, T.; Chen, T.H.; Qing, C.S.; Xie, J.J.; Frost, R.L. Development and application of Palygorskite porous ceramsite in a biological aerated filter (BAF). Desalination Water. Treat. 2016, 57, 1790–1803. [Google Scholar] [CrossRef]
- Liu, H.B.; Chen, T.H.; Frost, R.L. An overview of the role of goethite surfaces in the environment. Chemosphere 2014, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Huang, S.; Yang, Y.; Jiang, R. Experimental and modeling study on nitric oxide removal in a biotrickling filter using Chelatococcus daeguensis under thermophilic condition. Bioresour. Technol. 2012, 125, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Samuelsson, R.; Burvall, J.; Jirjis, R. Comparison of different methods for the determination of moisture content in biomass. Biomass Bioenergy 2006, 30, 929–934. [Google Scholar] [CrossRef]
- Kostura, B.; Kulveitova, H.; Lesko, J. Blast furnace slags as sorbents of phosphate from water solutions. Water Res. 2005, 39, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Huang, S.; Chow, A.T.; Yang, J. Nitric oxide removal from flue gas with a biotrickling filter using Pseudomonas putida. J. Hazard. Mater. 2009, 164, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Delhomenie, M.C.; Heitz, M. Biofiltration of air: A review. Crit. Rev. Biotechnol. 2005, 25, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Raj, I.; Vaidya, A.N.; Pandey, R.A.; Bansiwal, A.; Deshmukh, S.; Purohit, H.J. Recent advancements in the mitigation of obnoxious nitrogenous gases. J. Environ. Manag. 2018, 205, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Ragazzi, M.; Rada, E.C.; Torretta, V. Air pollution control through biotrickling filters: A review considering operational aspects and expected performance. Crit. Rev. Biotechnol. 2016, 36, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, M.; Zhang, L.; Zhao, J.; Xia, Y.; Liu, N.; Li, S.; Zhang, S. Enhanced NOx removal performance and microbial community shifts in an oxygen-resistance chemical absorption-biological reduction integrated system. Chem. Eng. J. 2016, 290, 185–192. [Google Scholar] [CrossRef]
- Lu, B.-H.; Jiang, Y.; Cai, L.-L.; Liu, N.; Zhang, S.-H.; Li, W. Enhanced biological removal of NOx from flue gas in a biofilter by Fe(II)Cit/Fe(II)EDTA absorption. Bioresour. Technol. 2011, 102, 7707–7712. [Google Scholar] [CrossRef] [PubMed]
- Okuno, K.; Hirai, M.; Sugiyama, M.; Haruta, K.; Shoda, M. Microbial removal of nitrogen monoxide (NO) under aerobic conditions. Biotechnol. Lett. 2000, 22, 77–79. [Google Scholar] [CrossRef]
- Zhou, Z.; Jing, G.; Zhou, Q. Enhanced NOx removal from flue gas by an integrated process of chemical absorption coupled with two-stage biological reduction using immobilized microorganisms. Process Saf. Environ. Prot. 2013, 91, 325–332. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, S.; Liang, W.; Zhang, Y.; Huang, H.; Xu, F. Microbial removal of NOx at high temperature by a novel aerobic strain Chelatococcus daeguensis TAD1 in a biotrickling filter. J. Hazard. Mater. 2012, 203–204, 326–332. [Google Scholar] [CrossRef] [PubMed]


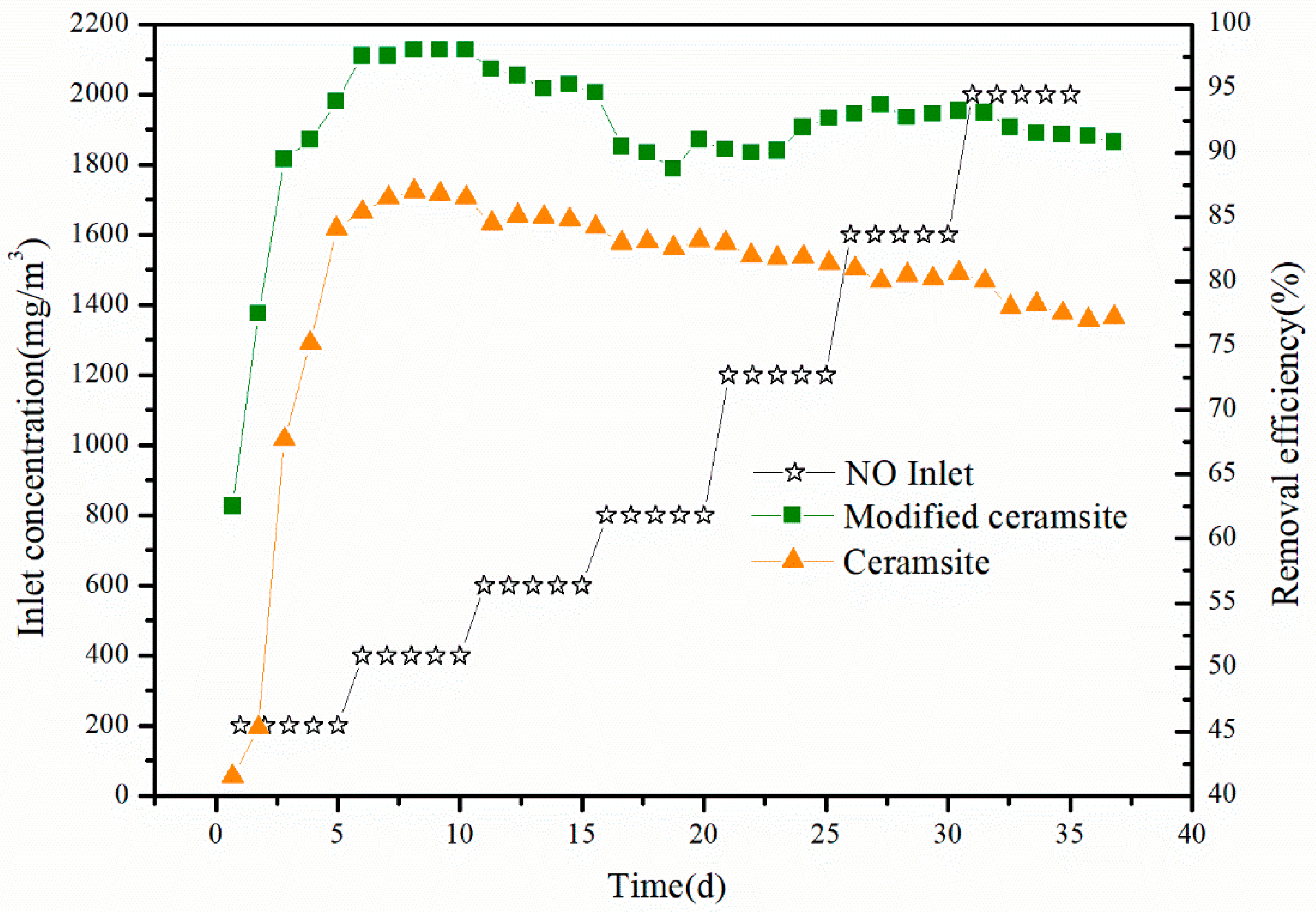
| Packing Material | Start Up (d) | NO3−-N Concentration (mg/L) | pH | Operation (d) | NO Inlet (g/m3) | EBRT (s) | T (°C) |
|---|---|---|---|---|---|---|---|
| Ceramsite | 22 | 136–145 | 7–7.5 | 35 | 0.2–2 | 88 | 50 ± 1 |
| Modified ceramsite | 8 | 136–145 | 7–7.5 | 35 | 0.2–2 | 88 | 50 ± 1 |
| Shape | Diameter (mm) | Coating Contents (mg/g) | Density (g/m3) | Surface Area (m2/m3) | Porosity (%) | PI | Surface pH | |
|---|---|---|---|---|---|---|---|---|
| Before | sphere | 3–5 | 0 | 1.98 | 398 | 48 | 0.7–3 | 6.95 |
| After | sphere | 3–5 | 42.1 | 2.36 | 398 | 55 | 8.5 | 3.46 |
| Filler | Temperature (°C) | O2 (%) | NO Inlet (mg/m3) | EBRT (min) | Inlet Loading (g/(m3·h)) | RE (%) | Reference |
|---|---|---|---|---|---|---|---|
| modified PVC | 50 ± 0.5 | 1–3 | 315 | 1 | 18.75 | 75 | [39] |
| soil | 20–37 | - | 335 | - | - | 60 | [40] |
| ceramics | 50 ± 0.5 | 2–20 | 800 | 1.8 | 26.67 | 80–92 | [41] |
| ceramics | 30 ± 0.5 | 2–20 | 800 | 1 | 48 | 63 | [42] |
| woven fiber | 50 ± 1 | 8 | 2000 | 0.7 | 163.6 | 89.8 | [34] |
| modified ceramsite | 50 ± 1 | 8 | 2000 | 1.5 | 80 | 91.1 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Guo, Z.; Wu, D.; Fan, J.; Huang, S.; Zhou, S. Synthesis and Performance of Iron Oxide-Coated Ceramsite in a Biotrickling Filter for Nitric Oxide Removal under Thermophilic Conditions. Materials 2018, 11, 359. https://doi.org/10.3390/ma11030359
Li H, Guo Z, Wu D, Fan J, Huang S, Zhou S. Synthesis and Performance of Iron Oxide-Coated Ceramsite in a Biotrickling Filter for Nitric Oxide Removal under Thermophilic Conditions. Materials. 2018; 11(3):359. https://doi.org/10.3390/ma11030359
Chicago/Turabian StyleLi, Han, Ze Guo, Dafu Wu, Jing Fan, Shaobin Huang, and Shaofeng Zhou. 2018. "Synthesis and Performance of Iron Oxide-Coated Ceramsite in a Biotrickling Filter for Nitric Oxide Removal under Thermophilic Conditions" Materials 11, no. 3: 359. https://doi.org/10.3390/ma11030359
APA StyleLi, H., Guo, Z., Wu, D., Fan, J., Huang, S., & Zhou, S. (2018). Synthesis and Performance of Iron Oxide-Coated Ceramsite in a Biotrickling Filter for Nitric Oxide Removal under Thermophilic Conditions. Materials, 11(3), 359. https://doi.org/10.3390/ma11030359




